Abstract
Once-weekly carfilzomib at 56 mg/m2 plus immunomodulatory drugs and dexamethasone has shown efficacy and tolerability treating early relapsed/refractory multiple myeloma (RRMM). The phase 2 SELECT study (NCT04191616) evaluated efficacy/safety of weekly carfilzomib, pomalidomide, and dexamethasone (KPd) in early RRMM patients refractory to lenalidomide. All 52 treated patients were refractory to prior treatment, and 19 (37%) were triple-class refractory. Overall response rate (ORR; primary endpoint) was 58% (35% ≥ very good partial response, 6% ≥ complete response); median response duration was 20.3 months. Minimal residual disease negativity (10−5) was achieved in 10% of patients. Median progression-free survival was 11.1 months; median overall survival was 18.8 months. Adverse events (AEs) were consistent with the known safety profile including grade ≥3 treatment-emergent AEs reported in 67% of patients. Although the primary endpoint of ORR was not met, KPd showed meaningful clinical benefits in lenalidomide-refractory RRMM patients, including those who were daratumumab-refractory and/or triple-class refractory.
Trial registration:
Introduction
Significant advances in the treatment of multiple myeloma (MM) over the past two decades have substantially improved patient survival outcomes [Citation1]. Treatment strategies for first-line therapy have expanded to include multi-drug regimens containing some combination of proteasome inhibitor (PI), lenalidomide, anti-CD38 monoclonal antibody (mAb), and dexamethasone [Citation2–4]. Many patients receive multi-agent induction and extended lenalidomide-based maintenance until disease progression. At relapse, it is probable for a patient to be refractory to up to 3 of these drugs as early as the second line of therapy [Citation1]. Refractoriness to bortezomib, lenalidomide, and/or an anti-CD38 mAb has been shown to correspond with worse outcomes, and increased use of these drugs in the frontline is leading to a growing need for effective regimens for early-line relapsed/refractory MM (RRMM) that include agents to which patients have not previously been exposed [Citation4–6]. Chimeric antigen receptor T-cell (CAR-T) and immune cell–redirecting therapies have recently become available, yet these have been approved for patients with ≥3 prior lines of therapy and are associated with logistical challenges and risk mitigation requirements that may limit their use to a subset of RRMM patients [Citation1,Citation7–10]. A gap therefore remains in addressing therapy for many patients with double- or triple-class refractory RRMM at first or second relapse.
Pomalidomide and carfilzomib are newer-generation immunomodulatory drugs (IMiD) and PI drugs, respectively, that have proven to be effective in patients previously exposed to both lenalidomide and bortezomib [Citation11–13]. Both drugs are approved separately in combination regimens for the treatment of RRMM. Carfilzomib has been evaluated in combination with pomalidomide and dexamethasone (KPd) in patients with RRMM at a low dose of 27 mg/m2 weekly, as well as a higher dose of 36 mg/m2 twice weekly in previous trials, resulting in overall response rates (ORRs) of 62 and 92%, respectively, and median progression-free survival (PFS) of 10.3 and 17.0 months, respectively [Citation14,Citation15]. The studies identified no emergent safety signals at the maximum tolerated doses and reported adverse event (AE) rates consistent with previously published reports. Previous studies have also demonstrated efficacy and tolerability with once-weekly dosing of carfilzomib plus lenalidomide and dexamethasone (KRd) for RRMM [Citation16]. Based on these results and the emerging unmet medical need in RRMM, the phase 2 SELECT study (NCT04191616) evaluated KPd with once-weekly carfilzomib (56 mg/m2) in patients with RRMM in first or second relapse who were refractory to lenalidomide. Here we report the results of the primary analysis of SELECT.
Materials and methods
Study design and eligible patients
SELECT (NCT04191616) is an open-label phase 2 study designed to evaluate the efficacy and safety of KPd. Eligible patients ≥ 18 years old had RRMM after receiving 1–2 prior treatment regimens and were refractory to lenalidomide with measurable disease. Patients need to have had at least a partial response (PR) to one prior line of therapy and an Eastern Cooperative Oncology Group performance status of 0–2. Patients who received prior carfilzomib were allowed if they achieved at least a PR, were not removed due to toxicity, did not relapse within 60 days from discontinuation of carfilzomib, and had at least a 6-month carfilzomib treatment-free interval from their last dose of carfilzomib. At study initiation, included patients must have completed at least two consecutive cycles of daratumumab; this criterion was removed in March 2021, ∼8 months before the last patient enrolled. Additional details are listed in the Supplemental Methods.
All patients provided written informed consent. The study protocol was approved by the institutional review boards or ethics committees of all participating institutions.
Procedures
Carfilzomib was administered intravenously over 30 ± 5 min on days 1, 8, and 15 (±2 days) of each 28-day cycle for up to 12 cycles or progression, then on days 1 and 15 for cycle 13 and beyond, and continuing until disease progression or end of study. A dose of 20 mg/m2 was administered on day 1 of cycle 1, and all subsequent doses were 56 mg/m2. Dosing rationale for carfilzomib was based on evidence from two phase 2 studies, the A.R.R.O.W. study and the CFZ013 trial (NCT02335983) [Citation17–19]. Pomalidomide was given at a dose of 4 mg/day orally on days 1–21 of each cycle. Dexamethasone was administered at least 30 min to 4 h before carfilzomib on days of carfilzomib administration. Dexamethasone was administered at a dose of 40 mg on days 1, 8, 15, and 22 of each 28-day cycle up to cycle 12 and at a dose of 20 mg on days 1 and 15 during cycle 13 onward. For patients ≥75 years old, the dose was reduced to 20 mg during cycles 1–12 and 10 mg from cycle 13 onward.
On-protocol therapy could be permanently discontinued because of an AE, pregnancy, death, loss to follow-up, noncompliance with study requirements, patient request, study termination by sponsor, investigator decision, protocol deviation, or disease progression. Dose reductions were permitted to manage AEs associated with these drugs (Supplemental Table 1).
Disease was assessed locally at study sites and by independent review committee according to details found in the Supplemental Methods. Minimal residual disease (MRD) measurement was performed by next-generation sequencing (NGS) at a sensitivity of 10−5 by a central laboratory. Bone marrow aspirates were collected to confirm a complete response (CR) or stringent CR (sCR). Patients with a suspected CR or better had bone marrow for MRD assessment at 12 months ± 4 weeks unless an MRD assessment was performed within 4 months before the scheduled assessment (e.g. coordinated with a bone marrow procedure to confirm CR).
Adverse events were collected for at least 30 (+3) days after the last dose of study treatment (safety follow-up) and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. All treatment-related AEs and serious AEs were followed up until resolution or stabilization. Long-term follow-up visits were conducted every 12 (±2) weeks after the safety follow-up visit until the end of the study. Further evaluations are detailed in the Supplemental Methods.
Outcomes
The primary endpoint was ORR, defined as achievement of PR, very good PR (VGPR), CR, or sCR according to International Myeloma Working Group (IMWG)-Uniform Response Criteria [Citation20] and was determined by an independent review committee (IRC). A key secondary endpoint was MRD-negative (MRD–) CR at 12 months at a sensitivity of 10−5 using NGS in the bone marrow. Other secondary endpoints included safety, MRD– at any time, duration of response (DOR; time from the earliest date of PR or better until the earliest date of confirmed progressive disease [PD] or death due to any cause), time to response (TTR; time from treatment initiation until the earliest date of PR or better), PFS (time from enrollment until disease progression or death due to any cause), overall survival (OS; time from enrollment until death due to any cause), and to estimate the rate of CR or better. Exploratory endpoints included MRD– CR at 10−4, 10−5, and 10−6 sensitivity by NGS, and PFS according to MRD assessment. Prespecified patient subgroups were assessed for overall response and PFS. These subgroups included number of prior lines of therapy, cytogenetic risk status, Revised Multiple Myeloma International Staging System (R-ISS) stage at baseline, prior daratumumab or bortezomib exposure, and being refractory to daratumumab or bortezomib.
Statistical analysis
Based on the results of previous studies evaluating KPd or KRd for the treatment of RRMM [Citation6,Citation14,Citation18,Citation19,Citation21,Citation22], the ORR was hypothesized to be >60%. The primary analysis required that the lower bound of the binomial 90% confidence interval (CI) of ORR exceed 60% to reject the null hypothesis. The 52 treated patients would have provided 80% power if the true ORR was >77%. An IRC monitored efficacy data. An independent data review team monitored safety data quarterly and every 6 months after the seventh review. Continuous variables were summarized by the non-missing sample size (n), mean, standard deviation, median, minimum, and maximum. Categorical variables were summarized by the number and percentage in each category. Time-to-event endpoints were summarized with Kaplan-Meier methods. Point estimates for efficacy endpoints were accompanied by 2-sided 90% CIs. SAS software (version 9.4) was used for the statistical analyses.
Results
Between 6 August 2020 and 30 November 2021, 54 patients were enrolled across seven countries in the United States and Europe. Of these, 52 patients received at least one dose of carfilzomib and were included in the primary analysis. Two patients did not receive treatment, as one had a myocardial infarction after enrollment and before starting treatment, and another was excluded due to workup resulting from a left iliac fossa lesion identified during screening. Baseline characteristics of the 52 patients are shown in . Median age at enrollment was 68 years (range, 35–87 years), and 46% of patients were male. A total of 38% of patients had high-risk disease, and 58% had undergone a prior hematopoietic cell transplantation (HCT). Most patients (n = 43; 83%) had received two prior lines of therapy. All 52 patients had prior exposure to lenalidomide (100% refractory), 45 (87%) had prior exposure to bortezomib (18 refractory; 35%), 40 (77%) had prior exposure to daratumumab (39 refractory; 75%), and 2 (4%) had prior exposure to carfilzomib (1 refractory; 2%). Overall, 39 (75%) patients were disease refractory to prior lenalidomide and daratumumab-containing regimen, including 20 (38%) patients who were double-class refractory (IMiD and anti-CD38), and 19 (37%) patients who were triple-class refractory (IMiD, PI, and anti-CD38).
Table 1. Baseline demographics.
As of the data cutoff on 30 November 2022, the study did not meet its primary endpoint, with an ORR among all patients of 58% (90% CI: 45.4, 69.3; ). Overall, 18 (35%) patients had a VGPR or better, with 3 (6%) reporting a CR. Among the 30 patients with PR or better, the median TTR was 1 month (range, 1–2 months). Median DOR was 20.3 months (90% CI, 9.2–not estimable [NE]). None of the subgroups evaluated, including patients grouped by number of prior lines of therapy, cytogenetic risk status, or R-ISS stage at baseline, showed a meaningful difference from the overall study population in ORR (Supplemental Table 2).
Table 2. Overall response.
An MRD– CR (at 10−5 sensitivity) at 12 months was achieved by two patients (4% [90% CI, 0.7–11.6]), and MRD– at any time was reported in five patients (3 in CR, 2 in VGPR; 10% [90% CI, 3.9–19.2]).
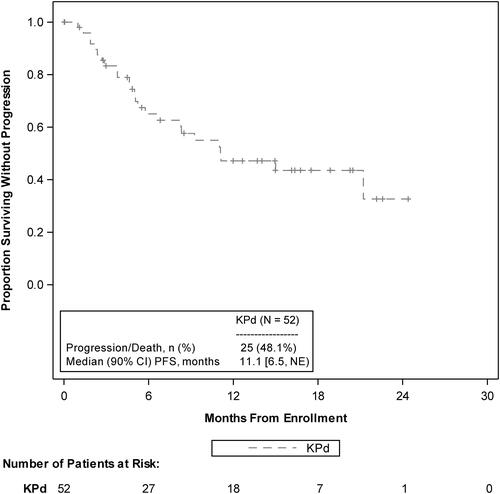
The median follow-up for PFS was 15.0 months (90% CI, 12.7–17.5), and median PFS among all patients was 11.1 months (90% CI, 6.5–NE; ). The estimated probability of PFS at 1 year was 47% (90% CI, 34.1–59.1) and at 2 years was 33% (90% CI, 15.7–50.8). Analysis of PFS by prespecified patient groups showed no meaningful differences from the total study patient population and was limited by small numbers of patients in the subgroups (Supplemental Table 2).
Figure 1. PFS KM curves as assessed by the IRC. IRC: independent review committee; KM: Kaplan-Meier; KPd: carfilzomib, pomalidomide, and dexamethasone; NE: not estimable; PFS: progression-free survival.
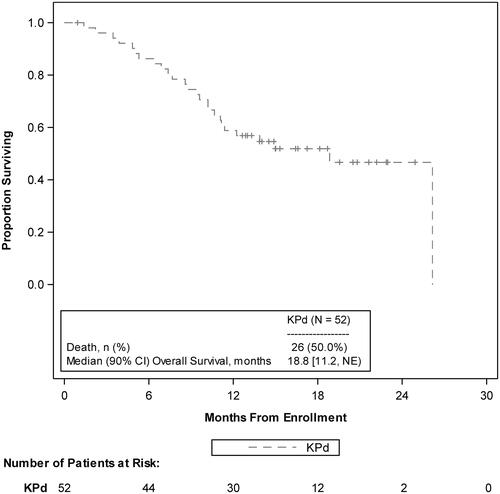
At a median follow-up of 16.6 months (90% CI, 15.0–19.6), median OS was 18.8 months (90% CI, 11.2–NE; ). The probability of OS at 12 months was 59% (90% CI, 46.6–69.1) and at 24 months was 47% (90% CI, 33.0–59.3).
Figure 2. OS KM curves. KM: Kaplan-Meier; KPd: carfilzomib, pomalidomide, and dexamethasone; NE: not estimable; OS: overall survival.
The median (range) treatment exposures were 29.8 (2.0–110.1) weeks carfilzomib, 32.7 (2.0–110.6) weeks pomalidomide, and 32.4 (2.0–110.1) weeks dexamethasone. Median (range) number of 28-day cycles received were 8.0 (1–28) cycles carfilzomib, 8.5 (1–28) cycles pomalidomide, and 8.5 (1–28) cycles dexamethasone. Median (range) relative dose intensity of carfilzomib was 90.5% (range, 29.5–102.1%), pomalidomide was 91.8% (range, 27.1–100.2%), and dexamethasone was 93.5% (range, 34.0–100.9%).
All-grade treatment-emergent AEs (TEAEs) were reported in 49 (94%) patients (Supplemental Table 3). Grade ≥3 TEAEs were reported in 35 (67%) patients. The most frequently reported grade ≥3 TEAEs were hematologic (neutropenia, 27%; anemia, 13%; thrombocytopenia, 13%). The most frequent non-hematologic grade ≥3 TEAEs were acute kidney injury, rash, asthenia, and respiratory failure, reported in three patients (6%) each. TEAEs of interest occurred in 42 (81%) patients (). The most frequently reported TEAE of interest (all grades) was neutropenia (33%), followed by dyspnea (15%), upper respiratory tract infection (12%), hypertension (10%), COVID-19 (10%), acute kidney injury (8%), and leukopenia (8%). Serious TEAEs were reported in 22 (42%) patients (), of which 11 (21%) patients reported TEAEs related to infections. These included COVID-19 (n = 2), COVID-19 pneumonia (n = 2), infection (n = 2), pneumonia (n = 2), sepsis (n = 2), Clostridium difficile (n = 1), cytomegalovirus infection (n = 1), and lower respiratory tract infection (n = 1). Fatal TEAEs were reported in five patients (10%). These included two patients with myeloma progressive disease, and one patient each with cardiac arrest, cerebrovascular accident, and pneumonia. All fatal TEAEs occurred within 30 days after the last dose of treatment. Four patients had no other TEAEs reported within a 7-day window around the onset of the event; one patient had grade 2 clostridium infection and grade 2 confusional state within 7 days of the onset of cardiac arrest. There were no treatment-related fatal TEAEs during the study. TEAEs leading to drug discontinuation are reported in Supplemental Results.
Table 3. TEAEsTable Footnote* of interest by preferred term.
Table 4. Serious TEAEs.Table Footnote*
Discussion
As standards of care evolve to include the most effective triplet and quadruplet drug combination regimens early in the treatment of MM, there is an emerging need for effective salvage therapies at subsequent relapses. This phase 2 study evaluated the efficacy and safety of once-weekly KPd for the treatment of second-line or third-line RRMM (17 and 83% of patients, respectively) who were refractory to lenalidomide. The initial study inclusion criterion of prior anti-CD38 exposure may have driven the preponderance of third-line patients. In this study, 69% of patients were triple-class exposed to regimens containing IMiD, PI, and anti-CD38, including 37% who were triple-class refractory.
With an ORR of 58% (90% CI: 45.4, 69.3) in this difficult-to-treat patient population, the study did not meet its primary endpoint, as the lower bound of the binomial 90% CI for the ORR estimate was not greater than the prespecified reference of 60%. This hypothesized threshold was based on preceding clinical trials of KPd and KRd in RRMM that included fewer multiple class–exposed and/or refractory patients [Citation6,Citation14,Citation18,Citation19,Citation21,Citation22]. Patients in these prior studies did not receive daratumumab as frontline or second-line treatment.
Nonetheless, KPd may be considered an attractive treatment option for an increasing number of patients with relapsed and multidrug-resistant MM [Citation23]. With lenalidomide as an SOC and increasing use of anti-CD38 mAbs in the frontline setting, effective treatment combinations for lenalidomide-refractory and CD38-refractory patients may be of increasing importance. In SELECT, 75% of enrolled patients were refractory to both lenalidomide and daratumumab (). Overall, patients with double-class or triple-class refractory disease in early-line relapse following the three commonly used upfront classes of agents have limited effective treatment options available, and outcomes remain poor [Citation23–27]. Although cross-trial comparisons should be approached with caution, the results of SELECT suggest that the clinical benefit of KPd for such patients compares favorably with other available regimens (Supplemental Table 4) [Citation4,Citation28–31]. For example, ORR (58%) and median PFS (11.1 months) with KPd were favorable compared with the SOC control arm of KarMMa-3 (42% and 4.4 months, respectively), although KarMMa-3 had a greater proportion of triple-class refractory patients (66 vs. 37% for SELECT).
Among responding patients in SELECT, responses were comparatively durable. The median DOR for KPd was 20.3 months, comparing favorably to outcomes with other available regimens (Supplemental Table 4) [Citation4,Citation29–31]. The majority of responders in SELECT with PR or better (n = 30) achieved a response of at least VGPR (n = 18), with CR confirmed in three patients. The MRD– (10−5) response rate was 10% in patients with either CR or VGPR, a measure that has been shown to correlate with improved PFS in several studies [Citation32–35]. No PFS events (progression or death) were noted in MRD– patients in SELECT.
Safety findings in this study were consistent with the known safety profiles of carfilzomib and pomalidomide, with no new safety concerns. Common TEAEs reported with KPd in this study were consistent with those previously reported for KRd [Citation6,Citation17], with the most frequent being hematologic AEs. Among non-hematologic events, fatigue was the most common followed by peripheral edema, asthenia, and diarrhea (Supplemental Table 3). Among TEAEs of interest, acute kidney injury, cardiac failure, hypertension, and pulmonary hypertension were similar to events reported previously [Citation6,Citation17]. Incidence of venous thromboembolic events (n = 1, 2%) and pulmonary embolism (n = 1, 2%) was lower than previous carfilzomib and carfilzomib lenalidomide combination studies, which may be attributable to adherence of recommended thromboprophylaxis guidelines implemented during SELECT (Supplemental Methods). Although no new safety concerns were identified, KPd is not without risk and, as such, patient selection and safety monitoring should always be taken into consideration.
These results suggest an overall favorable benefit:risk profile of KPd for RRMM patients with previous exposure to lenalidomide and daratumumab relative to currently available MM therapies. Immune cell–redirecting therapies have recently demonstrated promising efficacy in heavily pretreated later-line RRMM and may be approved in earlier lines of therapy, pending the results of ongoing clinical trials. Results from CARTITUDE 4, which had similar inclusion criteria as SELECT, showed that while SOC therapy (pomalidomide, bortezomib, and dexamethasone or daratumumab, pomalidomide, and dexamethasone) had similar ORR and median PFS outcomes (67% and 11.8 months, respectively) to the KPd arm of SELECT, superior outcomes were reported for the chimeric antigen receptor T-cell (CAR-T) cohort (ORR, 85%; median PFS, not reached, respectively) [Citation36]. Logistical challenges and risk mitigation requirements of CAR-T therapy may limit their availability to a subset of patients and specialized medical care facilities in the near term, suggesting an ongoing need for active therapies based on combinations of other available medicines for RRMM [Citation37]. The optimal role for KPd, a combination of the most active PI and IMID drugs, within the second- and third-line treatment setting will require further exploration as therapeutic options continue to evolve to potentially include CAR-T for appropriate patients.
Our study is limited in being a single-arm study without a comparator group, and its open-label design has the potential to introduce bias. The relatively small sample size, inclusive of mostly patients at second relapse, limits the interpretability of data across different lines of therapy.
In summary, there is an increasing medical need for effective therapies for lenalidomide-refractory, CD38-refractory, and triple-class–exposed patients in first or second relapse of MM. While the evolution of the treatment landscape for these patients continues, the use of emerging classes of therapy may be limited in the near term. The results of SELECT suggest that KPd is a safe and effective regimen that may be appropriate for a growing number of patients with relapsed MM.
Supplemental Material
Download MS Word (68.6 KB)Acknowledgments
We thank all patients and their families and the research nurses, study coordinators, and support staff who contributed to this study. This study was supported and funded by Amgen. Medical writing and editorial assistance were provided by Kevin Doty, PhD (Amgen Inc., Thousand Oaks, CA, USA). We thank all authors for their critical review of the manuscript.
Disclosure statement
A. Perrot received research grants from Bristol Myers Squibb, Sanofi, and Takeda and has served as a consultant for AbbVie, Amgen, Bristol Myers Squibb, Janssen, Pfizer, Sanofi, and Takeda. S. Delimpasi has served as a consultant and on the speakers bureau for Janssen, Takeda, Amgen, GSK, and BMS. E. Spanoudakis and U. Frølund have no disclosures. A. Belotti participated in advisory boards for Amgen, Janssen, Pfizer, and GSK. A. Oriol received honoraria from and served on a speakers bureau for GSK and BMS/Celgene and has served as a consultant for Amgen. P. Moreau received honoraria and has served as an advisory board member of Janssen, Celgene/Bristol Myers Squibb, Amgen, Takeda, Sanofi, Pfizer, and AbbVie. I. McFadden, Q. Xia, and M. Arora are employees of Amgen Inc. M. Dimopoulos has received research funding from BMS, Janssen, Amgen, Takeda, and Beigene; honoraria from BMS, Janssen, Amgen, Takeda, and Beigene; and consulting fees from BMS, Janssen, Amgen, Takeda, and Beigene.
Data availability statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
Additional information
Funding
References
- Elbezanti WO, Challagundla KB, Jonnalagadda SC, et al. Past, present, and a glance into the future of multiple myeloma treatment. Pharmaceuticals. 2023;16(3):415. doi:10.3390/ph16030415
- Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–2115. doi:10.1056/NEJMoa1817249
- Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2017;378:519–528. doi: 10.1056/NEJMoa1714678
- Richardson PG, Oriol A, Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(6):781–794. doi:10.1016/S1470-2045(19)30152-4
- Schjesvold FH, Dimopoulos MA, Delimpasi S, et al. Melflufen or pomalidomide plus dexamethasone for patients with multiple myeloma refractory to lenalidomide (OCEAN): a randomised, head-to-head, open-label, phase 3 study. Lancet Haematol. 2022;9(2):e98–e110. doi:10.1016/S2352-3026(21)00381-1
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. doi:10.1056/NEJMoa1411321
- Moreau P, Garfall A, van de Donk NWCJ, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495–505. doi:10.1056/NEJMoa2203478
- Munshi NC, Anderson LD Jr., Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. doi:10.1056/NEJMoa2024850
- US Food and Drug Administration. FDA approves ciltacabtagene autoleucel for relapsed or refractory multiple myeloma; 2022 [cited 2023 Jul 20]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ciltacabtagene-autoleucel-relapsed-or-refractory-multiple-myeloma
- US Food and Drug Administration. FDA approves teclistamab-cqyv for relapsed or refractory multiple myeloma; 2022 [cited 2023 Jul 20]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-teclistamab-cqyv-relapsed-or-refractory-multiple-myeloma
- Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. doi:10.1016/S1470-2045(13)70380-2
- Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–2825. doi:10.1182/blood-2012-05-425934
- Hájek R, Masszi T, Petrucci MT, et al. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia. 2017;31(1):107–114. doi:10.1038/leu.2016.176
- Bringhen S, Mina R, Cafro AM, et al. Once-weekly carfilzomib, pomalidomide, and low-dose dexamethasone for relapsed/refractory myeloma: a phase I/II study. Leukemia. 2018;32(8):1803–1807. doi:10.1038/s41375-018-0024-1
- Sonneveld P, Zweegman S, Cavo M, et al. Carfilzomib, pomalidomide, and dexamethasone as second-line therapy for lenalidomide-refractory multiple myeloma. Hemasphere. 2022;6(10):e786. doi:10.1097/HS9.0000000000000786
- Biran N, Siegel D, Berdeja JG, et al. Weekly carfilzomib, lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a phase 1b study. Am J Hematol. 2019;94(7):794–802. doi:10.1002/ajh.25498
- Moreau P, Mateos MV, Berenson JR, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19(7):953–964. doi:10.1016/S1470-2045(18)30354-1
- Shah JJ, Stadtmauer EA, Abonour R, et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015;126(20):2284–2290. doi:10.1182/blood-2015-05-643320
- Sonneveld P, Zweegman S, Cavo M, et al. Carfilzomib, pomalidomide and dexamethasone (KPd) in patients with multiple myeloma refractory to bortezomib and lenalidomide. The EMN011 trial. Blood. 2018;132(Supplement 1):801. doi:10.1182/blood-2018-99-114029
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi:10.1016/S1470-2045(16)30206-6
- Jakubowiak A, Jasielec J, Rosenbaum C, et al. Efficacy and safety of carfilzomib-pomalidomide-dexamethasone in relapsed and/or refractory multiple myeloma: pooled analysis of 2 single arm studies. Clin Lymphoma Myeloma Leuk. 2019;19(10):e34. doi:10.1016/j.clml.2019.09.050
- Jakubowiak A, Rosenbaum C, Stephens L, et al. P680: final results of phase 1/2 study of carfilzomib, pomalidomide, and dexamethasone (KPd) in patients with relapsed/refractory multiple myeloma: a multi-center MMRC study. Madrid: European Hematology Association; 2017.
- Wang P, Yee C, Gorsh B, et al. Treatment patterns and overall survival of patients with double-class and triple-class refractory multiple myeloma: a US electronic health record database study. Leuk Lymphoma. 2023;64(2):398–406. doi:10.1080/10428194.2022.2140284
- Davies F, Rifkin R, Costello C, et al. Real-world comparative effectiveness of triplets containing bortezomib (B), carfilzomib (C), daratumumab (D), or ixazomib (I) in relapsed/refractory multiple myeloma (RRMM) in the US. Ann Hematol. 2021;100(9):2325–2337. doi:10.1007/s00277-021-04534-8
- Sun Z, Zheng F, Wu S, et al. Triplet versus doublet combination regimens for the treatment of relapsed or refractory multiple myeloma: a meta-analysis of phase III randomized controlled trials. Crit Rev Oncol Hematol. 2017;113:249–255. doi:10.1016/j.critrevonc.2017.03.018
- Bal S, Malek E, Kansagra A, et al. Treatment outcomes of triple class refractory multiple myeloma: a benchmark for new therapies. Leukemia. 2022;36(3):877–880. doi:10.1038/s41375-021-01471-3
- Zanwar S, Ho M, Kapoor P, et al. Outcomes of triple class (proteasome inhibitor, IMiDs and monoclonal antibody) refractory patients with multiple myeloma. Leukemia. 2022;36(3):873–876. doi:10.1038/s41375-021-01433-9
- Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266–2275. doi:10.1038/s41375-019-0435-7
- Mateos MV, Weisel K, De Stefano V, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia. 2022;36(5):1371–1376. doi:10.1038/s41375-022-01531-2
- Moreau P, Weisel K, De Stefano V, et al. LocoMMotion: a prospective, observational, multinational study of real-life current standards of care in patients with relapsed/refractory multiple myeloma–final analysis at 2-year follow-up. Hemasphere. 2023;7(S3):e05307aa. doi:10.1097/01.HS9.0000970520.05307.aa
- Rodriguez-Otero P, Ailawadhi S, Arnulf B, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388(11):1002–1014. doi:10.1056/NEJMoa2213614
- Oliva S, Bruinink D, Rihova L, et al. Minimal residual disease assessment by multiparameter flow cytometry in transplant-eligible myeloma in the EMN02/HOVON 95 MM trial. Blood Cancer J. 2021;11(6):106. doi:10.1038/s41408-021-00498-0
- San-Miguel J, Avet-Loiseau H, Paiva B, et al. Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE. Blood. 2022;139(4):492–501. doi:10.1182/blood.2020010439
- Soh KT, Came N, Otteson GE, et al. Evaluation of multiple myeloma measurable residual disease by high sensitivity flow cytometry: an international harmonized approach for data analysis. Cytometry B Clin Cytom. 2022;102(2):88–106. doi:10.1002/cyto.b.22053
- Terpos E, Kostopoulos IV, Kastritis E, et al. Impact of minimal residual disease detection by next-generation flow cytometry in multiple myeloma patients with sustained complete remission after frontline therapy. Hemasphere. 2019;3(6):e300. doi:10.1097/HS9.0000000000000300
- San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. 2023;389(4):335–347. doi:10.1056/NEJMoa2303379
- Holstein SA, McCarthy PL. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs. 2017;77(5):505–520. doi:10.1007/s40265-017-0689-1
