Abstract
The efficacy of chimeric antigen receptor (CAR) T-cell therapy for large B-cell lymphoma (LBCL) is well-established. This study, using the Premier PINC AI Healthcare Database, assessed hospital costs and healthcare resource utilization (HRU) between CAR T-cell therapy and autologous hematopoietic cell transplant (AHCT) for 733 LBCL patients from 01/01/2017-04/30/2021 (166 CAR T and 567 AHCT from 37 US hospital systems. CAR T-cell therapy had higher index costs but lower non-pharmacy costs, shorter hospital stays, lower ICU utilization than AHCT. The CAR T-cell cohort also presented fewer preparatory costs and HRU. At a 180-day follow-up, AHCT had lower hospitalization rates and costs. Overall, despite higher index costs, CAR T-cell therapy has lower non-pharmacy costs and HRU during the index procedure and requires less preparation time with lower preparation HRUs and costs than AHCT. This has important implications for resource management and informed decision-making for stakeholders.
1. Introduction
Chimeric antigen receptor (CAR) T-cell therapy is a cutting-edge form of oncologic immunotherapy that utilizes the patient’s own T lymphocytes which are genetically modified ex vivo to target malignant cancer cells [Citation1,Citation2]. In recent years, the United States (US) Food and Drug Administration (FDA) has approved three CAR T-cell therapies for the treatment of relapsed or refractory large B-cell lymphoma (R/R LBCL): axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel) after initial treatment, and tisagenlecleucel (tisa-cel) after two or more lines of systemic therapies [Citation3–5]. The efficacy of CAR T-cell therapy in treating aggressive B-cell lymphomas has been well demonstrated in clinical trials and real-world studies, with higher overall response rates observed compared to alternative treatments.
Since the approval of CAR T-cell therapy to treat R/R LBCL, and the recent approval of second-line treatment for LBCL in April 2022 [Citation3,Citation4], there has been a growing interest in the future shift of treatment landscapes from autologous hematopoietic cell transplant (AHCT) to CAR T-cell therapies. However, the financial and other resource barriers to access for CAR T-cell therapies persist. Several reports have claimed that hospital providers could expect to see major financial losses in their CAR T-cell therapy programs [Citation6,Citation7]. Some studies have explored the costs and healthcare resource utilization (HRU) associated with CAR T-cell therapies and HCT from preparation through various follow-up periods from payer perspectives [Citation8,Citation9]. Despite the importance of understanding the financial impact of CAR T-cell therapy from the hospital perspective, to our knowledge, no previous research has quantified the comprehensive hospital delivery costs and HRU in the US or elsewhere where CAR T-cell therapies have been approved.
To thoroughly assess the net financial and HRU impact of CAR T-cell therapy and SCT for hospital providers, this study aimed to describe and compare the provider actuarial costs and hospital-reported HRU associated with CAR T-cell therapy and AHCT in treating patients with LBCL, from the preparation phase through a six-month post-procedure follow-up period. This study was to provide a current and comprehensive analysis of real-world US hospital system actuarial costs, filling a crucial gap in the literature with information needed for budgetary and resource allocation planning to establish new authorized treatment centers or for centers considering CAR T-cell therapy program expansion.
2. Materials and methods
2.1. Data source
This retrospective observational study used data from the Premier PINC AI Healthcare Database (PHD), containing inpatient and hospital-based outpatient discharge information from more than 1000 US hospitals and 20-25% of all US inpatient admissions from geographically diverse, non-governmental community and teaching hospitals [Citation10–12] (Supplemental Methods). The PHD included data on patient demographics, health insurance type, diagnoses, and costs from a hospital perspective. As per US Title 45 Code of Federal Regulations, Part 46, this study of fully deidentified data was exempted from institutional review board approval, as with prior PHD studies [Citation11–14].
2.2. Study population
The index period for this study spanned from January 1, 2017 through April 30, 2021, and patient-level data were evaluated for a 12-month look-back period and 180-day post-discharge follow-up period (Supplemental Figure). Patients were included if they met all of the following criteria: 1) age ≥18 years, 2) had an inpatient or outpatient visit of CAR T-cell infusion or AHCT transfusion during the index period, with the first such visit considered the index visit, 3) had discharge diagnosis of LBCL defined by ICD-10 diagnosis codes, C83.3x, C85.1x, C85.2x, C85.8x, C83.8x, and C83.9x, during the index visit, and 4) ≥1 inpatient or outpatient visit(s) with LBCL diagnosis defined as above within 90 days prior to the date of the index visit. Patients were excluded if 1) they had either ≥2 CAR T-cell or HCT infusions, or if they had received both CAR T-cell and HCT infusions 2) the treating hospital did not have continuous data for the patients during the 12-month look-back and 180-day follow-up periods, or 3) they were identified as clinical trial patients (i.e., having ICD-10 diagnosis code Z00.6x at the index visit). Patients were categorized into mutually exclusive cohorts (CAR T and AHCT) based on the index visit treatment.
The index date was the admission date of the index visit. The index discharge date was the discharge date of the index visit, which would be the same as the admission date for index procedures completed in the outpatient setting. Apheresis and bridging therapies were identified for CAR T-cell therapy patients. Apheresis visits were visits with apheresis codes (Supplemental Table 1) that occurred within 90 days prior to the CAR T-cell infusion procedure. Bridging therapies were defined as radiation or any of the pharmaceutical bridge therapy options specified in the National Comprehensive Cancer Network (NCCN) guideline [Citation15] (Supplemental Table 1) for LBCL given between the apheresis and CAR T-cell infusion procedure dates. Salvage chemotherapies (Supplemental Table 1) were identified for AHCT patients as those treatments received within 180 days before the HCT transfusion procedure.
2.3. Study measures
Baseline characteristics assessed at the index visit included demographics (age, sex, race/ethnicity), insurance type, primary payer, hospital characteristics (geographic region, urbanicity, teaching status, and number of beds). Comorbidities and the Deyo-modified Charlson Comorbidity Index (CCI) [Citation16,Citation17] were assessed using patient-level data from the index visit and the prior 12 months (Supplemental Table 2).
Costs were evaluated from the start of the preparation period through the end of the follow-up period. The preparation period was defined as the timeframe from the first apheresis (CAR T-cell therapy patients) and salvage chemotherapies (AHCT patients) up to the date before the index visit. Total costs summed all direct hospital costs incurred by and reported by the hospital systems where study patients were treated (Supplemental Methods) [Citation11,Citation18]. All costs were adjusted to 2021 US dollars using the Consumer Price Index for inpatient services [Citation19]. Index visit costs incurred were assessed in total and by type (i.e. pharmacy and non-pharmacy, variable and fixed). Pharmacy costs were defined as all costs incurred within the pharmacy department, whereas non-pharmacy costs were costs incurred under departments other than pharmacy. Variable costs include expenses that relate directly to or vary with the activity (volume) of the department such as supplies and hands-on patient care. Fixed costs include those that do not relate directly to or vary with the activity (volume) of the department such as depreciation, management, repair and maintenance and overhead. The total preparatory cost was defined as costs incurred during the preparation period. Total follow-up costs were those incurred within 180 days after the index discharge date. HRU, including inpatient hospitalization rate, inpatient hospital length of stay (LOS), intensive care unit (ICU) admission rate, ICU LOS, and emergency department (ED) and non-ED outpatient visits, were assessed from the start of the preparation period through the end of the follow-up period.
2.4. Statistical analysis
Descriptive statistical comparisons of baseline characteristics and outcomes were conducted to determine differences between the CAR T-cell therapy and AHCT cohorts. Continuous variables were presented as means and standard deviations, and compared using t-tests or Wilcoxon Rank Sum test for non-normal distributions. Categorical variables were expressed as counts and percentages, and compared using Chi-square tests. Additionally, we conducted adjusted analysis for cost outcomes, adjusting for patient age group, sex, race, CCI score, hospital characteristics, and primary payer. The adjusted mean cost ratios were quantified using a generalized linear model (GLM) with a log link and gamma distribution [Citation20]. The adjusted absolute incremental differences were calculated using the recycled prediction method with bootstrapped 95% confidence intervals (Supplemental Methods) [Citation21–23]. A p-value < 0.05 was considered significant for determining differences between cohorts. Analyses were performed using R 3.6.3 [Citation24].
3. Results
From January 1, 2017 through April 30, 2021, 94,403,470 patients aged ≥18 were identified (Supplemental Table 3). After applying inclusion and exclusion criteria, 733 adults with LBCL were included: 166 underwent CAR T-cell therapy and 567 received AHCT.
Demographic and clinical characteristics were similar between two cohorts (). The mean patient age was 60 ± 12 years (median = 62; 39.6% ≥ 65 years), with 39.6% females, and 76.3% white. Healthcare coverage varied (commercial: 43.2%; Medicare: 37.9%; Medicaid: 12.0%; other, uninsured, or unknown: 6.8%). The mean CCI was 4 in both cohorts () with similar prevalence of comorbidities with the most common comorbidities (other than malignancies) of diabetes (18.1%), chronic pulmonary disease (14.1%), and renal disease (10.2%).
Table 1. Demographic and clinical characteristics of CAR T and autologous HCT patients.
Patients were treated at 36 US hospital systems (). Most patients received treatment in urban teaching hospitals (85.3%) with >500 beds (70.9%). As compared to AHCT patients, more CAR T-cell patients were treated in hospital systems in the West.
Table 2. Hospital characteristics of CAR T and autologous HCT patients.
3.1. HRU and costs incurred at index procedure
Most treatments occurred in an inpatient setting (). The LOS for CAR T-cell cohort was shorter than for AHCT cohort (median: CAR T-cell therapy: 15 days and autologous HCT: 20 days; p < 0.01). ICU admission rates at index visit were 20.9% and 29.5% for CAR T-cell and AHCT cohorts. The median ICU LOS was 3 and 19 days for CAR T-cell and AHCT cohorts (p < 0.01).
Table 3. Healthcare resource utilization incurred during the index visit, preparation, and post-index periods.
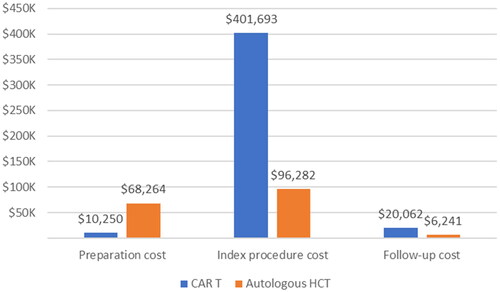
Total costs incurred during the index procedure were higher for the CAR T-cell cohort (, CAR T-cell therapy: $401,693 and AHCT: $96,282; p < 0.01), which was primarily due to the pharmacy costs including the CAR T-cell therapy acquisition (median: CAR T-cell therapy: $385,010 and AHCT: $35,063; p < 0.01). Non-pharmacy costs were lowest for CAR T-cell cohort (median: CAR T-cell therapy: $31,785, AHCT: $46,120; p < 0.01). Adjusted models show similar trends (Supplemental Tables 4 and 5). Fixed costs for the CAR T cohort were higher than for AHCT cohort (median: CAR T-cell therapy: $54,903 and AHCT: $35,129; p < 0.01). Among inpatient procedures, median total costs for CAR T-cell and AHCT were $421,578 and $87,843, higher than those in outpatient settings.
Table 4. Costs incurred during the index procedure: CAR T and autologous HCT (2021 USD).
3.2. Time, HRU, and costs during the preparation period
The median preparation time, which marks the span from initiating salvage chemotherapies to the index AHCT procedures was 78 days for AHCT. By contrast, the time from apheresis to CAR T-cell infusion was 29 days (p < 0.01). Preparation times varied based on the CAR T-cell product: axi-cel took 26 days, and tisa-cel took 34 days (p < 0.01).
The CAR T-cell cohort had a lower hospitalization rate with shorter LOS during the preparation period than AHCT patients (, hospitalization rate [median LOS]: CAR T-cell therapy: 11.2% [5] and AHCT: 24.8% [6]; p < 0.01). Preparatory costs for the CAR T-cell cohort were lower than the AHCT cohort (, median: CAR T-cell therapy: $10,250 and AHCT: $68,264; p < 0.01). After adjustment, CAR T-cell cohort’s preparatory costs remained significantly lower compared to AHCT cohort (Supplemental Tables 4 and 5).
3.3. HRU and costs during the follow-up period
In the 180-day follow-up after the index procedure, the AHCT cohort had the lower hospitalization rate (, CAR T-cell therapy: 41.6% and AHCT: 24.2%; p < 0.01), but with similar LOS between two cohorts. The ICU admission rate was slightly higher in the CAR T-cell cohort (CAR T-cell therapy: 10.2% and AHCT: 7.8%). No differences were observed in use of ED and non-ED outpatient visits between the two cohorts. The AHCT cohort incurred a lower cost during the follow-up (, CAR T-cell therapy: $47,624 and AHCT: $20,893; p < 0.01). Adjusted models showed the CAR T-cell therapy cohort had higher follow-up costs (+$22,156) than the AHCT (Supplemental Table 4).
4. Discussion
This study is the first to comprehensively evaluate the actuarial hospital costs and HRU associated with CAR T-cell therapy and AHCT in patients with LBCL. It demonstrated that CAR T-cell therapy had higher index procedure costs, largely due to pharmacy costs, including acquisition costs. However, CAR T-cell therapy had lower non-pharmacy costs and HRU, including shorter hospital LOS, lower ICU admission rates, and shorter ICU LOS. Care associated with inpatient settings had higher costs for both CAR T-cell and AHCT groups. Moreover, the CAR T-cell cohort had reduced preparatory costs and a shorter preparatory period compared to AHCT cohort. Follow-up costs were lower in the AHCT cohort.
Our finding of a shorter LOS for the CAR T-cell therapy procedures aligns with previous studies in the US [Citation8] and Europe [Citation25]. The published ICU admission rate during the CAR T-cell infusion inpatient encounter ranges from 9% to 33%, with a higher rate in the earlier years of CAR T-cell therapy [Citation26–30], suggesting improved clinical management of CAR T-cell patients with experience and better safety protocols.
With high acquisition costs for CAR T-cell therapy, over $350,000 per infusion [Citation31], our study found that the median costs for CAR T-cell therapy and AHCT was $412,207 and $85,955, respectively. Previous research has reported that, from a payer’s perspective, Medicare payments for CAR T-cell therapy may also be higher than that of HCT, with one real-world study reporting median payments for CAR T versus AHCT of $401,927 and $65,109, and median payments for CAR T versus allogenic HCT of $399,719 and $115,448 [Citation8]. Therefore, depending on reimbursement levels (which vary widely between institutions), the net financial impacts of CAR T-cell therapy programs have the potential to be similar to those of HCT.
This study provided a detailed cost breakdown using the actuarial costs reported by hospital systems. By breaking down the total hospital cost, this study found that non-pharmacy costs were lower for CAR T-cell therapy compared to AHCT (CAR T-cell therapy versus AHCT: $41,076 versus $51,579). Despite the initial challenges of marshaling capital and staff resources to establish new authorized treatment centers for CAR T-cell therapy [Citation8], these findings suggest that the hospital expenses excluding pharmacy costs may be lower for CAR T-cell therapy than for AHCT programs.
Additionally, this study demonstrated a difference between CAR T-cell therapy and AHCT in terms of preparation time and associated costs, which were lower for CAR T-cell therapy than for AHCT. HCT typically involves salvage therapy, harvesting, conditioning therapy in the lead-up to the transplant [Citation32]. The CAR T-cell therapy process also involves multiple steps, from leukapheresis through CAR T-cell infusion, during which patients might receive bridging chemotherapy and lymphodepleting chemotherapy [Citation33]. Manufacturing time (i.e. the time between leukapheresis to availability of the CAR T-cell therapy for infusion) is an important contributor to the preparation time, and of special concern for patients with aggressive disease [Citation34,Citation35]. The reported time from leukapheresis to CAR T-cell infusion varies by product and study type, with a median range of 21 to 45 days [Citation36–38], which provides useful context for the variations in preparation time found by product in our study. Our findings further suggested that in a real-world setting, the preparation time and associated costs were lower for CAR T-cell therapy than for AHCT.
This study found that the inpatient admission rate and cost during follow-up were higher for CAR T-cell therapy than for AHCT, which is consistent with previous real-world studies [Citation8,Citation9]. This observation aligns with clinical practice: CAR T-cell therapy patients are often admitted at the first sign of fever or for planned admissions about 7-10 days post-treatment and may require readmission due to complications, whereas AHCT patients typically have a continuous hospital stay for approximately 3.5 weeks. A previous study in the Medicare population reported that CAR T-cell therapy patients had a 7% higher six-month post-index inpatient admission rate than AHCT patients, but a 15% lower rate than allogenic HCT patients (CAR T versus AHCT 45% versus 38%; CAR T versus allogenic HCT 42% versus 57%) [Citation8]. Another study using commercial claims data in patients who had received three or more lines of therapy showed that the inpatient admission rate during the first six months post-procedure was higher for patients who received CAR T-cell therapy than for patients who received stem cell transplant, but that study did not differentiate between the different types of transplantation [Citation9].
In terms of follow-up costs, a real-world study showed that the six-month follow-up cost estimated using the allowed amount on administrative claims was higher for patients who received CAR T-cell therapy than for AHCT patients, and lower than for allogenic HCT patients [Citation8]. This cost difference may be due to the varying clinical follow-up protocols for each procedure, such as the recommended frequency of follow-up visits and the required imaging or laboratory tests [Citation39]. For example, patients who receive CAR T-cell therapies typically undergo positron emission tomography (PET) and computed tomography (CT) to monitor for disease progression and adverse events, while imaging tests for HCT patients are limited [Citation32]. The differences in follow-up costs and inpatient admission rates in the three treatment cohorts in our study could also be related to varying disease stages among the patients in each cohort. Most, if not all, of the CAR T-cell therapy patients in the current cohort received this treatment as a third line of therapy, as the FDA did not approve CAR T-cell therapy as a second-line therapy until April 2022 [Citation3,Citation4]. Further analysis is warranted to compare costs and HRUs while considering the lines of therapy and disease stage.
This study is the first to comprehensively describe and compare costs and HRU of patients with LBCL who have received CAR T-cell therapy and AHCT from preparation through six months of post-procedure follow-up. Our study also explored the allogeneic HCT patients, but they were ultimately excluded from the final analysis due to their limited numbers. CAR T-therapy and HCT treatments target patients refractory to one or two lines of therapy [Citation15]. Our findings have important implications for hospitals, payers, and policymakers in addressing factors that influence access to CAR T-cell therapy. The issue of access is gaining importance as evidence demonstrating the superior survival benefits of CAR T-cell therapy compared to HCT-intended treatments continues to accumulate [Citation40]. One of the major obstacles to improving access to CAR T-cell therapy is the limited number of authorized treatment centers [Citation7,Citation41]. Our study provides hospitals with valuable insights into the resource planning necessary to evaluate the option of offering CAR T-cell therapy for newly authorized or candidate centers or for existing centers that are considering if and how to scale up capacity to make this therapy more accessible to local patients and those living in different geographic regions. Additionally, as more CAR T-cell therapies are being approved in the US, the total cost of care reported in this study can help payers and policymakers make informed decisions about coverage and reimbursement for CAR T-cell therapy. The complex and uncertain reimbursement system for CAR T-cell therapies has, to some degree, become a disincentive to providing it, as some facilities may fear the potential financial burden for hospitals administering this treatment [Citation7]. Our up-to-date economic evaluation of CAR T-cell therapy in LBCL patients may, however, shed light for planners to better assess the benefits and risks, and to formulate more evidence-based and realistic strategies to alleviate some of the barriers to improving clinically-appropriate access to CAR T-cell therapy.
This study is not without limitations. First, treatment strategies and resource utilization may not fully represent real-world practices in the US, since the study used data from a sample of hospitals that may have a higher proportion of non-teaching facilities compared to the ratio suggested by the American Hospital Association survey data [Citation10]. Second while costs might differ across hospitals, direct comparisons within individual hospitals were not conducted due to the reduced sample size that would result from such stratification. Third, the study findings are limited to costs and HRU within the index hospital system without capturing data from outside referral sources. However, it is believed that any under-estimation of costs and HRU present in the data would not differ between two treatment cohorts. We also note that the hospital preparation costs and HRUs did not consider those patients who initiated the preparation stage but did not go on to receive either a CAR T-cell infusion or an HCT. The literature highlights this issue and an important differential in the treatment journey between HCT-intended patients and patients being prepared to receive CAR T-cell therapy. One recent, large-scale study, for example, reported that about one-third of AHCT-eligible patients proceeded to HCTs after initiating high-dose chemotherapy preparation therapies [Citation42] while more than 95% of patients undergoing preparation for CAR T-cell therapy proceeded to CAR T infusion after apheresis [Citation28]. Therefore, the total cost and HRU of preparation leading to a successful HCT procedure may be even higher than that for CAR T-cell therapy, and this uncertainty around the patient journey also may cause uncertainty around the expected HRUs and logistical considerations. Finally, the study defines on-label use as receiving CAR T-cell therapy after two or more lines of systemic therapies, but the data source lacks enough detail to accurately identify the specific lines of therapy or disease stage for each patient, which are important covariates that are associated with HRU. Specifically, given that CAR T-cell therapy is more likely to be used in later lines than AHCT during the study period of this study, the cost and HRU results estimated after adjusting for between-cohort differences in line of therapy would be more likely to favor the CAR T-cell therapy cohort.
Despite these limitations, this study uses real-world hospital-reported data to provide the first and most current evaluation of actual cost and healthcare resource utilization for patients with LBCL undergoing CAR T-cell therapy and AHCT. The results highlight that although the pharmacy costs for the CAR T-cell therapy infusion is higher than those for AHCT, non-pharmacy costs and HRU during the index procedure were lower for CAR T-cell therapy, and patients who received CAR T-cell therapy also required less preparation time with lower preparation costs. These findings have important implications for resource management and informed decision-making for hospitals, payers and policy makers.
Supplemental Material
Download PDF (250.1 KB)Acknowledgement
The authors would like to express their gratitude to Cate Polacek, who provided assistance with the submission of this manuscript.
Disclosure statement
The authors would like to declare the following financial interests and personal relationships which could potentially be seen as conflicts of interest. Dr. Chaoling Feng was employed by Kite, a Gilead Company at the time the study was conducted, and also holds equity in Gilead and Pfizer. Sally W. Wade, has served in consultancy roles for Kite, a Gilead Company, Abbvie, and Johnson & Johnson. Dr. Christine Fu is currently employed by Kite, a Gilead Company and holds stock options in this privately-held company. In addition, Dr. Fu holds stock options in Amgen, a publicly traded company, as well as Cellares, a privately-held company, and receives patents and royalties from intellectual property with Cellares. Dr. Gunjan L. Shah has received research funding from Janssen, Amgen, BMS, Beyond Spring, and GPCR. Lastly, Dr. Chendi Cui, Dr. Ning Rosenthal, and Dr. Laura Curry are employees of Premier, Inc. Dr. Chendi Cui and Dr. Laura Curry have provided consultancy services to BMS, GSK, CorMedix Inc. and bioMérieux.
Data availability statement
The data from this study are not available for sharing as they are from the PINC AI Health Database (PHD), which is a proprietary database. All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Additional information
Funding
References
- Barrett DM, Singh N, Porter DL, et al. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65(1):333–347. doi:10.1146/annurev-med-060512-150254
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi:10.1056/NEJMoa1215134
- U. S. Food and Drug Administration. FDA approves axicabtagene ciloleucel for second-line treatment of large B-cell lymphoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-second-line-treatment-large-b-cell-lymphoma
- U. S. Food and Drug Administration. FDA approves lisocabtagene maraleucel for second-line treatment of large B-cell lymphoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lisocabtagene-maraleucel-second-line-treatment-large-b-cell-lymphoma.
- U. S. Food and Drug Administration. FDA approves tisagenlecleucel for relapsed or refractory follicular lymphoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-relapsed-or-refractory-follicular-lymphoma.
- BioPharma Dive BJ. Hospital docs weigh in on CAR-T reimbursement: ‘It’s very complicated’. Accessed 4/17, 2023. https://www.biopharmadive.com/news/car-t-hospital-reimbursement-cancer-ash18/543626/.
- Kamal-Bahl S, Puckett JT, Bagchi I, et al. Barriers and solutions to improve access for chimeric antigen receptor therapies. Immunotherapy. 2022;14(9):741–753. doi:10.2217/imt-2022-0037
- Mohammadi I, Purdum AG, Wong AC, et al. Cost and healthcare utilization in relapsed/refractory diffuse large B-cell lymphoma: a real-world analysis of medicare beneficiaries receiving chimeric antigen receptor T-cell Vs. Autologous and allogeneic hematopoietic cell transplants. Blood. 2020;136(Supplement 1):4–4. doi:10.1182/blood-2020-134828
- Chen L, Xie J, Wu A, et al. Resource use and costs in patients with relapsed/refractory diffuse large C-cell lymphoma who initiated a third-line therapy in the post CAR-T era: a longitudinal outlook. J Clin Oncol. 2021;39(15_suppl):e19560–e19560. doi:10.1200/JCO.2021.39.15_suppl.e19560
- Premier Healthcare Database White Paper: Data that informs and performs. March 2, 2020., Available at: https://learn.premierinc.com/white-papers/premier-healthcare-database-whitepaper.
- Jeong IG, Khandwala YS, Kim JH, et al. Association of Robotic-Assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. Jama. 2017;318(16):1561–1568. doi:10.1001/jama.2017.14586
- Schneeweiss S, Seeger JD, Landon J, et al. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358(8):771–783. doi:10.1056/NEJMoa0707571
- Kadri SS, Gundrum J, Warner S, et al. Uptake and accuracy of the diagnosis code for COVID-19 among US hospitalizations. Jama. 2020;324(24):2553–2554. doi:10.1001/jama.2020.20323
- Moon RC, Mackey RH, Cao Z, et al. Is coronavirus disease 2019 (COVID-19) less deadly now? Trends in in-hospital mortality among hospitalized COVID-19 patients in the United States. Clin Infect Dis. 2022;74(12):2238–2242. doi:10.1093/cid/ciab830
- Zelenetz AD, Gordon LI, Chang JE, et al. NCCN guidelines® insights: b -cell lymphomas, version 5.2021: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2021;19(11):1218–1230. doi:10.6004/jnccn.2021.0054
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
- Prasad A, Rosenthal NA, Kartashov A, et al. Contemporary trend of acute kidney injury incidence and incremental costs among US patients undergoing percutaneous coronary procedures. Catheter Cardiovasc Interv. 2020;96(6):1184–1197. Nov doi:10.1002/ccd.28824
- US Department of Labor, Bureau of Labor Statistics. Inpatient hospital services consumer price index. http://www.bls.gov/cpi/.
- Mihaylova B, Briggs A, O'Hagan A, et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897–916. doi:10.1002/hec.1653
- Cao Z, Villa KF, Lipkin CB, et al. Burden of illness associated with sinusoidal obstruction syndrome/veno-occlusive disease in patients with hematopoietic stem cell transplantation. J Med Econ. 2017;20(8):871–883. doi:10.1080/13696998.2017.1336623
- Basu A, Rathouz PJ. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6(1):93–109. doi:10.1093/biostatistics/kxh020
- Koyner JL, Mackey RH, Rosenthal NA, et al. Health Care Resource Utilization and Costs of Persistent Severe Acute Kidney Injury (PS-AKI) Among Hospitalized Stage 2/3 AKI Patients. Kidney360. 2023;4(3):316–325. doi:10.34067/KID.0005552022
- R Core Team. 2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- Ring A, Grob B, Aerts E, et al. Resource utilization for chimeric antigen receptor T cell therapy versus autologous hematopoietic cell transplantation in patients with B cell lymphoma. Ann Hematol. 2022;101(8):1755–1767. doi:10.1007/s00277-022-04881-0
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2021;386(7):640–654. doi:10.1056/NEJMoa2116133
- Yang H, Bollu V, Lim S, et al. Healthcare resource use and reimbursement amount by site of care in patients with diffuse large B-cell lymphoma receiving chimeric antigen receptor T-cell (CAR-T) therapy - a retrospective cohort study using CMS 100% medicare claims database. Leuk Lymphoma. 2022;64(2):339–348. doi:10.1080/10428194.2022.2147395
- Riedell PA, Walling C, Nastoupil LJ, et al. A multicenter retrospective analysis of outcomes and toxicities with commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B-cell lymphomas. Biology of Blood and Marrow Transplantation. 2020/03/01/2020;26(3):S41–S42. doi:10.1016/j.bbmt.2019.12.108
- Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38(27):3119–3128. doi:10.1200/jco.19.02104
- Chacim S, Monjardino T, Cunha JL, et al. Costs, effectiveness, and safety associated with chimeric antigen receptor (CAR) T-cell therapy: results from a comprehensive cancer center. PLoS One. 2022;17(12):e0278950. doi:10.1371/journal.pone.0278950
- Fiorenza S, Ritchie DS, Ramsey SD, et al. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplant. 2020;55(9):1706–1715. doi:10.1038/s41409-020-0956-8
- Freedman SA, Friedberg WJ. Diffuse large B cell lymphoma (DLBCL): suspected first relapse or refractory disease in medically-fit patients. In: upToDate., Rosmarin AG (Ed), UpToDate, Waltham, MA (Accessed on March 03, 2023).
- Perica K, Curran KJ, Brentjens RJ, et al. Building a CAR garage: preparing for the delivery of commercial CAR T cell products at memorial sloan kettering cancer center. Biol Blood Marrow Transplant. 2018;24(6):1135–1141. doi:10.1016/j.bbmt.2018.02.018
- Tully S, Feng Z, Grindrod K, et al. Impact of increasing wait times on overall mortality of chimeric antigen receptor T-cell therapy in large B-cell lymphoma: a discrete event simulation model. J Clin Oncol Clin Cancer Inform. 2019;3:1–9. doi:10.1200/cci.19.00086
- Kansagra A, Farnia S, Majhail N. Expanding access to chimeric antigen receptor T-cell therapies: challenges and opportunities. Am Soc Clin Oncol Educ Book. 2020;40:1–8. doi:10.1200/edbk_279151
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi:10.1016/s0140-6736(20)31366-0
- Riedell PA, Hwang WT, Nastoupil LJ, et al. Patterns of use, outcomes, and resource utilization among recipients of commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B cell lymphomas. Transplant Cell Ther. 2022;28(10):669–676. doi:10.1016/j.jtct.2022.07.011
- Nastoupil LJ, Jain MD, Spiegel JY, et al. Axicabtagene ciloleucel (axi-cel) CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: real world experience. Blood. 2018;132(Supplement 1):91–91. doi:10.1182/blood-2018-99-114152
- Ibrahim Y-A, Christian C, Peter B, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the european society for blood and marrow transplantation (EBMT) and the joint accreditation committee of ISCT and EBMT (JACIE). Haematologica. 2020;105(2):297–316. doi:10.3324/haematol.2019.229781
- Business Wire. Kite’s Yescarta® CAR T-cell Therapy Demonstrates a Statistically Significant Improvement in Overall Survival for Initial Treatment of Relapsed/Refractory Large B-cell Lymphoma 2023 [Available from: https://www.businesswire.com/news/home/20230320005701/en/Kite%E2%80%99s-Yescarta%C2%AE-CAR-T-cell-Therapy-Demonstrates-a-Statistically-Significant-Improvement-in-Overall-Survival-for-Initial-Treatment-of-RelapsedRefractory-Large-B-cell-Lymphoma.]
- Gajra A, Zalenski A, Sannareddy A, et al. Barriers to chimeric antigen receptor T-cell (CAR-T) therapies in clinical practice. Pharmaceut Med. 2022;36(3):163–171. doi:10.1007/s40290-022-00428-w
- Snider JT, McMorrow D, Song X, Diakun D, Wade SW, Cheng P. Burden of Illness and Treatment Patterns in Second-line Large B-cell Lymphoma. Clin Ther. 2022;44(4):521–38.