Abstract
This study investigates COVID-19 outcomes and immune response in chronic myeloid leukemia (CML) patients post-SARS-CoV-2 vaccination, comparing effectiveness of various vaccine options. Data from 118 CML patients (85 in Brazil, 33 in the US) showed similar infection rates prior (14% Brazil, 9.1% US) and post-vaccination (24.7% vs. 27.3%, respectively). In Brazil, AstraZeneca and CoronaVac were the most commonly used vaccine brands, while in the US, Moderna and Pfizer-BioNTech vaccines dominated. Despite lower seroconversion in the Brazilian cohort, all five vaccine brands analyzed prevented severe COVID-19. Patients who received mRNA and recombinant viral vector vaccines (HR: 2.20; 95%CI 1.07–4.51; p < .031) and those that had achieved at least major molecular response (HR: 1.51; 95% CI 1.01–3.31; p < .0001) showed higher seroconversion rates. Our findings suggest that CML patients can generate antibody responses regardless of the vaccine brand, thereby mitigating severe COVID-19. This effect is more pronounced in patients with well-controlled disease.
Keywords:
1. Introduction
Patients with hematologic malignancies face a heightened risk of severe infection and mortality from COVID-19 [Citation1], due to pronounced immune dysregulation associated with the disease or its treatment [Citation2]. Aproximately 34% to 38% of patients fail to develop an effective immune response post-vaccination [Citation3,Citation4]. However, hematologic malignancies encompass a diverse range of neoplastic disorders with different immunogenicity levels [Citation5]. Patients with myeloid malignancies exhibit the highest antibody seroconversion with COVID-19 vaccines, while those with lymphoid disorders, such as chronic lymphocytic leukemia (CLL) and lymphoma, demonstrate the lowest rates [Citation6].
Data on COVID-19 vaccine-induced immune responses are largely biased toward the vaccine types used in the geographic areas where these studies have been conducted, predominantly the United States (US) and Europe [Citation3,Citation6,Citation7]. Therefore, the available evidence is primarily limited to the mRNA vaccines such as BNT162B2 (Pfizer–BioNTech) and MRNA-1273 (Moderna) [Citation3,Citation6,Citation7]. This focus neglects other vaccine types widely used in low- and middle-income countries, such as the recombinant adenoviral vector vaccine ChAdOx1 nCov-19 (AstraZeneca/Oxford University) and inactivated virus vaccine CoronaVac (Sinovac/Butantan), both of which were extensively administered to the Brazilian population during the pandemic declared in March 2020 [Citation8,Citation9].
COVID-19 remains prevalent thorough out the world, still claiming many deaths. Vaccine-induced seroconversion among a diverse population of patients thus remains an ongoing area of investigation and interest for hematologists worldwide. Therefore, we conducted a real-world analysis of COVID-19 clinical outcomes and humoral immunity in chronic myeloid leukemia (CML) patients. Our study spans two centers, one in Brazil and one in the US, each with their own distinct healthcare systems and where a total of five vaccine brand shave been used. We explored the influence of clinical factors on antibody titers, as assessed by immunoglobulin G (IgG) and neutralizing antibody (NAb) levels.
2. Methods
2.1. Study design and participants
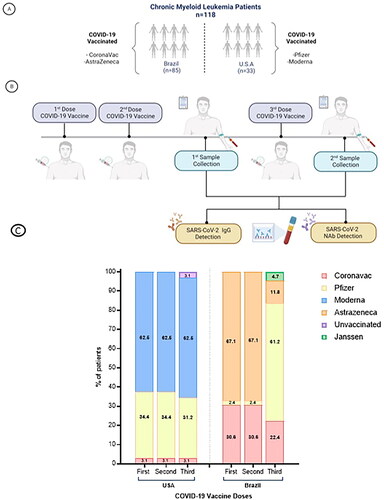
We conducted a comparative analysis of CML patients treated at Centro de Hematologia e Hemoterapia at Universidade Estadual de Campinas (HEMOCENTRO-UNICAMP) in Brazil and the Georgia Cancer Center (GCC) at Augusta University (AU) in the US. Both sites conducted observational studies enrolling CML patients aged 18 years or older, independent of disease phase, oncologic treatment, and prior SARS-CoV-2 infection (). The respective Institutional Review Board/Ethics Committee reviewed and approved the study protocols (UNICAMP # 4.861.688, AU-IRB #1858403, and WIRB #1663744). All patients provided written informed consent to participate, and the research adhered to the principles of the Declaration of Helsinki.
2.2. Data and sample collection
Demographic and clinical data were sourced from patients’ Electronic Medical Records (EMRs), while information related to COVID-19 history was retrieved through patient self-reporting via a questionnaire. Data collected included details on prior SARS-CoV-2 infection, confirmatory tests for SARS-CoV-2, the severity of COVID-19 symptoms according to the WHO clinical progression scale, and SARS-CoV-2 vaccination information. A minimum blood draw of 10 mL was collected at least twice, after patients received COVID-19 vaccine (). In the Brazilian cohort, samples were collected from one to three months after completing the vaccination schedule (receiving two doses).
2.3. SARS-CoV-2 antibody assays
SARS-CoV-2 antibody assays were performed separately at each study site. In the Brazilian cohort, patients initially underwent a qualitative screening serologic test using Chemiluminescent Microparticle Immunoassay (CMIA, SARS-CoV-2 IgM, IgG (Alinity System, Abbott Laboratories, Ireland)). Patients with reactive tests proceeded to a second analysis involving quantitative assessment of IgG antibodies specific to anti-S1 IgG (SARS-CoV-2 IgG II Quant, Alinity System, Abbott Laboratories, Ireland). Subsequently, for those patients who exhibited detectable IgG antibodies, NAb titers were gauged by assessing the ability of the antibodies to neutralize the cytopathic effect of the virus in culture (Vero CCL-81 cells).
In the US cohort, all patients’ serologic samples underwent testing for anti-spike IgG antibodies and anti-SARS-CoV-2 NAb, as previously reported [Citation4]. Briefly, the detection of serum IgG antibodies against SARS-CoV-2 was determined using the enzyme-linked immunosorbent assay (ELISA), (Anti-SARS-CoV-2 QuantiVac ELISA [IgG]; Euroimmun). Concurrently, levels of anti-SARS-CoV-2 NAb were assessed using the SuperFlex™ Anti-SARS-CoV-2 NAb Kit in conjunction with the SuperFlex™ Chemiluminescent Immunoassay System (PerkinElmer Inc.). The cutoff values for IgG and NAb seropositivity were 11 Ru/mL and 1 ng/ml, respectively. All tests were performed following the manufacturer’s instructions.
2.4. Statistical analysis
We performed descriptive statistics for demographic, clinical, and outcome characteristics of continuous variables, presenting absolute and relative frequencies. Quantitative variables were summarized using mean, median, and range measures. Considering the differences in the antibody assays used in both cohorts, our approach initially entailed categorizing the results as positive or negative based on the manufacturer’s instructions. Following this, we performed a comparative analysis using logistic regression to identify potential predictive factors of vaccination response. Bivariate analyses for comparing categorical variables utilized chi-square or Fisher’s exact tests. Statistical significance was set at p < .05. Data analysis was conducted using R (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria) [Citation10].
3. Results
3.1. Sociodemographic and clinical characteristics of CML patients
A total of 118consecutive CML patients were enrolled between August 2021 and September 2022 and were included in this analysis, 85 from Brazil and 33 from the US, with median age was 56.8 years in the Brazilian cohort (range 33–85) and 57.4 years in the US cohort (range 26–80) (p = .55). Overall, demographics and clinical features of patients from both centers were similar and are presented in .
Table 1. Sociodemographic and clinical characteristics of patients.
3.2. SARS-CoV-2 infection and vaccination
Before receiving the COVID-19 vaccine, 14.1% of patients in the Brazilian cohort and 9.1% from the US cohort reported a prior SARS-CoV-2 infection (p = .553). There were no significant differences in the severity of COVID-19, with most of the patients in both groups reporting mild disease (91.7% vs. 66.7%, p = .371) following infection. There was a slight increase in SARS-CoV-2 infection rates following COVID-19 vaccination with two or three doses in both groups (24.7% vs. 27.3%) (p = .816), with most of the patients reporting mild symptoms (95.2% vs. 100%, p = 1) ().
Table 2. SARS-CoV-2 infection and vaccination among CML patients.
We identified a significant difference in COVID-19 vaccine brands received between the two cohorts (p < .001). In Brazil, the most frequently administered brands for first and second doses were Oxford/AstraZeneca (68.2%) and Sinovac/Butatan (28.2%); all patients received the same brand for both doses. For the third dose, the majority received Pfizer–BioNTech (55.3%), followed by Sinovac/Butatan (17.6%) and Oxford/AstraZeneca (11.8%) (p < .001). In the US cohort, the brands Moderna and Pfizer-BioNTech were the most frequently administered for the first and second doses (Moderna (63.6%) and Pfizer–BioNTech (33.6%)). The same brand was administered for both doses to all patients. The distribution was similar for the third dose((Moderna (63.6%) and Pfizer–BioNTech (30.6%)). Notably, despite these differences, a significant proportion of patients in both cohorts had received three doses at the time of sample collection, with 63.5% in the Brazilian cohort and 77.3% in the US ().
3.3. Seroconversion rates after COVID-19 vaccination
3.3.1. Brazilian cohort
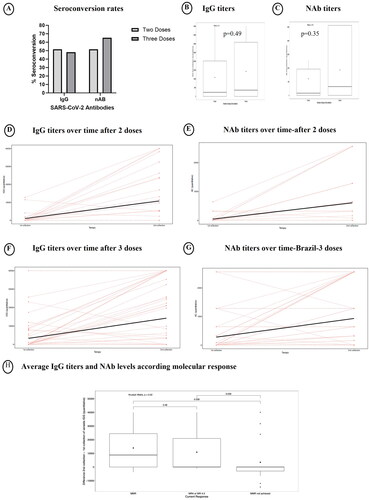
Patients had a first sample collection one to three months after receiving two doses of COVID-19 vaccine. A subsequent sample was obtained at least six months following the initial collection. At this moment, 31 patients had received two doses of the vaccine, while 54 had received a booster dose. We assessed seroconversion rates following vaccination and analyzed the outcome for patients who had received two doses (n = 31) with those who had received three doses (n = 54). SARS-CoV-2 antibodies, including IgG and NAb, were detected in 51.6% of the patients in the two-dose group. Among the patients that had received three doses, IgG antibodies were detected in 48.1% while NAb were detected in 65.2% ().
While patients who received three doses of vaccine showed higher IgG and NAbtiters, these differences were not statistically significant (IgG: 10,770.7 vs. 14,226.2, p = .49; NAb: 606.5 vs. 930.4, p = .35)(). We then examined the changes in antibody levels over time. The median time between the first and second collection was 158.6 days (range 47–329). The median time between the first collection and the antibodies evaluation of patients who received two and three doses were 77.9 and 82.5 days (p = .3). Regarding the second collection, the median time were 216.5 and 243.6 days (p = .067). Among patients who received two doses of vaccine, we observed a significant increase in both IgG and NAb titers from the first to the second vaccine, with a median of 9755.9UI/mL for IgG (p = .001) and 561.3 for NAb titers (p = .001) (). Similarly, there was a significant rise over time for patients who received three doses. The median difference in IgG titers between the collections was 10,940.9 UI/mL (p = .001) and for NAb titers, it was 653.3 (p = .001)(). Among the cohort of 22 patients who contracted COVID-19 subsequent to the initiation of vaccination, five patients had the infection prior to receiving the third vaccine dose. The brands of vaccines administered at the onset of vaccination and during the booster dose are shown in . No differences were observed in the average levels of IgG and Nab among different vaccine brands for any dose. The distribution of COVID-19 vaccine brands is showed in . The average IgG titers and NAb levels were 11,460.8, 10,953.2, −8550.9, and 0 IU/mL, respectively (p = .096). For NAb, the levels were 568.3, 695.2, −640, and 0, respectively (p = .282). The IgG titers and NAb levels were 6417.4, 9284.5, 13,259.7, 6701.5, and 6013.5 IU/mL, respectively (p = .813). For NAb, the levels were 341.3, 528, 772.8, 230, and 560, respectively (p = .984).
3.3.2. USA cohort
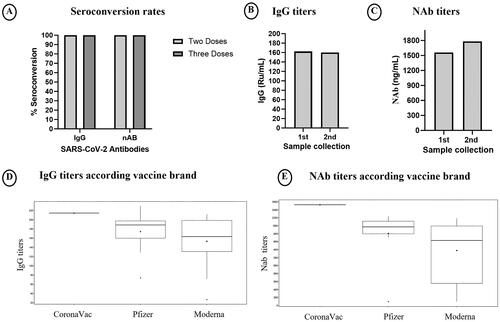
The majority (94%) of patients had already received three vaccine doses by the time we did the first sample collection. Interestingly, in this population, all patients exhibited detectable levels of IgG and NAb, whether they had received two or three doses (). Regarding the post-vaccination antibody levels, 33 patients were evaluated, and the average IgG titers were 162.2 Ru/mL, while NAb levels were 1560.5 ng/Ml (). For the analysis of antibody levels over time, we evaluated 22 patients; antibody levels remain at similar levels, with IgG at 160.2 IU/mL and NAbat 1782.9 ng/Ml (). The vaccine brands administered at the initiation of vaccination and during the booster dose are detailed in . The IgG and NAb titers were similar regarding the vaccine brand.
Regarding the brand of COVID-19 vaccine used, the average IgG titers were 214.4, 174.4, and 153.3 Ru/mL for the CoronaVac, Pfizer, and Moderna vaccines, respectively. The NAbtiters were 2526, 1805, and 1377 ng/mL for the same vaccines, respectively (p = .167; p = .097). The respective IgG and NAb levels were 214.4, 173.2, and 153.3 Ru/mL and 2526, 1769.9, and 1377.5 ng/mL, respectively (p = .31; p = .129). IgG titers were similar between the 21 patients who received three doses of the Moderna vaccine, compared to the 12 patients that received Pfizer or CoronaVac (p = .31) (). In the US cohort, out of nine patients with COVID-19 following the initiation of vaccination, only 1 exhibited infection prior to receiving the third dose.
3.4. Association of SARS-CoV-2 antibody titers and sociodemographic and clinical features
We evaluated whether there was an association between antibody titers and sociodemographic and clinical features. We also evaluated antibody titers over time, according to the variance between the second and first antibody collections. Consequently, patients exhibiting a decrease in antibody titers over time were categorized as having negative antibody titers in this analysis. Since for the US cohort all patients demonstrated seroconversion, this analysis was done only for the Brazilian cohort. The average IgG and NAb antibody titers, respectively, did not exhibit statistical significance based on patients’ age (p = .26; p = .17) and additional characteristics (Supplementary Table 1). There was a significant difference based on race, where patients of African descent exhibited lower antibody titers compared to other racial populations. Interestingly, the average IgG titers and NAb levels were higher in patients who achieved at least an major molecular response (MMR) compared to those who had not achieved MMR (). Predictive factors of SARS-CoV-2 antibody seroconversion.
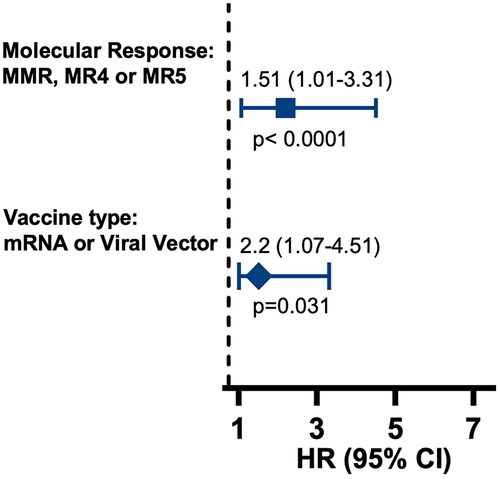
Given the differences in SARS-CoV-2 antibody seroconversion rates between Brazilian and US populations with similar clinical features, we investigated other associated factors. We analyzed quantitative IgG and NAb results, categorizing them as positive or negative based on manufacturer guidelines. Logistic regression identified vaccine type and current CML treatment response as independent predictors for higher seroconversion rates. Higher seroconversion rates were observed in patients who received mRNA vaccines (Moderna and Pfizer-BioNTech) and recombinant viral vector vaccines (AstraZeneca and Janssen) compared to those who received the inactivated virus vaccine (CoronaVac) (HR: 2.20; 95% CI 1.07–4.51; p < .031). Patients who achieved MMR or better response (MR 4.0, 4.5 or 5.0) showed significantly higher seroconversion incidence compared to those patients who did not achieve MMR (HR: 1.51; 95% CI 1.01–3.31; p < .0001) (). The median time between the vaccination and the first collection were 80.8 days in Brazil and 336.2 days in the US (p < .001). Regarding the second collection, the median were 233.7 days in Brazil and 436.8 days in the US (p < .001).
4. Discussion
Previous research focusing on patients with hematological malignancies has highlighted the crucial role of humoral responses in preventing SARS-CoV-2 infection, with the cellular response linked to the severity of the disease [Citation11]. Nevertheless, there is limited knowledge about vaccine-induced seroconversion according to a wide range of vaccine brands specifically in CML patients, a distinct hematologic malignancy. This study presents an assessment of a cohort of 118 CML subjects from two countries with distinct healthcare systems who received different brands of SARS-CoV-2 vaccines. Our findings demonstrated the efficacy of five different vaccines in mitigating the severity of COVID-19 in CML patients, despite variations in the vaccine brand administered. In addition, we showed that patients receiving mRNA and recombinant viral vector vaccines, as well as those with at least MMR, exhibit the highest seroconversion rates following vaccination.
We evaluated two cohorts of CML subjects treated at different cancer centers with similar clinical characteristics. Most of the patients were males in the sixth decade of life aligning with CML patients profile reported in the literature [Citation12]. Although comorbidities were more prevalent in American patients, a significant proportion (at least 30%) of Brazilian patients had comorbidities at the time of CML diagnosis, with rates increasing with age and correlating with the use of other medications [Citation13]. In both cohorts, most patients were in the chronic phase, as is common in most CML patient cohorts [Citation14]. CML treatment discrepancies were evident between the two countries; most Brazilian patients were receiving imatinib, reflecting its status as the first-line therapy in Brazil’s public healthcare system, while second-line TKIs were more commonly administered in the US where all second-generation TKIs are approved and available for first-line treatment [Citation15,Citation16]. Of note, despite these treatment variations, the majority of patients in both cohorts were on the first line of treatment and achieved at least aMMR.
Prior to vaccination, approximately 14.1% of the Brazilian cohort and 9.1% of US patients had contracted COVID-19, primarily experiencing mild severity of disease. Similar rates of infection were reported in other cohorts of CML patients from the UK (11.2%) and Brazil (12.4%) [Citation17,Citation18]. Conversely, broader Latin American patient data indicated a lower COVID-19 prevalence (2.3%) and a mortality rate of (11.9%) before vaccines, with a worse survival in patients not in MMR [Citation19]. The CANDID study reported a mortality rate of 13.7% after assessing 110 CML patients from 20 countries [Citation20]; this mortality rate was higher than observed in our study. This difference likely is derived from the wider range of institutions and countries represented in CANDID, while our study is based in two specialized academic institutions. Concerning post-vaccination COVID-19 incidence, we observed rates of 24.7% in Brazil and 27.3% in the US, with all patients experiencing mild infections. An analysis from Spain unveiled a 34.4% infection rate among vaccinated patients who contracted mild infections, mirroring our cohort findings [Citation21].
The variations in vaccine brands between the Brazilian and US cohorts are reflective of global differences based on each country’s access and regulatory decisions. Initially, Brazil administered CoronaVac and AstraZeneca vaccines, while the US predominantly utilized Moderna and Pfizer–BioNTech. The approved vaccine brands also differ in Brazil and are from Pfizer, CoronaVac, AstraZeneca, and Janssen [Citation22], whereas in the US, the brands approved are Moderna, Novavax, Pfizer-BioNTech, and Spikevax. According to World Health Organization, Pfizer–BioNTech and AstraZeneca are the most prevalent brands globally [Citation23].
Interestingly, differences were observed not only in the administered vaccine types but also in the seroconversion rates. The Brazilian cohort presented lower seroconversion rates compared to the US cohort and other populations reported in the broader literature. Despite similarities in baseline characteristics between cohorts, their distinctive use of DNA and inactivated virus vaccines sets them apart. There is limited literature addressing these specific vaccines, as most studies focus on mRNA vaccines potentially impacting vaccination effectiveness. Harrington et al. [Citation24] reported a seroconversion rate of 87.5% in 16 CML patients. A cohort from Spain with 29 CML patients and healthy individuals showed increased IgG levels and NAb after receiving Spikevax, Pfizer, or Vaxzevria [Citation21]. Notably, all CML patients in treatment-free remission (TFR) achieved neutralizing activity, surpassing 96% of those under TKI treatment and 85% of healthy individuals. Claudiani et al. evaluated 54 CML patients following two doses of Pfizer or AstraZeneca vaccine, revealing a seropositivity rate of 96%. While IgG levels decreased over time in patients, there was no significant association with the disease or TKI treatment [Citation25]. Another cohort of 43 CML patients showed a 96.3% seroconversion rate six weeks after CoronaVac or Pfizer vaccination. Despite higher IgG levels after Pfizer vaccination, the difference was not statistically significant, and both vaccines exhibited similar decreases in IgG levels over time. As found in our analysis, no association with TKI type was identified in other studies [Citation26].
Interestingly, we found that African American patients exhibited lower antibody titers compared to Caucasian and multiracial patients. Few studies have evaluated the efficacy of vaccines based on ethnicity. A meta-analysis showed 94–100% efficacy in non-Caucasian healthy individuals, although they constitute a minority of analyzed patients (less than 20% in most of the studies) [Citation27]. Concerning CML status, higher IgG titers were observed in patients who achieved at least aMMR. This result contrasts with findings from a UK cohort, where no differences were identified according to CML status for either AstraZeneca or Pfizer vaccines [Citation25].
Our analysis has limitations that need acknowledgment, several of which are intrinsic to the temporal context in which it was conducted. The number of patients is relatively small and there was variability across centers, underscoring the challenges encountered in patient recruitment during the pandemic period. The methodologies used to assess antibody response also varied. As a real-world study, we studied vaccines based on country availability, posing challenges for individual analysis. In addition, serological tests were not conducted before enrollment, potentially including COVID-19 asymptomatic cases. The timing of sequential testing varies between individuals and also in relation to vaccine administration, making full evaluation of the titers over time limited. Despite these limitations, our study is an extensive evaluation of serological responses to five different brands of SARS-CoV-2 vaccines in CML patients to date and a valuable comparison of patients in two different countries with very different patient populations and healthcare systems.
In conclusion, despite significant difference between the Brazilian and US healthcare systems, vaccinated CML patients generated antibody responses effectively protecting against severe disease. This effect is more pronounced in patients with well-controlled CML who achieve an MMR, emphasizing the value of vaccination for this patient population as COVID-19 remains prevalent throughout the world.
Supplemental Material
Download MS Word (16.9 KB)Acknowledgments
The authors express their sincere gratitude to Rhea-Beth Markowitz for her meticulous review of the English grammar and language usage in this manuscript. Special thanks are extended to all patients and collaborators from the Centro de Hematologia e Hemoterapia (Hemocentro) da Universidade Estadual de Campinas (UNICAMP)(Brazil) and the Georgia Cancer Center at Augusta University (US) who contributed to data collection and analyses.
Disclosure statement
K.P. reports participation in advisory boards for AbbVie, Sandoz, Novartis, and Pfizer; and honoraria (lecture) from Pint Pharma, EMS, Novartis, Teva, and Pfizer. J.C. is a consultant for Pfizer, Novartis, Takeda, Tern Pharma and Sunb Pharma; receives research support to his institution from Pfizer, Novartis, Sun Pharma, Tern Pharma. A.C.M.T., M.M.G., M.S., M.A.C., E.M., L.F., A.S.S.D., A.B., G.D, S.S.M., F.P., B.B., R.K., K.J., H.S., J.F., A.V., R.K., S.T.O.S., O.S, C.A.D., have no conflicts of interest with respect to this research study.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Langerbeins P, Hallek M. COVID-19 in patients with hematologic malignancy. Blood. 2022;140(3):236–252. doi:10.1182/blood.2021012251
- Pagano L, Salmanton-García J, Marchesi F, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):168. doi:10.1186/s13045-021-01177-0
- Teh JSK, Coussement J, Neoh ZCF, et al. Immunogenicity of COVID-19 vaccines in patients with hematologic malignancies: a systematic review and meta-analysis. Blood Adv. 2022;6(7):2014–2034. doi:10.1182/bloodadvances.2021006333
- Ahluwalia P, Vashisht A, Singh H, et al. Ethno-demographic disparities in humoral responses to the COVID-19 vaccine among healthcare workers. J Med Virol. 2023;95(9):e29067.
- Uaprasert N, Pitakkitnukun P, Tangcheewinsirikul N, et al. Immunogenicity and risks associated with impaired immune responses following SARS-CoV-2 vaccination and booster in hematologic malignancy patients: an updated meta-analysis. Blood Cancer J. 2022;12(12):173. doi:10.1038/s41408-022-00776-5
- Gong IY, Vijenthira A, Betschel SD, et al. COVID-19 vaccine response in patients with hematologic malignancy: a systematic review and meta-analysis. Am J Hematol. 2022;97(4):E132–E135. doi:10.1002/ajh.26459
- Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–953. doi:10.1038/s41562-021-01122-8
- Santos CVBD, Valiati NCM, Noronha TGd, et al. The effectiveness of COVID-19 vaccines against severe cases and deaths in Brazil from 2021 to 2022: a registry-based study. Lancet Reg Health Am. 2023;20:100465. doi:10.1016/j.lana.2023.100465
- Rotshild V, Hirsh-Raccah B, Miskin I, et al. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11(1):22777. doi:10.1038/s41598-021-02321-z
- Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018.
- Piechotta V, Mellinghoff SC, Hirsch C, et al. Effectiveness, immunogenicity, and safety of COVID-19 vaccines for individuals with hematological malignancies: a systematic review. Blood Cancer J. 2022;12(5):86. doi:10.1038/s41408-022-00684-8
- Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–984. doi:10.1038/s41375-020-0776-2
- Cortes J. How to manage CML patients with comorbidities. Hematology Am Soc Hematol Educ Program. 2020;2020(1):237–242. doi:10.1182/hematology.2020006911
- Rinaldi I, Winston K. Chronic myeloid leukemia, from pathophysiology to treatment-free remission: a narrative literature review. J Blood Med. 2023;14:261–277. doi:10.2147/JBM.S382090
- Saúde M. Protocolo clínico e diretrizes terapêuticas leucemia mieloide crônica do adulto. Diário Oficial da União, Portuguese. Publicação do DIÁRIO OFICIAL: Ministério da Saúde (BR), Gabinete do Ministro. PORTARIA CONJUNTA N° 4, DE 1° DE MARÇO DE 2021; 2021. Diário Oficial da União, Brasília, DF. Available from: https://www.gov.br/saude/pt-br/assuntos/protocolos-clinicos-e-diretrizes-terapeuticas-pcdt
- Held N, Atallah EL. Real-world management of CML: outcomes and treatment patterns. Curr Hematol Malig Rep. 2023;18(5):167–175. doi:10.1007/s11899-023-00703-w
- Claudiani S, Rosadas C, McClure M, et al. Prevalence of Sars-Cov-2 infection in patients with chronic myeloid leukemia. Blood. 2020;136(Supplement 1):20–20. doi:10.1182/blood-2020-142454
- Fechio L, Mota AVF, Bortolini J, et al. A survey conducted during COVID-19 pandemic among Brazilian chronic myeloid leukemia and philadelphia-negative myeloproliferative neoplasms patients. Blood. 2021;138(Supplement 1):4601–4601. doi:10.1182/blood-2021-151168
- Pagnano KB, Peralta EH, Navarro JR, et al. COVID-19 in chronic myeloid leukemia patients in Latin America. Leuk Lymphoma. 2021;62(13):3212–3218. doi:10.1080/10428194.2021.1950709
- Rea D, Mauro MJ, Cortes JE, et al. COVID-19 in patients (pts) with chronic myeloid leukemia (CML): results from the international CML foundation (iCMLf) CML and COVID-19 (CANDID) study. Blood. 2020;136(Supplement 1):46–47. doi:10.1182/blood-2020-140161
- Rodríguez-Mora S, Corona M, Solera Sainero M, et al. Regular humoral and cellular immune responses in individuals with chronic myeloid leukemia who received a full vaccination schedule against COVID-19. Cancers. 2023;15(20):5066. doi:10.3390/cancers15205066
- ANVISA. Vacinas - COVID-19; 2023. Available from: https://www.gov.br/anvisa/pt-br/assuntos/paf/coronavirus/vacinas
- Organization PAH. COVID-19 vaccination in the Americas; 2023. Available from: https://ais.paho.org/imm/IM_DosisAdmin-Vacunacion.asp
- Harrington P, Doores KJ, Radia D, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br J Haematol. 2021;194(6):999–1006. doi:10.1111/bjh.17568
- Claudiani S, Apperley JF, Parker EL, et al. Durable humoral responses after the second anti-SARS-CoV-2 vaccine dose in chronic myeloid leukaemia patients on tyrosine kinase inhibitors. Br J Haematol. 2022;197(1):e1–e4. doi:10.1111/bjh.18001
- Kuan JW, Tan CS, Su AT, et al. Antibody response post-COVID-19 vaccination in patients with chronic myeloid leukemia with comparison between comirnaty and CoronaVac vaccine. Asia Pac J Public Health. 2022;34(6-7):725–727. doi:10.1177/10105395221112836
- Salari N, Vepa A, Daneshkhah A, et al. Efficacy of COVID-19 vaccines by race and ethnicity. Public Health. 2022;208:14–17. doi:10.1016/j.puhe.2022.04.009