Abstract
To quantify the clinical unmet need of r/r MCL patients who progress on a covalent Bruton tyrosine kinase inhibitor (BTKi), we conducted a systematic review to identify studies that reported overall survival (OS), progression-free survival (PFS), or response outcomes of patients who received a chemo(immunotherapy) ± targeted agent standard therapy (STx) or brexucabtagene autoleucel (brexu-cel) in the post-BTKi setting. Twenty-six studies (23 observational; three trials) reporting outcomes from 2005 to 2022 were included. Using two-stage frequentist meta-analyses, the estimated median PFS/OS for patients treated with an STx was 7.6 months (95% CI: 3.9–14.6) and 9.1 months (95% CI: 7.3–11.3), respectively. The estimated objective response rate (ORR) was 45% (95% CI: 34–57%). For patients treated with brexu-cel, the estimated median PFS/OS was 14.9 months (95% CI: 10.5–21.0) and 32.1 months (95% CI: 25.2–41.2), with a pooled ORR of 89% (95% CI: 86–91%). Our findings highlight a significant unmet need for patients whose disease progresses on a covalent BTKi.
Introduction
Mantle cell lymphoma (MCL) is a rare, aggressive type of B-cell non-Hodgkin lymphoma (NHL) that is typically diagnosed in patients aged over 65 years and more often diagnosed in men [Citation1]. Despite advances in diagnostic technologies to reduce the number of false positives, the incidence of MCL in the United States has continued to trend upward since the 1990s and epidemiological estimates suggest that MCL accounts for 2–6% of all NHL diagnoses [Citation2–4]. It is heterogeneously characterized with a range of variants, relevant biological indicators and, consequently, varied treatment pathways [Citation5].
Front-line treatment is typically comprised of chemoimmunotherapy regimens, which have historically been followed by rituximab maintenance [Citation5,Citation6]. The course of treatment is typically risk-stratified according to the mantle cell international prognostic index (MIPI), a composite measure which factors in a patient’s age, performance status, lactase dehydrogenase (LDH), and leukocyte count [Citation7]. Other important biological qualifiers have been identified for MCL, such as the cell proliferation rate, measured as Ki-67, and the presence of a TP53 gene mutation, which are both considered reliable prognostic factors in predicting survival outcomes [Citation5,Citation6,Citation8].
Despite the typically aggressive front-line clinical course which results in adequate responses, patients whose treatments fail historically face a bleak prognosis [Citation7,Citation9]. The introduction of covalent Bruton tyrosine kinase inhibitors (BTKis), marked by the 2013 approval of ibrutinib for the second-line treatment of MCL, has improved clinical outcomes among high-risk patients who progress following front-line therapy [Citation10]. The approval for ibrutinib cited a pivotal single-arm trial (NCT01236391) and its results have been further supported by additional studies among patients with intermediate-to-high risk relapsed/refractory (r/r) MCL [Citation11]. Novel BTKis have emerged since, including acalabrutinib (second-generation BTKi) and zanubrutinib, which have demonstrated favorable efficacy and safety profiles [Citation12,Citation13]. Yet, treatment with BTKi is not without complications and, while treatment may yield initial success, long-term BTKi use, such as with ibrutinib, is characterized by difficulties in maintaining long-term remission, development of treatment resistance, and reports of off-target effects such as cardiac arrhythmias [Citation10,Citation14,Citation15].
While the development of covalent BTKis has improved the outcomes of patients who progress following front-line therapy, the issue of a poor prognosis continues to be a challenge for patients with r/r MCL who progress on a BTKi. For these patients, most chemotherapy salvage treatments are ineffective and durations of response, if achieved, are short [Citation14,Citation16]. Although this is well understood, the unmet clinical need for these patients is poorly quantified in the literature. As the use of BTKis has increased, studies which quantify this potential unmet need have become increasingly important to support clinicians and decision makers in understanding how emerging treatments are addressing this gap.
Until recently, impactful and reliable treatments for these patients have been elusive. The chimeric antigen receptor (CAR) T-cell therapy brexucabtagene autoleucel (brexu-cel) has been heralded as a breakthrough in this clinical space, with strong outcomes in the international, single-arm ZUMA-2 clinical trial [Citation17]. The success of this trial led to the approval of brexu-cel for adult patients with r/r MCL who had previously been treated with two lines of therapy, including a BTKi, by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2020 [Citation18,Citation19]. In addition to improving survival, brexu-cel appears to lead to a durable response. Despite the encouraging results, some researchers have expressed concern over the size and duration of the trial [Citation20]. To this end, a growing literature of the real-world use of brexu-cel in r/r MCL could help strengthen the conclusions of ZUMA-2.
The objective of this systematic review and meta-analysis was to characterize and quantify the unmet need in patients treated with a chemo(immunotherapy) ± targeted agent standard therapy (STx). Secondarily, to contrast these outcomes, studies of patients treated with brexu-cel in this setting were similarly identified and synthesized.
Methods
This systematic literature review and meta-analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation21].
Literature search
Systematic searches of MEDLINE, EMBASE, and Evidence-Based Medicine Reviews (EBM Reviews) were conducted on 26 October 2022. These searches were supplemented by hand searches of the conference proceedings from the 2022 American Society of Hematology (ASH) annual meeting which became available after the database search date but were anticipated to include several publications of relevance and importance. Study eligibility was determined using pre-defined PICOS (population, intervention, comparator, outcomes, and study design) criteria. Eligible studies included adults with r/r MCL who were receiving a post-BTKi STx (defined as chemo(immunotherapy) ± a targeted agent) or brexu-cel following treatment with a BTKi. To be considered a post-BTKi study, at least 80% of the patient cohort must have had a prior BTKi exposure [Citation22]. The list of eligible interventions was extended to include any non-experimental systemic therapy, with or without concomitant interventions such as radiotherapy. All prospective and retrospective designs, including both observational studies and trials, were eligible for inclusion. Our motivation for including both clinical trial designs and real-world data was that while clinical trials can provide insights into the efficacy and safety of treatments, real-world studies provide further insights into unmet needs by reflecting outcomes in routine clinical practice. The outcomes of interest included three time-to-event outcomes – overall survival (OS), progression-free survival (PFS), duration of response (DOR) – and three dichotomous outcomes – objective response rates (ORRs), complete response (CR), and partial response (PR). Study eligibility was restricted to records published in English. As this systematic literature review was designed as an extension and expansion to a previously published review and meta-analysis [Citation23], the search syntax was restricted to identify records published from November 2019 onward. A detailed search strategy is provided in Table S1.
Study selection and data extraction
All study selection and data extraction steps were conducted by two reviewers working independently and in duplicate in accordance with PRISMA guidelines [Citation24]. Titles and abstracts, followed by the full-text publications of included abstracts, were evaluated against the review’s eligibility criteria. In situations where consensus could not be reached between the two reviewers, a third reviewer provided arbitration. Following the identification of eligible publications, related publications reporting on common patient cohorts were linked or ‘mapped’ prior to data extraction to avoid double-counting of outcomes in the planned meta-analyses. These linkages were established based on characteristics such as references to a common study protocol, institution names, sample sizes, and interventions.
Data extraction of study characteristics, patient characteristics, and outcomes was completed at the study-level using standardized, piloted data extraction templates from all mapped publications. Where published Kaplan–Meier (KM) curves were available, curve data was digitized using WebPlotDigitizer-4.5 (https://automeris.io/WebPlotDigitizer) and the corresponding numbers at risk were recorded for reconstruction of pseudo-individual patient data (IPD) according to the Guyot algorithm [Citation25].
The quality of studies was assessed according to the Newcastle-Ottawa Scale (NOS), which is appropriate for observational prospective and retrospective studies as well as single-arm, non-comparative trials. The use of the NOS facilitated comparisons of methodological rigor across the evidence space as, while some studies may be initiated as randomized trials of BTKi, these also effectively become observational once patients progress to the post-BTKi setting. All data extraction and critical appraisal steps were completed independently and in duplicate by two reviewers. Discrepancies were resolved by discussion or, if a consensus could not be reached, through arbitration provided by a third reviewer.
Statistical analyses
Meta-analyses were used to estimate survival curves and response proportions. Whereas most meta-analyses aim to estimate treatment effects (i.e. to compare treatments), our aim was to estimate the clinical outcomes among r/r MCL patients receiving a post-BTKi STx – or receiving brexu-cel in the secondary analyses. Time-to-event outcomes (OS, PFS, and DOR) were analyzed using multivariate meta-analysis of survival function parameters as developed by Cope et al. [Citation26]. For each survival curve, the pseudo-IPD were analyzed using alternative survival distributions – namely Weibull, log-normal, log-logistic, Gompertz, and exponential distributions. For each distribution, the survival parameters were meta-analyzed to fit an OS curve for the collection of curves. The goodness-of-fit was statistically assessed according to the Akaike information criteria (AIC) and supplemented by visual inspection. Uncertainty about the point estimates was described with the 95% confidence interval (CI) based on bootstrapping with 1000 samples. While all studies reporting survival outcomes were included in the review and narrative summary, only studies with available KM curves were included in the meta-analysis. From the pooled survival distributions, estimates of median survival as well as survival proportions at 12 and 24 months were made.
Dichotomous response outcomes (ORR, CR, and PR) were meta-analyzed to generate a point estimate pooled proportion with 95% CI. Heterogeneity in the evidence for each outcome was assessed according to τ and further qualified by the I2 statistic [Citation27]. All meta-analyses were conducted under a frequentist framework and included evaluations of both random and fixed effects models. All analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Evidence base
The systematic searches identified 1460 publications from the bibliographic databases, with 37 records satisfying all eligibility criteria. The evidence selection process is summarized in . A further five publications were identified through conference hand searches and supplemental searches. Ten publications from an earlier iteration of this review were also included [Citation23]. In total, 52 publications describing 26 studies were included in the evidence base, with 19 and 7 studies describing outcomes of patients treated with STx and brexu-cel, respectively ().
Figure 1. PRISMA flow of information for study selection. *Search dates purposefully overlapped with the previous iteration of this literature review.
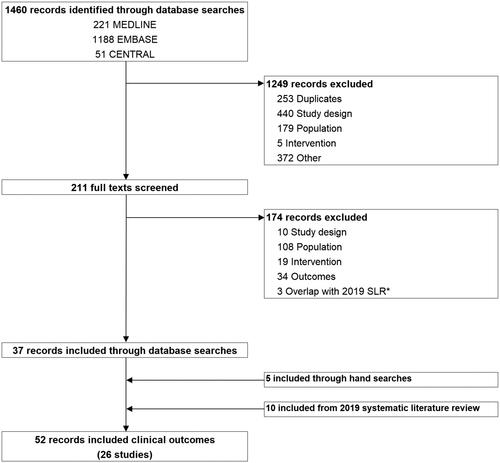
Table 1. Studies of chemo(immunotherapy) ± targeted agent standard therapy and brexucabtagene autoleucel in the post-BTKi setting.
Across the evidence base, most studies (17/26) were based at centers in North America. Two large international trials, ZUMA-2 [Citation66] and MCL-3001 [Citation34], were also included and recruited patients primarily from South America, North America, and Europe. Study protocols were initiated at different times, with 12 studies enrolling patients for BTKi administration and reporting downstream outcomes post-BTKi and 14 studies initiated in the post-BTKi setting. Most studies were of a retrospective observational design (23/26), with two (MCL-3001 [Citation34]; VALERIA [Citation56]) and one (ZUMA-2 [Citation66]) single-arm trials in the STx and brexu-cel settings, respectively. All studies of brexu-cel were initiated post-BTKi. Study outcome availability is summarized in Table S2.
Critical appraisal of the included studies did not reveal any methodological concerns that would distort outcome signals (Table S3).
Chemo(immunotherapy) ± targeted agents
For the primary analyses, outcomes from chemo(immunotherapy) ± targeted agent standard therapies were reported in 19 studies (N = 1238). A heterogeneous mix of interventions was reflected in the evidence base. Studies were comprised of cohorts treated with a common intervention as well as cohorts comprised of patients receiving one of several systemic treatments, including chemotherapy, immunotherapy, combination chemotherapy with immunotherapy, and with or without radiotherapy.
Fourteen studies (N = 1080) reported OS outcomes for post-BTKi standard therapies, where the median OS varied from 2.5 to 19.4 months. A log-normal distribution was fit to the published survival curves from 10 studies (n = 669), leading to an estimated median OS of 9.1 months (95% CI: 7.3–11.3), as illustrated in . Survival estimates at 12 and 24 months were 42% and 25%, respectively ().
Figure 2. Meta-analysis of Kaplan–Meier curves from reconstructed individual patient data for overall survival and progression-free survival. (A) Meta-analysis of overall survival outcomes from studies of chemo(immunotherapy) ± targeted agent standard therapy, summary curve based on a log-normal distribution; (B) meta-analysis of progression-free survival outcomes from studies of chemo(immunotherapy) ± targeted agent standard therapy, summary curve based on an exponential distribution; (C) meta-analysis of overall survival outcomes from studies of brexucabtagene autoleucel, summary curve based on an exponential distribution; (D) meta-analysis of progression-free survival outcomes from studies of brexucabtagene autoleucel, summary curve based on a log-normal distribution.
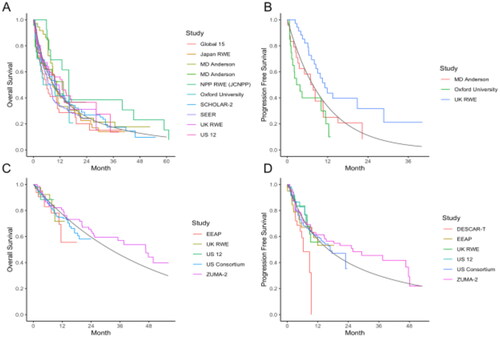
Table 2. Summary of available evidence and meta-analyses.
Progression-free survival was sparsely reported in the STx setting during the time period under review. Across the five studies (n = 169) where median PFS was reported, estimates varied from 1.9 to 10.1 months. Published KM curves were available from three studies (n = 80) and an exponential distribution was used to estimate a pooled median PFS of 7.6 months (95% CI: 3.9–14.6) as visualized in . The estimated PFS at 12 and 24 months was 33% and 11%, respectively (). Meta-analyses of OS and PFS were not sensitive to the selection of model distribution.
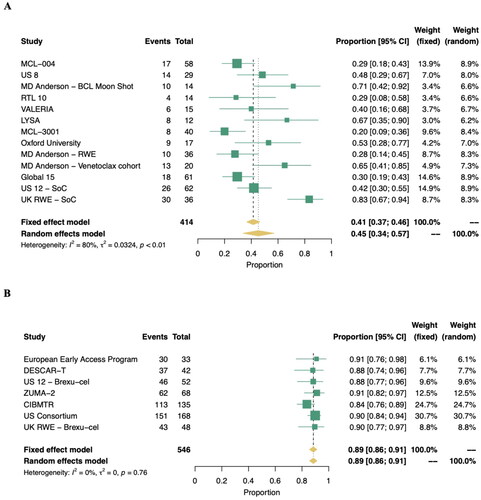
Thirteen studies (n = 414) were included in the meta-analysis of ORR, with a random effects pooled percentage of 45.3% (95% CI: 33.9–56.9%) as illustrated in . The pooled estimate was characterized by a significant level of heterogeneity (I2: 80%, p < .01). Eleven studies (n = 360) were included in meta-analyses of CR and PR, with 23.1% (95% CI: 13.9–33.6%) and 22.8% (95% CI: 16.3–30.0%) of patients achieving a complete or PR, respectively (). High heterogeneity was also observed in the analyses of both CR and PR, similar to the meta-analysis of ORR, and reflective of differences in standard therapies, treatment histories, and clinical settings. Among responders to a STx, the DOR was similar to PFS. Six studies reported DOR outcomes, and a KM survival curve was only available in a single publication [Citation43]. In this study, the 9/17 patients who achieved a response with venetoclax maintained response for a median 8.1 months (95% CI: 2.8–9.8).
Brexucabtagene autoleucel
For the secondary analyses, outcomes for patients treated with brexu-cel were available from seven studies (N = 607). While most studies enrolled only BTKi-exposed patients, two retrospective studies enrolled mixed cohorts with 86% and 89% of patients treated with a BTKi [Citation57,Citation62]. Most studies (4/7) were conducted in North America, with others enrolling patients at European sites. Primary analyses are based on outcomes for infused cohorts, as these were more consistently reported in the literature, with secondary analyses based on intent-to-treat populations.
Seven studies reported brexu-cel OS outcomes (N = 546), with observed 6-month survival estimates varying from 79% to 93%. The pooled median OS, when an exponential distribution was fit to the published survival curves from five studies, was 32.1 months (95% CI: 25.2–41.2; n = 371) as illustrated in . Survival estimates at 12 and 24 months were 77% and 60%, respectively (). Progression-free survival was available in all seven studies (N = 470), with observed 6-month PFS estimates varying from 58% to 83%. Based on the six studies with published KM curves (n = 418), a log-normal distribution was used to estimate a pooled median PFS of 14.9 months (95% CI: 10.5–20.9), as illustrated in . Estimates of PFS at 12 and 24 months were 56% and 37%, respectively (). Across all analyses, the estimates for OS and PFS were not sensitive to the selection of the survival model. Analyses of OS and PFS based on intent-to-treat populations, referring to patients who underwent leukapheresis, were feasible with data from three studies (N = 299) and generated similar estimates of effect (Table S4).
Response outcomes (ORR, CR, and PR) were available from all seven studies of brexu-cel (N = 546; ). The pooled ORR was 88.6% (95% CI: 85.7–91.2%) based on a random effects model and characterized by an absence of heterogeneity (I2: 0%) which is indicative of a strong and consistent signal of effect. This ORR estimate was primarily driven by CR, with a pooled rate of 74% (95% CI: 68–80%; I2: 68%, p = .03). Duration of response KM curves was available from two studies, with a pooled median estimate of 22.3 months (95% CI: 16.1–30.1; n = 213) based on a log-normal distribution. An estimated 64% and 48% of patients maintained response at 12 and 24 months, respectively.
Discussion
Our systematic literature review and meta-analysis quantified and confirmed a clear and persistent unmet clinical need. Treatment options have historically been limited for patients in the r/r MCL setting. For patients treated with a chemo(immunotherapy) ± targeted agent STx, median survival estimates were consistently short and fewer than half of patients responded to treatment. These observations are based on the collective findings of several studies, which together reflect the heterogeneous and diverse clinical nature of this setting. Not surprisingly, these up-to-date findings echo that of earlier research in this field that has maintained urgent calls for more effective treatments.
The emergence of BTKi therapies has considerably improved patient outcomes among r/r MCL patients and provides a genuine second-line option. Indeed, clinical guidelines for the treatment of MCL, including guidelines by the National Comprehensive Cancer Network (NCCN), list BTKi as a preferred second-line treatment for advanced MCL [Citation76]. Since the introduction of ibrutinib, further developments have been made, with the approval of second-generation and next-generation BTKis [Citation77]. Second-generation BTKi therapies provide less off-target activity, which translates to fewer adverse events, while next-generation BTKis have been shown to be effective across high-risk groups. Nonetheless, numerous studies have suggested that patients who progress while receiving BTKi therapy have diminished outcomes, culminating in short survival. The aforementioned NCCN guidelines do not provide a preferred third-line treatment, but do acknowledge the use of brexu-cel for this setting [Citation76]. Our study provides a definitive, contemporary overview of the unmet need for these patients, by identifying and synthesizing all available evidence. While the studies were heterogeneous, and the source of this heterogeneity was not immediately clear, they were consistent in demonstrating an unmet clinical need. Our secondary analyses also demonstrated the potential role that brexu-cel can play in addressing this need.
The promising clinical outcomes of patients treated with brexu-cel mark a significant improvement over historically poor clinical outcomes in this therapeutic space. The results of our systematic literature review show that there is a growing evidence base that supports the strong improvements in OS and DOR observed in patients treated with brexu-cel. Not only does this review offer a much larger sample size relative to ZUMA-2, but the results were consistent and outcomes in the real-world settings aligned well with those reported in the trial setting. This reinforces the clinical utility of brexu-cel across the broader clinical setting found in the real-world where patients have different comorbidities, prognostic and risk factors, and treatment histories, as well as responses to those previous treatments. To this end, clinicians managing patients in the r/r MCL setting often weigh the value of clinical predictors in decision-making. Several host factors, disease characteristics, and pathobiologic factors that may be prognostic for an increased risk in non-response or a loss-of-response to BTKi have been identified [Citation78]. For instance, recent literature exploring prognostic factors following ibrutinib monotherapy found that many patients were considered high-risk based on the simplified MIPI (s-MIPI), blastoid morphology, and/or a TP53 mutation [Citation79,Citation80]. Similarly, patients with a Ki-67 index greater than 30% or 50% may have inferior response outcomes [Citation8,Citation47,Citation81]. Further research is needed to identify and understand prognostic factors in the post-BTKi setting, particularly when considering the use of CAR T-cell therapy such as brexu-cel. Some evidence suggests a reduced effect of CAR T-cell therapy on some outcomes among patients with a TP53 mutation [Citation62]. However, other emerging evidence, such as from the ZUMA-2 trial of brexu-cel in r/r MCL, suggests that the impact of many typical front-line prognostic factors, such as those listed above, may not carry as much weight in the context of cellular therapy [Citation17,Citation82]. These large, robust improvements in clinical outcomes mirror those shown by CAR T-cell therapies for other lymphoma indications, such as large B-cell lymphoma [Citation83,Citation84].
Looking forward, we can expect a shift in the post-BTKi population. In the current evidence base, patients treated with ibrutinib and other BTKi have primarily been second-line or higher, meaning that patients in the post-BTKi setting have primarily been in the third- or later line of therapy setting. Recently, first-line approaches with fixed-duration ibrutinib have been published [Citation85,Citation86]. This shift toward first-line use opens the possibility of other solutions to the unmet need. For example, in later relapses after a limited duration treatment, a BTKi re-exposure may be reasonable. However, whether the other salvage treatments (CAR T-cells vs. conventional) display different efficacy, as shown in the current meta-analysis, remains to be seen. Other treatment alternatives, such as allogeneic stem cell transplantation, have demonstrated durable remission for patients in post-BTKi and high-risk feature settings [Citation87,Citation88].
This literature review was designed with several methodological considerations to strengthen the confidence and credibility of its findings. In establishing the scale of the evidence base, deliberate efforts were made to identify studies which may report the outcomes of patients post-BTKi but may be missed through more traditional search and screening strategies. That is, the search strategy was designed primarily around BTKi terminology, with an understanding that investigators in this space would speak to patient treatment exposure in defining their clinical questions. Furthermore, publication of studies which enrolled patients for treatment with a BTKi was eligible for inclusion during the title and abstract screening phase so the full-text publication could be reviewed for post-BTKi outcomes. This approach led to the identification of six publications which would have been otherwise excluded. Several analytical approaches were adopted, including the consideration of multiple survival extrapolation models which were evaluated based on robust criteria. Finally, study credibility was assessed according to the NOS. This instrument was chosen as it is well-suited for evaluating both observational studies as well as non-comparative single-arm trials, making it an ideal tool for this clinical context. Evaluations with the NOS returned few points of methodological concern in the included studies.
While the findings and conclusions of this review are based on robust methodology, there are limitations to consider. Observational studies hold an inherent risk of selection bias in how patients are assigned to treatments, and, in the case of the current review, post-BTKi treatment protocols were not disclosed in the publications. This is primarily applicable to studies which initiate in the BTKi space and then report post-BTKi treatments and outcomes. Similarly, patients who continue to receive treatment following BTKi discontinuation may be healthier and more fit as they are able to continue with further lines of therapy. Despite this supposed clinical advantage, however, studies of chemo(immunotherapy) ± targeted agent STx repeatedly demonstrated failures in treatments achieving their intended effects. It is possible that this represents a selection bias for patients who were recommended to and received CAR T-cell therapy, as these patients may be more clinically fit for this treatment. Similarly, era effects may distort efficacy and effectiveness signals for the chemo(immunotherapy) ± targeted agents STx treatments which were collected over a longer time horizon. Indeed, there have been multiple advances in both active treatment options for earlier lines of therapy as well as for diagnostics and supportive care which contribute to better outcomes today than in the past [Citation89]. Time-to-event analyses were restricted to studies where a published KM curve was available, thus restricting the number of studies which contributed to the meta-analysis. In meta-analyses of KM survival curves, a single survival extrapolation model must be selected to reflect all evidence inputs and, while the chosen model may be the best fit for most studies, it is possible it would not be the best fit for each individual study.
The treatment landscape for post-BTKi r/r MCL is prominently defined by an urgent unmet clinical need, with treatments that fail to generate responses and diminished survival expectations. A growing body of evidence consistently reflects the promising impact of brexu-cel, with high response and duration rates and extended survival. Its real-world uptake and study outside of clinical trials further reinforces the positive impact this novel treatment has had across a more diverse and clinically heterogeneous population. Further research, particularly outside of clinical trial settings, will further the profile for brexu-cel as an answer to the repeated calls for innovative and impactful treatments in the post-BTKi setting.
Author contributions
All authors had full access to all data in the study. All authors take responsibility for the integrity of the data, the accuracy of the data analysis, and the final decision to submit for publication. Study concept and design: all authors. Acquisition, analysis, or interpretation of data: JW, SK, and MJZ. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: all authors. Administrative, technical, or material support: JW, TI, and MW. Study supervision: MD, BS, and MW.
Supplemental Material
Download PDF (167.1 KB)Acknowledgements
David Wennersbusch and Fang Zhu assisted with some of the systematic literature review steps.
Disclosure statement
SK and MJZ report being employed by RainCity Analytics. JW, SWW, TI, JC, IK, and WP report being employed by Kite, a Gilead Company.
Data availability statement
All non-confidential data are available upon request.
Additional information
Funding
References
- Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl. 4):iv62–iv71. doi:10.1093/annonc/mdx223
- Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113(4):791–798. doi:10.1002/cncr.23608
- Epperla N, Hamadani M, Fenske TS, et al. Incidence and survival trends in mantle cell lymphoma. Br J Haematol. 2018;181(5):703–706. doi:10.1111/bjh.14699
- Aschebrook-Kilfoy B, Caces DBD, Ollberding NJ, et al. An upward trend in the age-specific incidence patterns for mantle cell lymphoma in the USA. Leuk Lymphoma. 2013;54(8):1677–1683. doi:10.3109/10428194.2012.760041
- Silkenstedt E, Linton K, Dreyling M. Mantle cell lymphoma – advances in molecular biology, prognostication and treatment approaches. Br J Haematol. 2021;195(2):162–173. doi:10.1111/bjh.17419
- Kumar A, Eyre TA, Lewis KL, et al. New directions for mantle cell lymphoma in 2022. Am Soc Clin Oncol Educ Book. 2022;42:1–15. doi:10.1200/EDBK_349509
- Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–565. doi:10.1182/blood-2007-06-095331
- Scheubeck G, Jiang L, Hermine O, et al. Clinical outcome of mantle cell lymphoma patients with high-risk disease (high-risk MIPI-c or high p53 expression). Leukemia. 2023;37(9):1887–1894. doi:10.1038/s41375-023-01977-y
- Al-Mansour M. Treatment landscape of relapsed/refractory mantle cell lymphoma: an updated review. Clin Lymphoma Myeloma Leuk. 2022;22(11):e1019–e1031. doi:10.1016/j.clml.2022.07.017
- Stephens DM, Spurgeon SE. Ibrutinib in mantle cell lymphoma patients: glass half full? Evidence and opinion. Ther Adv Hematol. 2015;6(5):242–252. doi:10.1177/2040620715592569
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. doi:10.1056/NEJMoa1306220
- Zhou K, Zou D, Zhou J, et al. Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol. 2021;14(1):167. doi:10.1186/s13045-021-01174-3
- Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659–667. doi:10.1016/S0140-6736(17)33108-2
- Cheah CY, Chihara D, Romaguera JE, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol. 2015;26(6):1175–1179. doi:10.1093/annonc/mdv111
- Burger JA. Bruton tyrosine kinase inhibitors: present and future. Cancer J. 2019;25(6):386–393. doi:10.1097/PPO.0000000000000412
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27(8):1209–1213. doi:10.1200/JCO.2008.19.6121
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi:10.1056/NEJMoa1914347
- Food and Drug Administration. FDA approves brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma; 2020. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-brexucabtagene-autoleucel-relapsed-or-refractory-mantle-cell-lymphoma
- O'Reilly MA, Sanderson R, Wilson W, et al. Brexucabtagene autoleucel for relapsed/refractory mantle cell lymphoma: real-world outcomes in the United Kingdom. Blood. 2022;140(Suppl. 1):7519–7521. doi:10.1182/blood-2022-165031
- Mohty R, Kharfan-Dabaja MA. CAR T-cell therapy for follicular lymphoma and mantle cell lymphoma. Ther Adv Hematol. 2022;13:20406207221142133.doi:10.1177/20406207221142133
- Shaw ML. Second-generation BTK inhibitors hit the treatment bullseye with fewer off-target effects. Am J Manag Care. 2020;26(7 Spec No.):SP226–SP227. doi:10.37765/ajmc.2020.88475
- IQWIG general methods. Version 6.1; 2022.
- Dreyling M, Shah B, Wu JJ, et al. Efficacy outcomes following treatment with Bruton tyrosine kinase inhibitors (BTKI) for relapsed/refractory mantle cell lymphoma (R/R MCL): a literature-based meta-analysis. Value Health. 2022;25(7):S609–S610. doi:10.1016/j.jval.2022.04.1695
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(1):b2700. doi:10.1136/bmj.b2700
- Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12(1):9. doi:10.1186/1471-2288-12-9
- Cope S, Chan K, Jansen JP. Multivariate network meta-analysis of survival function parameters. Res Synth Methods. 2020;11(3):443–456. doi:10.1002/jrsm.1405
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
- Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–1563. doi:10.1182/blood-2015-10-673145
- Rai S, Tanizawa Y, Cai Z, et al. Outcomes for recurrent mantle cell lymphoma post-ibrutinib therapy: a retrospective cohort study from a Japanese Administrative Database. Adv Ther. 2022;39(10):4792–4807. doi:10.1007/s12325-022-02258-3
- Rai S, Tanizawa Y, Cai Z, et al. MCL-041: outcomes for recurrent mantle cell lymphoma post-BTK inhibitor therapy in Japan: an administrative database study. Clin Lymph Myeloma Leuk. 2021;21(Suppl. 1):S407–S408. doi:10.1016/S2152-2650(21)01918-2
- Yi JH, Kim SJ, Yoon DH, et al. Real-world outcomes of ibrutinib therapy in Korean patients with relapsed or refractory mantle cell lymphoma: a multicenter, retrospective analysis. Cancer Commun. 2021;41(3):275–278. doi:10.1002/cac2.12150
- Regny C, Oberic L, Guillaume M, et al. Clinical efficacy of the RIBVD regimen for refractory/relapsed (R/R) mantle cell lymphoma (MCL) patients: a retrospective study of the LYSA Group. HemaSphere. 2019;3(S1):193. doi:10.1002/j.2572-9241.2019.tb00081.x
- Wang M, Schuster SJ, Phillips T, et al. Observational study of lenalidomide in patients with mantle cell lymphoma who relapsed/progressed after or were refractory/intolerant to ibrutinib (MCL-004). J Hematol Oncol. 2017;10(1):171. doi:10.1186/s13045-017-0537-5
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778. doi:10.1016/S0140-6736(15)00667-4
- Jain P, Kanagal-Shamanna R, Zhang S, et al. Outcomes of relapsed mantle cell lymphoma patients after discontinuing acalabrutinib. Am J Hematol. 2021;96(5):E137–E140. doi:10.1002/ajh.26109
- Jain P, Zhang S, Kanagal-Shamanna R, et al. Outcomes of acalabrutinib failures in relapsed mantle cell lymphoma. Blood. 2020;136(Suppl. 1):9–10. doi:10.1182/blood-2020-137440
- Jain P, Kanagal-Shamanna R, Zhang S, et al. Outcomes, causes of discontinuation and mutation profile of patients with mantle cell lymphoma who progressed on acalabrutinib. Blood. 2018;132(Suppl. 1):4151. doi:10.1182/blood-2018-99-115179
- Jain P, Kanagal-Shamanna R, Zhang S, et al. Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol. 2018;183(4):578–587. doi:10.1111/bjh.15567
- Jain P, Zhao S, Kanagal-Shamanna R, et al. Non-Bcl2 mutations are predominant in patients (pts) with venetoclax resistant mantle cell lymphoma (MCL) – response and clinical outcomes in ultra-refractory MCL. Blood. 2019;134(Suppl. 1):2815. doi:10.1182/blood-2019-126175
- Zhao S, Kanagal-Shamanna R, Navsaria L, et al. Efficacy of venetoclax in high risk relapsed mantle cell lymphoma (MCL) – outcomes and mutation profile from venetoclax resistant MCL patients. Am J Hematol. 2020;95(6):623–629. doi:10.1002/ajh.25796
- Tucker D, Morley N, MacLean P, et al. The 5-year follow-up of a real-world observational study of patients in the United Kingdom and Ireland receiving ibrutinib for relapsed/refractory mantle cell lymphoma. Br J Haematol. 2021;192(6):1035–1038. doi:10.1111/bjh.16739
- Tucker D, Morley N, Vandenburghe E, et al. Five years on: a real-world observational study of patients receiving ibrutinib for relapsed/refractory MCL from the UK and Ireland. Br J Haematol. 2020;189(Suppl. 1):29.
- Eyre TA, Walter HS, Iyengar S, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica. 2019;104(2):E68–E71. doi:10.3324/haematol.2018.198812
- Cencini E, Mecacci B, Morelli F, et al. Ibrutinib in patients with relapsed/refractory mantle cell lymphoma: a real-life, retrospective, multicenter trial on behalf of the "RTL" (Regional Tuscan Lymphoma Network). Am J Blood Res. 2021;11(4):373–383.
- Hess G, Dreyling M, Oberic L, et al. Real-world experience among patients with relapsed/refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor failure in Europe: the SCHOLAR-2 retrospective chart review study. Br J Haematol. 2022;202(4):749–759. doi:10.1111/bjh.18519
- Di M, Long JB, Kothari SK, et al. Real-world practice patterns and outcomes following Bruton tyrosine kinase inhibitors (BTKi) in older patients with mantle cell lymphoma (MCL): a population-based analysis. Blood. 2022;140(Suppl. 1):10904–10906. doi:10.1182/blood-2022-164959
- McCulloch R, Lewis D, Crosbie N, et al. Ibrutinib for mantle cell lymphoma at first relapse: a United Kingdom real-world analysis of outcomes in 211 patients. Br J Haematol. 2021;193(2):290–298. doi:10.1111/bjh.17363
- McCulloch R, Rule S, Eyre T, et al. Ibrutinib at first relapse for mantle cell lymphoma: a United Kingdom real world analysis of outcomes in 185 Patients. Br J Haematol. 2020;189(Suppl. 1):104–105.
- McCulloch R, Rule S, Eyre TA, et al. Ibrutinib at first relapse for mantle cell lymphoma: a United Kingdom real world analysis of outcomes in 169 patients. Blood. 2019;134(Suppl. 1):3993. doi:10.1182/blood-2019-125947
- McCulloch R, Visco C, Eyre TA, et al. Efficacy of R-BAC in relapsed, refractory mantle cell lymphoma post BTK inhibitor therapy. Br J Haematol. 2020;189(4):684–688. doi:10.1111/bjh.16416
- McCulloch R, Visco C, Frewin R, et al. R-BAC maintains high response rate in mantle cell lymphoma following relapse on BTK inhibitor therapy. Blood. 2019;134(Suppl. 1):3989. doi:10.1182/blood-2019-129829
- Sawalha Y, Goyal S, Switchenko JM, et al. Outcomes of patients with relapsed mantle cell lymphoma treated with venetoclax: a multicenter retrospective analysis. Blood. 2020;136(Suppl. 1):4–6. doi:10.1182/blood-2020-138878
- Epperla N, Hamadani M, Cashen AF, et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma—a "real world" study. Hematol Oncol. 2017;35(4):528–535. doi:10.1002/hon.2380
- Rai S, Hess L, Chen Y, et al. Outcomes for patients with mantle cell lymphoma post-CBTK inhibitor therapy in the United States and Japan: a study of two real-world databases. HemaSphere. 2022;6(Suppl. 3):1024–1025. doi:10.1097/01.HS9.0000847404.28922.1a
- Rai S, Hess LM, Chen Y, et al. Outcomes for patients with mantle cell lymphoma post-covalent BTK inhibitor therapy in the United States and Japan: a study of two real-world databases. Blood. 2021;138(Suppl. 1):4523. doi:10.1182/blood-2021-148265
- Jerkeman M, Kolstad A, Hutchings M, et al. Venetoclax, lenalidomide and rituximab for patients with relapsed or refractory mantle cell lymphoma – the Nordic Lymphoma Group NLG-MCL7 (VALERIA) Phase Ib-II Trial. Blood. 2022;140(Suppl. 1):184–185. doi:10.1182/blood-2022-155654
- Locke F, Hu ZH, Gerson J, et al. Real-world outcomes of brexucabtagene autoleucel (Brexu-Cel) for the treatment of relapsed or refractory (R/R) mantle cell lymphoma (MCL) in the United States (US). HemaSphere. 2022;6(Suppl. 3):1336–1337. doi:10.1097/01.HS9.0000848672.43673.8a
- Herbaux C, Bret C, Di Blasi R, et al. Kte-x19 in relapsed or refractory mantle-cell lymphoma, a ‘real-life’ study from the DESCAR-T Registry and LYSA Group. Blood. 2021;138(Suppl. 1):743. doi:10.1182/blood-2021-148626
- Iacoboni G, Rejeski K, Camacho L, et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood. 2021;138(Suppl. 1):2827. doi:10.1182/blood-2021-153087
- Iacoboni G, Rejeski K, Villacampa G, et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2022;6(12):3606–3610. doi:10.1182/bloodadvances.2021006922
- Romancik JT, Goyal S, Gerson JN, et al. Analysis of outcomes and predictors of response in patients with relapsed mantle cell lymphoma treated with brexucabtagene autoleucel. Blood. 2021;138(Suppl. 1):1756. doi:10.1182/blood-2021-153277
- Wang Y, Jain P, Locke FL, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in standard-of-care practice: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2023;41(14):2594–2606.
- Jain P, Wang Y, Locke FL, et al. Brexucabtagene autoleucel for relapsed/refractory mantle cell lymphoma: real-world experience from the United States lymphoma CAR T consortium. J Clin Oncol. 2022;40(16 Suppl.):e19583. doi:10.1200/JCO.2022.40.16_suppl.e19583
- Wang Y, Jain P, Locke F, et al. Brexucabtagene autoleucel for relapsed/refractory mantle cell lymphoma in routine practice: updated report from the US Lymphoma CAR T Consortium. HemaSphere. 2022;6(Suppl. 3):1042–1043. doi:10.1097/01.HS9.0000847488.86807.da
- Wang Y, Jain P, Locke FL, et al. Brexucabtagene autoleucel for relapsed/refractory mantle cell lymphoma: real world experience from the US Lymphoma CAR T Consortium. Blood. 2021;138(Suppl. 1):744. doi:10.1182/blood-2021-147563
- Wang M, Munoz J, Goy A, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2022;41(3):555–567. doi:10.1200/JCO.21.02370
- Munoz J, Reagan PM, Goy AH, et al. Assessment of durable responses after brexucabtagene autoleucel (KTE-X19) in the ZUMA-2 study in relapsed/refractory mantle cell lymphoma (R/R MCL). Blood. 2022;140(Suppl. 1):9320–9322. doi:10.1182/blood-2022-158097
- Wang ML, Munoz J, Goy AH, et al. One-year follow-up of ZUMA-2, the multicenter, registrational study of KTE-X19 in patients (Pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL). Transplant Cell Ther. 2021;27(3):S342. doi:10.1016/S2666-6367(21)00440-1
- Wang ML, Munoz J, Goy A, et al. KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in patients (Pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL): results of the Phase 2 ZUMA-2 Study. Blood. 2019;134(Suppl. 1):754. doi:10.1182/blood-2019-126064
- Wang ML, Rossi JM, Munoz J, et al. Pharmacological profile and clinical outcomes of KTE-X19 by prior Bruton tyrosine kinase inhibitor (BTKi) exposure or mantle cell lymphoma (MCL) morphology in patients (Pts) with relapsed/refractory (R/R) MCL in the ZUMA-2 Trial. Transplant Cell Ther. 2021;27(3):S341–S342. doi:10.1016/S2666-6367(21)00439-5
- Wang Y, Munoz J, Goy A, et al. One-year follow-up of ZUMA-2, the multicenter, registrational study of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma. Blood. 2020;136(Suppl. 1):20–22. doi:10.1182/blood-2020-136382
- Wang M, Munoz J, Goy A, et al. KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in patients (Pts) with relapsed/refractory mantle cell lymphoma (R/R MCL): results from Phase 2 of ZUMA-2. Mol Ther. 2020;28(4 Suppl. 1):573.
- Wang M, Munoz J, Goy A, et al. KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in patients (Pts) with relapsed/refractory mantle cell lymphoma (R/R MCL): results of the Phase 2 ZUMA-2 Study. Biol Blood Marrow Transplant. 2020;26(3):S1. doi:10.1016/j.bbmt.2019.12.135
- Wang M, Rossi JM, Munoz J, et al. Pharmacological profile and clinical outcomes of KTE-X19 by prior Bruton tyrosine kinase inhibitor (BTKi) exposure or mantle cell lymphoma (MCL) morphology in patients with relapsed/refractory (R/R) MCL in the ZUMA-2 Trial. Blood. 2020;136(Suppl. 1):29. doi:10.1182/blood-2020-136831
- Wang M, Rossi JM, Munoz J, et al. Pharmacological profile and clinical outcomes of KTE-X19 by prior Bruton tyrosine kinase inhibitor exposure or mantle cell lymphoma morphology in patients with relapsed/refractory mantle cell lymphoma in the ZUMA-2 trial. Br J Haematol. 2021;193(Suppl. 1):43–44.
- National Comprehensive Cancer Network. Mantle cell lymphoma. Plymouth (PA): National Comprehensive Cancer Network; 2021.
- Burkart M, Karmali R. Relapsed/refractory mantle cell lymphoma: beyond BTK inhibitors. J Pers Med. 2022;12(3):376. doi:10.3390/jpm12030376
- Jain P, Dreyling M, Seymour JF, et al. High-risk mantle cell lymphoma: definition, current challenges, and management. J Clin Oncol. 2020;38(36):4302–4316. doi:10.1200/JCO.20.02287
- Rule S, Dreyling M, Goy A, et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. Br J Haematol. 2017;179(3):430–438. doi:10.1111/bjh.14870
- Rule S, Dreyling M, Goy A, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica. 2019;104(5):e211–e214. doi:10.3324/haematol.2018.205229
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):48–56. doi:10.1016/S1470-2045(15)00438-6
- Wang M, Munoz J, Goy A, et al. Outcomes with KTE-X19 in patients (pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL) in ZUMA-2 who had progression of disease within 24 months of diagnosis (POD24). J Clin Oncol. 2021;39(15 Suppl.):7547. doi:10.1200/JCO.2021.39.15_suppl.7547
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2021;386(7):640–654. doi:10.1056/NEJMoa2116133
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi:10.1016/S0140-6736(20)31366-0
- Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized triangle trial by the European MCL Network. Blood. 2022;140(Suppl. 1):1–3. doi:10.1182/blood-2022-163018
- Giné E, Medina-Herrera A, Cruz F, et al. Five-year update of the first-line imcl-2015 GELTAMO study. Prolonged molecular and clinical responses were observed after MRD-driven ibrutinib discontinuation. Hematol Oncol. 2023;41(S2):148–150. doi:10.1002/hon.3163_98
- Arcari A, Morello L, Vallisa D, et al. Allogeneic stem cell transplantation in patients with mantle cell lymphoma: results from the MANTLE-FIRST study on behalf of Fondazione Italiana Linfomi. Leuk Lymphoma. 2021;62(14):3474–3483. doi:10.1080/10428194.2021.1961238
- Lew TE, Cliff ERS, Dickinson M, et al. T-cell replete allogeneic stem cell transplant for mantle cell lymphoma achieves durable disease control, including against TP53-mutated disease. Bone Marrow Transplant. 2021;56(11):2857–2859. doi:10.1038/s41409-021-01418-3
- Ip A, Della Pia A, Goy AH. SOHO state of the art updates and next questions: treatment evolution of mantle cell lymphoma: navigating the different entities and biological heterogeneity of mantle cell lymphoma in 2024. Clin Lymphoma Myeloma Leuk. 2024. doi:10.1016/j.clml.2024.02.010