Abstract
This report focuses on part 3 of a multicenter, open-label, phase 1 study (NCT03198650) assessing the safety, pharmacokinetics (PK), pharmacodynamics (PD), and antitumor activity of acalabrutinib plus obinutuzumab in Japanese patients with treatment-naive (TN) chronic lymphocytic leukemia (CLL). Ten patients were included; median age was 68 years. With a median treatment duration of 27.2 months, treatment-emergent adverse events (AEs) occurred in all patients (grade ≥3, 70%), and the most common AEs were anemia and headache (40% each). One patient had a grade 4 AE of neutropenia (the only dose-limiting toxicity). PK results suggested no marked effects of concomitant obinutuzumab treatment on the exposure of acalabrutinib. PD assessment indicated that combination therapy provided >98% Bruton tyrosine kinase (BTK) occupancy. Overall response rate (ORR) was 100% with median duration of response (DoR) and median progression-free survival (PFS) not reached. Treatment with acalabrutinib plus obinutuzumab was generally safe and efficacious in adult Japanese patients with TN CLL.
Introduction
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are B-cell malignancies that have historically been considered incurable diseases [Citation1]. CLL/SLL predominantly affects older adults, with a median age of 69 years at diagnosis [Citation2]. In Japan, CLL accounted for 3.2% of all cases of malignant lymphomas diagnosed in 2003–2008, with an annual age-adjusted incidence of 0.2 cases per 100,000 people in 2008 [Citation3]. The incidence of CLL is considerably lower in the Japanese population than in the Western world [Citation3]. According to a nationwide, prospective, observational study in Japan that enrolled patients from January 2011 to March 2015, the median age at diagnosis of CLL was 66 years and 65% were male [Citation4].
Bruton tyrosine kinase (BTK) inhibitors are targeted, chemotherapy-free options for the treatment of CLL/SLL that have improved survival outcomes compared with standard chemoimmunotherapy [Citation5,Citation6]. Acalabrutinib, a second-generation, highly selective, potent, covalent BTK inhibitor, was approved by the Food and Drug Administration for the treatment of CLL/SLL based on the results of the phase 3, randomized, controlled ELEVATE-TN and ASCEND clinical studies [Citation7]. In the ELEVATE-TN study [Citation6,Citation8], acalabrutinib monotherapy and acalabrutinib plus obinutuzumab showed significant progression-free survival (PFS) benefit versus obinutuzumab plus chlorambucil in patients with treatment-naive (TN) CLL. In the ASCEND study [Citation9,Citation10], acalabrutinib monotherapy significantly improved PFS versus investigator’s choice of idelalisib plus rituximab or bendamustine plus rituximab in patients with relapsed/refractory (R/R) CLL. Acalabrutinib has also been approved in Japan for the treatment of CLL/SLL based on the results of the ELEVATE-TN and ASCEND clinical studies, as well as this phase 1 study in Japanese patients [Citation11].
The multicenter, open-label, phase 1 study was conducted to assess the safety, tolerability, antitumor activity, pharmacokinetics (PK), and pharmacodynamics (PD) of acalabrutinib in adult Japanese patients with B-cell malignancies (NCT03198650). Part 1 (dose-confirmation phase) and part 2 (dose-expansion phase) of this study were previously reported [Citation11]. Herein, we report the results of part 3, a dose-confirmation phase for the combination therapy of acalabrutinib plus obinutuzumab in patients with TN CLL.
Methods
Patients
Japanese patients ≥65 years of age with a diagnosis of CD20+ CLL, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and adequate organ function were enrolled. Patients aged ≥20 to <65 years of age were eligible if they met at least one of the following criteria: creatinine clearance 30–69 mL/min, or a Cumulative Illness Rating Scale-Geriatric (CIRS-G) score >6. Patients were also required to meet ≥1 International Workshop on Chronic Lymphocytic Leukemia 2018 criteria for active disease requiring treatment [Citation12]. Patients who received prior systemic treatment for CLL (except for localized radiotherapy) or ongoing immunosuppressive therapy, or who had known central nervous system involvement of lymphoma/leukemia, known prolymphocytic leukemia, or history of (or currently suspected) Richter’s syndrome were excluded from the study. Baseline cytogenetic abnormalities were assessed by fluorescence in situ hybridization.
Study design and treatments
This was a multicenter, open-label, 3-part, phase 1 study of acalabrutinib in adult Japanese patients with B-cell malignancies (NCT03198650). Parts 1 and 2 of the study were previously reported [Citation11]. Part 3 of the study assessed acalabrutinib plus obinutuzumab treatment in adult Japanese patients with TN CLL. The primary endpoint of the study was safety and tolerability. Secondary endpoints included PK, PD, overall response rate (ORR), duration of response (DoR), and PFS. Minimal residual disease (MRD) status was an exploratory efficacy endpoint.
Patients received oral (capsule) acalabrutinib in combination with intravenous (IV) obinutuzumab. Acalabrutinib 100 mg twice daily (BID) was continuously administered from day 1 of cycle 1 until disease progression or unacceptable toxicity (28 d per cycle). A loading dose of obinutuzumab 1000 mg was administered on day 1 and day 2 of cycle 2 (100 mg on day 1 and 900 mg on day 2) and a single dose of obinutuzumab 1000 mg was administered on days 8 and 15 of cycle 2 and day 1 of subsequent cycles for a total of 6 treatment cycles. Dose-limiting toxicities (DLTs) were assessed during the first cycle of combination therapy (cycle 2) and were defined as grade ≥3 nonhematologic toxicity lasting for ≥7 d (except those considered to be a response to supportive therapy, such as alopecia and grade 3 nausea, vomiting, and diarrhea), grade ≥3 prolongation of the QTc interval, absolute neutrophil count (ANC) <500/μL lasting ≥7 d after discontinuation of therapy without growth factors or lasting ≥5 d after discontinuation of therapy with growth factors in patients with pretreatment ANC ≥ 1 × 109/L, grade ≥3 febrile neutropenia, platelet count <20,000/μL lasting ≥7 d after discontinuation of therapy or requiring transfusion in patients with pretreatment platelet count >50,000/μL, or a toxicity-related dosing delay for >14 consecutive days.
Study oversight
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki that are consistent with International Conference on Harmonization/Good Clinical Practice, applicable regulatory requirements, and the AstraZeneca policy on bioethics. The study protocol was approved by the institutional review board and all patients provided written informed consent.
Safety outcomes
Adverse events (AEs) and events of clinical interest (ECI) were summarized based on the Medical Dictionary for Regulatory Activities (MedDRA) system organ class, MedDRA preferred term, and Common Terminology Criteria for Adverse Events grade. ECIs included cardiac events, cytopenias, hemorrhage, hepatotoxicity, hypertension, infection, interstitial lung disease/pneumonitis, second primary malignancies (SPM; excluding nonmelanoma skin malignancies), and tumor lysis syndrome.
Pharmacokinetic outcomes
Single-dose PK of acalabrutinib and its metabolite (ACP-5862) were assessed after the first dose of acalabrutinib monotherapy on day 1 of cycle 1. Multiple-dose PK of acalabrutinib in combination with obinutuzumab was assessed from day 1 of cycle 2 (first dose of obinutuzumab) up to day 1 of cycle 4.
Pharmacodynamic outcomes
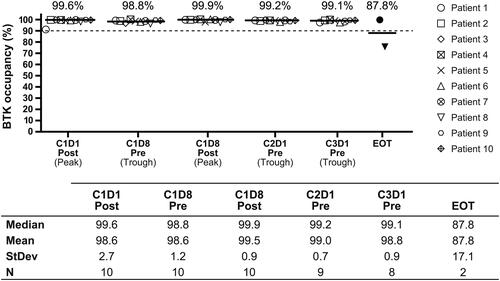
To evaluate the PD profile of acalabrutinib, BTK occupancy was measured using enzyme-linked immunosorbent assay. BTK occupancy was measured in patients with TN CLL on BID dosing followed by a total of 6 obinutuzumab IV treatment cycles starting at cycle 2. Blood samples for BTK occupancy assessment were collected predose and 4 h (±10 min) postdose on days 1 and 8 of cycle 1, and predose on day 1 of cycles 2–3 and end of treatment. The percent occupied BTK was calculated in each peripheral blood (PB) mononuclear cell sample relative to the patient’s baseline sample.
Efficacy outcomes
To evaluate the antitumor activity of acalabrutinib, ORR, DoR, and PFS were assessed. ORR was defined as the proportion of patients who achieved a response (including complete response [CR], CR with incomplete bone marrow (BM) recovery [CRi], and partial response [PR]) during the study period, as assessed by the investigator. Best objective response was assessed at 9 months, or later after the last patient who was recruited started study treatment. DoR was defined as the interval from the first documentation of response to the first documentation of definitive disease progression or death from any cause. PFS was defined as the interval from the start of acalabrutinib therapy to the first documentation of objective disease progression or death from any cause. Computed tomography (CT) scans were performed for tumor assessments every 12 weeks (±14 d) with the first on-treatment radiologic assessment occurring on day 1 of cycle 4, the second on-treatment scan on day 1 of cycle 7, and so on through cycle 25, and then every 24 weeks (±14 d) thereafter.
Minimal residual disease
MRD negativity rate indicating molecular remission was defined as the proportion of patients having <1 CLL cell per 104 leukocytes as assessed by flow cytometry in PB and/or in BM after initiation of treatment with acalabrutinib plus obinutuzumab.
When BM aspiration and biopsy were performed to confirm CR/CRi, PB, or BM MRD samples were also collected. For patients with BM-confirmed CR/CRi, PB was collected for MRD assessment every 24 weeks until confirmation of disease progression or death, consent withdrawal, or loss to follow-up. If CR was not confirmed, PB and BM MRD were assessed during the next CR/CRi confirmation.
Statistical analyses
The target enrollment for part 3 of the study was at least 9 patients, with 6 DLT-evaluable patients required for DLT assessment. To evaluate ORR using an exact test with one-sided significance level of 5% (i.e. exact 2-sided 90% confidence interval [CI]), 9 patients would provide 99% power, assuming a threshold ORR of 30% and an expected ORR of 90% for patients with TN CLL. The safety analysis set included all patients who received at least 1 dose of acalabrutinib. The tumor response analysis set included patients who received at least 1 dose of both acalabrutinib and obinutuzumab. For ORR, 80%, 90%, and 95% CIs were calculated. The PK analysis set included all patients who received at least 1 dose of acalabrutinib with at least 1 measurable plasma concentration of acalabrutinib and PK parameter data, and with no important AEs or protocol deviations that could impact PK. PK analysis of obinutuzumab was not included in the study. The PD analysis set included all patients who received at least 1 dose of acalabrutinib with baseline PD data. All data were listed and summarized using descriptive statistics and no formal statistical hypothesis testing was performed.
Results
Patients and exposure
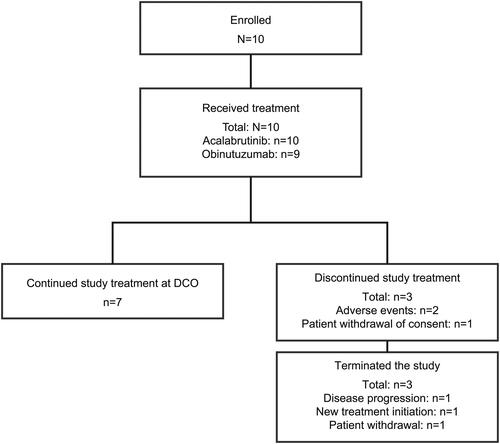
In total, 10 patients were enrolled from 13 study sites in Japan (). All 10 patients received acalabrutinib treatment, but 1 patient discontinued study prior to the start of obinutuzumab due to a grade 2 AE of maculopapular rash, which was considered to be related to acalabrutinib treatment by the investigator. At the data cutoff (DCO) of October 27, 2022, the median study follow-up duration was 27.2 months (range: 1.8–37.3). Of the 10 patients, 7 (70%) continued treatment at DCO. Three (30%) patients discontinued study treatment and were eventually withdrawn from the study. One patient discontinued study treatment and was withdrawn from the study after withdrawing consent. The second patient discontinued study treatment due to an AE (grade 4 neutropenia determined by the investigator to be related to both acalabrutinib and obinutuzumab) and was eventually withdrawn from the study due to disease progression. The third patient also discontinued study treatment, prior to the start of obinutuzumab, due to an AE (grade 2 maculopapular rash determined by the investigator to be related to acalabrutinib as mentioned above) and was withdrawn from the study before initiating next treatment other than study treatment.
All 10 patients were included in the safety, PK, and PD analysis sets, and 9 patients were included in the tumor response analysis set. The patient who did not receive obinutuzumab treatment was excluded from the response analysis set.
The median age of patients was 68.0 years (range: 57–77), 4 (40%) patients were male, median body mass index was 23.4 kg/m2 (range: 18.6–28.4), and 9 (90%) patients had an ECOG PS of 0 at baseline (). Among the patients in the tumor response analysis set, the number of cytogenetic abnormalities at baseline was: 13q14 deletion (n = 6), 17p13 deletion (n = 2), 11q22 deletion (n = 2), and trisomy 12 (n = 2). Some patients harbored multiple cytogenic abnormalities.
Table 1. Baseline demographics and patient characteristics.
Safety
The median acalabrutinib and obinutuzumab treatment duration was 27.2 months (range: 0.9–37.3) and 5.6 months (range: 1.4–6.4), respectively. The mean percentage of the intended dose of acalabrutinib and obinutuzumab administered to patients was 94.5% and 90.4%, respectively.
Study treatment was discontinued in 2 (20%) patients due to AEs; there were no patients who required dose reductions in this study. One (10%) patient had a DLT, reported as a grade 4 nonserious event of neutropenia 36 d after treatment initiation, and was considered related to both acalabrutinib and obinutuzumab treatment by the investigator. Both study treatments were withdrawn because of the event. Another patient had a grade 2 AE of maculopapular rash that occurred 14 d after treatment initiation and was considered related to acalabrutinib treatment by the investigator.
All patients experienced an AE; the most common any-grade AEs were anemia (40%) and headache (40%) (). Six (60%) patients had grade 3 AEs, including hypochromic anemia, angina pectoris, maculopapular rash, alanine aminotransferase increased, pancreatic enzymes abnormal, and platelet count decreased in one patient each, and anemia in two patients. One (10%) patient had a grade 4 AE of neutropenia (the DLT described previously). There were no grade 5 AEs, and no patient experienced a serious AE (Supplementary Table 1). One patient experienced a grade 2 COVID-19 infection that was not related to acalabrutinib or obinutuzumab and had resolved 13 d after onset.
Table 2. Treatment-emergent adverse events (≥2 patients [any grade]).
Nine (90%) patients had at least 1 ECI (Supplementary Table 2). Atrial fibrillation (grade 1) occurred in 1 patient. There were no patients with hypertension or tumor lysis syndrome. Hepatotoxicity was reported in three patients and included events of alanine aminotransferase increased, aspartate aminotransferase increased, and gamma-glutamyltransferase increased.
Pharmacokinetics
Single-dose acalabrutinib was rapidly absorbed, with time to maximum plasma concentration (tmax) values ranging from 0.48 to 1.90 h (). High interpatient variability was observed in acalabrutinib maximum plasma concentration (Cmax), with a coefficient of variation value of 151.4%. Peak concentrations of ACP-5862 were observed at 1.01 h postdose, indicating rapid formation of the metabolite; elimination of ACP-5862 was apparently slower than for acalabrutinib. Individual plasma concentration–time curves were variable after single administration; 1 patient demonstrated lower Cmax and slower elimination compared with other patients (Supplementary Figure 1). Plasma concentration–time curves for ACP-5862 showed variability similar to those of acalabrutinib (Supplementary Figure 2). The elimination half-life of ACP-5862 was also highly variable and affected by the longer half-lives observed in the patient who had slower elimination of acalabrutinib.
Table 3. Summary of pharmacokinetic profiles of acalabrutinib and ACP-5862 following single-dose and multiple-dose acalabrutinib in combination with obinutuzumab.
Pharmacodynamics
Acalabrutinib and obinutuzumab combination therapy provided near-complete BTK occupancy (>98%), which was maintained over the BID dosing interval with low interpatient variation ().
Efficacy
All 9 response-evaluable patients had a response, with an ORR of 100%, including 6 patients (67%) with a CR/CRi (CR, n = 5; CRi, n = 1) and 3 (33.3%) with a PR (). Time to response in the 5 patients who achieved CR ranged between 247 and 337 d from the first dose of study drug (median, 256 d). No patients had a PR with lymphocytosis (PRL). The 2 patients with 17p13 deletion at baseline had PR. At the time of DCO, median DoR and median PFS were not reached.
Table 4. Overall response rate.
Minimal residual disease
Among the 6 patients with CR/CRi based on investigator assessment, all 6 (100%) had PB MRD data available and 5 (83.3%) had BM MRD data available for evaluation by flow cytometry (Supplementary Table 3). Treatment with acalabrutinib plus obinutuzumab achieved sustained undetectable MRD (uMRD, in ≥3 consecutive assessments) at any timepoint at and/or after the first CR/CRi confirmation in 4 (66.7%) patients in PB and in 2 (33.3%) patients in BM. Two patients achieved PB and BM uMRD at the first clinical CR/CRi. There was an overall 100% concordance (6 of 6 matched samples) of MRD status between PB and BM as assessed by flow cytometry.
Discussion
This study demonstrated that treatment with acalabrutinib plus obinutuzumab was safe and well tolerated in Japanese patients with TN CLL. AEs were mostly mild in nature, with no patients experiencing hypertension or tumor lysis syndrome. No patients experienced grade ≥3 infusion-related reactions or atrial fibrillation. These findings are consistent with those reported in the global phase 3 ELEVATE-TN study [Citation8], which included 179 patients with TN CLL treated with acalabrutinib plus obinutuzumab. In that study, grade ≥3 hypertension, tumor lysis syndrome, infusion-related reactions, and atrial fibrillation occurred in 2.8%, 1.1%, 2.2%, and 0.6% of patients, respectively, at a median treatment duration of 27.7 months. Overall, the safety findings were consistent with that of previously reported safety data for acalabrutinib and obinutuzumab, and no new safety concerns were identified [Citation6,Citation8,Citation13,Citation14].
PK results indicated that the single-dose PK (exposure) of acalabrutinib and ACP-5862 in patients with TN CLL receiving acalabrutinib in combination with obinutuzumab in part 3 and patients with R/R B-cell malignancies receiving acalabrutinib monotherapy in part 1 [Citation11] had no marked difference of Cmax and AUC0-12 of acalabrutinib or ACP-5862 by therapeutic line. The comparison of the exposure of acalabrutinib and ACP-5862 observed at steady state in patients receiving acalabrutinib BID in combination with obinutuzumab in part 3 of this study was similar to that reported in part 1 [Citation11], suggesting no marked effect of concomitant obinutuzumab treatment on the exposure of acalabrutinib or ACP-5862.
PD evaluation demonstrated that there was a high level of BTK receptor occupancy after treatment with acalabrutinib plus obinutuzumab, consistent with findings of acalabrutinib monotherapy studies [Citation11,Citation13]. Because treatment with obinutuzumab does not affect target engagement of acalabrutinib, PD data support the use of acalabrutinib 100 mg BID in combination with 6 cycles of obinutuzumab to achieve high target coverage (>95%).
Antitumor activity of acalabrutinib plus obinutuzumab was demonstrated by an ORR of 100%. No patient had PRL. These findings are also consistent with those reported in the ELEVATE-TN study [Citation6,Citation8], in which patients treated with acalabrutinib plus obinutuzumab had an ORR of 94% at a median follow-up of 28.3 months and 96.1% at a median follow-up of 46.9 months. The median PFS was not reached in this study. In the ELEVATE-TN study [Citation6,Citation8], the median PFS was also not reached at a median follow-up of 28.3 months nor with longer follow-up of 46.9 months in the acalabrutinib plus obinutuzumab arm.
PB or BM MRD negativity was observed in 4 of 6 MRD-evaluable patients (66.7%) with investigator-assessed CR/CRi who were treated with acalabrutinib plus obinutuzumab. Similarly, the PB or BM MRD-negative rate reported in MRD-evaluable patients with investigator-assessed CR/CRi who were treated with acalabrutinib plus obinutuzumab in ELEVATE-TN [Citation8] was 56% at a median follow-up of 28.3 months.
Although the results of this study of acalabrutinib and obinutuzumab treatment in Japanese patients with TN CLL are consistent with the results of the global phase 3 ELEVATE-TN study, results should be interpreted with caution due to the limited number of patients treated. In ELEVATE-TN, 11% of patients developed SPM after a median follow-up of 28.3 months, and 15.7% of patients after a median follow-up of 46.9 months. Although SPMs were not detected in this study, careful monitoring of patients is required, and SPMs should be further investigated in larger studies. Other limitations of this study include its nonrandomized, open-label study design, the lack of independent central review-assessed efficacy endpoints, lack of time-to-event analysis, and relatively short follow-up period.
Conclusions
This phase 1 study demonstrated that acalabrutinib plus obinutuzumab treatment in Japanese patients with TN CLL was generally safe and well tolerated, with clinical activity consistent with the findings observed in a global phase 3 study of acalabrutinib plus obinutuzumab in patients with TN CLL.
Supplemental Material
Download PDF (276.9 KB)Acknowledgments
We thank all the patients, their families, and the investigators and their study teams who participated in this study.
Disclosure statement
JT: Research Funding: AstraZeneca, AbbVie, Janssen, Novartis, Chugai, Yakult, Kyowa Kirin, Ono, Eisai, Nippon Shinyaku, Asahi Kasei, and Nippon Kayaku; Honoraria: AstraZeneca, AbbVie, Janssen, Novartis, Chugai, Kyowa Kirin, Ono, Eisai, and Nippon Kayaku.
TT: Grant: Chugai, Astellas, Fuji Pharma, Nippon Shinyaku, Kyowa Kirin, Eisai, Sumitomo Pharma, Ono, Asahi Kasei Pharma, Shionogi, and Otsuka; Honoraria: Astellas, AbbVie, Nippon Shinyaku, Kyowa Kirin, Novartis, Bristol Myers Squibb, Sumitomo Pharma, MSD, Celgene, and Chugai, Janssen.
DE: Research Grant: Eisai, Nippon Shinyaku, and Chugai; Honoraria: Bristol Myers Squibb, AstraZeneca, Janssen, Eisai, Kyowa Kirin, Chugai, Symbio, Ono Pharmaceutical, and Nippon Shinyaku.
SI: Research Funding: AstraZeneca and BeiGene.
RS: Grant: Chugai, Kyowa Hakko Kirin, Shionogi, Taiho, Eisai, Otsuka, and Meiji Seika Pharma; Honoraria: Chugai, Kyowa Hakko Kirin, AbbVie, Bristol Meyers Squibb, Eisai, Otsuka, MSD, Janssen, Takeda, Meiji Seika Pharma, Novartis, and AstraZeneca.
AK: Employment: AstraZeneca K.K.
YT: Employment: AstraZeneca K.K.
NH: Employment: AstraZeneca K.K.
HK: Employment: AstraZeneca K.K.
KM: Employment: AstraZeneca K.K.
PC: Employment: AstraZeneca.
TK: Employment: AstraZeneca K.K.
KI: Grant: MSD, AstraZeneca, AbbVie, Eisai, Incyte, Bristol Myers Squibb, Novartis, Pfizer, Janssen, Yakult, Kyowa Kirin, Ono, Daiichi Sankyo, and LOXO Oncology; Consultant: Bristol Myers Squibb, AstraZeneca, Zenyaku, Kyowa Kirin, MSD, Nippon Shinyaku, AbbVie, Ono, Genmab, and Mitsubishi Tanabe Pharma; Honoraria: Bristol Myers Squibb, AstraZeneca, Janssen, Eisai, Kyowa Kirin, Takeda, Chugai, Novartis, MSD, Symbio, AbbVie, Ono, Pfizer, Eli Lilly, and Daiichi Sankyo.
Data sharing statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Additional information
Funding
References
- Rainone M, Siddiqi T. Management of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma in the era of targeted therapies. Curr Hematol Malig Rep. 2022;17(1):39–45. doi:10.1007/s11899-021-00652-2
- Cancer stat facts. NHL—chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Bethesda (MD): National Cancer Institute; 2023. 26 September. Available from: https://seer.cancer.gov/statfacts/html/cllsll.html.
- Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164(4):536–545. doi:10.1111/bjh.12659
- Takizawa J, Suzuki R, Izutsu K, et al. Characteristics of chronic lymphocytic leukemia in Japan: comprehensive analysis of the CLLRSG-01 study. Int J Hematol. 2024;119(6):686–696. doi:10.1007/s12185-024-03741-z
- Shanafelt TD, Wang XV, Hanson CA, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022;140(2):112–120. doi:10.1182/blood.2021014960
- Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022;36(4):1171–1175. doi:10.1038/s41375-021-01485-x
- Calquence. [package insert]. Wilmington (DE): AstraZeneca Pharmaceuticals; 2022.
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291. doi:10.1016/S0140-6736(20)30262-2
- Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–2861. doi:10.1200/JCO.19.03355
- Ghia P, Pluta A, Wach M, et al. Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results. Hemasphere. 2022;6(12):e801. doi:10.1097/HS9.0000000000000801
- Izutsu K, Ando K, Ennishi D, et al. Safety and antitumor activity of acalabrutinib for relapsed/refractory B-cell malignancies: a Japanese phase I study. Cancer Sci. 2021;112(6):2405–2415. doi:10.1111/cas.14886
- Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi:10.1182/blood-2017-09-806398
- Byrd JC, Woyach JA, Furman RR, et al. Acalabrutinib in treatment-naïve chronic lymphocytic leukemia. Blood. 2021;137(24):3327–3338. doi:10.1182/blood.2020009617
- Woyach JA, Blachly JS, Rogers KA, et al. Acalabrutinib plus obinutuzumab in treatment-naive and relapsed/refractory chronic lymphocytic leukemia. Cancer Discov. 2020;10(3):394–405. doi:10.1158/2159-8290.CD-19-1130