Abstract
This study used COTA de-identified data (2010–2021) of patients in the US to explore outcomes of novel therapies in relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) in real-world settings. Demographics, clinical characteristics, and clinical outcomes of patients with R/R DLBCL who received novel treatments including chimeric antigen receptor T-cell (CAR T) therapy and tafasitamab- or polatuzumab-based therapies were evaluated. Overall, 175 patients with R/R DLBCL were analyzed; 73, 69, and 27 received CAR T therapy, polatuzumab-based regimens, and tafasitamab-based regimens, respectively. In patients who had ≥1 prior lines of therapy (i.e. starting second-line or later therapy; 2 L+), CAR T, polatuzumab-based regimens, and tafasitamab-based regimens achieved a median overall survival of 26.5, 7.8, and 6.3 months, respectively. Outcomes were particularly poor for patients with relapse following CAR T, indicating that polatuzumab- and tafasitamab-based regimens in 2 L + R/R DLBCL have suboptimal outcomes in the real world. Additional treatment options are needed.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for approximately 25% of cases [Citation1]. While the majority of patients can be cured with frontline chemoimmunotherapy, approximately 35–40% of patients will have relapsed or refractory (R/R) disease [Citation2]. Outcomes are poor for these patients, particularly if they have primary refractory DLBCL [Citation2,Citation3]. Prior to the availability of chimeric antigen receptor T-cell (CAR T) therapy, retrospective data demonstrated that patients with refractory DLBCL had an objective response rate of 26% to the next line of therapy (LOT) and a median overall survival (OS) of only 6.3 months [Citation2,Citation3]. Other studies have indicated that response rates decrease with increasing LOTs in the relapsed setting as well [Citation4].
Second-line chemoimmunotherapy followed by autologous stem cell transplantation (ASCT) was the standard of care for the past 2 decades [Citation5,Citation6], although it had been restricted to young, fit patients with no major comorbidities, owing to the risk of therapy-related toxicity [Citation7]. Furthermore, among those who respond and are eligible to undergo ASCT, up to 50% relapse after transplantation [Citation8,Citation9]. Treatment options for patients ineligible for, or who relapsed after, ASCT were previously limited [Citation6].
Recently, there has been a paradigm shift in the management of R/R DLBCL with the advent of novel treatments including CAR T therapy [Citation6]. Three CAR T products are currently approved by the United States (US) Food and Drug Administration (FDA) for patients with R/R DLBCL who have received ≥2 LOTs (2L+) [Citation6]. Additionally, axicabtagene ciloleucel and lisocabtagene maraleucel have been approved for second-line therapy based on the results of the phase III ZUMA-7 and TRANSFORM studies, respectively [Citation10,Citation11]. These randomized studies demonstrated improved outcomes with CAR T therapy compared with standard of care (salvage chemotherapy and ASCT) in patients with primary refractory or early relapse of DLBCL [Citation12,Citation13].
In addition to CAR T therapy, there have been other treatment regimens approved by the US FDA for R/R DLBCL, including polatuzumab vedotin plus bendamustine and rituximab (BR), selinexor, tafasitamab plus lenalidomide, and loncastuximab tesirine [Citation6,Citation14–20]. Polatuzumab vedotin is an antibody drug conjugate (ADC) that targets CD79b, a B-cell receptor component [Citation18]. The phase Ib/IIGO29365 trial included a randomized component comparing polatuzumab-BR with BR alone in patients with R/R DLBCL and ≥1 prior LOTs; treatment with polatuzumab BR resulted in improved outcomes compared with BR alone [Citation18]. Selinexor, an oral selective inhibitor of XPO1-mediated nuclear export, was approved by the US FDA based on the results of the phase IIb SADAL trial, which included patients with R/R DLBCL and 2–5 prior LOTs [Citation21,Citation22]. The phase II L-MIND trial examined the combination of tafasitamab, a monoclonal anti-CD19 antibody, plus lenalidomide in patients with R/R DLBCL and 1–3 prior LOTs [Citation23]. Lastly, loncastuximab, a CD19-directed ADC, received US FDA approval for patients with R/R DLBCL and ≥2 prior LOTs based on the results of the phase II LOTIS-2 trial [Citation24,Citation25].
Despite promising results in phase II trials, eligibility criteria were thought to be restrictive in some studies, raising concern regarding the applicability of these findings to real-world settings. Furthermore, given differences in trial design and patient selection across studies, it remains difficult to compare outcomes among available agents in single-arm trials. This study aims to describe the demographic and clinical characteristics of patients with R/R DLBCL who had ≥1 prior lines of therapy (i.e. starting second-line or later therapy; 2 L+), and to characterize DLBCL treatment patterns in academic and community practice sites in the US. This study also aims to examine clinical outcomes among 2 L + patients with R/R DLBCL receiving novel therapies, stratified by treatment and LOT.
Methods
Study design
This was a retrospective observational cohort study of patients with R/R DLBCL receiving a US FDA-approved 2 L + novel therapy (Supplementary Figure S1). The start date of the relevant novel therapy was the index date. Patient demographics and clinical characteristics were assessed from the time of first DLBCL diagnosis to the index date (pre-index baseline period), and clinical endpoints were evaluated from the index date to the end of follow-up, death, or end of data availability, whichever occurred first (post-index observational period). No minimum follow-up was required. To be included, patients were required to have ≥1 post-index evaluable response assessment and outcome or a date of death.
Data source and collection
The study utilized data from the COTA US electronic health records (EHR) database that provides de-identified patient-level data with detailed demographic and clinical oncology information. The database includes EHR data from 91 academic centers and 287 community sites in the US. For this study, data were collected from patients with a diagnosis of, and treatment for, DLBCL between January 2010 and December 2021. A diagnosis of DLBCL was based on a record of pathological confirmation of initial DLBCL diagnosis. Diagnoses prior to 2010 were excluded owing to EHRs not being mandated before this time, thus creating potential for missing data.
Study population
A flow diagram showing the patient identification and selection process is presented in . Adult patients (≥18 years of age) diagnosed with R/R DLBCL treated with an approved novel DLBCL therapy as 2 L + were included in the study; DLBCL included de novo disease or disease histologically transformed from any indolent subtype and could be double-hit or triple-hit high-grade B-cell lymphoma. Patients must also have had ≥1 line of prior systemic antineoplastic therapy (including ≥1 anti-CD20 monoclonal antibody [mAb]-containing therapy) and Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Relapsed disease was defined as disease that had recurred ≥6 months after completion of therapy, and refractory disease was defined as disease progression during therapy or <6 months after therapy completion.
Figure 1. Patient attrition flow diagram.
CAR T, chimeric antigen receptor T-cell; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; HIV, human immunodeficiency virus; LBCL large B-cell lymphoma; lonca, loncastuximab; LOT, line of therapy; NHL, Non-Hodgkin Lymphoma; pola, polatuzumab vedotin; R/R, relapsed/refractory; tafa, tafasitamab; WHO, World Health Organization.
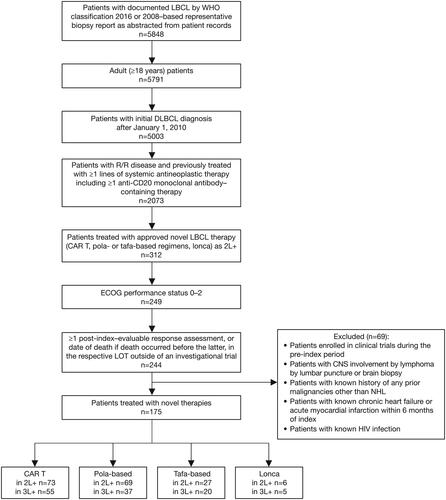
Patients were excluded if they were enrolled in clinical trials during the index period and if they had central nervous system involvement, known history of previous malignancies other than NHL, chronic heart failure or acute myocardial infarction within 6 months of the study index period, or human immunodeficiency virus.
Study variables of interest
Line of therapy was defined by COTA’s proprietary algorithm, which used curated and unstructured data elements. The index LOT must have comprised a regimen of interest in the 2 L + setting. If the patient had records indicating >1 qualifying LOT, one of those LOTs was randomly selected as the index LOT.
Study endpoints
The study had 2 primary endpoints: demographic and clinical characteristics of patients with R/R DLBCL receiving 2 L + therapy, and patterns of novel treatments (including CAR T, tafasitamab, polatuzumab vedotin, and loncastuximab tesirine) in the same population, with patient demographic/clinical characteristics stratified by novel treatment type and LOT.
Secondary endpoints were real-world overall response rate (rwORR; proportion of patients with complete response [CR] or partial response [PR] of any duration as the best documented response for a given LOT in the real world), real-world CR (rwCR; proportion of patients with CR of any duration as the best documented response for a given LOT in the real world), and OS (time from start of index LOT to death from any cause) in 2 L + patients with R/R DLBCL receiving novel therapies and stratified by LOT.
Statistical analysis
Descriptive statistics (mean, standard deviations, and median values for continuous variables, and frequency and proportion for categorical variables) were used to describe patient demographics and clinical characteristics during the pre-index period or on the index date.
The total number and proportion, including 95% confidence interval (CI), of patients who achieved CR, PR, stable disease, and progressive disease were determined, and the rwORR was calculated for each cohort.
Overall survival was calculated for patients with the relevant LOT and described using Kaplan–Meier curves. For each endpoint, the median time to event and corresponding 95% CI were reported. Subgroup analyses among patients with DLBCL were performed, including evaluation of outcome by prior number of LOTs (2, 3, 4+), response status to first LOT, response status to last LOT, exposure status to ASCT, and exposure status to CAR T therapy.
Results
Demographic and clinical characteristics
2L + patient cohort
A total of 175 patients (median age 63 years; 60.6% male; 67.4% White) diagnosed with R/R DLBCL had received ≥1 prior LOT and therefore met the inclusion criteria for the 2 L + cohort (i.e. patients starting second-line or later therapy). When stratified by novel treatment type, 41.7% (73/175) received CAR T therapy, 39.4% (69/175) received polatuzumab-based regimens, 15.4% received tafasitamab-based regimens (27/175), and 3.4% (6/175) received loncastuximab (). Overall, 61.7% (108/175) of patients in the 2 L + cohort were reported to have Ann Arbor stage III or IV; 65.7% (115/175) had primary refractory disease, and 80.6% (141/175) and 81.7% (143/175) had refractory disease during their previous LOT and most recent anti-CD20 therapy, respectively. Previous CAR T therapy was recorded in 12.0% (21/175) of patients (). Baseline demographic and clinical characteristics were largely comparable between the two main cohorts (CAR T and polatuzumab-based), with the exception of age. In the polatuzumab-based cohort, 55.1% of patients were over 65 years age at diagnosis compared with 24.7% in the CAR T cohort.
Table 1. Demographics, treatment history, and clinical characteristics by novel treatment type; 2L + patient cohort.
3L + patient cohort
The 3 L + patient cohort (i.e. patients starting third-line or later therapy) comprised 117 patients (median age 61.0 years; 65% male; 64% White) with R/R DLBCL and ≥2 prior LOTs. Novel treatments were distributed as follows: 47.0% (55/117) of patients received CAR T therapy, 31.6% (37/117) received polatuzumab-based regimens, 17.1% (20/117) received tafasitamab-based regimens, and 4.3% (5/117) received loncastuximab (). Of the patients in the 3 L + cohort, 64.1% (75/117) had Ann Arbor stage III or IV; 66.7% (78/117) had primary refractory disease, and 88.9% (104/117) and 90.1% (106/117) had refractory disease during their previous LOT and most recent anti-CD20 therapy, respectively; 17.9% (21/117) of patients had previous CAR T therapy (). Baseline demographics and clinical characteristics were largely comparable between the two main cohorts (CAR T and polatuzumab-based).
Table 2. Demographics, treatment history and clinical characteristics, by novel treatment type; 3 L + patient cohort.
Clinical outcomes
2L + patient cohort
Treatment regimens for the 2 L + patient cohort (n = 175) are presented in Supplementary Table S1. The majority (63.0%) of CAR T patients (n = 73) received axicabtagene ciloleucel. Over two-thirds (68.1%) of patients treated with polatuzumab-based regimens (n = 69) had polatuzumab vedotin plus bendamustine and rituximab and 88.9% of tafasitamab-based patients (n = 27) received tafasitamab plus lenalidomide.
The median (IQR) follow-up times were 10.8 (4.6, 23.1) months for CAR T, 6.5 (3.8, 10.2) months for polatuzumab-based regimens, and 2.3 (1.2, 11.7) months for tafasitamab-based regimens.
The rwORR was 76.7%, 59.4%, and 40.7% for CAR T, polatuzumab-based regimens, and tafasitamab-based regimens, respectively; the rwCR rate was 52% for CAR T therapy, 18.8% for polatuzumab-based regimens, and 11.1% for tafasitamab-based regimens (; ). Median OS was longer in the CAR T cohort at 26.5 months, compared with that for the polatuzumab-based regimens and tafasitamab-based regimens (7.8 months and 6.3 months, respectively) (; ). Overall survival at 2 years was 55.4% for CAR T and 14.7% and 0% for polatuzumab- and tafasitamab-based regimens, respectively.
Figure 2. Real-world response rates.
CAR T, chimeric antigen receptor T-cell; CR, complete response; pola, polatuzumab; PR, partial response; tafa, tafasitamab.
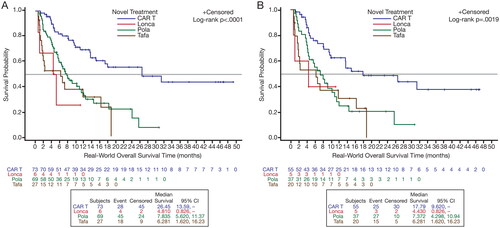
Figure 3. OS Kaplan–Meier curves, by treatment type, for the 2L+ (A) and 3 L+ (B) patient cohorts.
CAR T, chimeric antigen receptor T-cell; lonca: loncastuximab; OS, overall survival; pola, polatuzumab; tafa, tafasitamab.
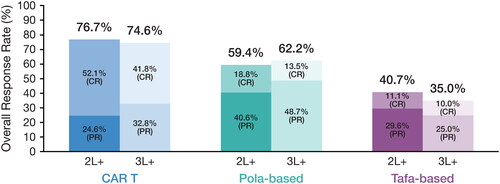
Table 3. Clinical outcomes by LOT and novel treatment type.
3L + patient cohort
Treatment regimens for the 3 L + patient cohort (n = 117) are presented in Supplementary Table S1. Among patients treated with CAR T therapy (n = 55), 60% received axicabtagene ciloleucel; 64.9% of patients treated with polatuzumab-based regimens (n = 37) received polatuzumab vedotin plus bendamustine and rituximab, and 85% of patients treated with tafasitamab regimens (n = 20) received tafasitamab plus lenalidomide.
The median (IQR) follow-up times were 10.0 (4.0, 19.8) months for CAR T, 6.5 (3.8, 10.9) months for polatuzumab-based regimens, and 4.3 (1.4, 13.1) months for tafasitamab-based regimens, respectively.
Similar response estimates to those reported for patients in the 2 L + cohort were found for the 3 L + cohort. Among patients who received CAR T therapy, polatuzumab-based or tafasitamab-based regimens in this setting, rwORRs were 74.6%, 62.2%, and 35.0%, respectively; those in the CAR T cohort had a higher rwCR rate at 41.8% compared with that observed in the polatuzumab-based (13.5%) and tafasitamab-based (10.0%) cohorts (; ). Median OS was longer in the CAR T cohort at 17.8 months compared with that reported for the polatuzumab-based and tafasitamab-based cohorts (7.4 months and 6.3 months, respectively) (; ). Overall survival at 2 years was 48.8% for CAR T and 20.7% and 0% for polatuzumab- and tafasitamab-based regimens, respectively.
Outcomes of polatuzumab- and tafasitamab-based regimens following CAR T relapse were associated with a high risk of relapse, with median OS of 2.3 months.
Discussion
This real-word study analyzed the demographic and clinical characteristics, as well as the outcomes, of patients with R/R DLBCL who received novel treatments (defined as CAR T and tafasitamab- or polatuzumab-based therapies) in the 2 L + setting in the US. While responses to CAR T therapy were encouraging and consistent with other real-world data sets [Citation26,Citation27], outcomes with other novel agents showed inferior responses compared with those seen in clinical trials [Citation18,Citation23]. Novel agents in the real-world setting were used in patients with high-risk disease who may not have met eligibility for clinical trials. For example, polatuzumab and tafasitamab pivotal studies did not include primary refractory patients [Citation18,Citation23], whereas in this real-world analysis, 62.3% and 48.1% of patients in the polatuzumab-based and tafasitamab-based treatment cohorts, respectively, had primary refractory disease. In the present study, 14.5% and 33.3% of the patients in the polatuzumab- and tafasitamab-based cohorts, respectively, were previously treated with CAR T therapy, and 76% of these patients were CAR T refractory. Outcomes of novel agents in this subset of patients were particularly poor, as demonstrated by a median OS of 2.3 months, which is consistent with that reported in other studies [Citation28].
Prior database studies have shown encouraging real-world outcomes with CAR T therapy. A real-world prospective study of 1297 patients with R/R DLBCL showed durable response with CAR T therapy, with an ORR of 73% and a CR rate of 56%, comparable to those reported in the pivotal ZUMA-1 clinical trial [Citation26]. In a retrospective cohort analysis of Medicare Fee-For-Service beneficiaries treated with CAR T therapy, real-world patients demonstrated similar survival at 12 months and experienced fewer adverse events compared with CAR T patients in the JULIET, TRANSCEND NHL 001, and ZUMA-1 clinical trials [Citation27]. While these results show promise for CAR T therapy utilization in real-world patients with DLBCL, they are based on registries that do not reflect intent-to-treat patient populations. Additionally, access to CAR T therapy remains an issue in clinical practice, owing to complex logistics, manufacturing challenges, toxicities, and cost [Citation29].
The results of this study are consistent with those of other small real-world studies demonstrating unfavorable outcomes of novel agents outside of clinical trials. An observational study of 55 patients with R/R DLBCL in Italy showed an ORR rate of 32.7% (after a median of 4 LOTs) for polatuzumab-based regimens [Citation30]. A similar ORR rate (33%) was reported in a small (n = 21) real-world study of patients with R/R DLBCL in the US [Citation31]. The present study included a larger sample of 69 patients receiving polatuzumab-based regimens in the 2 L + setting and found a higher ORR (59.4%); however, nearly half of polatuzumab-based patients had only one prior LOT. It is also important to note the absence of bendamustine treatment in 32–35% of patients treated with polatuzumab-based regimens in our study, raising the possibility that this may have impacted efficacy. The response and survival outcomes associated with tafasitamab-based regimens were similar to those observed in a recently published EHR analysis using data from 9 institutions in the US, which reported an ORR and CR rate of 27% and 17%, respectively, and an OS of 6.8 months [Citation32].
This study is not without limitations that are inherent to the use of EHR databases. Due to the nature of the database, there is the potential for some degree of missingness of follow-up data, especially if patients changed care setting (e.g. if a patient moved from an academic center included in the COTA to a community oncology practice outside the COTA database). In addition, progressive disease (PD) information can be underreported in EHR databases. Due to the above limitations, especially among the CAR T population, rwDOR, rwDOCR, and rwPFS were not included in this analysis out of concern for over- or underestimating the endpoint. In terms of sample size, when inclusion/exclusion criteria were applied, the sample size was greatly reduced relative to the entire DLBCL cohort, as the inclusion criteria required patients to remain in the database for a specified amount of time to ensure ample follow-up data. Additionally, this study evaluated patients treated with novel therapies, most of which were approved after 2019. Therefore, despite the 12-year study span from initial DLBCL diagnosis, the novel therapy index period was limited to the time after FDA approval of each of the novel treatments. Limitations of this study also include the utilization of real-world outcomes, for which assessment and monitoring may not be performed consistently across patients and physicians, unlike in clinical trials. Additionally, due to the small sample size, comparative analyses were not feasible among all cohorts; to counteract this, sample size feasibility numbers were identified prior to analysis to determine the final cohorts for comparison. Furthermore, owing to the retrospective and observational nature of the data, this study had potential for incomplete data (such as clear documentation of a clinical event), variability in the quality of information recorded by physicians, difficulty in verifying database information, and differences in clinical practices across study sites. Finally, if the overall real-world population of patients with DLBCL differs systematically in terms of demographic or clinical characteristics, the findings may not be truly representative of the overall population of patients with DLBCL.
Notwithstanding the above limitations, the COTA EHR database is a rich source of demographic and clinical information relating to real-world patients with R/R DLBCL. The database represents both clinical and academic practices around the US. As such, the present retrospective study provides insights into recent trends in treatment patterns by LOT, and associated outcomes among US patients. Moreover in the COTA database, mortality data is validated with commercially available obituary data. These insights may have practical applicability in that they can inform clinical decision-making by potentially assisting in the identification of patients with R/R DLBCL who are less likely to benefit from novel treatments and for whom alternative approaches may be needed.
Conclusion
In patients with R/R DLBCL, real-world outcomes of polatuzumab-based regimens and of tafasitamab-based regimens are suboptimal and particularly poor when these agents are used after CAR T therapy relapse. While treatment options for DLBCL continue to expand, these findings indicate an unmet medical need for additional treatment approaches for patients with R/R disease.
Disclosure of interest
Jennifer Crombie: Consulting: Karyopharm, Incyte, MorphoSys, Kite, ADC Therapeutics, Seagen, Regeneron; Research Funding: AbbVie, Bayer, Merck, Genentech/Roche.
Monika Jun, Tongsheng Wang, Alex Mutebi, Anindit Chhibber, Jon Ukropec, Julie Blaedel, and Anupama Kalsekar: Genmab: Current Employment.
Anthony Wang and Rajesh Kamalakar: AbbVie: Current Employment.
Supplemental Material
Download PDF (39.4 KB)Acknowledgments
Medical writing was provided by Lorena Tonarelli, MSc, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by Genmab. Genmab A/S and AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of this article. All authors had access to relevant data and participated in the drafting, review, and approval of this article. No honoraria or payments were made for authorship.
Data sharing statement
De-identified individual participant data collected during the trial will not be available upon request for further analyses by external independent researchers. Aggregated clinical trial data from the trial is provided via publicly accessible study registries/databases as required by law. For more information, please contact [email protected].
Additional information
Funding
References
- Padala SA, Kallam A. Diffuse Large B Cell Lymphoma. Treasure Island, FL: StatPearls Publishing; 2023.
- Duarte C, Kamdar M. Management considerations for patients with primary refractory and early relapsed diffuse large B-cell lymphoma. Am Soc Clin Oncol Educ Book. 2023;43:e390802. doi:10.1200/EDBK_390802
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi:10.1182/blood-2017-03-769620
- Radford J, White E, Castro FA, et al. Treatment patterns and outcomes in patients with relapsed or refractory diffuse large B-cell lymphoma: experience from a single UK centre [abstract]. Blood. 2019;134(Supplement_1):2917–2917. doi:10.1182/blood-2019-123664
- Philip T, Chauvin F, Armitage J, et al. Parma international protocol: pilot study of DHAP followed by involved-field radiotherapy and BEAC with autologous bone marrow transplantation. Blood. 1991;77(7):1587–1592. doi:10.1182/blood.V77.7.1587.1587
- Sawalha Y. Relapsed/refractory diffuse large B-cell lymphoma: a look at the approved and emerging therapies. JPM. 2021;11(12):1345. doi:10.3390/jpm11121345
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi:10.1056/NEJM199512073332305
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. doi:10.1200/JCO.2010.28.1618
- Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1729–1736. doi:10.1016/j.bbmt.2014.06.036
- FDA approves axicabtagene ciloleucel for second-line treatment of large B-cell lymphoma [press release]; 2022. U.S. Food and Drug Administration; [20 September 2023]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-second-line-treatment-large-b-cell-lymphoma.
- FDA approves lisocabtagene maraleucel for second-line treatment of large B-cell lymphoma [press release]; 2022. U.S Food and Drug Administration; [20 September 2023]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lisocabtagene-maraleucel-second-line-treatment-large-b-cell-lymphoma.
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. doi:10.1016/S1470-2045(18)30864-7
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi:10.1016/S0140-6736(22)00662-6
- Kochenderfer JN, Somerville RPT, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25(10):2245–2253. doi:10.1016/j.ymthe.2017.07.004
- Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019;10:2040620719841581. doi:10.1177/2040620719841581
- Sermer D, Batlevi C, Palomba ML, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4(19):4669–4678. doi:10.1182/bloodadvances.2020002118
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi:10.1016/S0140-6736(20)31366-0
- Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155–165. doi:10.1200/JCO.19.00172
- Calabretta E, Hamadani M, Zinzani PL, et al. The antibody-drug conjugate loncastuximab tesirine for the treatment of diffuse large B-cell lymphoma. Blood. 2022;140(4):303–308. doi:10.1182/blood.2021014663
- Nedved A, Maddocks K, Nowakowski GS. Clinical treatment guidelines for tafasitamab plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Oncologist. 2023;28(3):199–207. doi:10.1093/oncolo/oyac256
- Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7(7):e511–e522. doi:10.1016/S2352-3026(20)30120-4
- FDA approves selinexor for relapsed/refractory diffuse large B-cell lymphoma [press release]; 2020. U.S. Food & Drug Administration; [10 July 2023]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selinexor-relapsedrefractory-diffuse-large-b-cell-lymphoma.
- Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. doi:10.1016/S1470-2045(20)30225-4
- Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790–800. doi:10.1016/S1470-2045(21)00139-X
- FDA grants accelerated approval to loncastuximab tesirine-lpyl for large B-cell lymphoma. 2021. [press release]: U.S. Food & Drug Administration; [10 July 2023]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-loncastuximab-tesirine-lpyl-large-b-cell-lymphoma.
- Jacobson CA, Locke FL, Ma L, et al. Real-world evidence of axicabtagene ciloleucel for the treatment of large B cell lymphoma in the United States. Transplant Cell Ther. 2022;28(9):581.e581–581.e588.
- McBride K, Snyder S. Real-world versus clinical trial CAR T outcomes among patients diagnosed with diffuse large B-cell lymphoma. [abstract 413]. J Clin Oncol. 2022;40(28_suppl):413–413. doi:10.1200/JCO.2022.40.28_suppl.413
- Caballero AC, Escribà-Garcia L, Alvarez-Fernández C, et al. CAR T-cell therapy predictive response markers in diffuse large B-cell lymphoma and therapeutic options after CART19 failure. Front Immunol. 2022;13:904497. doi:10.3389/fimmu.2022.904497
- Gajra A, Zalenski A, Sannareddy A, et al. Barriers to chimeric antigen receptor T-cell (CAR-T) therapies in clinical practice. Pharmaceut Med. 2022;36(3):163–171. doi:10.1007/s40290-022-00428-w
- Argnani L, Broccoli A, Pellegrini C, et al. Real-world outcomes of relapsed/refractory diffuse large B-cell lymphoma treated with polatuzumab vedotin-based therapy. Hemasphere. 2022;6(12):e798. doi:10.1097/HS9.0000000000000798
- Vodicka P, Benesova K, Janikova A, et al. Polatuzumab vedotin plus bendamustine and rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma in the real world. Eur J Haematol. 2022;109(2):162–165. doi:10.1111/ejh.13784
- Qualls D, Buege MJ, Dao P, et al. Tafasitamab and lenalidomide in relapsed/refractory large B cell lymphoma (R/R LBCL): real world outcomes in a multicenter retrospective study [abstract. ]. Blood. 2022;140(supplement 1):787–789. doi:10.1182/blood-2022-167620
