 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The overall value of treatments for chronic lymphocytic leukemia (CLL) depends on several factors, including preferences of the general population, who contributes to the financing of health systems. This study investigated societal preferences for attributes of CLL treatments in Italy. An online large-scale survey was designed using a discrete choice experiment (DCE) methodology and delivered to the Italian adult general population. Ten treatment attributes were identified, covering efficacy, safety, operational aspects and (hypothetical) out-of-pocket cost. DCE data were analyzed using a mixed logit regression model, estimating the willingness-to-pay for attribute levels’ change. The general population significantly preferred more effective treatments, with shorter duration, administered orally rather than orally + intravenously. Changes in therapy duration, frequency of checkups and organ damage risk had the greatest impact on preferences. The integration of societal preferences in the value judgments of CLL therapies may help health authorities in establishing priority setting and taking pricing-reimbursement decisions.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in Western countries [Citation1,Citation2]. It affects mainly the elderly population, with a median age at diagnosis of 72 years [Citation3,Citation4]. CLL is characterized by a high variability in its clinical presentation and course. Some patients may present an indolent form that will not require treatment during their lifetime, while others develop a progressive disease that will eventually need treatment [Citation5].
Currently, there are several therapies available for CLL. In particular, over the past years, novel targeted therapies for CLL have been developed (e.g. ibrutinib, idelalisib, acalabrutinib, duvelisib, venetoclax) [Citation6,Citation7] and have challenged the role of chemoimmunotherapy, resulting in improved progression-free survival (PFS) and overall survival (OS) [Citation8]. Overall, CLL treatments present different profiles in terms of clinical efficacy (possibility to achieve undetectable minimal residual disease – uMRD) and safety (with differences in tolerability profiles), but also operational aspects (e.g. modes and frequency of administration, need of hospitalization) and costs. The choice of the optimal therapy for a patient with CLL requires consideration of disease-related factors (prognostic/predictive) but also patient-related factors like age, comorbidities and patient preferences [Citation9].
Integrating patient preferences in medical decision-making is becoming increasingly important in order to comprehensively assess the value of a particular treatment [Citation10]. In addition, over the last years, health technology assessment (HTA) organizations have started promoting the involvement of both patients and members of the general population in some aspects of their HTA processes (patient and public involvement – PPI) [Citation11]. Several authors stress the importance of involving the public in the decisions related to service design and delivery, especially in a publicly financed health system [Citation12–14]. Moreover, considering the value judgments of members of society in policy-making is in line with the democratic debate about the development and uptake of health technologies [Citation15]. The inclusion of societal preferences could be particularly relevant for high-spending therapeutic areas, such as cancer [Citation16], for which health authorities are continuously struggling in establishing priority setting and in taking decisions about pricing and reimbursement of medicines. Despite the recognized relevance of integrating different perspectives of value in health-related decisions and policy-making, there is still little systematic research in this area.
This study aims at filling the knowledge gap on societal preferences regarding CLL treatments by conducting a discrete choice experiment (DCE) in Italy among the public. The prioritization of the most valuable treatments for CLL is crucial considering current resource constraints and the increased spending for cancer treatments. Within a publicly-funded healthcare system, as the Italian one, empirical evidence on the value judgments of members of society can help policy-makers to establish priority setting and foster an efficient allocation of resources.
Materials and methods
An online large-scale survey among the adult general population was designed using a discrete choice experiment (DCE) methodology to investigate the preferences of the Italian general population for characteristics of CLL treatments. The online questionnaire was implemented using a web-based survey tool, Qualtrics XM. The study obtained the approval of the Ethics Committee of Bocconi University.
Attributes and levels definition
DCEs are a stated preference method based on the random utility theory [Citation17]. This theory proposes that a person has an ‘utility’ (or preference) for each choice alternative, which is latent and cannot be observed by researchers. However, the frequency of choices between alternatives provides an estimate of the utility associated with each element on a scale of preferences [Citation18]. DCEs show the participant a series of hypothetical scenarios, obtained as a unique combinations of attributes and levels, and allows to estimate the preferences for the different attributes and levels composing them [Citation19].
For this study, attributes (i.e. characteristics of CLL treatments) and the respective levels (i.e. range of variation of the attributes) were derived according to a two-step procedure. First, a comprehensive review of the literature, both grey and published, was performed to retrieve data on available CLL treatments [Citation3,Citation20–27], and previous studies investigating preferences in CLL were collected [Citation28–33]. A preliminary set of candidate attributes and levels was identified and discussed with an internationally-recognized clinical expert in CLL to mirror the main CLL treatments available in Italy as of 2022. Three rounds of discussion were performed, which allowed to refine the list of attributes (e.g. we added an attribute not retrieved through the literature review, namely the frequency of clinical checkups), revise the levels (and their wording) in order to carefully reflect clinical practice in Italy, and formulate a lay description of the attributes. Following the discussion with clinical expert, ten attributes were included, each with three or four levels (). The attributes cover different domains, namely, treatment efficacy, safety, operational aspects (e.g. frequency and mode of administration) and cost.
Table 1. List of attributes and levels.
Regarding the cost attribute, there is currently limited guidance in the literature on how to determine its levels [Citation34]. It is generally advisable to ensure that the cost levels are neither excessively high nor excessively low for the treatment/condition considered, as this would make the attribute either prohibitive or irrelevant. Following these general indications, we selected some levels of monthly out-of-pocket cost that were plausible for a patient in Italy, including a 0-cost level to align with the free-of-charge provision of health services in our country. Overall, we selected a range of costs that could generate enough elasticity in respondents’ preferences, subsequently tested in a pilot study (see ‘Experiment design’ section), and which was deemed credible by the clinical expert involved in the study.
A preliminary pilot test of the questionnaire was conducted with a small sample of colleagues (n = 4) who have limited or no expertise in both DCEs and the clinical condition under scrutiny. The feedbacks received were predominantly positive, and only minor adjustments to the description of attributes were suggested.
Experiment design
An unlabeled design with two alternatives (Treatment A and B) was generated using Ngene software (version 1.2, ChoiceMetrics 2018) [Citation35]. A fractional factorial design was applied in order to obtain a manageable number of choice questions from all possible combinations of attributes and levels [Citation36].
First, a pilot study involving a small sample drawn from the general population (n = 100) was performed to pretest the questionnaire. The pilot relied on an efficient design with a basic multinomial logit model (MNL), that assumes fixed parameters and near-zero initial priors. Completion time, drop-out rates and the extent of straight-lining [Citation37] were analyzed in order to assess the feasibility of the DCE. As no major issues were detected from these analyses, the questionnaire format was considered definitive.
Parameter estimates from the pilot were used as priors for the final design, obtained using a mixed multinomial logit (MMNL). This model has random parameters, for which we assumed a normal distribution. Dummy coding was applied to categorical variables (i.e. mode and frequency of administration, therapy duration, frequency of clinical checkups). A set of 140 DCE questions was created, divided into 10 blocks of 14 questions. The number of questions in each block was the minimum number of choice situations needed to estimate the parameters of interest. This allowed to lower the cognitive burden for respondents as much as possible. An example of a DCE question is reported in Supplemental Figure 1.
A heuristic rule of thumb was used for the calculation of the minimum sample size [Citation38]. The rule of thumb suggests that the sample (N) required depends on the number of decision questions presented in the questionnaire (t = 14), the number of scenarios in each question (a = 2) and the maximum number of levels for each factor (Lk = 4), according to the relation: N > 500 * Lk/(t * a). Therefore, the minimum sample size is equal to:
A sample of approximately 1,000 respondents from the adult general population was recruited by an Italian commercial online panel provider (Pepe Research s.r.l.) in July 2022. A non-probabilistic proportional quota sampling approach was used [Citation39]. With this approach, the population is divided into relevant strata (i.e. quota controls). For this study, the sample was selected to be representative of the Italian general population in terms of age, sex and macro-geographical area of residence. The number of elements in each stratum in the population was estimated using census results.
Data collection and analysis
After providing consent, the respondents accessed the survey and were asked some background questions, including some personal information (e.g. age and sex) and other information about their health (Supplemental File 1). After the background questions, respondents were presented with a brief description of CLL (Supplemental File 2) and with the instruction to complete the DCE exercise. A synthetic explanation of the attributes was made available before the DCE exercise, and, in case of doubt, could also be accessed again through a link at the end of each DCE question (Supplemental File 3). The respondents were randomly assigned to one of the 10 blocks using the function provided by Qualtrics XM. Within each block, questions were randomized in order to rule out any possible effect that the ordering may have on the estimation.
Data analysis
The DCE data were analyzed using a mixed logit regression model [Citation40,Citation41]. Mixed logit models are particularly useful for choice data because they are based on random coefficients, which allow the alternatives to be correlated. In other words, they relax the assumption of independence of irrelevant alternatives, which states that the relative probability of someone choosing between two options is independent of any additional alternative in the choice set.
The dependent variable was the dichotomous choice of the hypothetical scenarios, and independent variables were the attribute/level combinations. For dummy-coded variables, the worst level of each factor resulted from the pilot was considered as the reference case, and it was omitted from the regression. Parameter estimates from the model can be interpreted as relative preference weights that indicate the average relative preference for one attribute level compared with other attribute levels. In other words, they indicate the increment in utility associated with moving from the reference level of each attribute (the worst level) to the other levels. All attributes were specified as random parameters. Interaction terms between the respondents’ observable characteristics and attribute levels were added to the model to assess how they affect respondents’ preferences. Akaike information criterion (AIC), Bayesian information criterion (BIC), and conditional Akaike information criterion (CAIC) statistics were computed to assess models’ fit.
The willingness-to-pay (WTP) for a change in each attribute level (€/month) was computed as the negative ratio of mean coefficients to cost coefficient from the model without interactions [Citation42]. Positive coefficients should be interpreted as the investment that a citizen would be willing to make in order to observe a certain change (i.e. an improvement) in attribute levels. Negative coefficients should be interpreted as the amount of money that a citizen would receive as a compensation for observing a certain change (i.e. a worsening) in attribute levels.
Four dummy variables (i.e. age 65+, gender, presence of a disease, presence of a chronic disease) were added separately to the main model in order to investigate heterogeneity in preferences. The age and gender variables were selected to compare preference estimates across relevant population strata and to reflect the subgroup analyses carried out by a published Italian study investigating preferences of patients and clinicians for CLL treatments [Citation43], and a study carried out in Sweden and Germany on the general population [Citation31]. The rationale behind incorporating the presence of a disease and chronic disease as covariates stems from our interest in exploring the potential influence of individuals’ prior experiences with illness and treatment regimens on their preferences. The interaction coefficients represent the additional utility of each attribute (or level) for the subgroup considered (e.g. males vs. females).
A relative attribute importance (RAI) analysis was performed in order to estimate the change in utility associated with each attribute in the DCE [Citation44]. The relative attribute importance was computed as the difference in preference weights between the most and least preferred level in each attribute divided by the sum of preference weight ranges of all attributes [Citation45].
Analyses were conducted using Stata17 (College Station, TX: StataCorp LLC). A p-value of 0.05 was considered statistically significant.
Results
The final sample, after database cleaning, consisted of 1.000 respondents aged 18+. The sample descriptive statistics are provided in . The respondents’ mean age is 51.4 years, with 28.4% of the sample represented by people aged 65+ (6.3% aged 75+), and 48.0% are males. The 42.9% of respondents declared to be affected by at least one disease, and the 35.6% by one or more chronic diseases. When compared to the data collected by the Italian Institute of Statistics (ISTAT), which estimates approximately 40% of Italians living with at least one chronic disease [Citation46], our sample appears to be slightly healthier.
Table 2. Sample descriptive characteristics.
shows the results of the DCE. The directions of the coefficients are all in accordance with our hypotheses, and the majority are statistically significant. The general population significantly prefers more effective treatments (b = 0.021; p < 0.001), administered orally every day (b = 0.190; p < 0.001), and with a 1-year fixed duration (b = 0.246; p < 0001). Clinical checkups with a lower frequency (i.e. every 3-6 months) are preferred over more frequent checkups (b = 0.237; p < 0.001). Finally, the higher the probability of adverse events (all negative coefficients; p < 0.001) and out-of-pocket costs (b=-0.001; p < 0.001), the lower the preference.
Table 3. Results of the DCE.
The monthly WTP for changes in attributes’ levels is shown graphically in (coefficient details are reported in Supplemental Table 1). Overall, the three characteristics of CLL treatments whose change has the greatest impact on societal preferences are therapy duration, frequency of clinical checkups and risk of organ damage. In particular, the Italian general population would be willing to pay 169.3 €/month and 140.9 €/month to switch from a continuous therapy to a 1-year and 6-months fixed therapy respectively. Moreover, the general population would be willing to pay significantly more for a treatment with less frequent checkups (e.g. every 3-6 months). The adverse event for which respondents would require the highest compensation is organ damage (151.1 €/month for a 1% increase in probability), while diarrhea is the adverse event that registered the lowest willingness to be compensated (12.1 €/month for a 1% increase in probability). shows the ranking of levels in absolute terms (reference levels were omitted) according to WTP estimates, and the respective domain (i.e. safety, efficacy, operational).
Figure 1. WTP estimates (€/month) for changes in attributes’ levels.
Note. Non-significant results are reported with a striped pattern. Grey bars represent the 95% confidence intervals.
Abbreviations: n.s.: not significant
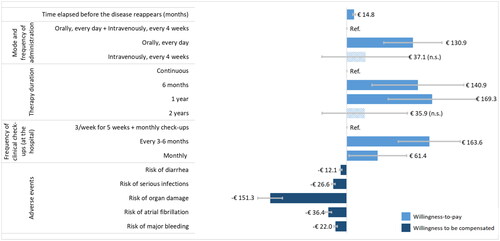
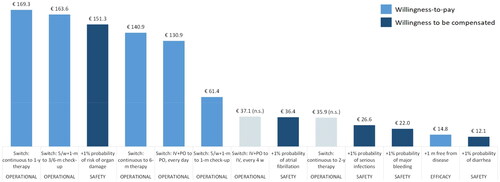
Figure 2. Levels ranking according to WTP estimates (€/month).
Note. Non-significant results are reported with a striped pattern.
Abbreviations: IV: intravenuous. m: month. n.s.: not significant. y: year. PO: oral. w: week.
Results of interaction analysis suggest that preferences are quite stable across different subgroups. Preferences do not differ between respondents aged 65+ and younger respondents (Supplemental Table 2). Similar results are obtained when using gender as an interaction term (Supplemental Table 3). However, females seem to attribute a lower importance than males to the oral mode of administration (b=-0.219; p = 0.011) and a higher importance to the risk of serious infections (b=-0.008; p = 0.047), the latter possibly related to differences in risk aversion. Respondents affected by a disease show a higher preference than healthy people for less frequent clinical checkups, both at 3-6 months (b = 0.273; p = 0.003) and at 1 month (b = 0.229; p = 0.008) (Supplemental Table 4). The same evidence was found for people with chronic diseases (Supplemental Table 5). Moreover, respondents with a chronic disease seem to attribute a lower importance than healthy people to the risk of serious infection (b = 0.009; p = 0.031) and to the risk of atrial fibrillation (b = 0.015; p = 0.040).
The results of the RAI analysis are shown in Supplemental Figure 2. The three attributes with the highest relative importance over the range of levels included in the experiment are monthly out-of-pocket cost, risk of serious infections and risk of major bleeding of CLL treatments.
Discussion
The recent expansion of the therapeutic landscape in CLL (e.g. oral targeted agents) has substantially complicated treatment decision-making [Citation47]. Moreover, the high cost of novel therapies raises budget concerns for payers [Citation48]. Understanding the importance that society (i.e. tax payers) attributes to CLL treatment characteristics may be helpful to inform HTA and related evaluations of these therapies.
To our knowledge, this is the first large-scale DCE survey to investigate societal preferences for CLL treatments. The study by Landfeldt and colleagues (2016) [Citation31] estimated the preferences for treatment characteristics of different target populations, including the general one, in relapsed or refractory CLL in Sweden and Germany using conjoint analysis. However, the number of respondents from the general population was limited (100 individuals for each country), hindering the generalizability of results. Despite the involvement of the general population, the study by Tolley et al. (2013) [Citation49] did not focus on CLL treatment characteristics but investigated preferences for progression-free and progressive states of late-stage CLL. The target populations of the majority of published studies were patients and/or clinicians [Citation28–30, Citation32,Citation33,Citation43].
Our results revealed that the Italian general population is sensitive to changes in the characteristics of CLL treatments, as suggested by the statistical significance of coefficients. In particular, respondents put a great emphasis on changes in ‘operational aspects’ of treatments, such as the mode of treatment administration (oral administration is preferred), the frequency with which an individual should go to the hospital for follow-up visits (less frequent checkups are preferred) and the duration of therapy (fixed therapies are preferred over continuous ones). For the attribute related to therapy duration, a non-monotonic effect was found, i.e. a 1-year therapy was more preferred than both a 6-months and 2-year therapy. A possible explanation might be that, on the one hand, people prefer to have short treatments (e.g. 1 year preferred to 2 years) but, on the other, a shorter duration therapy (e.g. 6 months) might be interpreted as a proxy of low treatment efficacy. Overall, the results for attributes concerning operational aspects suggest that the lower the perceived burden of treatments for individuals, the higher the preference.
In accordance with the study by Landfeldt and colleagues (2016) [Citation31], when looking at the WTP estimates, we found that changes in efficacy, expressed as PFS, have a relatively low importance compared to other attributes. This evidence was also confirmed by the RAI analysis. Interestingly, this result is in line with the preferences for treatment characteristics expressed by a cohort of Italian CLL patients and clinicians, as reported by Laurenti and colleagues (2022) [Citation43]. Focusing on patients’ preferences, the authors estimated that PFS represents the third treatment attribute in terms of importance for patients, after safety (e.g. risk of severe infection) and operational (e.g. mode/timing of administration) aspects.
Our study revealed that the societal preferences for CLL treatments were highly influenced by the risk of experiencing an AE. More specifically, considering the WTP estimates, the general population showed the highest aversion for a change in the risk of organ damage, followed by atrial fibrillation, serious infections, major bleeding and diarrhea. Compared to the other AEs presented, organ damage might be the least well known and therefore the consequences of this event could be perceived as particularly serious by the general population. When it comes to actual treatment choice, appropriate information to individuals about the effects and consequences of drugs is needed to increase individuals’ awareness around the risks and benefits of treatments. Although the AEs in our study coincide only partially with those in Laurenti and colleagues (2022) [Citation43] (e.g. serious infections, diarrhea, atrial fibrillation), some considerations can be made. CLL patients judge serious infections as the least preferred AE, followed by atrial fibrillation and diarrhea. When looking at the marginal effects, and in particular at the willingness to be compensated for a 1% increase in the risk of experiencing an AE, the Italian general population displays a higher aversion to a 1% increase in the risk of atrial fibrillation compared to serious infections. This discrepancy is not surprising, as direct experience with the disease and therapies, with their unintended consequences, is likely to influence the perceived value of treatment characteristics. Infections might be perceived by the general population as more likely to be managed successfully than cardiac issues, which, in the common perception, are generally considered life-threatening conditions.
As policy decisions are (or at least should be) influenced by a range of social values, it is crucial for policy-makers to be aware of full spectrum of stakeholders’ preferences, especially when conflicting, in order to take decisions that maximize the value obtainable with scarce resources. WTP estimates provided in this study can be used to infer the overall value (in monetary terms) of switching from a treatment with certain attributes to another, characterized for example by the same efficacy but with a different profile in terms of mode and frequency of administration, therapy duration, frequency of clinical checkups and risk of adverse events. An example of WTP calculation for treatment A to treatment B switch is provided in .
Table 4. Example of WTP calculation for treatment switch (€/month).
This study is not exempted from limitations. First, for survey administration, we relied on an online market research panel, and adopted a quota sampling approach rather than a probabilistic one. This may have caused some selection biases and therefore the selected sample may not be fully representative of the population, limiting the generalizability of results. However, using more representative sampling frames (e.g. from the electoral roll) may result in very low response rates, hindering representativeness, and may be very costly and time consuming [Citation50]. Moreover, the reliance on an online market research panel allowed us to administer the survey also to the elderly population (aged 65+), which is usually hard to reach, especially with online questionnaires.
Second, an inherent limitation of the DCE method is its external validity: when faced with similar choices in real life, individuals may choose differently than what they reported during the experiment. However, as underlined by Lancsar and Swait (2014) [Citation51], a more comprehensive conceptualization of external validity should be used when talking about DCEs: external validity is not only a comparison of final outcomes, but it is an objective pursued from the initial conceptualization and design of any DCE (e.g. process validity). The authors believe that as the process validity of a model representation (e.g. the verisimilitude between model and reality) is increased, so will the quality of inferences and their external validity. As regards experiment internal validity, only 0.9% of respondents consistently chose only Treatment A or Treatment B, indicating that the majority of participants had engaged constructively with the task and effectively considered tradeoffs between the presented scenarios. The extent of straight-lining in our study resulted to be far below the mean and median values reported in the literature [Citation37] (7% and 2% respectively).
Third, the high number of attributes included in the DCE may have increased its cognitive demandingness. According to a systematic review by Soekhai and colleagues (2019) [Citation52], the number of attributes in DCEs ranges from 2 to 21, with a median equal to 5. Having acknowledged that the number of attributes in our study was higher than the median, we tried to decrease the cognitive burden of respondents by creating blocks and keeping the number of questions presented to each respondent at its minimum according to the generated design [Citation53]. The median completion time was 9.4 min (25th percentile = 6.2 min; 75th percentile = 14.5 min), suggesting that the completion of choice tasks was feasible. Moreover, in order to further minimize the respondents’ cognitive effort, the attributes and levels were exhaustively explained at the beginning of the DCE, and, in case of doubt, respondents could access the explanations at every question. Finally, the attributes were selected after a careful process, which included a literature review and three rounds of discussion with a clinical expert.
Fourth, seven out of ten attributes were treated as continuous variables in our analyses, thus assuming their linearity. Although this prevented us from considering the existence of nonlinear effects for these attributes, we deemed this assumption necessary in order to reduce the number of parameters to be estimated, consequently minimizing the number of choice questions for respondents and the DCE’s cognitive demandingness.
Finally, in the present study we did not perform any analyses to investigate the attribute nonattendance, i.e. the tendency of respondents to make their choices by using only a subset of attributes [Citation54,Citation55]. Future work might explore this aspect, either with a stated approach, i.e. by using follow-up questions asking respondents to state which attributes they ignored when making their choices, or with a modeling approach, by using latent class models to infer attribute nonattendance from the choice data [Citation55]. These further analyses would allow on one hand to assess whether and to what extent attribute nonattendance affected the significance and strengths of preferences, and on the other to better understand the respondents’ motives for ignoring attributes.
Conclusions
This study aimed at filling the knowledge gap on societal preferences regarding CLL treatments by conducting a large-scale survey with a DCE methodology in Italy. To our knowledge, this is the first study collecting data from an extensive sample of the general population to infer public preferences for the characteristics of CLL treatments. Integrating the public perspective into policy decisions-making is becoming increasingly important, but is still an underdeveloped and under-investigated area. Within a publicly-funded healthcare system, such as the Italian one, empirical evidence on societal preferences can inform policy-makers on the value assigned to different therapeutic options, and foster the prioritization of the most valuable treatments.
Supplemental Material
Download PDF (492.4 KB)Disclosure statement
LB, FC, CM, GS and PA declare no conflicts of interest. PG declares the following competing interests: AbbVie, AstraZeneca, Janssen (research support); AbbVie, AstraZeneca, BeiGene, BMS, Janssen, Lilly Loxo Oncology, MSD, Roche (honoraria).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
- Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi:10.1182/blood-2017-09-806398
- Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33. doi:10.1016/j.annonc.2020.09.019
- Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–3734. doi:10.1182/blood-2010-05-282632
- Cramer P, Hallek M. Prognostic factors in chronic lymphocytic leukemia—what do we need to know? Nat Rev Clin Oncol. 2011;8(1):38–47. doi:10.1038/nrclinonc.2010.167
- Kipps TJ, Choi MY. Targeted therapy in chronic lymphocytic leukemia. Cancer J. 2019;25(6):378–385. doi:10.1097/PPO.0000000000000416
- Shustik C, Bence-Bruckler I, Delage R, et al. Advances in the treatment of relapsed/refractory chronic lymphocytic leukemia. Ann Hematol. 2017;96(7):1185–1196. doi:10.1007/s00277-017-2982-1
- Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524–1537. doi:10.1016/S0140-6736(18)30422-7
- Walewska R, Parry-Jones N, Eyre TA, et al. Guideline for the treatment of chronic lymphocytic leukaemia. Br J Haematol. 2022;197(5):544–557. doi:10.1111/bjh.18075
- Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology Value Framework: revisions and Reflections in Response to Comments Received. J Clin Oncol. 2016;34(24):2925–2934. doi:10.1200/JCO.2016.68.2518
- Weeks L, Polisena J, Scott AM, et al. Evaluation of patient and public involvement initiatives in health technology assessment: a survey of international agencies. Int J Technol Assess Health Care. 2017;33(6):715–723. doi:10.1017/S0266462317000976
- Souliotis K. Public and patient involvement in health policy: a continuously growing field. Health Expect. 2016;19(6):1171–1172. doi:10.1111/hex.12523
- Andreasen PB. Consensus conferences in different countries. Aims and perspectives. Int J Technol Assess Health Care. 1988;4(2):305–308. doi:10.1017/s0266462300004104
- Ong BN. The lay perspective in health technology assessment. Int J Technol Assess Health Care. 2009;12(3):511–517.
- Gauvin FP, Abelson J, Giacomini M, et al. "It all depends": conceptualizing public involvement in the context of health technology assessment agencies. Soc Sci Med. 2010;70(10):1518–1526. doi:10.1016/j.socscimed.2010.01.036
- Luengo-Fernandez R, Leal J, Gray A, et al. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–1174. doi:10.1016/S1470-2045(13)70442-X
- McFadden D. The choice theory approach to market research. Marketing Science. 1986;5(4):275–297. doi:10.1287/mksc.5.4.275
- Louviere J, Hensher D, Swait J. Stated choice methods: analysis and application, Vol. 17. Cambridge: Cambridge University Press; 2000.
- Louviere JJ, Flynn TN, Carson RT. Discrete choice experiments are not conjoint analysis. J Choice Model. 2010;3(3):57–72. doi:10.1016/S1755-5345(13)70014-9
- Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–3452. doi:10.1200/JCO.21.01210
- Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–2861. doi:10.1200/JCO.19.03355
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291. doi:10.1016/S0140-6736(20)30262-2
- Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi:10.1056/NEJMoa1713976
- European Medicines Agency (EMA). Riassunto delle Caratteristiche del Prodotto - Calquence. Available from: https://www.ema.europa.eu/en/documents/product-information/calquence-epar-product-information_it.pdf.
- European Medicines Agency (EMA). Riassunto delle Caratteristiche del Prodotto - Imbruvica. Available from: https://www.ema.europa.eu/en/documents/product-information/imbruvica-epar-product-information_it.pdf.
- European Medicines Agency (EMA). Riassunto delle Caratteristiche del Prodotto - Venclyxto. Available from: https://www.ema.europa.eu/en/documents/product-information/venclyxto-epar-product-information_it.pdf.
- Patel K, Pagel JM. Current and future treatment strategies in chronic lymphocytic leukemia. J Hematol Oncol. 2021;14(1):69. doi:10.1186/s13045-021-01054-w
- Mansfield C, Masaquel A, Sutphin J, et al. Patients’ priorities in selecting chronic lymphocytic leukemia treatments. Blood Adv. 2017;1(24):2176–2185. doi:10.1182/bloodadvances.2017007294
- Koffman B, Stewart C, Avruch L, et al. Awareness, knowledge, and preferences of United States (US) patients with chronic lymphocytic leukemia (CLL) and their caregivers related to finite duration (FD) therapy and minimal (measurable) residual disease (MRD). Blood. 2021;138(Supplement 1):1927–1927. doi:10.1182/blood-2021-145046
- Molica S, Laurenti L, Ghia P, et al. COVID-19 pandemic impact on chronic lymphocytic leukemia (CLL) patients’ preferences towards therapies: the Italian experience (CHOICE study). Blood. 2021;138(Supplement 1):4690–4690. doi:10.1182/blood-2021-148308
- Landfeldt E, Eriksson J, Ireland S, et al. Patient, physician, and general population preferences for treatment characteristics in relapsed or refractory chronic lymphocytic leukemia: a conjoint analysis. Leuk Res. 2016;40:17–23. doi:10.1016/j.leukres.2015.11.006
- Le H, Pendergraft T, Wahlstrom SK, et al. Understanding clinician and patient preferences about novel agents in chronic lymphocytic leukemia. Blood. 2019;134(Supplement_1):4730–4730. doi:10.1182/blood-2019-129606
- Le H, Ryan K, Wahlstrom SK, et al. Oncologist and patient preferences for novel agents in first-line treatment for chronic lymphocytic leukemia: commonalities and disconnects. Patient Prefer Adherence. 2021;15:99–110. doi:10.2147/PPA.S289139
- Rowen D, Stevens K, Labeit A, et al. Using a discrete-choice experiment involving cost to value a classification system measuring the quality-of-life impact of self-management for diabetes. Value Health. 2018;21(1):69–77. doi:10.1016/j.jval.2017.06.016
- ChoiceMetrics. Ngene (version 1.2); 2018.
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–677. doi:10.2165/00019053-200826080-00004
- Johnson FR, Yang J-C, Reed SD. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health. 2019;22(2):157–160. doi:10.1016/j.jval.2018.07.876
- de Bekker-Grob EW, Donkers B, Jonker MF, et al. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–384. doi:10.1007/s40271-015-0118-z
- Sedgwick P. Proportional quota sampling. BMJ. 2012;345(sep26 3):e6336–e6336. doi:10.1136/bmj.e6336
- McFadden D, Train K. Mixed MNL models for discrete response. J Appl Econ. 2000;15(5):447–470. doi:10.1002/1099-1255(200009/10)15:5<447::AID-JAE570>3.0.CO;2-1
- Mixed logit modeling in Stata – an overview. Hole AR, editor. Stata users’ group meetings 2013. United Kingdom: Stata Users Group; 2013.
- Lancsar E, Fiebig DG, Hole AR. Discrete choice experiments: a guide to model specification, estimation and software. Pharmacoeconomics. 2017;35(7):697–716. doi:10.1007/s40273-017-0506-4
- Laurenti L, Gaidano G, Mauro FR, et al. What are the attributes prioritized in the choice of therapy in chronic lymphocytic leukemia? A patient-physician cross-matching analysis of a discrete choice experiment. Hemasphere. 2022;6(9):e771. doi:10.1097/HS9.0000000000000771
- Gonzalez JM. A guide to measuring and interpreting attribute importance. Patient. 2019;12(3):287–295. doi:10.1007/s40271-019-00360-3
- Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004
- Italian Institute of Statistics (ISTAT). Statistiche Istat [22 Feb 2024]. Available from: http://dati.istat.it/.
- Stevenson FK, Forconi F, Kipps TJ. Exploring the pathways to chronic lymphocytic leukemia. Blood. 2021;138(10):827–835. doi:10.1182/blood.2020010029
- Shanafelt TD, Borah BJ, Finnes HD, et al. Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract. 2015;11(3):252–258. doi:10.1200/JOP.2014.002469
- Tolley K, Goad C, Yi Y, et al. Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur J Health Econ. 2013;14(5):749–759. doi:10.1007/s10198-012-0419-2
- Giles EL, Becker F, Ternent L, et al. Acceptability of financial incentives for health behaviours: a discrete choice experiment. PLoS One. 2016;11(6):e0157403. doi:10.1371/journal.pone.0157403
- Lancsar E, Swait J. Reconceptualising the external validity of discrete choice experiments. Pharmacoeconomics. 2014;32(10):951–965. doi:10.1007/s40273-014-0181-7
- Soekhai V, de Bekker-Grob EW, Ellis AR, et al. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226. doi:10.1007/s40273-018-0734-2
- Jonker MF, Donkers B, de Bekker-Grob EW, et al. Effect of level overlap and color coding on attribute non-attendance in discrete choice experiments. Value Health. 2018;21(7):767–771. doi:10.1016/j.jval.2017.10.002
- Lagarde M. Investigating attribute non-attendance and its consequences in choice experiments with latent class models. Health Econ. 2013;22(5):554–567. doi:10.1002/hec.2824
- Heidenreich S, Watson V, Ryan M, et al. Decision heuristic or preference? Attribute non-attendance in discrete choice problems. Health Econ. 2018;27(1):157–171. doi:10.1002/hec.3524
