ABSTRACT
Avoiding genetic interactions between wild and farmed Atlantic salmon is regarded as one of the major requirements for a sustainable salmon aquaculture industry. For this reason, farming functional sterile triploids has been suggested as a possible solution. However, knowledge about how triploids cope under commercial conditions is lacking. In the present study, we compared the performance of diploid and triploid Atlantic salmon among four Norwegian aquaculture companies. Diploid and triploid groups of the same genetic line were farmed in western, mid, and northern Norway under commercial conditions from seawater transfer until slaughter.Overall, triploid salmon exhibited reduced survival, higher incidence of emaciated fish, and scored, on average, a lower quality rating during primary processing. The results highlight the need for further research on how to improve the welfare and performance of triploid salmon in commercial aquaculture operations.
Introduction
One of the greatest challenges in the aquaculture of Atlantic salmon (Salmon salar L.) is escape of domestic specimens (Føre and Thorvaldsen Citation2021). The main concern is that hybridization of domesticated salmon with wild conspecifics has the potential to alter the genetic integrity of wild populations, reduce local adaptation, and negatively affect population fitness (Taranger et al. Citation2015). The farming of sterile salmon has been proposed as a possible solution to minimize the impacts on wild populations and thus a way to make the salmon aquaculture industry more sustainable (Aarset et al. Citation2020; Benfey Citation2016). Although there exist several different sterilization techniques (Güralp et al. Citation2020), to date, the only commercially available method of producing sterile salmon is to triploidise newly fertilized eggs (Golpour et al. Citation2016).
Triplodisation of salmon is normally induced by exposing newly fertilized eggs to hydrostatic pressure, which causes retention of the second polar body (Benfey and Sutterlin Citation1984). This results in triploid eggs with two sets of chromosomes from the female and one set from the male, rendering the offspring sterile. Comparative studies have shown that triploid salmon have a higher prevalence of ocular cataracts (Fraser et al. Citation2012; Olsvik et al. Citation2020; Oppedal, Taranger, and Hansen Citation2003; Sambraus et al. Citation2017a), skeletal deformities such as those of the lower‐jaw (Amoroso et al. Citation2016; Benfey Citation2001; Sadler, Pankhurst, and King Citation2001; Sutterlin, Holder, and Benfey Citation1987) and vertebral compression or fusion (Fjelldal and Hansen Citation2010), compared to diploids. However, triploid salmon production can be optimized by fulfilling their triploid-specific requirements, including a lower thermal optimum (Fraser et al. Citation2015; Sambraus et al. Citation2017b) and higher requirements for key nutrients such as phosphorus (Fjelldal et al. Citation2016; Sambraus et al. Citation2020; Smedley et al. Citation2016, Citation2018) and histidine (Taylor et al. Citation2015b)
The performance of triploid salmon has been monitored during full production cycles in Scotland (Taylor et al. Citation2013) and Norway (Fraser et al. Citation2013). However, these studies were performed under semi-commercial conditions with small research sea cages, and there is no scientific evaluation of triploids under commercial conditions. Therefore, the current study was designed with the aim of documenting and comparing the welfare of triploid salmon in sea cages at four different commercial farms.
Materials and methods
Fish and farms
The diploid and triploid eggs were produced by the same group of broodstock in 2017 at AquaGen AS (Postboks 1240, Sluppen, 7462 Trondheim, Norway). To ensure that the triploid and diploid groups at each farm had the same genetic origin, sperm and eggs were mixed and, 37 min (300 degree-minutes) post-fertilization, half of the eggs from each female were subjected to hydrostatic pressure of 655 bar for 5 min to induce sterile triploids (Benfey and Sutterlin Citation1984).
The diploid and triploid eyed eggs were transferred to hatcheries near the respective farms for hatching and growth in freshwater until smoltification. Between April and June 2018, the smoltified salmon were transferred to the respective farms for growth until slaughter. One group of diploid and one group of triploid Atlantic salmon were transferred to sea cages at four different commercial farms. Farm A was located in the southwestern part of Norway, B and D in the middle, and farm C in the north.
The numbers of diploid and triploid salmon transferred into the sea cages of each farm, the months of transfer, and the average weights of specimens are shown in . The success rate of triploidisation for the salmon received by Farms A, B, and C was verified by microsatellite analysis (Jacq Citation2020) and rate for the Farm D was tested by measuring the red blood cell size from blood smears (Benfey, Sutterlin, and Thompson Citation1984; Sumpter, Dye, and Benfey Citation1986). The success rate was 100% for all specimens (). Salmon were reared for standard commercial production. The diploid and triploid salmon were fed a standard commercial feed (Appendix 1). Information about the significant handling operations during the seawater phase is included in Appendices 2 and 3.
Table 1. Diploid and triploid Atlantic salmon transferred to four commercial sea-cage farms in 2018. Site ID, cage no., ploidy, month, smolt type, number of fish (/1000) and estimated average weight (g) .
Table 2. Triploidisation efficiency test for genetic analyses and blood cell size on four triploid groups employed in each of the four farms.
Sampling procedure
Samples were collected in tandem with the obligatory weekly salmon lice (Lepeophtheirus salmonis) counts. Norwegian salmon farmers are required to count lice on 10 to 20 representative fish from each cage every week or every second week, depending on the time of year (Ministry of Trade Industry and Fisheries Citation2012; Stien et al. Citation2020). The farmers had slightly different solutions on how to obtain the best representative sample. In short, the fish were fasted for 24 h, attracted close to the surface with feed, and netted out. On farm A, the fish were simply dipnetted. Farm B used a net held by a steel-frame cylindric basket (~Ø2 m and 1.5 deep) that was placed to first sink in the cage and then pulled to the surface by a hydraulic crane when fish again started to school after the disturbance. Farms C and D used a 6 m × 6 m seine, with a rope in each corner, which was hauled against the wall of the net pen to capture fish for sampling. The fish were then anaesthetized (15–20 ml 100 L-1 Benzoak vet, EuroPharma, Leknes, Norway) in a tailored-lice counting tank (Tellekar, MarinHelse AS, Norway). For each sedated fish, the number of salmon lice, the weight, and the fork length were recorded, then the welfare status was scored.
Welfare scoring of sampled fish
Scoring of individual fish according to the FISHWELL morphological welfare indicator (WI) scoring scheme (Noble et al. Citation2018) was performed either by the fish farmer or by a scientist from the current project. The FISHWELL scheme employs standardized scoring of WI levels from 0 to 3, and is a simplification of the Salmon Welfare Index Model (Pettersen et al. Citation2014; Stien et al. Citation2013). The scoring standards were provided to the farmers along with photo examples of each WI level from the FISHELL-handbook (see Appendix 4). The WIs were: emaciation level, skin bleeding, scale loss, eye bleeding/damage, eye protrusion, opercula deformity, snout damage, vertebral deformity, fin status, upper and lower jaw deformity, cataract, and sea lice infestation. The score for each category was set according to the level of evidence of damage/defect/symptom: (i) score 0, no evidence; (ii) score 1, minor/suspected evidence; (iii) score 2, severe evidence; and (iv) score 3, extreme evidence (see Appendix 4). After the welfare assessment, the fish were allowed to recover from the anesthesia and swim freely back into the cage via a pipe from the lice counting tank.
Production and slaughter data
At each farm, the fish farmers reported water temperature, monthly mortality (Appendix 5), and estimated growth rate. At the end of the production the slaughter data was reported by commercial slaughterhouses where the fish were processed. These data included the total number of salmon harvested, the average specimen weight, and the percent of farmed fish ranked as high (“Superior”), moderate (“Ordinary”) and low (“Production”) quality. The number of fish reported at the start and at the end of the production period was used to calculate the total percent mortality in each sea cage (). Fish weight at seawater transfer and at slaughter and time spent in the sea cage were used to calculate the daily specific growth rate as described by Lugert et al. (Citation2016):
Table 3. Comparison of the percentage distribution of FISHWELL welfare score for diploid and triploid groups sampled from the beginning of the production up to slaughter. Percentage of fish with WI≥2 for each category.*Farm A not included in statistical test due to the severe disease outbreak at this farm. Percentage of not emaciated fish with at least one other WI scored as severe (WI≥2) is also calculated.
Table 4. Data report production harvest follow slaughter. “Weight at end” refers to weight after fish have been slaughtered. *Farm A not included in statistical test owing to the severe disease outbreak at this farm. P1 = t-test. Daily SGR = daily specific growth rate.
Daily SGR = [(ln final body weight − ln initial body weight)/days]×100
In addition, accumulated mortality for the first 15 months, based on the reported monthly mortalities, was calculated to obtain a standardized measure of mortality, independent of production length.
Health reports
At each farm, fish farmers reported all disease outbreaks identified by fish health services. However, the veterinary reports are not enclosed in the manuscript, as farmers requested document confidentiality. Health information from the reports, important to describe group performances is contextualized here with the results, side by side with mortality data.
Statistical analyses
Comparison of diploid and triploid WI-scores, first 15-month accumulated mortality, total mortality, production loss, and production quality grade at slaughter were tested using a paired t-test, with the significance level set at 5%. For farm B, the WI scoring data from the last three sampling episodes were excluded from the statistical analyses because only one group was available (the farmers could not sample both groups) for each sampling point, making the comparison impossible. Percentage data were arcsine transformed (sin−1∏[0.001 × p]) before being submitted to t-tests, as recommended by Crawley (Citation2013).
Results
Descripton of production at farm A
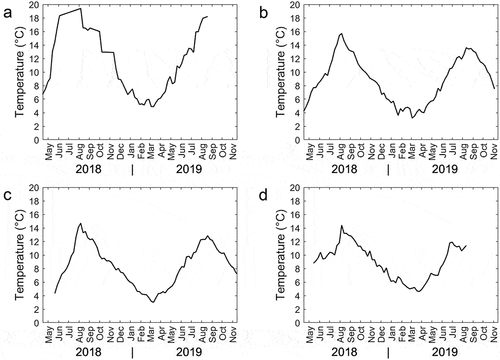
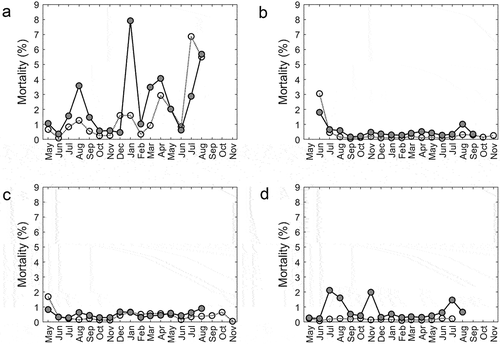
Diploid (average weight 115 g) and triploid (average weight 132 g) smolts were transferred to sea cages in April 2018. Water temperature at 5 m quickly rose from 7°C in April to above 18°C during the summer 2018, with a slow decline during the autumn months to nearly 5°C in the winter before increasing again in April 2019 (). Groups of both ploidy showed a similar pattern in reported monthly mortality, but with generally higher levels in triploids than in diploids ( and Appendix 4). There were four periods with peaks in mortality: (1) July–September 2018, (2) January 2019, (3) March and April 2019, and (4) July and August 2019. Plausible reasons for this mortality include high water temperature during the summer months and the presence of sluggish fish, typically referred to by farmers as “loser fish”, which are characterized by stunted growth and listless floating at the surface. In May 2018, both ploidy groups were deloused with Ectoban (Appendix 2), and there were several delousing operations in early winter 2019. Warm water treatment for delousing was performed four times for diploids (January (32°C), March (32°C), May (30°C), August (34°C)), and three times for triploids (January (32°C), March (32°C), July (34°C)). Farm A did not allow access to veterinary reports, but informed us that pancreatic disease (PD) and cardiomyopathy syndrome (CMS) were detected in May 2019. All fish were slaughtered in August 2019.
Descripton of production at farm B
Diploid (average weight 67 g) and triploid (average weight 55 g) smolts were transferred to sea cages in June 2018. Temperatures never exceeded 16°C, but fell below 4°C in the winter period (). After transfer, sluggish moribund “loser fish” without appetite occupied much of the surface layer in both sea cages, and the mortality in the first month was 3.5% in diploids and 2.5% in triploids (). Whether this is related to incomplete smoltification is unclear. After the first month, mortality was low in both groups. The only exception was in August 2019, when triploids exhibited elevated mortality (1.4%), possibly related to lice infestation and Hydrolicer de-lousing treatment (Appendix2, ). Farm B did not allow access to veterinary reports; however, the veterinarian informed us that on 25 January 2018, a bacterial wound infection by Tenacibaculum sp. and Moritella viscosa was identified in diploids. Triploids were harvested in October 2019, while diploids were harvested in November 2019.
Descripton of production at farm C
Diploid (90 g) and triploid (90 g) smolts were transferred to sea cages in May 2018. The temperature quickly rose from about 4°C in May to above 14°C in August, and then decreased to about 4°C during the following winter, before rising again in May (). The mortality rate during the first month was 1.6% in diploids and 0.84% in triploids (). This mortality was probably related to mechanical damage during transfer. Thereafter, mortality was low in both the ploidy groups. There was a small increase in mortality in December 2018 and January 2019 in both ploidy (), which was suspected to be related to cardiovascular and skeletal muscle inflammation (HSMB), particularly in the diploids. In August 2019, the last month before slaughter, the triploids showed higher mortality (0.91%). This may have been due to delousing operations (Hydrolicer in July 2019 and Alphamax in August 2019; Appendix 2), and/or to possible mechanical damage caused when for logistic reasons, the triploid fish were split into two equal halves and transferred to two new sea cages during the same month (August 2019; Appendix 3). Diploids were transferred to a new sea cage at the same time. The veterinarian reported that triploids exhibited a higher occurrence of emaciated loser fish (and moribund fish in the surface layer of the sea cage, and/or of fish displaying aberrant behavior, i.e. random swimming). Triploids were slaughtered in August and September 2019, while diploids were slaughtered in September 2019.
Descripton of production at farm D
Diploid (average weight 83 g) and triploid (average weight 80 g) smolts were transferred to sea cages in May 2018. This farm had a relatively stable temperature throughout the production (), with a peak temperature of about 14°C, but a relatively long period below 6°C during the winter (). Compared to the diploids, which exhibited low mortality throughout the production period in sea cages, the triploid group had three periods with elevated mortality: (1) July (2.1%) and August (1.6%) 2018, (2) November 2018 (2.0%), and (3) July 2019 (1,5%) (). The first period was attributed by the veterinarian to liver changes compatible with the effect of a toxic algae (not specified) bloom, skeletal and eye deformity (microphthalmy), and the presence of loser fish. The second period may have been related to a warm water delousing treatment (Appendix 2), followed by transfer to a new sea cage. In fact, the veterinarian reported several fish with physical damage during this period. Deaths during the third triploid mortality period may also be related to sea lice since elevated lice levels in triploids required a mechanical delousing treatment and transfer to a new sea cage (Appendix 2). Diploids did not require delousing treatment during production. Diploids were slaughtered in mid-July 2019, and triploids were slaughtered in late August 2019.
Triploid vs. Diploid (analysis of farm B,C,D)
Farm A was excluded in the following ploidy comparison because of the generally high mortality rate in the farm caused by fish infection with PD and CMS, which compromised possible ploidy effects.
Productions ended between 429 and 558 days after sea transfer (). The differences in mortality after 15 months between groups at farms B, C, and D were not significant (p = .09, ). For the whole production period, the total mortality was higher in triploids than in diploids on farms B and D, and equal between ploidy groups in farm C ().
In each ploidy and farm group, total fish loss for the entire production period was calculated based on the reported number of smolts transferred and the number of fish harvested. Total fish loss was higher in triploids than in diploids on all farms (p = .02, ).
At slaughter, diploids on all farms exhibited a significantly higher (p = .03) occurrence of fish with a “superior” quality grade (). Regarding “ordinary” and “production” quality grades, there was no systematic difference between diploid and triploid groups ().
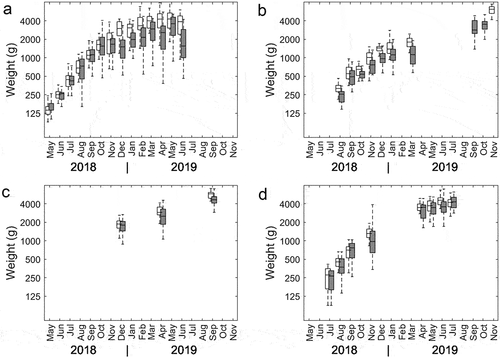
There were few observations and data points on individual fish weights recorded by fish farmers but based on available data, the average body weight of diploids was higher than that of triploids at almost all sampling points (). The daily specific growth rates (SGRs) were similar between ploidys on farms C and D, while it was slightly higher for triploids than for diploids on farm B, possibly owing to the smaller size of triploids compared to diploids when transferred to the sea cages (). There was no significant difference in SGR between diploids and triploids across farms (p = .26).
Figure 3. Body weights for the triploid (dark) and diploid salmon (white) measured at sampling at the four respective farms a, b, c and d.
The welfare scoring showed that the sampled triploid groups were more severely affected by emaciation (p = .013) than the diploid group (). Furthermore, the sampling revealed that the triploid groups had a significantly higher incidence of fish with scores ≥2 (thus severe) for at least one WI (p = .02, ).
Discussion
The present study aimed to compare production results from four aquaculture companies raising triploid and diploid Atlantic salmon from the same genetic line, all farmed under standard commercial conditions. Overall, triploid salmon exhibited lower performance and welfare than diploid.
Although the sampled triploid fish were generally smaller than the sampled diploids, there were no statistically significant differences in SGR. This is in contrast to earlier studies showing that triploid growth rates in seawater are typically lower than those of diploids (Fjelldal and Hansen Citation2010; Fraser et al. Citation2014, Citation2013; Leclercq et al. Citation2011; Taylor et al. Citation2014, Citation2015a, Citation2013). However, it is well established that smaller fish have a higher SGR than larger fish; thus, the smaller size at the start may explain some of this difference. This size effect was further amplified by two farms slaughtering the triploid groups earlier than the diploids. It is, therefore, not possible to make strong conclusions about the differences in growth between the diploid and triploid groups in the present study.
Recording mortality and precise quantities of fish in commercial sea cages is notoriously difficult (Aunsmo, Skjerve, and Midtlyng Citation2013; Ellis et al. Citation2012). The mortality analysis is, therefore, strengthened by both the 15 month accumulated mortality (calculated as total counted dead divided by number of fish put into the cage) and the percentage of production loss (calculated by the difference between what was transferred in the sea cage at the start of production and what was reported at slaughter), both pointing toward higher mortality in triploids. Most of the triploid monthly mortality seems to be correlated with delousing treatments, possibly indicating a lower resistance to handling and severe stressors, a theory suggested in other studies (Benfey Citation1999; Cotter et al. Citation2002). On farm A, the combination of lice infestation, delousing treatments, and PD and CMS outbreaks were assumed responsible for high mortality in both groups.
The incidence of emaciated fish was significantly higher in the sampled triploid groups than in the sampled diploid groups. During early sampling, these growth-stunted fish, also known as loser fish, displayed aberrant behaviors and low or null appetite, and were, therefore, characterized by a lean body (Vindas et al. Citation2016). It is thought that these loser fish are those poorly adapted to seawater, but salmon can also become stunted as a result of injury and disease (Noble et al. Citation2018). This is in line with the present observations, as both the sampled diploid and the sampled triploid groups exhibited high incidence of poor WI-scores. However, emaciation was the only WI attribute that exhibited a pattern of significant difference between the two groups. Sick and emaciated fish typically swim close to the surface, and may be easier to catch than other fish, potentially skewing samples. After accounting for this, the triploid groups still had a higher incidence of poor WI scores than the diploid groups. This, combined with the increased mortality, suggests that the triploid groups did exhibit overall lower welfare than the diploid groups. The present finding of more loser fish among triploids compared to diploids is in line with that of Fraser et al. (Citation2013), who followed diploid and triploid salmon to slaughter and reported a high incidence of loser fish among discharged triploids during primary processing. In diploid Atlantic salmon, reduced hypo-osmoregulatory ability during transfer to seawater creates loser fish, but these individuals survive up to harvest size, often retaining normal growth rates and condition factors after a period in seawater (unpublished personal results). Recently, Glover et al. (Citation2020) reported that triploid salmon display chromosomal aberrations. If the triploid loser fish phenomenon is related to osmoregulatory dysfunction, infections and/or genetic aberrations need to be further investigated to fully understand the suitability of triploid salmon in commercial aquaculture
Conclusion
The results of this study highlight important aspects of the production and welfare of triploid fish farmed under commercial conditions on four farms along the Norwegian coast. Working within commercial facilities made it impossible to standardize rearing conditions across companies. This was mainly linked to differences in the geographical positions, local physical (e.g. weather, temperature) and biological conditions (e.g. lice infestation/delousing treatments and disease) and feeding practices. In addition, within companies/sites, the farmers in some cases adopted different strategies for each cage, for example, timing of delousing or slaughter. However, since we followed several productions, the present results are representative of a strong indicator of triploid performance under commercial production. We found that the attempt to use triploid fish in order to increase environmental sustainability of the Atlantic salmon aquaculture industry is challenged by several factors: For a welfare perspective, the frequency of fish displaying moderate/severe welfare indicators and the current mortality rates is still too high. In addition, from an economic perspective, the higher loss in triploids results in lower income for the fish farmers, who may be unwilling to accept this in their production.
For the first time, triploid induction has been successfully conducted on a large scale, suggesting the possibility of wider use of sterile fish in commercial aquaculture. Moreover, success of triploidisation on this scale has not been recorded in commercial egg production. Further research is required in order to improve triploid performance and welfare, particularly during production in sea cages. Future studies should focus on optimization of husbandry conditions with the aim of making triploid farming more feasible for fish farmers.
Supplemental Material
Download MS Word (44.9 KB)Acknowledgments
The authors are grateful to all those who contributed to this work, in particular to all the fish farms and their staff, which contributed to the planning of the experiment, data collection, and reporting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Aarset, B., S. G. Carson, H. Wiig, I. E. Måren, and J. Marks. 2020. Lost in translation? Multiple discursive strategies and the interpretation of sustainability in the Norwegian Salmon farming industry. Food Ethics 5:1–21. doi:10.1007/s41055-020-00068-3.
- Amoroso, G., J. M. Cobcroft, M. B. Adams, T. Ventura, and C. G. Carter. 2016. Concurrence of lower jaw skeletal anomalies in triploid Atlantic salmon (Salmo salar L.) and the effect on growth in freshwater. Journal of Fish Diseases 39:1509–21. doi:10.1111/jfd.12492.
- Aunsmo, A., E. Skjerve, and P. J. Midtlyng. 2013. Accuracy and precision of harvest stock estimation in Atlantic salmon farming. Aquaculture 396–399:113–18. doi:10.1016/j.aquaculture.2013.03.001.
- Benfey, T. J. 1999. The Physiology and Behavior of Triploid Fishes. Reviews in Fisheries Science 7:39–67. doi:10.1080/10641269991319162.
- Benfey, T. J. 2001. Use of sterile triploid Atlantic salmon (Salmo salar L.) for aquaculture in New Brunswick, Canada. ICES Journal of Marine Science 58:525–29. Academic Press. doi:10.1006/jmsc.2000.1019.
- Benfey, T. J. 2016. Effectiveness of triploidy as a management tool for reproductive containment of farmed fish: Atlantic salmon (Salmo salar) as a case study. Reviews in Aquaculture 8:264–82. doi:10.1111/raq.12092.
- Benfey, T. J., and A. M. Sutterlin. 1984. Triploidy induced by heat shock and hydrostatic pressure in landlocked Atlantic salmon (Salmo salarL.). Aquaculture 36:359–67. doi:10.1016/0044-8486(84)90328-4.
- Benfey, T. J., A. M. Sutterlin, and R. J. Thompson. 1984. Use of erythrocyte measurements to identify triploid salmonids. Canadian Journal of Fisheries and Aquatic Sciences 41:980–84. doi:10.1139/f84-112.
- Cotter, D., V. O’Donovan, A. Drumm, N. Roche, E. N. Ling, and N. P. Wilkins. 2002. Comparison of freshwater and marine performances of all-female diploid and triploid Atlantic salmon (Salmo salar L.). Aquaculture Research 33:43–53. doi:10.1046/j.1355-557X.2001.00643.x.
- Crawley, M. J. 2013. The R book. John Wiley & Sons, Ltd. Chichester, UK.
- Ellis, T., I. Berrill, J. Lines, J. F. Turnbull, and T. G. Knowles. 2012. Mortality and fish welfare. Fish Physiology and Biochemistry 38:189–99. doi:10.1007/s10695-011-9547-3.
- Fjelldal, P. G., and T. Hansen. 2010. Vertebral deformities in triploid Atlantic salmon (Salmo salarL.) underyearling smolts. Aquaculture 309:131–36. doi:10.1016/j.aquaculture.2010.09.027.
- Fjelldal, P. G., T. J. Hansen, E.-J. Lock, A. Wargelius, T. W. K. Fraser, F. Sambraus, A. El-Mowafi, S. Albrektsen, R. Waagbø, and R. Ørnsrud. 2016. Increased dietary phosphorous prevents vertebral deformities in triploid Atlantic salmon (Salmo salar L.). Aquaculture Nutrition 22:72–90. doi:10.1111/anu.12238.
- Føre, H. M., and T. Thorvaldsen. 2021. Causal analysis of escape of Atlantic salmon and rainbow trout from Norwegian fish farms during 2010–2018. Aquaculture 532:736002. doi:10.1016/j.aquaculture.2020.736002.
- Fraser, T. W. K., P. G. Fjelldal, T. Hansen, and I. Mayer. 2012. Welfare considerations of triploid fish. Reviews in Fisheries Science 20:192–211. doi:10.1080/10641262.2012.704598.
- Fraser, T. W. K., T. Hansen, M. S. Fleming, and P. G. Fjelldal. 2015. The prevalence of vertebral deformities is increased with higher egg incubation temperatures and triploidy in Atlantic salmon Salmo salar L. Journal of Fish Diseases 38:75–89. doi:10.1111/jfd.12206.
- Fraser, T. W. K., T. Hansen, I. Mayer, J. E. Skjæraasen, K. A. Glover, F. Sambraus, and P. G. Fjelldal. 2014. The effect of triploidy on vaccine side-effects in Atlantic salmon. Aquaculture 433:481–90. doi:10.1016/j.aquaculture.2014.07.009.
- Fraser, T. W. K., T. Hansen, J. E. Skjæraasen, I. Mayer, F. Sambraus, and P. G. Fjelldal. 2013. The effect of triploidy on the culture performance, deformity prevalence, and heart morphology in Atlantic salmon. Aquaculture 416–417:255–64. doi:10.1016/j.aquaculture.2013.09.034.
- Glover, K. A., A. C. Harvey, T. J. Hansen, P. G. Fjelldal, F. N. Besnier, J. B. Bos, F. Ayllon, J. B. Taggart, and M. F. Solberg. 2020. Chromosome aberrations in pressure-induced triploid Atlantic salmon. BMC Genetics 21:59. doi:10.1186/s12863-020-00864-0.
- Golpour, A., M. A. M. Siddique, D. H. Siqueira-Silva, and M. Pšenička. 2016. Induced sterility in fish and its potential and challenges for aquaculture and germ cell transplantation technology: A review. Biologia (Bratisl) 71:853–64. doi:10.1515/biolog-2016-0118.
- Güralp, H., K. O. Skaftnesmo, E. Kjærner-Semb, A. H. Straume, L. Kleppe, R. W. Schulz, R. B. Edvardsen, and A. Wargelius. 2020. Rescue of germ cells in dnd crispant embryos opens the possibility to produce inherited sterility in Atlantic salmon. Scientific Reports 10:18042. doi:10.1038/s41598-020-74876-2.
- Jacq, C. (2020) NOFSal-MP10: A single hypervariable STR 12-plex for accurate verification of triploidy in Atlantic salmon (Salmo salarL.). bioRxiv 2020. 12.22.423910
- Leclercq, E., J. F. Taylor, D. Fison, P. G. Fjelldal, M. Diez-Padrisa, T. Hansen, and H. Migaud. 2011. Comparative seawater performance and deformity prevalence in out-of-season diploid and triploid Atlantic salmon (Salmo salar) post-smolts. Comparative Biochemistry and Physiology- A Molecular and Integrative Physiology 158:116–25. doi:10.1016/j.cbpa.2010.09.018.
- Lugert, V., G. Thaller, J. Tetens, C. Schulz, and J. Krieter. 2016. A review on fish growth calculation: Multiple functions in fish production and their specific application. Rev Aquac 8:30–42. doi:10.1111/raq.12071.
- Ministry of Trade Industry and Fisheries. 2012. Regulation on the operation of aquaculture production sites (In Norwegian: Forskrift om drift av akvakulturanlegg (Akvakulturdriftsforskriften)), FOR-2008-06-17-822, lovdata.no (2008). https://lovdata.no/dokument/SF/forskrift/2008-06-17-822, Accessed 31st Jul 2019.
- Noble, C., K. Gismervik, J. Iversen, Martin H. Kolarevic, J. Nilsson, L.H. Stien, J.F. Turnbull. (2018). Welfare Indicators for Farmed Atlantic Salmon: Tools for Assessing Fish Welfare FHF – Norwegian Seafood Research Fund. Trondheim, Norway: Google Scholar
- Olsvik, P. A., R. N. Finn, S. C. Remø, P. G. Fjelldal, F. Chauvigné, K. A. Glover, T. Hansen, and R. Waagbø. 2020. A transcriptomic analysis of diploid and triploid Atlantic salmon lenses with and without cataracts. Experimental Eye Research 199:108150. doi:10.1016/j.exer.2020.108150.
- Oppedal, F., G. L. Taranger, and T. Hansen. 2003. Growth performance and sexual maturation in diploid and triploid Atlantic salmon (Salmo salarL.) in seawater tanks exposed to continuous light or simulated natural photoperiod. Aquaculture 215:145–62. doi:10.1016/S0044-8486(02)00223-5.
- Pettersen, J. M., M. B. M. Bracke, P. J. Midtlyng, O. Folkedal, L. H. Stien, H. Steffenak, and T. S. Kristiansen. 2014. Salmon welfare index model 2.0: An extended model for overall welfare assessment of caged Atlantic salmon, based on a review of selected welfare indicators and intended for fish health professionals. Reviews in Aquaculture 6:162–79. doi:10.1111/raq.12039.
- Sadler, J., P. M. Pankhurst, and H. R. King. 2001. High prevalence of skeletal deformity and reduced gill surface area in triploid Atlantic salmon (Salmo salarL.). Aquaculture 198:369–86. doi:10.1016/S0044-8486(01)00508-7.
- Sambraus, F., P. G. Fjelldal, S. C. Remø, E. M. Hevrøy, T. O. Nilsen, A. Thorsen, T. J. Hansen, and R. Waagbø. 2017a. Water temperature and dietary histidine affect cataract formation in Atlantic salmon (Salmo salarL.) diploid and triploid yearling smolt. Journal of Fish Diseases 40:1195–212. doi:10.1111/jfd.12594.
- Sambraus, F., T. Hansen, B. S. Daae, A. Thorsen, R. Sandvik, L. H. Stien, T. W. K. Fraser, and P. G. Fjelldal. 2020. Triploid Atlantic salmon Salmo salar have a higher dietary phosphorus requirement for bone mineralization during early development. Journal of Fish Biology 97:137–47. doi:10.1111/jfb.14338.
- Sambraus, F., R. E. Olsen, M. Remen, T. J. Hansen, T. Torgersen, and P. G. Fjelldal. 2017b. Water temperature and oxygen: The effect of triploidy on performance and metabolism in farmed Atlantic salmon (Salmo salarL.) post-smolts. Aquaculture 473:1–12. doi:10.1016/j.aquaculture.2017.01.024.
- Smedley, M. A., B. G. J. Clokie, H. Migaud, P. Campbell, J. Walton, D. Hunter, D. Corrigan, and J. F. Taylor. 2016. Dietary phosphorous and protein supplementation enhances seawater growth and reduces severity of vertebral malformation in triploid Atlantic salmon (Salmo salarL.). Aquaculture 451:357–68. doi:10.1016/j.aquaculture.2015.10.001.
- Smedley, M. A., H. Migaud, E. L. McStay, M. Clarkson, P. Bozzolla, P. Campbell, and J. F. Taylor. 2018. Impact of dietary phosphorous in diploid and triploid Atlantic salmon (Salmo salarL.) with reference to early skeletal development in freshwater. Aquaculture 490:329–43. doi:10.1016/j.aquaculture.2018.02.049.
- Stien, L. H., M. B. M. Bracke, O. Folkedal, J. Nilsson, F. Oppedal, T. Torgersen, S. Kittilsen, P. J. Midtlyng, M. A. Vindas, Ø. Øverli, et al. 2013. Salmon Welfare Index Model (SWIM 1.0): A semantic model for overall welfare assessment of caged Atlantic salmon: Review of the selected welfare indicators and model presentation. Reviews in Aquaculture 5:33–57. doi:10.1111/j.1753-5131.2012.01083.x.
- Stien, L. H., B. Tørud, K. Gismervik, M. E. Lien, C. Medaas, T. Osmundsen, T. S. Kristiansen, and K. V. Størkersen. 2020. Governing the welfare of Norwegian farmed salmon: Three conflict cases. Marine Policy 117:103969. doi:10.1016/j.marpol.2020.103969.
- Sumpter, J. P., H. M. Dye, and T. J. Benfey. 1986. The effects of stress on plasma ACTH, α-MSH, and cortisol levels in salmonid fishes. General and Comparative Endocrinology 62:377–85. doi:10.1016/0016-6480(86)90047-X.
- Sutterlin, A. M., J. Holder, and T. J. Benfey. 1987. Early survival rates and subsequent morphological abnormalities in landlocked, anadromous and hybrid (landlocked× anadromous) diploid and triploid Atlantic salmon. Aquaculture 64:157–64. doi:10.1016/0044-8486(87)90351-6.
- Taranger, G. L., Ø. Karlsen, R. J. Bannister, K. A. Glover, V. Husa, E. Karlsbakk, B. O. Kvamme, K. K. Boxaspen, P. A. Bjørn, B. Finstad, et al. 2015. Risk assessment of the environmental impact of Norwegian Atlantic salmon farming. ICES Journal of Marine Science 72:997–1021. doi:10.1093/icesjms/fsu132.
- Taylor, J. F., P. Bozzolla, B. Frenzl, C. Matthew, D. Hunter, and H. Migaud. 2014. Triploid Atlantic salmon growth is negatively affected by communal ploidy rearing during seawater grow-out in tanks. Aquaculture 432:163–74. doi:10.1016/j.aquaculture.2014.05.014.
- Taylor, J. F., L. Martinez-Rubio, J. Del Pozo, J. M. Walton, A. E. Tinch, H. Migaud, and D. R. Tocher. 2015a. Influence of dietary phospholipid on early development and performance of Atlantic salmon (Salmo salar). Aquaculture 448:262–72. doi:10.1016/J.AQUACULTURE.2015.06.012.
- Taylor, J. F., F. Sambraus, J. Mota-Velasco, D. R. Guy, A. Hamilton, D. Hunter, D. Corrigan, and H. Migaud. 2013. Ploidy and family effects on Atlantic salmon (Salmo salar) growth, deformity and harvest quality during a full commercial production cycle. Aquaculture 410–411:41–50. doi:10.1016/j.aquaculture.2013.06.004.
- Taylor, J. F., R. Waagbø, M. Diez-Padrisa, P. Campbell, J. Walton, D. Hunter, C. Matthew, and H. Migaud. 2015b. Adult triploid Atlantic salmon (Salmo salar) have higher dietary histidine requirements to prevent cataract development in seawater. Aquaculture Nutrition 21:18–32. doi:10.1111/anu.12130.
- Vindas, M. A., I. B. Johansen, O. Folkedal, E. Höglund, M. Gorissen, G. Flik, T. S. Kristiansen, and Ø. Øverli. 2016. Brain serotonergic activation in growth-stunted farmed salmon: Adaption versus pathology. Royal Society Open Science 3:160030. doi:10.1098/rsos.160030.