?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Atlantic salmon and rainbow trout with initial weights of 460–468 g were fed two diets either high in fish oil (FO) or rapeseed oil (RO) content. The abilities of the fish to metabolize and deposit lipid and n-3 fatty acids were compared. Growth rates, FCR, and fat digestibility were similar in both species. Rainbow trout showed higher condition factors and lower dress-out percentage and hepatosomatic index than salmon. Whole-body content of EPA and DHA were higher in both species fed the FO diet than in the RO diet, but indicated higher capacities to produce DHA in rainbow trout than in salmon. The total fat content in the RO group was lower and DHA higher in rainbow trout liver than in salmon, indicating a greater ability of rainbow trout to increase metabolism to DHA.
Introduction
Salmonids in aquaculture are routinely fed diets based on vegetable oils (VOs) as a replacement for fish oil (FO). VOs contain minimal amounts of fatty acids with chain lengths longer than 18 carbons and more than three double bonds, and lack long-chain n-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA; C20:5 n-3) and docosahexaenoic acid (DHA; C22:6 n-3) that are abundant in FOs. The use of VOs does affect the fatty acid composition of the edible portion of the fish (Bell et al. Citation2002, Citation2001, Citation2003a, Citation2003b; Regost, Jakobsen, and Rørå Citation2004; Rosenlund et al. Citation2001; Torstensen et al. Citation2005, Citation2004; Waagbø et al. Citation1991, Citation1993). Several studies in salmonids have shown that replacement of FO with VO can be used with good growth results. However, reducing the levels of EPA and DHA below the dietary requirement levels may reduce the growth of Atlantic salmon (Bou et al. Citation2017a, Citation2017b; Rosenlund et al. Citation2016), while rainbow trout growth was less affected by dietary fatty acid composition (Caballero et al. Citation2002). Feeding VO to rainbow trout reduced the body content of EPA and DHA less than expected compared with the reduction in the dietary content (Yıldız et al. Citation2018). The fatty acid composition of the fish also depends on factors such as fatty acid digestibility (Sigurgisladottir et al. Citation1992; Torstensen, Lie, and Frøyland Citation2000), transport, and deposition in tissues, elongation, and desaturation processes (Bell et al. Citation2002, Citation2001), and β-oxidation (Frøyland, Lie, and Berge Citation2000; Torstensen, Lie, and Frøyland Citation2000).
Atlantic salmon (Ruyter et al. Citation2000a, Citation2000b) and rainbow trout (Thanuthong et al. Citation2011) are, to some extent, able to convert dietary α-linolenic acid (ALA; C18:3 n-3) and linoleic acid (C18:2 n-6) to C20 and C22 PUFAs, and this conversion mainly takes place in the liver. It has been shown in both species that dietary inclusion levels of VO and FO influence the capacity for this conversion (Kjær et al. Citation2016; Randall, Reaney, and Drew Citation2013; Tocher et al. Citation2001), however, to our knowledge, no studies have directly compared the conversion capacity between the two species when fed the same diets.
Several studies have shown that increased dietary levels of VO in salmon diets lead to reduced levels of DHA and increased levels of C18 fatty acid from VO in the liver as well as increased total liver fat content (Bou et al. Citation2017c; Ruyter et al. Citation2006; Sissener et al. Citation2017). Similar responses have been observed in rainbow trout (Fonseca‐madrigal et al. Citation2005).
The aim of the present study was to compare the ability of Atlantic salmon and rainbow trout to utilize dietary n-3 fatty acids and deposit EPA and DHA in the whole body and liver when fed two diets with different VO and FO content.
Materials and methods
Fish and facilities
Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) with mean initial weights of 460 g and 468 g, respectively, were used in the feeding experiment. All fish were individually tagged (PIT-tags; Passive Integrator Responder, Biosonic, Seattle, USA) to record individual weights. Groups of 30 fish were kept in 1-m2 fiberglass tanks with a water depth of 60 cm. During the experiment, each tank was supplied with salt water (32.9 g/L salinity, standard deviation = 0.8), with an average temperature of 8.3°C (range, 7.2°C to 9.9°C). Both species were fed the same commercial diet (Skretting, Stavanger, Norway) for 4 weeks prior to beginning the experiment. During the experimental period of 73 days, two different experimental diets were fed to duplicate groups of salmon and trout. The feed was distributed by electrically driven disc-feeders (Akvaprodukter AS, Sunndalsøra, Norway). The tanks were designed to accommodate the collection of waste feed from the effluent water in wire mesh boxes. Uneaten feeds were collected to monitor daily feed intake in each tank (Helland, Grisdale-Helland, and Nerland Citation1996). The feeding procedure aimed to achieve the optimal voluntary feed intake in all groups of fish. The feeding trial was performed at Nofima Sunndalsøra Research Station. The feeding study was performed in compliance with the Norwegian regulation for the use of experimental animals, FOR-1996.01–1523 Forskrift om forsøk med dyr, which was valid from 1996 to 2015. The conclusion of the local competent authority was that the experiment was exempt from the requirement for a specific license, with reference to the criteria listed in §2 in the regulation.
Diets
The experimental diets were provided by BioMar AS, Brande, Denmark. The composition of the two diets (6-mm extruded pellets) was identical except for the oil fraction, one diet contained only fish oil (FO), while the other diet contained 70% of the oil as rapeseed oil (RO). Yttrium oxide was added to the diets as an inert marker to determine digestibility. The formulations and proximate compositions of the diets are shown in , and the dietary fatty acid profiles are shown in .
Table 1. Feed components (g kg−1) and proximate compositions of the experimental diets (% of diet as is).
Table 2. Fatty acid composition (% of total fatty acids) of the experimental diets.
Data collection
All fish were individually weighed at the start and end of the experimental period. Initial samples of 2 × 10 fish from each species, collected from the same population as fish in the experiment, were used for whole-body chemical composition analyses at the beginning of the experiment. At the end of the experiment, five representative fish were pooled to one sample per tank for whole-body chemical analyses. Another 10 fish per tank were sampled and dissected, and liver and gutted weights were recorded. Livers were collected for fat content and fatty acid profile analyses. Fish sampled for dissection and for chemical analyses were given a lethal dose of metacaine prior to being sacrificed. The remaining fish were stripped to collect pooled fecal samples, one sample per tank, to determine digestibility according to the procedure described previously by (Austreng, Citation1978). The fish were anaesthetized using 60 mg/L metacaine (MS-222, Sipsy, Avrille, France) before weighing and collecting feces by stripping.
Analyses and calculations
Both diets and fecal samples were analyzed to determine dry matter (105°C until constant weight), total fat content (Folch, Lees, and Stanley Citation1957), fatty acid composition [lipid extraction (Folch, Lees, and Stanley Citation1957), methylation (Mason and Waller Citation1964), and separation by gas chromatography (Røsjø et al. Citation1994)], and yttrium [inductively coupled plasma (ICP) mass spectroscopy, as described by (Refstie, Helland, and Storebakken Citation1997)]. Diets were also analyzed for nitrogen content using a Kjeltech Auto Analyzer (Tecator, Höganäs, Sweden; crude protein: N × 6.25), starch was analyzed by enzymatic hydrolysis (Megazyme, Bray, Ireland) prior to measuring glucose (GOD/POD method), gross energy was measured by bomb calorimetry (Parr 1271, Parr, Moline, IL, USA), and ash was determined by flame combustion (at 550°C until constant weight). Total fat and fatty acids were measured using the methods described above in homogenized whole fish sampled at the beginning and end of the experiment as well as in livers sampled at the end of the experiment.
Specific growth rate (SGR, %/day) and thermal unit growth coefficient (TGC) were calculated based on individual recordings of weights (g) as follows:
where W0 represents the start weight, W1 represents the final weight, d represents the number of feeding days, and d° represents the total sum of day degrees during the feeding experiment.
The feed conversion ratio (FCR), condition factor (CF), dress-out percentage (DP), and hepatosomatic index (HSI) were calculated as follows:
The apparent digestibility coefficient (ADC) was calculated as described by (Austreng, Citation1978) as follows:
The apparent retention coefficient (ARC) was calculated as follows:
where Fa1 represents the amount of fat or fatty acid in fish whole body at end of the experiment, Fa0 represents the amount of fat or fatty acid in fish at start of the experiment, and Fai represents the amount of fat or fatty acid ingested during the experiment.
Individual recordings of the initial and final weights, SGR, TGC, and CF (n = 30) were used for initial statistical analysis using a nested model to assess any dietary effect. Other parameters were based on a subsample of representative fish (n = 10 for dissecting, n = 5 for whole body analyses) from each tank averaged by tank (HSI, DP, body composition) or pooled samples by tank (FCR, ADC, ARC). Weights and growth rates were also averaged by tanks, and all data were subjected to analysis of variance. A factorial model including the effect of species, oil source, and the interaction between species and oil source was tested as follows:
Y = μ + species + oil + (species*oil) + error
Significant effects were indicated at a 5% level.
Results from nested analyses of individual weights and growth rates were similar to results for averaged data, and therefore all data were presented based on analyses of data averaged per tank (n = 2). All statistical analyses were performed using the software package SAS System for Windows Release 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Fish performance
The average initial weights of the experimental fish were similar for Atlantic salmon and rainbow trout (460 g and 468 g, respectively). During the experimental period, the fish grew to final weights of 879–954 g and lengths 36.0–38.7 cm, with an SGR of 0.98–1.05%/day, TGC of 3.4–3.7, and FCR of 0.88–0.89 (). No significant effects of diet or species were observed for any of these parameters, except the final length. The model was significant for both CF, DP, and HSI, and species was the factor that explained the majority of the variation. CF was higher in rainbow trout than Atlantic salmon, while DP and HSI were higher in Atlantic salmon than in rainbow trout. No mortality was observed during the experimental period.
Table 3. Weight, length, growth rate (SGR, and TGC), feed conversion ratio (FCR), condition factor (CF), dress-out percentage (DP), and hepatosomatic index (HSI) of fish fed the experimental diets (mean ± SEM, n = 2).
Apparent digestibility
The mean ADC for total lipids, ALA, EPA, DPA (docosapentaenoic acid; C22:5 n-3), and DHA, and the main groups of fatty acids are shown in . The overall ADC levels were high for both total fat and fatty acids. ADC was lowest for saturated fatty acids and highest for PUFAs. The ADC of fat or fatty acid showed no significant differences between the species, and there was no effect on the oil source.
Table 4. Apparent digestibility coefficients of total lipids and some fatty acids in Atlantic salmon and rainbow trout fed the experimental diets (mean ± SEM, n = 2).
Whole-body fat and fatty acid content
The final total whole-body fat content () did not significantly differ between the two species although the average values tended to be higher in rainbow trout compared with Atlantic salmon. Both species showed a significant increase in whole-body fat content compared with the initial values. The whole-body content of EPA, DPA, and DHA, and the sum of EPA and DHA were significantly higher in both Atlantic salmon and rainbow trout fed the FO diet compared with those fed the RO diet and there were no significant differences between the two species. Whole-body saturated fatty acid (SFA) content was higher in fish fed the FO diet compared with those fed the RO diet, and was also higher in rainbow trout compared with Atlantic salmon. Whole-body monounsaturated fatty acid (MUFA) content was higher in fish fed the RO diet compared with those fed the FO diet, and was also higher in Atlantic salmon compared with rainbow trout. Whole-body PUFA content was only affected by oil sources and was higher in fish fed the RO diet compared with those fed the FO diet.
Table 5. Whole-body total lipid and fatty acid profiles (% of total fatty acids), and quantitative content of EPA and DHA (g/100 g), in Atlantic salmon and Rainbow trout fed the experimental diets. Initial values are included, but statistical comparisons include the final content of fish from the different dietary groups only (mean ± SEM, n = 2).
Whole-body retention
The mean apparent whole-body ARC values for the total amount of ingested fat, n-3 fatty acids, and groups of fatty acids during the experiment are presented in . The ARC of total fat was 70%–73% for Atlantic salmon and around 86% for rainbow trout. Due to high variation in some groups, the model was not significant for ARCs of either total fat or fatty acids except ALA. The ARC of ALA was significantly affected by both species and oil sources, with a higher ARC observed in rainbow trout than in Atlantic salmon, and higher ARC for the FO diet compared with the RO diet. The ARC of the EPA was 32%–41% in Atlantic salmon and 50%–60% in rainbow trout. Species accounted for 57.7% of the total variation in the ARC of EPA, while oil source only accounted for 15.4%. The ARC of DHA was 125%–157% for rainbow trout and 93%–115% for Atlantic salmon. Species accounted for 33.9% of the total variation in the ARC of DHA, while oil sources accounted for 18.6%. The ARC of EPA tended to be lower in fish fed the RO diet compared with the FO diet, while the ARC of DHA tended to be higher with the RO diet than the FO diet. This finding was observed in both species.
Table 6. Apparent retention coefficients of total fat and fatty acids ingested in Atlantic salmon and rainbow trout fed the experimental diets (mean ± SEM, n = 2).
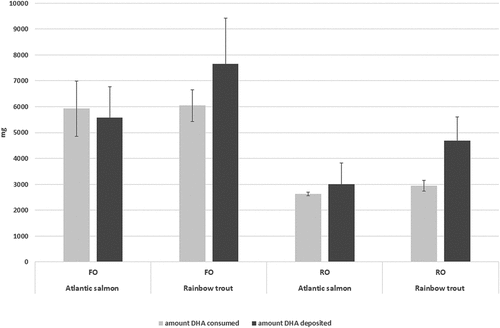
To visualize the deposition relative to the amount consumed, the total amount (mg) of DHA deposited in an average fish was shown together with the amount (mg) consumed by an average fish () for both species and with both diets. If the amount of DHA deposited in the fish exceeded the amount consumed, the ARC was >100%. When salmon were fed the FO diet, with a relatively high DHA content, the amount deposited was less than the amount ingested, whereas the opposite was observed when salmon were fed the RO diet, which contained less DHA. Rainbow trout were able to deposit on average more DHA than the amount consumed in both the FO and RO diets, but the differences were not significant due to the high variation.
Liver fat and fatty acid content
The liver fat and fatty acid contents are presented in . The factorial model was highly significant for nearly all fatty acids. Overall, oil sources could account for the majority of the variation in both total fat and fatty acid profiles. Interestingly, species and interactions between oil sources and species explained a larger proportion of variations in total fat and some fatty acids. Interaction between oil source and species explained 59.5% of the variation in total liver fat, indicating that Atlantic salmon and rainbow trout responded differently to the two oil sources. Liver fat content in salmon fed the RO diet was nearly two-fold higher than that of salmon fed the FO diet, and a similar increase in fat content was not seen in rainbow trout liver. Interaction between oil source and species was important for total amount of n-3 fatty acids, particularly DHA, and MUFA. Liver from salmon fed the RO diet showed reduced n-3 content and increased MUFA content compared with liver from salmon fed the FO diet. The liver content of total n-3 and MUFA were similar for rainbow trout fed both diets. For some fatty acids (C18:0, C 16:1 n-7, C18:1 n-7, C20:1 n-11, and DHA), species accounted for a larger (>10%) proportion of the variation, indicating differences in metabolism between the two species.
Table 7. Liver total lipid (%) and fatty acid profile (% of total fatty acids) in Atlantic salmon and Rainbow trout fed the experimental diets (mean ± SEM, n = 2).
Discussion
The high survival and growth rate (TGC) and low FCR observed for fish in both dietary groups in the present study indicated that RO showed no negative overall effect on fish performance as long as the dietary content of EPA and DHA was sufficient (Bou et al. Citation2017a, Citation2017b; Rosenlund et al. Citation2016). The difference in final length and CF between the two species reflected the difference in body shape, with rainbow trout being shorter relative to weight compared with Atlantic salmon, and thus the CF was higher in rainbow trout than in Atlantic salmon. HSI and DP were both lower in rainbow trout compared with Atlantic salmon. These findings could indicate that rainbow trout had more excess abdominal fat.
The present study found no difference in total lipid digestibility between the two species although there was a slight trend toward higher fat digestibility in the RO group compared with the FO group. The findings were in accordance with (Caballero et al. Citation2002) that the digestibility of neither total fat nor EPA and DHA was affected by dietary inclusion of RO.
Most studies presenting fish fillet or whole-body fatty acid composition have reported that the composition of fish reflects the fatty acid profile of the diet although in some cases fatty acids could be overrepresented in fish compared with diets (Bou et al. Citation2017b; Glencross Citation2009; Yıldız et al. Citation2018). (Caballero et al. Citation2002) fed different lipid sources to rainbow trout and found that although RO caused a reduction in the relative content of tissue EPA and DHA compared with FO, the relative tissue content of these fatty acids was higher than in the corresponding diets. In the present study, dietary oil was a major factor affecting the fatty acid profile in whole-body of the two species of fish. RO caused reduced levels of EPA and DHA in both species.
In the present study, the total lipid ARC was 70%–73% for Atlantic salmon and almost 86% for rainbow trout, and species was the major factor accounting for the variation in lipid retention. A relatively high content of dietary lipids relative to proteins in the present experiment may have contributed to the high total lipid ARC. Our findings on retention of total lipids in Atlantic salmon whole body were in the high range but still in accordance with previously reported ARC values of 55%–75% of the ingested lipids observed in a study with Atlantic salmon fed differing dietary contents of a bacterial protein meal (Aas et al. Citation2006).
When the ARC of the DHA is >100%, this may indicate the ability of the species to convert ALA to DHA. It is well known that elongase and desaturase activities are increased when fish are fed lipid sources that are low in long-chain PUFAs (Bell et al. Citation2001; Bou et al. Citation2017c; Francis et al. Citation2007; Kjær et al. Citation2016; Stubhaug et al. Citation2005). Fatty acid productivity values (FAPVs), which correspond to the ARC, in Atlantic salmon fed diets containing FO or a blend of VOs, were previously reported (Stubhaug, Lie, and Torstensen Citation2007) in a long-term feeding trial with salmon growing from 160 g to 2500 g. The FAPVs of both EPA and DHA were higher in salmon fed VO, indicating more efficient deposition of DHA when the diet was low in these fatty acids. However, the increased conversion to EPA and DHA is not sufficient to compensate for the lower dietary content of these fatty acids, and the tissue level of these fatty acids in fish is reduced. When dietary levels of these essential fatty acids are reduced, the conservation, and thus the ARC will be increased (i.e., the rate of utilization will be higher) as shown by (Bou et al. Citation2017a). This trend was also observed in the present study although no significant differences were seen. Numerically higher values for the ARC of DHA were seen when rainbow trout were fed diets containing RO, with retention values of 124.9% (FO) and 157.4% (RO) indicating net production of DHA. In Atlantic salmon, the ARCs of DHA were 93.5% (FO) and 115.3% (RO), indicating that salmon were able to produce DHA, although at a lower capacity than rainbow trout. Species was the most important factor accounting for the total variation in ARC of both EPA and DHA. DPA, which is an intermediate product in the conversion from EPA to DHA, showed ARC values >300, indicating that this was a limiting step in the pathway to DHA, since DPA levels accumulated. Accumulation of DPA was also seen in salmon fed diets containing an EPA-rich yeast (Berge et al. Citation2013; Hatlen et al. Citation2012). In the present study, accumulation of DPA was seen in both species and with both diets. However, the level of DPA was low compared to the level of EPA and DHA in the diets (), and the low dietary level could give reduced accuracy in the ARC values of DPA.
The highest average ARC of DHA was seen in rainbow trout fed the RO diet, which had the lowest DHA content. Although the average amount (mg) of DHA consumed during the experimental period was similar for the two species, the amount deposited was higher in rainbow trout than Atlantic salmon (). The present results supported a hypothesis that rainbow trout have a higher capacity than Atlantic salmon to deposit DHA, in agreement with a tendency toward a higher degree of deposition of total fat (as measured by total lipid retention) and lower DP.
Turchini and Francis (Citation2009) reported that rainbow trout fed a diet containing linseed oil would actively convert ALA to DHA, but the proportion was low. The largest proportion (58.1%) was deposited in whole body as ALA, while 29.5% was used for energy. In the present study, the ARC of ALA was 87.5% and 61.8% for rainbow trout fed the FO and RO diets, respectively. These results support the findings of Turchini and Francis (Citation2009) who reported that the majority of ALA is deposited in the body of rainbow trout. However, conversion to DHA occurred as the ARC values of DHA were >100. The higher ARC values for DHA also indicated that DHA is used less commonly for energy compared with ALA.
The possibility of transforming salmonid aquaculture into a net producer of long-chain n-3 fatty acids has been discussed extensively (Bendiksen et al. Citation2011; Bou et al. Citation2017a, Citation2017b; Crampton et al. Citation2010; Sanden et al. Citation2011; Turchini et al. Citation2011; Ytrestøyl, Aas, and Åsgård Citation2015). In a case study Turchini et al. (Citation2011) showed that the input of n-3 long-chain PUFA far exceeded the output (4.5-fold) in rainbow trout fed a FO diet. When the fish were fed a diet with linseed oil, the output was twice that of the input. The fillet content of n-3 long-chain PUFA was still significantly lower in the linseed group compared with the FO group. The results from our study showed ARC > 100% of EPA+DHA in rainbow trout fed the RO diet, thus supporting the findings reported by (Turchini et al. Citation2011). However, ARC < 100% for EPA+DHA was observed in Atlantic salmon in our study. The ARC of DHA was >100% in both species fed the RO diet as well as rainbow trout fed the FO diet.
Previous studies have shown that the liver has a relatively high capacity for converting ALA to EPA and DHA in Atlantic salmon (Bou et al. Citation2017c; Kjær et al. Citation2016; Ruyter et al. Citation2000a; Ruyter and Thomassen Citation1999) and rainbow trout (Mourente and Tocher Citation1998), and that this capacity increases with increased dietary levels of VO. In the present study, the ratio of n-3 fatty acids (20:5 + 22:5 + 22:6)/18:3 was 11.1 in rainbow trout liver compared with 5.2 in salmon liver when both species were fed the RO diet, indicating a two-fold higher capacity of rainbow trout liver to convert ALA to C20–22 products when fed a VO diet. Salmon liver showed a marked increase in fat content from 5.2% to 8.9%, while the liver fat content in trout remained unchanged and was approximately 6% for the two dietary groups. The fact that interaction between species and oil source was the most important factor accounting for the variation in liver fat content supports that the two species respond differently to RO. The differences between the species may indicate that the higher capacity for EPA and DHA production in rainbow trout compared with salmon may result in less deposition of fat in the liver of trout compared with salmon. Several studies have shown that feeding plant oil to Atlantic salmon increases the deposition of fat in the liver, particularly at low water temperatures (Bou et al. Citation2017b; Ruyter et al. Citation2006; Sissener et al. Citation2017). However, in agreement with our findings, previous studies have indicated that increased levels of dietary plant oils do not affect liver lipogenesis or lipid droplet content in rainbow trout (Figueiredo‐Silva et al. Citation2005; Richard et al. Citation2006).
Conclusions
In summary, the present study found that the growth rate and feed conversion ratio in Atlantic salmon and rainbow trout were not affected by the dietary oil source, and both species showed similar high digestibility of fat and fatty acids. The ARC of n-3 fatty acids did not significantly differ between the two species. However, our results indicated that rainbow trout tended to have a better capacity to deposit dietary EPA and DHA in whole body compared with Atlantic salmon. Rainbow trout showed a higher liver capacity to convert ALA to DHA when fed a high-VO diet. Rainbow trout had a higher CF and reduced DP compared with Atlantic salmon, indicating a greater excess of abdominal fat in rainbow trout. The species differences indicated are probably linked to genetic differences in metabolism of fat and fatty acids. Further studies are required to better understand the mechanisms, and further to make use of rainbow trout as an efficient net producer of long-chain n-3 fatty acids.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aas, T. S., B. Grisdale-Helland, B. F. Terjesen, and S. J. Helland. 2006. Improved growth and nutrient utilisation in Atlantic salmon (Salmo salar) fed diets containing a bacterial protein meal. Aquaculture 259 (1–4):365–76. doi:10.1016/j.aquaculture.2006.05.032.
- Austreng, E. 1978. Digestibility determination in fish using chromic oxide marking and analysis of contents from different segments of the gastrointestinal tract. Aquaculture 13 (3):265–72. doi:10.1016/0044-8486(78)90008-X.
- Bell, J. G., R. J. Henderson, D. R. Tocher, F. McGhee, J. R. Dick, A. Porter, R. P. Smullen, and J. R. Sargent. 2002. Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. The Journal of Nutrition 132 (2):222–30. doi:10.1093/jn/132.2.222.
- Bell, J. G., J. McEvoy, D. R. Tocher, F. McGhee, P. J. Campbell, and J. R. Sargent. 2001. Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. The Journal of Nutrition 131 (5):1535–43. doi:10.1093/jn/131.5.1535.
- Bell, J. G., F. McGhee, P. J. Campbell, and J. R. Sargent. 2003a. Rapeseed oil as an alternative to marine fish oil in diets of post-smolt Atlantic salmon (Salmo salar): Changes in flesh fatty acid composition and effectiveness of subsequent fish oil “wash out”. Aquaculture 218 (1–4):515–28. doi:10.1016/S0044-8486(02)00462-3.
- Bell, J. G., D. R. Tocher, R. J. Henderson, J. R. Dick, and V. O. Crampton. 2003b. Altered fatty acid compositions in Atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet. The Journal of Nutrition 133 (9):2793–801. doi:10.1093/jn/133.9.2793.
- Bendiksen, E. Å., C. A. Johnsen, H. J. Olsen, and M. Jobling. 2011. Sustainable aquafeeds: Progress towards reduced reliance upon marine ingredients in diets for farmed Atlantic salmon (Salmo salar L.). Aquaculture 314:132–39.
- Berge, G., B. Hatlen, J. Odom, and B. Ruyter. 2013. Physical treatment of high EPA Y arrowia lipolytica biomass increases the availability of n‐3 highly unsaturated fatty acids when fed to A tlantic salmon. Aquaculture Nutrition 19:110–21. doi:10.1111/anu.12092.
- Bou, M., G. M. Berge, G. Baeverfjord, T. Sigholt, T. K. Ostbye, O. H. Romarheim, B. Hatlen, R. Leeuwis, C. Venegas, and B. Ruyter. 2017a. Requirements of n-3 very long-chain PUFA in Atlantic salmon (Salmo salar L): Effects of different dietary levels of EPA and DHA on fish performance and tissue composition and integrity. British Journal of Nutrition 117 (1):30–47. doi:10.1017/S0007114516004396.
- Bou, M., G. M. Berge, G. Baeverfjord, T. Sigholt, T.-K. Østbye, and B. Ruyter. 2017b. Low levels of very-long-chain n-3 PUFA in Atlantic salmon (Salmo salar) diet reduce fish robustness under challenging conditions in sea cages. Journal of Nutritional Science 6:e32. doi:10.1017/jns.2017.28.
- Bou, M., T.-K. Østbye, G. M. Berge, and B. Ruyter. 2017c. EPA, DHA, and lipoic acid differentially modulate the n-3 fatty acid biosynthetic pathway in Atlantic salmon hepatocytes. Lipids 52 (3):265–83. doi:10.1007/s11745-017-4234-5.
- Caballero, M., A. Obach, G. Rosenlund, D. Montero, M. Gisvold, and M. Izquierdo. 2002. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 214 (1–4):253–71. doi:10.1016/S0044-8486(01)00852-3.
- Crampton, V. O., D. A. Nanton, K. Ruohonen, P. O. Skjervold, and A. El‐Mowafi. 2010. Demonstration of salmon farming as a net producer of fish protein and oil. Aquaculture Nutrition 16 (4):437–46. doi:10.1111/j.1365-2095.2010.00780.x.
- Figueiredo‐Silva, A., E. Rocha, J. Dias, P. Silva, P. Rema, E. Gomes, and L. Valente. 2005. Partial replacement of fish oil by soybean oil on lipid distribution and liver histology in European sea bass (Dicentrarchus labrax) and rainbow trout (Oncorhynchus mykiss) juveniles. Aquaculture Nutrition 11 (2):147–55. doi:10.1111/j.1365-2095.2004.00337.x.
- Folch, J., M. Lees, and G. S. Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry 226 (1):497–509. doi:10.1016/S0021-9258(18)64849-5.
- Fonseca‐madrigal, J., V. Karalazos, P. Campbell, J. G. Bell, and D. R. Tocher. 2005. Influence of dietary palm oil on growth, tissue fatty acid compositions, and fatty acid metabolism in liver and intestine in rainbow trout (Oncorhynchus mykiss). Aquaculture Nutrition 11 (4):241–50. doi:10.1111/j.1365-2095.2005.00346.x.
- Francis, D. S., G. M. Turchini, P. L. Jones, and S. S. De Silva. 2007. Dietary lipid source modulates in vivo fatty acid metabolism in the Freshwater Fish, Murray Cod (Maccullochella peelii peelii). Journal of Agricultural and Food Chemistry 55 (4):1582–91. doi:10.1021/jf062153x.
- Frøyland, L., Ø. Lie, and R. Berge. 2000. Mitochondrial and peroxisomal β-oxidation capacities in various tissues from Atlantic salmon, Salmo salar. Aquaculture Nutrition 6 (2):85–89. doi:10.1046/j.1365-2095.2000.00130.x.
- Glencross, B. D. 2009. Exploring the nutritional demand for essential fatty acids by aquaculture species. Reviews in Aquaculture 1:71–124.
- Hatlen, B., G. M. Berge, J. M. Odom, H. Mundheim, and B. Ruyter. 2012. Growth performance, feed utilisation and fatty acid deposition in Atlantic salmon, Salmo salar L., fed graded levels of high-lipid/high-EPA Yarrowia lipolytica biomass. Aquaculture 364:39–47. doi:10.1016/j.aquaculture.2012.07.005.
- Helland, S., B. Grisdale-Helland, and S. Nerland. 1996. A simple method for the measurement of daily feed intake of groups of fish in tanks. Aquaculture 139 (1–2):157–63. doi:10.1016/0044-8486(95)01145-5.
- Kjær, M. A., B. Ruyter, G. M. Berge, Y. Sun, and T.-K.-K. Østbye. 2016. Regulation of the omega-3 fatty acid biosynthetic pathway in Atlantic salmon hepatocytes. PloS One 11 (12):e0168230. doi:10.1371/journal.pone.0168230.
- Mason, M. E., and G. R. Waller. 1964. Dimethoxypropane induced transesterification of fats and oils in preparation of methyl esters for gas chromatographic analysis. Analytical Chemistry 36 (3):583–86. doi:10.1021/ac60209a008.
- Mourente, G., and D. R. Tocher. 1998. The in vivo incorporation and metabolism of [1-14 C] linolenate (18: 3n-3) in liver, brain and eyes of juveniles of rainbow trout Oncorhynchus mykiss L and gilthead sea bream Sparus aurata L. Fish Physiology and Biochemistry 18 (2):149–65. doi:10.1023/A:1007717312480.
- Randall, K., M. Reaney, and M. Drew. 2013. Effect of dietary coriander oil and vegetable oil sources on fillet fatty acid composition of rainbow trout. Canadian Journal of Animal Science 93 (3):345–52. doi:10.4141/cjas2013-001.
- Refstie, S., S. J. Helland, and T. Storebakken. 1997. Adaptation to soybean meal in diets for rainbow trout, Oncorhynchus mykiss. Aquaculture 153 (3–4):263–72. doi:10.1016/S0044-8486(97)00025-2.
- Regost, C., J. V. Jakobsen, and A. M. B. Rørå. 2004. Flesh quality of raw and smoked fillets of Atlantic salmon as influenced by dietary oil sources and frozen storage. Food Research International 37 (3):259–71. doi:10.1016/j.foodres.2003.12.003.
- Richard, N., S. Kaushik, L. Larroquet, S. Panserat, and G. Corraze. 2006. Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). British Journal of Nutrition 96 (2):299–309. doi:10.1079/BJN20061821.
- Rosenlund, G., A. Obach, M. Sandberg, H. Standal, and K. Tveit. 2001. Effect of alternative lipid sources on long‐term growth performance and quality of Atlantic salmon (Salmo salar L.). Aquaculture Research 32:323–28. doi:10.1046/j.1355-557x.2001.00025.x.
- Rosenlund, G., B. E. Torstensen, I. Stubhaug, N. Usman, and N. H. Sissener. 2016. Atlantic salmon require long-chain n-3 fatty acids for optimal growth throughout the seawater period. Journal of Nutritional Science 5:e19. doi:10.1017/jns.2016.10.
- Røsjø, C., T. Berg, K. Manum, T. Gjøen, S. Magnusson, and M. S. Thomassen. 1994. Effects of temperature and dietary n-3 and n-6 fatty acids on endocytic processes in isolated rainbow trout (Oncorhynchus mykiss, Walbaum) hepatocytes. Fish Physiology and Biochemistry 13 (2):119–32. doi:10.1007/BF00004337.
- Ruyter, B., C. Moya-Falcón, G. Rosenlund, and A. Vegusdal. 2006. Fat content and morphology of liver and intestine of Atlantic salmon (Salmo salar): Effects of temperature and dietary soybean oil. Aquaculture 252 (2–4):441–52. doi:10.1016/j.aquaculture.2005.07.014.
- Ruyter, B., C. Roesjoe, K. Måsøval, O. Einen, and M. Thomassen. 2000a. Influence of dietary n-3 fatty acids on the desaturation and elongation of [1-14C] 18: 2 n-6 and [1-14C] 18: 3 n-3 in Atlantic salmon hepatocytes. Fish Physiology and Biochemistry 23 (2):151–58. doi:10.1023/A:1007893317923.
- Ruyter, B., C. Røsjø, O. Einen, and M. Thomassen. 2000b. Essential fatty acids in Atlantic salmon: Effects of increasing dietary doses of n-6 and n-3 fatty acids on growth, survival and fatty acid composition of liver, blood and carcass. Aquaculture Nutrition 6 (2):119–27. doi:10.1046/j.1365-2095.2000.00137.x.
- Ruyter, B., and M. Thomassen. 1999. Metabolism of n− 3 and n− 6 fatty acids in Atlantic salmon liver: Stimulation by essential fatty acid deficiency. Lipids 34 (11):1167–76. doi:10.1007/s11745-999-0468-3.
- Sanden, M., I. Stubhaug, M. H. Berntssen, Ø. Lie, and B. E. Torstensen. 2011. Atlantic Salmon (Salmo salar L.) as a Net Producer of Long-Chain Marine ω-3 fatty acids. Journal of Agricultural and Food Chemistry 59 (23):12697–706. doi:10.1021/jf203289s.
- Sigurgisladottir, S., S. P. Lall, C. C. Parrish, and R. G. Ackman. 1992. Cholestane as a digestibility marker in the absorption of polyunsaturated fatty acid ethyl esters in Atlantic salmon. Lipids 27 (6):418. doi:10.1007/BF02536382.
- Sissener, N., B. Torstensen, M. Owen, N. Liland, I. Stubhaug, and G. Rosenlund. 2017. Temperature modulates liver lipid accumulation in Atlantic salmon (Salmo salar L.) fed low dietary levels of long-chain n-3 fatty acids. Aquaculture Nutrition 23 (4):865–78. doi:10.1111/anu.12453.
- Stubhaug, I., Ø. Lie, and B. Torstensen. 2007. Fatty acid productive value and β‐oxidation capacity in Atlantic salmon (Salmo salar L.) fed on different lipid sources along the whole growth period. Aquaculture Nutrition 13 (2):145–55. doi:10.1111/j.1365-2095.2007.00462.x.
- Stubhaug, I., D. R. Tocher, J. G. Bell, J. R. Dick, and B. E. Torstensen. 2005. Fatty acid metabolism in Atlantic salmon (Salmo salar L.) hepatocytes and influence of dietary vegetable oil. Biochimica Et Biophysica Acta (Bba)-molecular and Cell Biology of Lipids 1734:277–88.
- Thanuthong, T., D. S. Francis, E. Manickam, S. D. Senadheera, D. Cameron-Smith, and G. M. Turchini. 2011. Fish oil replacement in rainbow trout diets and total dietary PUFA content: II) Effects on fatty acid metabolism and in vivo fatty acid bioconversion. Aquaculture 322:99–108. doi:10.1016/j.aquaculture.2011.09.026.
- Tocher, D. R., J. G. Bell, P. MacGlaughlin, F. McGhee, and J. R. Dick. 2001. Hepatocyte fatty acid desaturation and polyunsaturated fatty acid composition of liver in salmonids: Effects of dietary vegetable oil. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 130 (2):257–70. doi:10.1016/S1096-4959(01)00429-8.
- Torstensen, B. E., J. G. Bell, G. Rosenlund, R. J. Henderson, I. E. Graff, D. R. Tocher, Ø. Lie, and J. R. Sargent. 2005. Tailoring of Atlantic Salmon (Salmo salar L.) flesh lipid composition and sensory quality by replacing fish oil with a vegetable oil blend. Journal of Agricultural and Food Chemistry 53 (26):10166–78. doi:10.1021/jf051308i.
- Torstensen, B. E., L. Frøyland, R. Ørnsrud, and Ø. Lie. 2004. Tailoring of a cardioprotective muscle fatty acid composition of Atlantic salmon (Salmo salar) fed vegetable oils. Food Chemistry 87 (4):567–80. doi:10.1016/j.foodchem.2004.01.009.
- Torstensen, B. E., Ø. Lie, and L. Frøyland. 2000. Lipid metabolism and tissue composition in Atlantic salmon (Salmo salar L.)—effects of capelin oil, palm oil, and oleic acid‐enriched sunflower oil as dietary lipid sources. Lipids 35 (6):653–64. doi:10.1007/s11745-000-0570-6.
- Turchini, G. M., and D. S. Francis. 2009. Fatty acid metabolism (desaturation, elongation and β-oxidation) in rainbow trout fed fish oil-or linseed oil-based diets. British Journal of Nutrition 102 (1):69–81. doi:10.1017/S0007114508137874.
- Turchini, G. M., D. S. Francis, R. S. Keast, and A. J. Sinclair. 2011. Transforming salmonid aquaculture from a consumer to a producer of long chain omega-3 fatty acids. Food Chemistry 124 (2):609–14. doi:10.1016/j.foodchem.2010.06.083.
- Waagbø, R., K. Sandnes, A. Sandvin, and Ø. Lie. 1991. Feeding three levels of n-3 polyunsaturated fatty acids at two levels of vitamin E to Atlantic salmon (Salmo salar), Growth and Chemical Composition. Fisk. Dir. Skr., Ser. Ernæring IV (l): 51–63.
- Waagbø, R., K. Sandnes, O. J. Torrissen, A. Sandvin, and Ø. Lie. 1993. Chemical and sensory evaluation of fillets from Atlantic salmon (Salmo salar) fed three levels of n-3 polyunsaturated fatty acids at two levels of vitamin E. Food Chemistry 46 (4):361–66. doi:10.1016/0308-8146(93)90005-Z.
- Yıldız, M., T. O. Eroldoğan, S. Ofori-Mensah, K. Engin, and M. A. Baltacı. 2018. The effects of fish oil replacement by vegetable oils on growth performance and fatty acid profile of rainbow trout: Re-feeding with fish oil finishing diet improved the fatty acid composition. Aquaculture 488:123–33. doi:10.1016/j.aquaculture.2017.12.030.
- Ytrestøyl, T., T. S. Aas, and T. Åsgård. 2015. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 448:365–74. doi:10.1016/j.aquaculture.2015.06.023.