ABSTRACT
Blood was collected from alligators at a large alligator farm in Louisiana to compare stress levels to those in wild alligators. The measurement of corticosterone (CORT) levels and heterophil/lymphocyte ratios were used to assess stress levels because both parameters become elevated during periods of physiological stress. We compared CORT concentrations and H/L ratios in wild animals that were either the same size or the same age class as farmed alligators. Plasma CORT levels and H/L ratios were no higher than those in wild alligators. To ensure consistent results across the farm, we compared plasma hormone concentrations in animals of the same size classes maintained in different enclosures and also in different buildings and found no differences. We believe that results such as these obtained from independent laboratories can be used as evidence for regulatory agencies that crocodylian farms raise their animals in low stress environments under Best Management Practices guidelines.
Introduction
In the early 1980s, the results of research in Louisiana showed a high mortality in wild alligators and alligator eggs, as only 17% of viable eggs hatched and the resulting animals reached a length of 1 m (Taylor and Neal Citation1984). These results led to the development of an alligator ranching program in which alligator eggs were collected from private lands, hatched, and grown for commercial and conservation purposes. This program was initiated in 1986 and was closely regulated by the Louisiana Department of Wildlife and Fisheries. As a condition of this harvest of eggs, the alligator farmer was obligated to release 17% of the hatched alligators back into the marsh within two years of egg harvest (Louisiana Department of Wildlife and Fisheries Citation2011). This conservation measure has resulted in a large, continual growth of the alligator population in Louisiana (Joanen and Merchant Citation2018), and the required alligator release has been steadily decreased to the current 5% based on the population growth and survivorship of the released animals, and continues to undergo periodic review.
The most recent compiled crocodylian world trade report showed an average of 1,423,436 hides traded annually, from 30 different countries, from 2006 to 2013 (Caldwell Citation2017). These hides are from farming and ranching operations, and from regulated harvest of wild animals. During this same time period, the annual production of alligator hides in the United States was 337,838. These hides were tanned and used to produce leather items (Thorbjarnarson Citation1999). In 2019, the 438,577 hides and meat from farmed alligator in Louisiana were worth more than 86 million $US (Louisiana Department of Wildlife and Fisheries Citation2020).
In order to adhere to best management practices of animal husbandry, alligator farmers must raise their animals in a low stress environment. Farmers work to provide growing conditions that allow animals to be free from 1) hunger and thirst, 2) discomfort, 3) pain, injury and disease, 4) inability to express normal behavior, and 5) fear and distress (Manolis and Webb Citation2016). Clean water, quality food, and moderate stocking densities are utilized to keep the animals healthy and disease-free (Nickum et al. Citation2018). Poor growing conditions can lead to stress, which in turn leads to low growth performance, low reproductive success, diseased animals, and potentially increased mortality (Elsey et al. Citation1990; Masser Citation1993). Alternatively, alligators raised in controlled, stress-free environments in well-designed communal or individual controlled aquatic enclosures can produce unaggressive, fast-growing animals with unmarred hides (Joanen and McNease Citation1987). Louisiana law and associated regulations govern the conditions under which all alligators are housed and managed in their state (Louisiana Department of Wildlife and Fisheries Citation2011).
Plasma corticosteroids (CORTs) are increased in vertebrates by physiological stressors due to increased secretion of adrenocorticotropic hormone and corticotropin releasing hormone (Dallman and Jones Citation1973). Corticosteroids have been measured in a variety of tissues in wildlife to determine stress levels (Sheriff et al. Citation2011). Corticosterone is the hormone of choice for the determination of stress in most lower vertebrates (Breuner, Delehanty, and Boonstra Citation2013), and is the primary stress hormone in crocodylians (Silvestre Citation2014). There are a multitude of studies that link stress in crocodylians to the expression of CORT hormones (Mahmoud et al. Citation1996). Restraint stress induces increases in plasma CORTs in alligators (Lance and Elsey Citation1999). Stress has also been linked to high stocking densities in captive American alligators in research studies at Rockefeller Refuge (Elsey et al. Citation1990). The measurement of the stress-related hormones (CORTs) is believed to be an efficient method for the determination of physiological and biochemical stress in crocodylians (Elsey et al. Citation1990; Finger et al. Citation2015; Franklin et al. Citation2003; Isberg and Shilton Citation2013; Isberg, Shilton, and Thompson Citation2009; Silvestre Citation2014; Turton et al. Citation1997). Low levels of CORTs can be measured in small samples of plasma, making this an ideal technique for the measurement of CORTs in crocodylian plasma. We conducted this study to compare the levels of stress between wild alligators in a federal wildlife refuge to those in a large commercial alligator farm in Louisiana.
Materials and methods
Farm conditions
Alligators were hatched from wild eggs collected from private land in south Louisiana. The eggs were incubated at approximately 31.7°C and 98–100% humidity. After hatching, the hatchlings were fed a commercial extruded pelletized feed (56% protein, Cargill Inc., Minneapolis, MN) ad libitum. Alligators of approximate length 46–89 cm were stocked at a density of 33 animals/m2 in a water depth of 25–30 cm. Alligators of medium size (approximately 90–150 cm) were grown at a stocking density of 10 animals/m2 in a water depth of 46–51 cm, and larger animals (151–183 cm) were maintained in individual enclosures (0.6 × 2.0 m) with a water depth of 20–23 cm. The larger two alligator groups were fed commercial feed (Cargill Inc., Minneapolis, MN) that contained 45% protein. All alligators were maintained in dark environments in water of 29.5°C. The water was circulated through the tanks a minimum of 3–5 times per day. Water from the alligator tanks was released into large retention ponds that contained water hyacinths and other aquatic plants. For the larger two alligator groups, surface water was mixed with ground water (from a deep well) to achieve the desired temperature (as defined by LDWF farming regulations and Best Management Practices (BMP) before it was circulated back into the tanks. Groundwater, maintained at temperature ranges defined in the LDWF BMP document, was used in tanks with hatchlings.
Collection of blood
Blood was collected from captive alligators of three different size classes (46–89 cm, 90–150 cm, and 150–181 cm) from multiple locations on a large alligator farm in Louisiana, USA. The alligators were captured from enclosures by hand (≤150) and by electroimmobilization (>151 cm, Franklin et al. Citation2003). Wild alligators were captured from a boat with the use of a spotlight by hand (<1.4 m) or by cable noose (>1.4 m) from McFaddin National Wildlife Refuge west of Sabine Pass, Texas. This location was selected because it was a short distance (100 km, same latitude and same type of marsh habitat) from where the wild alligator eggs were collected for the farm. The collection of blood samples from all 192 farmed alligators occurred on the same day, and the samples collected from the wild alligators occurred on two consecutive nights one week later. Approximately 0.5 mL of whole blood were collected from the spinal vein (Oslon, Hessler, and Faith Citation1975) of both captive and wild alligators using a 3 mL syringe and a 2 cm, 22 ga. needle within 2 minutes of initial restraint. For CORT determination, whole blood samples were immediately transferred to heparinized blood collection tubes and stored on ice for less than 24 hrs. Samples were centrifuged at 2500xg and the plasma was transferred to 0.5 mL microcentrifuge tubes and stored at −20°C.
To compare stress parameters across different enclosures, plasma samples were collected from alligators housed in 15 different enclosures within the same building, with 6–8 alligators sampled per enclosure. To compare stress parameters in alligators housed in different buildings, blood was collected from animals housed in 10 enclosures, all located in different buildings (6–8 alligators/enclosure).
CORT determination
The corticosterone (CORT) concentration was determined in each plasma sample using an enzyme-linked immunoassay kit purchased from Enzo Life Sciences (Farmingdale, NY, catalog # ADI-900-097). Prior to measurement by ELISA, the CORT was extracted via the previously described methods of Al-Dujaili et al. (Citation2009). Each sample was analyzed in duplicate according to directions from the manufacturer and the results were presented as an average of the two trials for each sample. Ten replicates of a high and low range alligator plasma sample (1.39 and 5.26 ng/mL) were analyzed each in ten independent trials ().
Table 1. Accuracy and precision of CORT Determination-Two alligator plasma samples, at the low and high end of the range for this study, were analyzed for CORT concentrations by ELISA. The results are presented as the means ± standard deviations for ten independent determinations, and the coefficients of variance are shown to represent analytical precision.
Heterophil/lymphocyte ratios
Immediately after collection, approximately 5 μL of whole blood were spotted onto the end of a glass microscope slide smeared across the slide using the edge of a second microscope slide. The blood was allowed to dry and the cells were then stained using Giemsa-Wright stain and viewed under oil immersion lens at 1000× magnification. Leukocytes (100/slide) were counted manually, and H/L ratios were obtained.
Statistics and controls
Prior to data collection, sample size estimates were performed to determine if mean stress levels were different between farmed and wild alligators in different size classes. Sample sizes were calculated for conducting three two-sample t-tests. To keep experiment-wise Type I error at 0.05, an adjusted alpha of .017 was used to determine the sample size for each size class.
Sample sizes were calculated using a Power and Sample Size applet (Lenth Citation2006) with parameter estimates derived from previous studies (Elsey et al. Citation1990). Estimates used for mean (and standard deviation) were 0.69 ng/ml (2.6 ng/ml) for wild alligators and 2.69 ng/ml (3.5 ng/ml) for captive alligators. A true mean difference to detect of 2 ng/ml was also used. Estimates required that blood be collected from at least 64 farmed alligators and at least 32 wild alligators for each size group. The collection of less than 32 wild samples per size group likely resulted in a reduction of power of the chosen statistical tests.
Where necessary, assumptions were checked for performing the appropriate analyses. The use of Levene’s test (Levene Citation1960) revealed no violation of the homogeneity of variance assumption, and a Shapiro–Wilk analysis (Shapiro and Wilk Citation1965) showed that the assumption of normality could be used for all but one test. The Kruskal–Wallis procedure (Kruskal and Wallis, Citation1952) was used for analysis violating the normality assumption.
To ensure analytical accuracy and precision across a large range of CORT concentrations, plasma samples were spiked with known concentrations of CORT hormone (10, 30, or 50 ng/mL). The results for five replicates in each spike range were averaged and the results were presented as mean % recovery ± standard deviations (Microsoft Excel, Microsoft Office 16). In addition, 10 replicates of alligator plasma samples from the high and low ends of the spectrum were analyzed for CORT to examine the coefficient of variance for the analytical method (Microsoft Excel, Microsoft Office 16). The results were expressed as mean ng/mL ± standard deviations. CORT from blood samples of alligators housed in different tanks in the same building, alligators from different buildings, and alligators of different size classes were compared using two-tailed t-tests and the level of significance was set at 0.05 (R Core Team Citation2021).
The abundance of heterophils and lymphocytes were expressed as the means of three independent determinations of H/L ratios. The results of comparison of H/L ratios of farmed vs wild alligators, farmed alligators from different size classes, and animals housed in different buildings were compared using two-tailed t-tests and the level of significance was set at 0.05 using Jamovi software package (R Core Team Citation2021; The jamovi project Citation2021). The normality of distribution of all H/L data was tested using a Shapiro–Wilk test (Shapiro and Wilk Citation1965).
Results
A comparison of plasma CORT levels from farmed animals of different size classes revealed no statistical differences (P > .05 for all comparisons; ). Farmed alligators in the small size class (46–89 cm, n = 64) exhibited CORT levels of 3.66 ± 0.89 ng/mL, while animals in the medium (90–150 cm, n = 64) and larger (151–183 cm, n = 64) size classes had 3.83 ± 0.70 and 3.72 ± 0.89 ng/mL, respectively. Likewise, plasma CORT concentrations in the plasma of wild alligators from different size classes did not vary (P > .05, ). Wild animals in the smaller size class exhibited CORT levels of 3.99 ± 0.73 ng/mL, while the medium and large size classes showed 3.83 ± 0.60 and 3.92 ± 0.9 ng/mL, respectively. In addition, there was also no statistical difference in the CORT levels of farmed animals of any size class compared to wild animals in any size class (P > .05, ).
Figure 1. Plasma CORT levels (A) and H/L ratios (B) in alligators from three different size classes. There was no statistical difference (P > .05) in CORT levels for captive alligators in any two size classes for either parameter. In addition, there was no difference (P > .05) in hormone concentrations or H/L ratios of captive and wild animals of the same age class. The data represent the means ± SD for 64 (CORT) or 10 (H/L ratios) different animals.
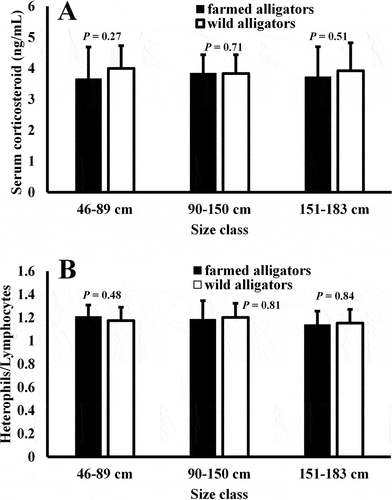
An evaluation of heterophil/lymphocyte ratios in the whole blood of farmed alligators from the size classes (n = 10 for each size class) revealed that there were no differences (P > .05) for all comparisons (). Farmed alligators in the 46–89 cm size class exhibited H/L ratios of 1.21 ± 0.10, while wild alligators of the same size class had similar H/L ratios of 1.18 ± 0.12. There were also no differences between H/L ratios in farmed (1.19 ± 0.16) and wild alligators (1.20 ± 0.13) of the 90–150 cm size class, or farmed (1.14 ± 0.12) and wild (1.15 ± 0.12) alligators of the 151–180 cm size class.
Because farmed alligators are maintained in controlled environments under constant temperatures (27.5–30°C) and fed high nutrient diets ad libitum, they grow much faster than wild alligators (Chabreck and Joanen Citation1979). Farmed alligators typically reach 100–112 cm long at one year of age, while wild alligators are normally 51–56 cm in length at one year in south Louisiana marshes (Elsey et al. Citation1992; Rootes et al. Citation1991; Saalfeld et al. Citation2008). Therefore, the plasma corticosteroid levels and H/L ratios of one-year old farmed alligators were compared to that of wild alligators that were estimated to be three years of age (100–110 cm) due to the similarity in size. Likewise, these same parameters in two-year old farmed alligators were compared to those in wild alligators that were estimated to be five–six years old (approximately 125–140 cm). The comparisons of plasma corticosteroid levels or H/L ratios in age-matched farmed and wild alligators revealed no differences (P > .05).
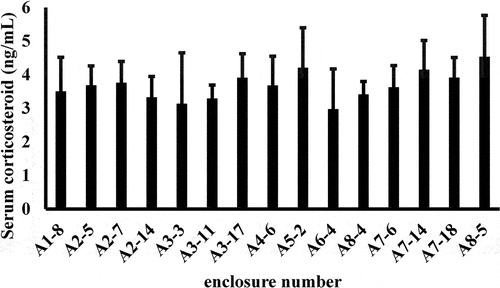
Alligators in the 46–89 cm size class that were housed in separate enclosures within the same building did not express different plasma CORT concentrations (; P > .05, n = 15 enclosures). The mean CORT concentration in alligators from all 15 enclosures were between 3.37 and 4.20 ng/mL. In addition, the small ranges of values and low standard deviations showed no statistical variability in stress levels among gators in these enclosures (P > .05; 1). Furthermore, animals in different enclosures within the same building did not exhibit different H/L ratios (, P > .05, n = 4 enclosures). The mean H/L ratio in animals from all of the enclosures was 1.21, with a range of 1.14 −1.30.
Figure 2. Plasma CORT concentrations in alligators from 15 different enclosures within the same building were not statistically discernible. (P > .05; n = 4 alligators sampled from each enclosure).
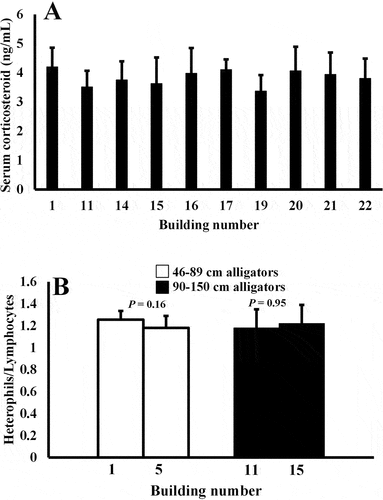
Alligators in the same size class (90–150 cm) that were housed in 10 different enclosures, all located in different buildings, did not exhibit different plasma CORT concentrations (P > .05; ). Animals housed in 10 enclosures, all located in different buildings, were sampled. In addition, blood from animals housed in four different buildings did not show differences in H/L ratios (P > .05, ). Alligators in the size class 46–89 cm housed in Buildings 1 and 5 exhibited 1.26 ± 0.08 and 1.21 ± 0.10 H/L ratios, while alligators in the size class 90–150 cm housed in Buildings 11 and 15 showed 1.19 ± 0.16 and 1.14 ± 0.13 H/L, respectively ().
Figure 3. Plasma CORT concentrations (A) in alligators housed in 10 different buildings were not statistically discernable. (P > .05; n = 6–8 alligators sampled from each building). Plasma H/L ratios (B) were also not different in animals housed in different buildings (P > .05, 2 enclosures from each building, n = 5 animals sampled per enclosure).
Ten replicates of a high and low range alligator plasma sample (1.39 and 5.26 ng/mL) were analyzed each in ten independent trials (). The low CORT sample showed 1.370 ± 0.053, for a coefficient of variance of 3.83%. Likewise, the high concentration sample exhibited a concentration of 5.141 ± 0.235 with a coefficient of variance of 4.56%.
A sample of alligator plasma (derived from a 115 cm animal) that contained 2.69 ng/mL of CORT was spiked with 10 ng/mL of CORT standard in five replicates. The replicates were analyzed independently and the recovery of total CORT was 94.25 ± 3.85% (). Likewise, the same plasma sample spiked with 30 and 50 ng/mL exhibited 93.78 ± 1.86% and 96.10 ± 3.66% recoveries, respectively.
Table 2. Reproducibility of CORT Determination at Different Concentrations – An alligator plasma sample was spiked with three different concentrations of CORT (five independent spikes per concentration) and subjected to ELISA to determine hormone levels. The results are presented as the means ± standard deviations of the mean of the spike recovery of five independent determinations. The results indicate high analytical precision and low variance.
Discussion
There are a few different methods for the determination of stress in crocodylians. The comparison of heterophil/lymphocyte (H/L) ratios is an inexpensive method, as stress has been shown to induce higher H/L ratios in alligators (Lance et al. Citation2010; Merchant et al. Citation2006; Murray et al. Citation2013). Because this method relies on manual quantitation of cells, it is relatively easy and inexpensive, and the results are comparable across different laboratories as long as the technician is versed in leukocyte identification (Winter et al. Citation2019). However, this method lacks the sensitivity and analytical accuracy and precision of CORT determination. In addition, CORTs can be measured in a variety of tissues. The most common tissue in which CORTs is blood; however, these hormones have also been analyzed in scutes (Hamilton et al. Citation2018) and even fecal samples (Ganswindt et al. Citation2014). Although CORTs can be measured in scutes, the sensitivity is far less than that in plasma (Hamilton et al. Citation2018). The use of fecal samples would not allow for individual analysis in a farm setting. This technique would also not allow for direct comparison to wild alligators as fecal samples cannot be collected because alligators defecate in the water.
Corticosteroids can be measured by a variety of methods in crocodylian blood samples. The easiest and perhaps best way to measure CORT is by enzyme-linked immunoassay (ELISA). The analyses of CORT conducted for this study represented a direct comparison of plasma concentrations in plasma of both size- and age-matched farmed and wild alligators. Analyses using different methods (i.e., radioimmunoassay, HPLC, ELISA, etc.) often yield different results that are not directly comparable. However, the analyses presented in this study were conducted using the same ELISA kit from the same manufacturer (same lot number). In addition, the analyses were performed by the same technician during the same three-day time frame, and these analytical conditions provided a direct comparison of results for plasma CORT concentrations in different groups of animals. Furthermore, blood samples were collected within 2 minutes of restraint, which is important because unpublished results in our laboratory have shown that CORTs are not elevated until approximately 15–20 minutes after restraint in alligators. In addition, Romero and Reed (Citation2005) and Isberg and Shilton (Citation2013) recommend collection of blood samples within three minutes of restraint. Therefore, the CORT levels measured in both wild and farmed animals in this study were basal levels that were less likely to be affected by restraint stress.
Although the evaluation of H/L ratios is analytically inferior to the measurement of CORT levels, it is potentially a better indicator of long-term, chronic stress in ectothermic vertebrates (Davis and Maney Citation2018). The results of several studies have shown that physiological stressors elevate plasma CORT levels temporarily, and they return to near baseline levels within 24 hours as the response is attenuated; however, H/L ratios can remain elevated for several days (Goessling et al. Citation2015; Müller, Jenni-Eiermann, and Jenni Citation2011). For instance, stressors elevate CORT in green sea turtles (Chelonia mydas) approximately three-fold after one hour, but these levels are reduced dramatically within 24 hours while H/L ratios remain elevated 10-fold at 24 hrs (Aguirre et al. Citation1995). Likewise, CORT levels in eastern hellbenders (Cryptobranchus alleganiensis) after capture increased almost 3-fold after one hour but were reduced notably after 2–3 days while H/L ratios remained elevated 10-fold after 50 hrs. These results were also similar for captive chickens (Gallus gallus, McFarlane and Curtis Citation1989), Although these experiments have not been conducted with crocodylians, it is reasonable to assume that the results may be similar.
The studies described in this report were designed to determine the stress levels of wild alligators and those raised on a large commercial alligator farm, using analysis of plasma CORT concentrations and heterophil/lymphocyte ratios as indicators of stress. It is a common practice for farmed alligators to be raised together in size classes, with reduced stocking densities for animals in larger size classes. The comparison of plasma CORT levels and H/L ratios in animals from different size classes (34–89 cm, 90–150 cm, and 151–183 cm) showed no differences (P > .05), indicating the stocking densities, along with other factors that may cause stress, are sufficiently low as to prevent chronic stress (). In addition, wild and farmed animals of the same size class did not exhibit different CORTs, suggesting that the farmed animals exhibit no more chronic stress than wild animals in a National Wildlife Refuge (). It should be noted that blood was collected from the farmed and wild alligators only six days apart, thus avoiding potential seasonal variations in CORT expression (Davis and Maney Citation2018; Guillette, Cree, and Rooney Citation1995).
The determination of steroid hormone levels in some tissues can be problematic due to a variety of factors. The “matrix effect” is a concept that describes problems with molecules such as proteins that bind and inhibit the detection of steroid hormones in biological samples, thus resulting in artificially low analytical values (Liu et al. Citation2019; Voegel et al. Citation2021). The ELISA method has been shown to be a sensitive, accurate, precise, and relatively reliable method for the determination of steroid hormone content in samples (Manickum and Wilson Citation2015). The problem of the matrix effect was avoided in this study by the extraction of the hormones in the blood by the use of organic solvents that destroy proteins, thus releasing the hormone molecules and making them available for detection by the ELISA method. The use of the spike-recovery analytical control method ensured that the recovery and detection of CORT was high throughout a large range of hormone concentration. In addition, the inclusion of the analytical spike to the serum sample provided evidence that this system was not hindered by the matrix effect, and that all of the hormone was detected. Furthermore, the results of repeated analysis of samples in the low and high end of the range for plasma CORT showed a high level of reproducibility with low coefficients of variation.
Conclusions
A comparison of CORT samples in blood drawn from alligators of different size classes resulted in hormone levels statistically indistinguishable from those in wild alligators of the similar size or age. Comparison of stress hormone levels of animals from different enclosures within the same building, or from different buildings also yielded no statistical difference in stress hormone levels. The CORT concentrations from alligator of different size classes (46–89 cm, 90–150 cm, and 151–180 cm) were the same (P > .05 for all comparisons), and also the same when compared to stress hormone levels in wild alligators of the same size or age class ().
Alligators, in their natural environments, can be territorial animals that exhibit conspecific aggression which can lead to serious injuries (Merchant et al. Citation2003). Therefore, alligator farmers strive to create a low-stress growing environment to reduce the probability that animals will fight and create injuries that will eventually scar the hides. The fact that a high proportion (approximately 90%) of the hides produced at the alligator farm are Grade 1 suggests that the animals are not stressed and there is minimal amount of aggression. Furthermore, the high growth rate exhibited provides another indicator that the animals are maintained in a low-stress environment because elevated CORT concentrations have been correlated with low growth performance (Morici, Elsey, and Lance Citation1997). This site-specific study at the alligator farm, based on its findings, suggests the animals on the farm are being grown in an environment that is experiencing no more chronic stress than wild animals in a National Wildlife Refuge.
The analyses conducted for this study should suffice to provide evidence to any regulatory agency or authority that the farming methods practiced are sufficient such that alligators are maintained in a low stress environment. Regulatory agencies should strive for data such as those presented in this study to be provided by farmers for certification, compliance, approval, verification, etc. Although the data set in this study was collected for a large alligator farm in Louisiana, the size of the data set can be scaled to fit any facility, with a proper power analysis (Lenth Citation2006), to retain statistical validity.
Once an initial data set is collected and analyzed, the farm can be certified, approved, verified, etc., for a specific amount of time (i.e., ten years) before new data are required. However, if farming parameters are changed (such as water temperature, enclosure design, feed composition, stocking density, etc.) or when a new building is constructed, then the farm should be required to have data collected and analyzed, by an independent laboratory, within a specific time frame to determine the effects of the changed atmosphere on plasma CORT concentrations in the farmed animals.
Acknowledgments
The authors would like to thank the owner of the alligator farm who allowed us to collect samples. In addition, we would like to acknowledge the help of the farm employees who assisted in capturing and collecting blood from the farmed alligators. This study was funded by a McNeese State University Endowed Professorship grant awarded to M. Merchant (EP0073). Both state (Texas Parks and Wildlife SPR-0402-207) and federal (USFWS SUP 21525-20-0002) permits to capture and collect blood from animals on McFaddin National Wildlife Refuge land were held by M. Merchant. The methods of alligator capture, handling, and blood collection were approved be the McNeese State University Institutional Animal Care and Use Committee.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aguirre, A. A., G. H. Balazs, T. R. Spraker, and T. S. Gross. 1995. Adrenal and hematological responses to stress in juvenile green turtles (Chelonia mydas) with and without fibropapillomas. Physiological Zoology 68 (5):831–54. doi:10.1086/physzool.68.5.30163934.
- Al-Dujaili, E., L. Mullins, M. Bailey, R. Andrew, and C. Kenyon. 2009. Physiological and pathophysiological applications of sensitive ELISA methods for urinary deoxycorticosterone and corticosterone in rodents. Steroids 74 (12):938–44. doi:10.1016/j.steroids.2009.06.009.
- Breuner, C., B. Delehanty, and R. Boonstra. 2013. Evaluating stress in natural populations of vertebrates: Total CORT is not good enough. Functional Ecology 27 (1):24–36. doi:10.1111/1365-2435.12016.
- Caldwell, J. 2017. World trade in crocodile skins 2013-2015. UNEP-WCMC technical report, Cambridge.
- Chabreck, R. H., and T. Joanen. 1979. Growth rates of alligators in Louisiana. Herpetologia 35:51–57.
- Dallman, M., and M. Jones. 1973. Corticosteroid feedback control of ACTH secretion: Effect of stress-induced corticosterone secretion on subsequent stress responses in the rat. Endocrinology 92 (5):1367–75. doi:10.1210/endo-92-5-1367.
- Davis, A., and D. Maney. 2018. The use of glucocorticoid hormones or leucocyte profiles to measure stress in vertebrates: What’s the difference? Methods in Ecology and Evolution 9 (6):1556–68. doi:10.1111/2041-210X.13020.
- Elsey, R. M., T. Joanen, L. Mcnease, and N. Kinler. 1992. Growth rates and body condition factors of Alligator mississippiensis in coastal Louisiana wetlands: A comparison of wild and farm-released juveniles. Comparative Biochemistry and Physiology Part A: Physiology 103 (4):667–72. doi:10.1016/0300-9629(92)90164-L.
- Elsey, R. M., T. Joanen, L. McNease, and V. Lance. 1990. Stress and plasma corticosterone levels in the American alligator—relationships with stocking density and nesting success. Comparative Biochemistry and Physiology Part A: Physiology 95 (1):55–63. doi:10.1016/0300-9629(90)90009-H.
- Finger, J. W., Jr., P. C. Thomson, A. L. Adams, S. Benedict, C. Moran, and S. R. Isberg. 2015. Reference levels for corticosterone and immune function in farmed saltwater crocodiles (Crocodylus porosus) hatchlings using current Code of Practice guidelines. General and Comparative Endocrinology 212:63–72. doi:10.1016/j.ygcen.2015.01.023.
- Franklin, C. E., B. M. Davis, S. K. J. Peucker, H. Stephenson, R. Mayer, J. Whittier, J. Lever, and G. C. Grigg. 2003. Comparison of stress induced by manual restraint and immobilisation in the estuarine crocodile,Crocodylus porosus. Journal of Experimental Zoology 298A (2):86–92. doi:10.1002/jez.a.10233.
- Ganswindt, S., J. Myburgh, E. Cameron, and A. Ganswindt. 2014. Non-invasive assessment of adrenocortical function in captive Nile crocodiles (Crocodylus niloticus). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 177:11–17. doi:10.1016/j.cbpa.2014.07.013.
- Goessling, J. M., H. Kennedy, T. Mendonca, A. E. Wilson, and J. Grindstaff. 2015. A meta-analysis of plasma corticosterone and heterophil: Lymphocyte ratios – Is there conservation of physiological stress responses over time? Functional Ecology 29 (9):1189–96. doi:10.1111/1365-2435.12442.
- Guillette, L. J., A. Cree, and A. A. Rooney. 1995. Biology of stress: Interactions with reproduction, immunology and intermediary metabolism. In Health and welfare of captive reptiles, ed. C. Warwick, F. L. Frye, and J. B. Murphy, 32-81. Dordrecht: Springer. doi:10.1007/978-94-011-1222-2_3.
- Hamilton, M., J. Finger, R. M. Elsey, G. Mastromonaco, and T. Tuberville. 2018. Corticosterone in American alligator (Alligator mississippiensis) tail scutes: Evaluating the feasibility of using unconventional samples for investigating environmental stressors. General and Comparative Endocrinology 268:7–13. doi:10.1016/j.ygcen.2018.07.008.
- Isberg, S., and C. Shilton. 2013. Stress in farmed saltwater crocodiles (Crocodylus porosus): No difference between individually- and communally-housed animals. SpringerPlus 2 (1):1–6. doi:10.1186/2193-1801-2-381.
- Isberg, S., C. Shilton, and P. Thompson. 2009. Improving Australia’s crocodile farming industry: Understanding runtism and survival. RIRDC Publication No. 09/135. Canberra, Australia: RIRDC.
- Joanen, T., and L. McNease. 1987. Alligator farming research in Louisiana, USA. In Wildlife Management: Crocodiles and Alligators, ed. G. J. W. Webb, S. C. Manolis, and P. J. Whitehead, 332–37. Chipping Norton, Australia: Surrey Beatty & Sons.
- Joanen, T., and M. Merchant. 2018. The history and development of the alligator industry: A historical perspective. In American Alligators: Habitats, behaviors, and threats, ed. C. Eversole and S. Henke, 349–74. New York, USA: Nova Science Publications.
- Kruskal, W. H., and W. A. Wallis. 1952. Use of ranks in one-criterion variance. Analysis. Journal of the American Statistical Association 47 (260):583–621. doi:10.1080/01621459.1952.10483441.
- Lance, V., and R. M. Elsey. 1999. Plasma catecholamines and plasma corticosterone following restraint stress in juvenile alligators. Journal of Experimental Zoology 283 (6):559–65. doi:10.1002/(SICI)1097-010X(19990501)283:6<559::AID-JEZ7>3.0.CO;2-4.
- Lance, V., R. M. Elsey, G. Butterstein, P. Trosclair III, and M. Merchant. 2010. The effects of Hurricane Rita and subsequent drought on alligators in southwest Louisiana. Journal of Experimental Zoology Part A 313A:106–13.
- Lenth, R. V. 2006. Java applets for power and sample size [Computer software]. Retrieved December 4, 2019, from http://www.stat.uiowa.edu/~rlenth/Power.
- Levene, H. 1960. Robust tests for equality of variances. In Contributions to probability and statistics: Essays in honor of Harold Hotelling, ed. S. G. Iolkin, G. W. Hoeffding, W. G. Madow, and H. B. Mann, 278–92. Stanford, CA: Stanford University Press.
- Liu, Q., Q. Chi, R. Fan, H. Tian, and X. Wang. 2019. Quantitative-profiling method of serum steroid hormones by hydroxylamine-derivatization HPLC–MS. Natural Products and Bioprospecting 9 (3):201–08. doi:10.1007/s13659-019-0204-3.
- Louisiana Department of Wildlife and Fisheries. 2011. Best management practices for Louisiana alligator farming. https://cdn2.hubspot.net/hub/152878/file-12758065-pdf/docs/best_management_practices_allig_farms_-_june_2011.pdf
- Louisiana Department of Wildlife and Fisheries. 2020. Louisiana’s alligator management program: 2019-2020 annual report. https://www.wlf.louisiana.gov/assets/Resources/Publications/Alligator/2019-2020_Alligator_Annual_Report.pdf
- Mahmoud, I., K. Vliet, L. Guillette, and J. Plude. 1996. Effect of stress and ACTH1–24 on hormonal levels in male alligators. ,Alligator Mississippiensis. Comparative Biochemistry and Physiology Part A: Physiology 115 (1):57–62. doi:10.1016/0300-9629(96)00002-3.
- Manickum, T., and J. Wilson. 2015. The current preference for the immuno-analytical ELISA method for quantitation of steroid hormones (endocrine disruptor compounds) in wastewater in South Africa. Analytical and Bioanalytical Chemistry 407 (17):4949–70. doi:10.1007/s00216-015-8546-0.
- Manolis, S. C., and G. J. W. Webb. 2016. Best management practices for crocodilian farming. Version 1. Darwin, Australia: IUCN-SSC Crocodile Specialist Group.
- Masser, M. 1993. Alligator production: Grow-out and harvest, 232. Southern Region Aquaculture Center Publication.
- McFarlane, J. M., and S. E. Curtis. 1989. Multiple concurrent stressors in chicks. 3. Effects on plasma corticosterone and the heterophil‐lymphocyte ratio. Poultry Science 68 (4):522–27. doi:10.3382/ps.0680522.
- Merchant, M., C. Roché, D. Thibodeaux, J. Prudhomme, and R. Elsey. 2003. Antibacterial properties of serum from the American alligator (Alligator mississippiensis). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 136 (3):505–13. doi:10.1016/S1096-4959(03)00256-2.
- Merchant, M., K. Mills, S. Williams, F. Kleckley, A. Sims, R. Elsey, and J. Bushnell. 2006. Effects of bacterial lipopolysachharide on peripheral leukocytes in the American alligator (Alligator mississippiensis). Veterinary Immunology and Immunopathology 111 (3–4):315–20. doi:10.1016/j.vetimm.2006.01.018.
- Morici, L., R. M. Elsey, and V. Lance. 1997. Effects of long-term corticosterone implants on growth and immune function in juvenile alligators,Alligator mississippiensis. The Journal of Experimental Zoology 279 (2):156–62. doi:10.1002/(SICI)1097-010X(19971001)279:2<156::AID-JEZ6>3.0.CO;2-N.
- Müller, C., S. Jenni-Eiermann, and L. Jenni. 2011. Heterophils/Lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Functional Ecology 25 (3):566–76. doi:10.1111/j.1365-2435.2010.01816.x.
- Murray, C., M. Easter, M. Merchant, A. Cooper, and B. Crother. 2013. Can reproductive allometry assess population marginality in crocodilians? A comparative analysis of gulf coast American alligator (Alligator mississippiensis) populations. Copeia 2013 (2):268–76. doi:10.1643/CH-11-136.
- Nickum, M., M. Masser, R. Reigh, and J. Nickum. 2018. Alligator (Alligator mississippiensis) aquaculture in the United States. Reviews in Fisheries Science & Aquaculture 26 (1):86–98. doi:10.1080/23308249.2017.1355350.
- Oslon, G., J. Hessler, and R. Faith. 1975. Technics for blood collection and intravascular infusion of reptiles. Laboratory Animal Science 25:783–86.
- R Core Team. (2021). R: A language and environment for statistical computing. (Version 4.0) [Computer software]. https://cran.r-project.org. (R packages retrieved from MRAN snapshot 2021-04-01).
- Romero, L. M., and J. M. Reed. 2005. Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 140 (1):73–79. doi:10.1016/j.cbpb.2004.11.004.
- Rootes, W., R. Chabreck, V. Wright, B. Brown, and T. Hess. 1991. Growth rates of American alligators in estuarine and palustrine wetlands in Louisiana. Estuaries 14 (4):489. doi:10.2307/1352272.
- Saalfeld, D., K. Webb, W. Conway, G. Calkins, and J. Duguay. 2008. Growth and condition of American alligators (Alligator mississippiensis) in an inland wetland of east Texas. Southeastern Naturalist 7 (3):541–50. doi:10.1656/1528-7092-7.3.541.
- Shapiro, S. S., and M. B. Wilk. 1965. An analysis of variance test for normality (complete samples). Biometrika 52 (3–4):591–611. doi:10.1093/biomet/52.3-4.591.
- Sheriff, M., B. Dantzer, B. Delehanty, R. Palme, and R. Boonstra. 2011. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 166 (4):869–87. doi:10.1007/s00442-011-1943-y.
- Silvestre, A. 2014. How to assess stress in reptiles. Journal of Exotic Pet Medicine 23 (3):240–43. doi:10.1053/j.jepm.2014.06.004.
- Taylor, D., and W. Neal. 1984. Management implications of size class frequency distributions in Louisiana alligator populations. Wildlife Society Bulletin 12:312–19.
- The jamovi project (2021). jamovi. (Version 1.8) [Computer Software]. https://www.jamovi.org.
- Thorbjarnarson, J. 1999. Crocodile tears and skins: International trade, economic constraints, and limits to the sustainable use of crocodilians. Conservation Biology 13 (3):465–70. doi:10.1046/j.1523-1739.1999.00011.x.
- Turton, J. A., P. W. Ladds, S. C. Manolis, and G. J. W. Webb. 1997. Relationship of blood corticosterone, immunoglobulin and haematological values in young crocodiles (Crocodylus porosus) to water temperature, clutch of origin and body weight. Australian Veterinary Journal 75 (2):114–19. doi:10.1111/j.1751-0813.1997.tb14170.x.
- Voegel, C., M. Baumgartner, T. Kraemer, S. Wust, and T. Binz. 2021. Simultaneous quantification of steroid hormones and endocannabinoids (ECs) in human hair using an automated supported liquid extraction (SLE) and LC-MS/MS – Insights into EC baseline values and correlation to steroid concentrations. Talanta 222:121499. doi:10.1016/j.talanta.2020.121499.
- Winter, J., N. Stacy, L. Adamovicz, and M. Allender. 2019. Investigating the analytical variability and agreement of manual leukocyte quantification methods in eastern box turtles (Terrapene carolina carolina). Frontiers in Veterinary Science 12. doi:10.3389/fvets.2019.00398.
