ABSTRACT
A nontoxic method that results in distinct and persistent marks in Haliotis midae juveniles is fundamental for field-based studies, abalone ranching, and stock enhancement programs. Developed (6 days after fertilization) H. midae larvae were batch-tagged with 25 and 50 mg L−1 of calcein and xylenol orange for 24 and 48 h to determine the effect on survival, settlement, and the persistence of the mark on the shell. Epifluorescence was detected in the shell spire of larvae tagged with either 25 mg L−1 or 50 mg L−1 of calcein for 24 and 48 h immediately after the staining (t0) and was visible after 51 days when the experiment was terminated. Survival of calcein stained larvae was higher after 24 h compared to 48 h immersion. However, percentage settlement was higher in the 48 h treatments when compared to 24 h treatments for both concentrations and dyes. Therefore, bright and persistent epifluorescence marks can be induced in H. midae shells using calcein (25 mg L−1 for 24 h) without negatively affecting the survival and settlement of larvae, post-immersion.
Introduction
The South African wild abalone (Haliotis midae, commonly known as perlemoen) fishery has experienced a considerable decline in population abundance over the last three decades, primarily due to heavy fishing pressure from the illegal catch (Witte Citation2017). Two techniques have been evaluated to restore depleted stocks of this commercially valuable species. Stock enhancement using cultured juveniles has been practiced in Japan for several decades, while pilot-scale feasibility studies have been conducted in South Africa (Godfrey Citation2003; Witte Citation2017). The associated high cost of seeding juvenile abalone has led to an interest in the possibility of larval seeding (Preece et al. Citation1997). Studies on the larval seeding of abalone have been conducted on a small-scale, experimental level (Mills-Orcutt, Bouma, and Donovan Citation2020; Preece et al. Citation1997; Tong, Moss, and Illingworth Citation1987) on a variety of species. Results have shown high variation in survival for the different species seeded which is likely influenced by the different seeding methods, suggesting that strategies for out-planting larvae are probably site and species-specific.
When conducting field-based studies or stock enhancement programs, released individuals must be unambiguously identifiable from wild animals, and therefore, a reliable, effective tagging method is required. However, tagging methods to identify individuals uniquely can often be problematic due to extensive handling, which is also labor intensive if a large number is tagged and can also negatively affect the survival and growth of the individuals (Chick Citation2010). Free swimming abalone larvae are small in size (H. midae 207 × 265 µm) (Genade, Hirst, and Smit Citation1988), which makes it challenging to tag them individually, and larvae are also sensitive to handling and manipulation (Chick Citation2010). The batch-tagging method is an effective method for tagging many individuals simultaneously. The marking tag can either be added to water in which the animals are then marked by immersion, or it can be incorporated into the feed (Chick Citation2010). The immersion marking method is the most suitable for small life stages (Purcell and Blockmans Citation2009), such as abalone larvae, since they may not begin be feeding until settlement.
Fluorochromes are calcium-binding substances that are incorporated at the site of active mineralization of bone, known as calcification front at the site of new bone formation (Day, Williams, and Hawkes Citation1995; Kaehler and McQuaid Citation1999; Moran Citation2000; Rahn and Perren Citation1971; Stuart and Smith Citation1992). After binding, the dye provides a bright epifluorescence reference which remains embedded in the growing shell at the top of the spire (Chick Citation2010). Fluorochromes dyes have been used for batch-tagging small life stages such as class Gastropoda abalone larvae (Chick Citation2010; Day, Williams, and Hawkes Citation1995), snails (Moran Citation2000) and class Bivalvia (van der Geest et al. Citation2011). Studies have shown that fluorochrome dye does not appear to have a detrimental effect on aquatic larvae when administered at an appropriate concentration (Day, Williams, and Hawkes Citation1995; Moran Citation2000; Moran and Marko Citation2005; Uggeri et al. Citation2004) and extended immersion periods at the appropriate concentration can result in higher levels of epifluorescence brightness (Purcell and Blockmans Citation2009). However, fluorochrome dyes can be toxic in marine animals if used at excessively high concentrations for a prolonged period (Purcell and Blockmans Citation2009).
Fluorochromes commonly used for marking the shell of marine species include calcein, xylenol orange, alizarin red S, oxytetracycline, and tetracycline. Fluorochrome calcein is a nonfluorescent, cell-permeant compound converted by intracellular esterases into calcein, the anionic fluorescent form that fluoresces bright green when combined with calcium (Uggeri et al. Citation2004). Calcein has been used successfully to mark the shell of different species in numerous growth studies, i.e., abalone larval, Haliotis rubra (Chick Citation2010; Day, Williams, and Hawkes Citation1995), juvenile scallop, Amusium balloti (Lucas et al. Citation2008); green-lipped mussel larvae, Perna canaliculus (Fitzpatrick, Jeffs, and Dunphy Citation2013); and the bivalve, Loripes lacteus (van der Geest et al. Citation2011). The calcein mark retained within the shell during settlement and metamorphosis remain embedded in the growing shell at the top of the spire (Collin and Voltzow Citation1998). Xylenol orange is fixed in newly formed calcified tissues where it remains throughout the growth of the animal (Rahn and Perren Citation1971) and has been successfully used to mark abalone larvae, H. rubra (Day, Williams, and Hawkes Citation1995).
The release of hatchery-produced larvae has been identified as an alternative option to building depleted wild abalone stock; millions of larvae can be produced in a hatchery on demand and cost-effectively. However, research still needs to be done to test the viability of using abalone larvae in terms of potential economic benefit, including tracking the survival and growth of released larvae. One of the critical factors required to determine the feasibility of this stock enhancement technique is the unambiguous identification of the released abalone from the wild stock. Marked recapture allows quantification of fundamental aspects of biology such as mortality, growth, population size, age at maturity, and possible track movement, which is essential information for stock enhancement programs (Lucas et al. Citation2008). Identifying hatchery-produced larvae will also enable proof of resource ownership for abalone ranching programs, which is also critical for the further development of abalone ranching. This study aimed to compare and identify an ideal dosage of two fluorochrome dyes (calcein and xylenol orange) in staining the shell of abalone (H. midae) larvae and assessing their effect on initial survival and settlement as well as brightness and longevity of resultant mark. The objectives of this study were to determine (1) whether the concentration of the chemical dyes have any effect on larval survival and settlement; and (2) if the combination of immersion time and epifluorescence dye concentrations can induce a persistent marker in the larval shell which is detectable using fluorescent light. The results from this study could be used in abalone larvae reseeding or stock-enhancement programs to help identify the hatchery-produced juveniles upon recapture.
Materials and methods
The study was conducted at Wild Coast Abalone Farm (Pty) Ltd (32º45’05’“S 28º16’27”’ E), South Africa. Abalone larvae were raised at the WCA farm hatchery in a flow-through rearing system, with each aquarium receiving independent aeration and filtered (1 µm) UV-treated seawater. Larvae were developed until they reached a stage where they could settle, 6 days after fertilization. Two fluorochrome dyes were calcein-AM (Sigma-Aldrich, Johannesburg, South Africa) and xylenol orange tetrasodium salt (Reflecta Laboratory Supplies, Johannesburg, South Africa).
Experimental setup
The experiment was conducted in 20 L tanks filled with 1 µm filtered seawater mixed with sodium bicarbonate (0.1 g. L−1) to buffer the pH in the seawater. Calcein and xylenol orange were used as fluorescent markers with each at aconcentration of either 25 mg. L−1 or 50 mg. L−1. The chemical powder was dissolved in the water before adding the abalone larvae. Larvae were held in each tank for 24 h and 48 h, with aeration but without water flow. Two controls containing no fluorochrome dye for the 24 h and 48 h periods were included. All treatments had three replicates.
Approximately 40,000 six-day-old larvae that were competent to settle were collected from the larval rearing tanks onto an 80 µm screen and transferred into the tanks (2 larvae mL−1) containing calcein or xylenol orange. Larvae were immersed in the marking baths for 24 h and 48 h, with both treatments starting at the same time (t0), and then held for their respective immersion times (24 h and 48 h). The tanks were in a temperature-controlled room maintained at 18 ± 0.5°C.
Evaluating the effect of calcein and xylenol orange immersion period on abalone larval survival
Immediately after the respective immersion periods, patches of dead larvae, which tend to accumulate in dark clumps at the bottom of the rearing tank (referred to in abalone hatcheries as “spotting”), were siphoned from each tank. The remaining larvae were gently poured through an 80 µm screen and placed into a tank with flow-through water to remove excess dye. Once clean, the larvae were gently poured into 10 L tanks containing filtered (1 µm) seawater. Each tank received water (6 L per hour) and gentle aeration. In each tank, two plastic sheets (15 × 15 cm = 900 cm2 total surface area per tank) pre-coated with natural diatom film were suspended as inducers for settlement. The sheets had been conditioned by placing them in outside abalone weaning tanks supplied with incoming seawater and allowed to develop a film of natural diatoms.
Post-exposure larval survival was determined by taking 10 mL water samples from each tank in triplicate. The number of swimming larvae was counted under a dissecting microscope (Best Scope, NSZ-606) as a function of the initial stocking density (2 larvae per mL).
Determining the effect of calcein and xylenol orange immersion period on abalone larvae initial settlement
To determine the effect of calcein and xylenol orange on the settlement of larvae at different concentrations and immersion times, after 24 and 48 h post-transfer to the clean 10 L tank, each sheet was removed and photographed (Canon camera, EOS 2000D) at high resolution (≈24.1 megapixels) so that the number of settled larvae could be counted on both sides of each sheet. The experiment ended after 48 h because the settlement sheet was small and insufficient to provide food for an extended period. The data presented in was recorded after 48 h and 24 hours post 24- and 48-h immersion periods, respectively, to ensure that larvae were in the same developmental stage.
Determining the persistence of epifluorescent dose in the shell of juvenile H. midae
The experiment was conducted following the same method described in the first experiment to determine the persistence of calcein and xylenol orange stain in the shell of juvenile H. midae. After the respective immersion period, 3500 and 4500 larvae were placed into small (26 cm2) and large (31.8 cm2) abalone settlement bags, respectively. Bags were randomly placed in the hatchery larval culture room with two replicates for each treatment. The inside of the bags were coated by filling the plastic bag with seawater, and allowing a natural diatom film to attach and grow in the bags for 2 weeks before the experiment. The stained larvae were then introduced into the bags where they settled and started feeding on the diatom layer. During the first 5 days of settlement, the water flow was switched off to avoid flushing the swimming larvae out of the bags. Each bag received aeration while the temperature was maintained at 18 ± 0.2°C. Larvae were raised in the settlement bags until food became limiting which was at 51 days after transfer into the bag. The persistence and level of epifluorescence were determined by taking 15 individuals from each bag at t0 (start of the experiment), and subsequent samples were collected after 24-days, 38-days, 47-days, and 51-days post-transfer to the bags. Samples were stored at −20°C, and after thawing, the shell of each juvenile was observed under a fluorescence microscope (Olympus BX-61) using the FITC set (green fluorescent), DAPI set (blue fluorescent) and Red set (Red fluorescent) to visualize the larval shell. Images of the abalone shell were captured using a Leica DFC 490 camera.
Images were then analyzed for brightness, where the intensity of fluorochrome (brightness of the band) was quantified using “ImageJ” image processing software’s freehand selection tool and histogram analysis function (developed by Wayne Rasband at the National Institutes of Health (NIH)). This function quantifies the brightness of each pixel within the selected area. The number of pixels for each value is plotted on a histogram, and the overall mean brightness of the selected area is established. The mean brightness was recorded to measure the total chemical fluorescence for each abalone’s shell band (Fitzpatrick, Jeffs, and Dunphy Citation2010).
Statistical analysis
All data were analyzed to check if they met parametric test assumptions using Shapiro–Wilk test for normality and Levene’s test for homogeneity. To test the effect of different concentrations of the fluorochrome dyes on larval survival and initial settlement, Levene’s test revealed non-homogeneity of variances and the data were log transformed. Then the data were analyzed using Welch’s analysis of variance (ANOVA), which is robust to non-equal variance among treatments. The mean epifluorescence brightness between fluorochrome (exposure period and different concentrations) met the parametric test assumption and was analyzed using a one-way ANOVA. For all data, when significant differences (p < .05) were found, Tukey’s multiple comparisons test was used to determine specific differences among treatments. An independent sample t-test was also used to compare the overall mean between exposure period treatments. The IBM SPSS version 26 statistical program was used for all data analyses with all data presented as mean ± SE.
Results
Determining the effect of calcein and xylenol orange immersion period on abalone larval survival and initial settlement
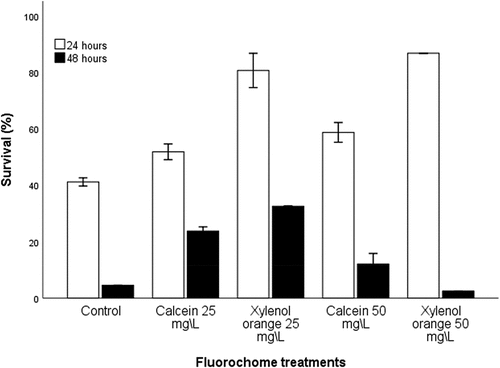
Survival of H. midae larvae was significantly affected by both immersion periods and different concentrations of fluorochromes. Abalone larvae immersed for 24 hours had an overall higher percentage survival than those exposed to 48 hours immersion (t (28) = 7.96, p < .001). After 24 h immersion, the highest percentage survival of 86.78 ± 4.83% and 80.65 ± 10.54% was recorded in the xylenol orange 50 mg L−1 and 25 mg L−1 treatments, respectively. The overall survival of abalone larvae declined after 48 h immersion for both fluorescent marker treatments with survival below 35%. The highest percentage survival of 32.54 ± 1.17% and 23.73 ± 1.50% were recorded for xylenol orange 25 mg L−1 and calcein 50 mg L−1, respectively, while xylenol orange 50 mg L−1 had the lowest survival (2.35 ± 0.98%) compared to the other treatments, including the control ().
Determining the effect of calcein and xylenol orange immersion period on abalone larvae initial settlement
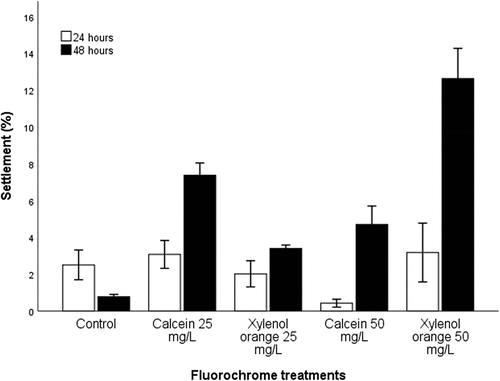
The initial settlement of abalone larvae was significantly affected by the immersion period and different concentrations of fluorochrome dyes. The immersion period had a significant effect on the percentage settlement of H. midae larvae, with the overall higher percentage of settled larvae recorded (averaged across fluorescent marker type) after 48 h immersion (5.89 ± 0.58%) compared to 24 h immersion (2.25 ± 0.55%) (t(78) = −4.45, p < .001). After 24 h immersion, there was no difference in the larval percentage settlement. While after 48 h immersion period, the percentage settlement of larvae was different between treatments (F(4) = 46.02, p < .001). After 48 h immersion, the xylenol orange 50 mg L−1 treatment had the highest percentage settlement, followed by calcein 25 and 50 mg L−1, while the control treatment had the lowest settlement ().
Determining the persistence of epifluorescence in the shell of juvenile H. midae
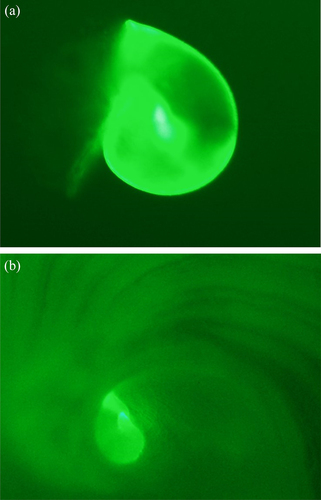
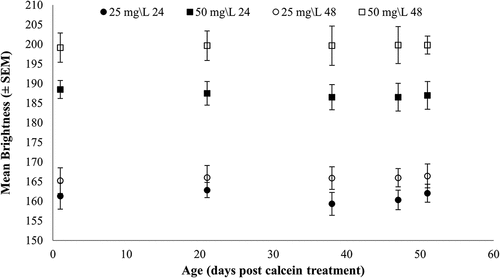
There was 100% marking success of larvae immersed in the calcein treatment at both concentrations and immersion periods (). Epifluorescence bands were evident in all juvenile abalone shells after 51 days. The control treatments also had no natural epifluorescence and there was no successful marking of larval shells in either of the xylenol orange treatments. Higher and more consistent levels of mean brightness were observed in the 50 mg L−1 calcein batch-tagged juvenile shells, irrespective of the immersion period (F (3) = 137, p < .001). The difference in the mean brightness among calcein concentrations varied with immersion time, and 48 h had highest mean brightness level of both concentrations (t (397) = 2.182, p = .030). Larvae immersed for 48 h at 50 mg L−1 calcein had highest mean brightness (199.59 ± 1.57 SEM) compared to those immersed for 24 h at 25 mg L−1 (161.16 ± 1.63 SEM). There was no difference in the level of mean brightness associated with time (decrease of the mean brightness level) of H. midae juveniles sampled before and post-settlement ().
Discussion
The choice of fluorescent dye for batch-tagging abalone larvae should not be based solely on tag brightness; post-staining survival is also essential. Larval survival is crucial for both abalone hatcheries and stock enhancement programe. Longer immersion (48 h) of H. midae larvae on calcein or xylenol orange affected the survival, ranging between 10% and 20% compared to 40% and 95% after 24 h immersion, respectively. This survival percentage was different from that found by Chick (Citation2010), who observed that staining Haliotis rubra larvae with calcein does not appear to affect larval survival. These differences could be attributed to (1) different methods used in these studies to determine survival, Chick (Citation2010) is based on assumptions while survival was measured in this study. (2) Species-specific abalone settlement variability may have resulted in some larvae settling on the side surface of the tank after 48 h immersion; hence, fewer larvae were swimming in the water column.
Fluorescent dyes can be toxic to marine organisms (Day, Williams, and Hawkes Citation1995) when administered at high concentrations or for extended periods (Purcell and Blockmans Citation2009). Fluorescent dyes positively affected the survival of H. midae larvae, and in both immersion periods, survival was higher in all treatments than in control. Similar results were reported by Moran and Marko (Citation2005), who tested the survival of bivalve larvae Mytilus trossulus marked with calcein which was significantly higher in 7-day post-marked larvae (38 ± 3% (SD)) compared to unmarked larvae (21 ± 4% (SD)). The underlying mechanism for this positive effect on survival is unknown. Moran and Marko (Citation2005) stated that one of the significant causes of mortality in larval culture is microbiological contamination, predominantly bacteria and viruses. While microorganism concentration was not evaluated in this study, pausing UV-treated water flow during the staining period might increase the chance for microorganism growth. The positive effect of calcein and xylenol orange on the survival of H. midae larvae suggests that both fluorochrome dyes possibly had an antibiotic or antiviral effect.
Abalone larvae must survive the planktonic stage and seek a suitable substrate where they change from swimming larvae into benthic juveniles (Leighton Citation2008). All abalone larvae settled post-immersion across all treatments. However, more extended immersion periods influenced the settlement of H. midae larvae, resulting in a higher percentage settlement. This may have resulted from the stress caused to abalone larvae during the longer (48 h) immersion periods. Larvae (Haliotis laevigata) are sensitive to poor water quality which stresses the abalone and slows growth, in the worst case, water quality can deteriorate to the extent of causing mortalities (Hindrum et al. Citation2001). While Yu, Yan, and Li (Citation2010) reported that abalone larvae fall out of suspension and consequently attach to the substratum under the stressed condition. After 48 h immersion, H. midae were possibly stressed, forcing more larvae to settle. It is possible that abalone larvae were more developed after 48 h immersion which might have resulted in higher percentage settlement (Moss and Tong Citation1992).
Different fluorescent dye concentrations also play a vital role in settlement of H. midae larvae. While there was no difference between the treatments and the control after 24 h immersion, xylenol orange (50 mg. L−1) and calcein (50 mg. L−1) had higher settlement after 48 h immersion. It has been shown in previous small-scale experiments that abalone larvae can continue to settle for up to 94 h post-initial settlement (Mzozo, Hugo, and Vine Citation2023). Unfortunately, the percentage settlement of H. midae post-settlement was not measured beyond 48 h. It is, therefore, unclear what might have happened with unsettled larvae from the control treatment. Therefore, conclusions on the effect of fluorescent dye on the long-term settlement of H. midae larvae should be made with caution.
During the batch-tagging of abalone larvae, the epifluorescence mark is embedded in the spiral of the growing shell of abalone (Chick Citation2010). The incorporation of epifluorescent markers in the shell increases with an increase in dye concentration of fluorescent and immersion period (Pirker and Schiel Citation1993). This study showed that the use of calcein enabled unambiguous identification of tagged H. midae larvae from untagged larvae (control treatment) post immersion, and the mark is a visible bright green, fluorescent FITC band on the shell when observed under the fluorescence microscope. Chick (Citation2010) reported that H. rubra juveniles batch-tagged with calcein demonstrated a visible mark was in the shell spire regardless of the concentration or immersion period. The results of this study clearly indicate staining H. midae larvae with 50 mg L−1 calcein results in a brighter epifluorescent mark on the shell than using 25 mg L−1, regardless of the immersion period. Similar results were obtained by Day, Williams, and Hawkes (Citation1995), who reported that fluorescent marks in the shell of abalone juveniles, H. rubra stained with calcein, were brightest at a concentration of 60 mg L−1 compared to 10 mg L−1 irrespective of the immersion time. Furthermore, van der Geest et al. (Citation2011) reported that the percentage of successfully marked bivalve, Loripes lacteus increased with higher calcein concentrations (100 − 800 mg L−1).
The most appropriate protocol for quality marking may vary between species (Eads and Layzer Citation2002). The xylenol orange treatments did not produce a mark on the shell of H. midae larvae when reviewed under the FITC filter sets of the fluorescence microscope. These results contradict the claims of Day, Williams, and Hawkes (Citation1995), who reported H. rubra larvae marked with xylenol orange at concentrations between 20 and 120 mg L−1 produced a visible mark in the shell after 48 h immersion. The method used by Day, Williams, and Hawkes (Citation1995) was different from this study in that the H. rubra shells were put through a series of water, acetone, and resin baths to facilitate resin penetration. The shell was then embedded in non-fluorescing resin before being viewed under a fluorescence microscope. This may have helped increase the contrast or brightness of the mark, making it easier to be detected by fluorescence microscope. Other research has used xylenol orange to mark dermal spicules of sea cucumbers (Purcell and Blockmans Citation2009) and the bone of rabbit (Rahn and Perren Citation1971) supporting the use of methods that do not implement embedding samples in resin. Day, Williams, and Hawkes (Citation1995) suggested that 60 mg L−1 was the best concentration for H. rubra; so it is possible that the xylenol orange concentrations used in this study were too low to result in a visible mark in the shell of H. midae juveniles. Eads and Layzer (Citation2002) reported that the marking success of batch tags varies between species. The authors showed that calcein (250 mg L−1) resulted in a distinct marking with rings around the whole shell for bivalves Actinonaias pectorosa while the marking was indifferent to that of Lampsilis cardium.
The persistence of an epifluorescence mark in the abalone shell is significant for the long-term use of the marking methodology (Eads and Layzer Citation2002). Consistency in the intensity epifluorescence is important when batch tagging for stock enhancement, as all marked individuals must be quickly identified among wild stock (Fitzpatrick, Jeffs, and Dunphy Citation2013). Calcein-produced epifluorescent marks were clearly visible on tagged H. midae juveniles raised under culture conditions after 51 days. Also, there was no difference in the mean brightness of epifluorescence between larvae (before settlement) and juveniles after 51 days post-immersion, suggesting there was no epifluorescence loss over the period. Similar results were obtained by Chick (Citation2010), who reported that up to 95% of H. rubra larval shells were easily distinguishable and that there was no difference in the level of epifluorescence after 260 days. Although this experiment was terminated after 51 days due to food limitations in the settlement bags, this study suggests that calcein at concentrations of 25 and 50 mg L−1 are suitable for the successful long-term tagging of H. midae juveniles.
In conclusion, it is possible to produce bright and persistent epifluorescence marks using calcein in H. midae shells that do not negatively affect the larvae’s survival during immersion or at settlement. A 24-h calcein immersion period results in higher larvae survival and is recommended as it subsequently increases the chances of settlement. Although 50 mg L−1 produced high mean brightness, at 25 mg L−1 it was still clearly visible and persistent after 51 days, making it easy to identify marked individuals. Calcen is expensive, and therefore, if budget is limited, 25 mg L−1 is recommended over higher concentrations (50 mg L−1). These results are of value to studies investigating larval dispersal dynamics and where larvae are released and recaptured, such as stock enhancement or ranching programs. Further research should further refine the optimal calcein concentration to create a persistent mark as well as determine the long-term effect of calcein staining on the survival and settlement of early-stage juvenile H. midae in the natural environment.
Acknowledgments
We acknowledge the South African Institute for Aquatic Biodiversity’s ACEP Phuhlisa Programme for a bursary for Z. B. M. Wild Coast Abalone (Pty) Ltd farm (WCA), in particular the Hatchery Manager, Stephen Ashlin for infrastructural support and Rhodes University, South Africa for access to the Electron Microscope facilities. We thank Dr. D. van Niekerk and Mr. M. Randall for training and guidance with data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chick, R. 2010. Batch-tagging blacklip abalone (haliotis rubra) for identification of hatchery-reared individuals on natural coastal reefs in new South Wales, Australia. Journal of Shellfish Research 29 (1):209–15. doi:10.2983/035.029.0117.
- Collin, R., and J. Voltzow. 1998. Initiation, calcification, and form of larval “archaeogastropod” shells. Journal of Morphology 235 (1):77–89. doi:10.1002/(SICI)1097-4687(199801)235:1<77:AID-JMOR6>3.0.CO;2-L.
- Day, R., M. Williams, and G. Hawkes. 1995. A comparison of fluorochromes for marking abalone shells. Marine and Freshwater Research 46 (3):599–605. doi:10.1071/mf9950599.
- Eads, C., and J. B. Layzer. 2002. How to pick your mussels out of a crowd: Using fluorescence to mark juvenile freshwater mussels. The University of Chicago Press Journals 21 (3):476–86. doi:10.2307/1468484.
- Fitzpatrick, M. P., A. G. Jeffs, and B. J. Dunphy. 2010. Identification of the optimal fluorochrome for marking larvae of the pulmonate limpet siphonaria australis. Journal of Shellfish Research 29 (4):941–44. doi:10.2983/035.029.0427.
- Fitzpatrick, M. P., A. G. Jeffs, and B. J. Dunphy. 2013. Efficacy of calcein as a chemical marker of green-lipped mussel (Perna canaliculus) larvae and its potential use for tracking larval dispersal. Aquaculture Research 44 (3):345–53. doi:10.1111/j.1365-2109.2011.03034.x.
- Genade, A. B., A. L. Hirst, and C. J. Smit. 1988. Observations on the spawning, development and rearing of the South African abalone haliotis midae linn. South African Journal of Marine Science 6 (1):3–12. doi:10.2989/025776188784480465.
- Godfrey, B. P. 2003. The potential of abalone stock enhancement in the Eastern Cape province of South Africa. MSc Thesis., Rhodes University. 1–170. http://core.ac.uk
- Hindrum, S. M., C. Burke, and S. J. Edwards. 2001. Growth reduction in greenlip (haliotis laevigata) and blacklip (H. rubra) abalone resulting from chronic exposure to sublethal combination of elevated ammonia and low dissolved oxygen levels. Environmental Requirements of Abalone 48–57.
- Kaehler, S., and C. D. McQuaid. 1999. Use of the fluorochrome calcein as an in situ growth marker in the brown mussel Perna perna. Marine Biology 133 (3):455–60. doi:10.1007/s002270050485.
- Leighton, P. 2008. Abalone hatchery manual. Irish Sea Fisheries Board 1–89.
- Lucas, T., P. J. Palmer, S. Wang, R. Scoones, and E. O’Brien. 2008. Marking the shell of the saucer scallop amusium balloti for sea ranching using oxytetracycline, calcein and alizarin red S. Journal of Shellfish Research 27 (5):1183–88. doi:10.2983/0730-8000-27.5.1183.
- Mills-Orcutt, K. A., J. V. Bouma, and D. A. Donovan. 2020. Outplanting larval pinto abalone Haliotis kamtschatkana kamtschatkana (Jonas) as a recovery tool in the Salish sea. Journal of Shellfish Research 39 (2):381–88. doi:10.2983/035.039.0220.
- Moran, A. L. 2000. Calcein as a marker in experimental studies newly-hatched gastropods. Marine Biology 137 (5–6):893–98. doi:10.1007/s002270000390.
- Moran, A. M. Y. L., and P. B. Marko. 2005. A simple technique for physical marking of larvae of marine bivalves. Journal of Shellfish Research 24:567–71. doi:10.2983/0730-8000(2005)24[567:ASTFPM]2.0.CO;2.
- Moss, G. A., and L. J. Tong. 1992. Effect of stage of larval development on the settlement of the abalone, Haliotis iris. New Zealand Journal of Marine and Freshwater Research 26 (1):69 ̶ 73. doi:10.1080/00288330.1992.9516501.
- Mzozo, Z. B., S. Hugo, and N. G. Vine. 2023. The use of chemical and biological settlement cues in enhancing the larval settlement of abalone (haliotis midae): Implications for hatcheries and ocean ranching. Journal of the World Aquaculture Society 54 (6):1702–17. doi:10.1111/jwas.13001.
- Pirker, J. G., and D. R. Schiel. 1993. Tetracycline as a fluorescent shell-marker in the abalone haliotis iris. Marine Biology 116 (1):81–86. doi:10.1007/BF00350734.
- Preece, P. A., S. A. Shepherd, S. M. Clarke, and J. K. Keesing. 1997. Abalone stock enhancement by larval seeding: Effect of larval density on settlement and survival. Molluscan Research 18 (2):265–73. doi:10.1080/13235818.1997.10673700.
- Purcell, S. W., and B. F. Blockmans. 2009. Effective fluorochrome marking of juvenile sea cucumbers for sea ranching and restocking. Aquaculture 296 (3–4):263–70. doi:10.1016/j.aquaculture.2009.08.027.
- Rahn, B. A., and S. M. Perren. 1971. Xylenol orange, a fluorochrome useful in polychrome sequential labeling of calfiying tissue. Stain Technology 4 (3):125–29. doi:10.3109/10520297109067836.
- Stuart, A. A. J., and D. A. Smith. 1992. Use of the fluorochromes xylenol orange, calcein green, and tetracycline to document bone deposition and remodeling in healing fractures in chickens. American Association of Avian Pathologists 36 (2):447–49. doi:10.2307/1591527.
- Tong, L. J., G. A. Moss, and J. Illingworth. 1987. Enhancement of a natural population of the abalone, haliotis iris, using cultured larvae. Aquaculture 62 (1):67–72. doi:10.1016/0044-8486(87)90185-2.
- Uggeri, J., R. Gatti, S. Belletti, R. Scandroglio, R. Corradini, B. M. Rotoli, and G. Orlandini. 2004. Calcein-AM is a detector of intracellular oxidative activity. Histochemistry and Cell Biology 122 (5):499–505. doi:10.1007/s00418-004-0712-y.
- van der Geest, M., J. A. van Gils, J. van der Meer, H. Oiff, and T. Piersma. 2011. Suitability of calcein as an in situ growth marker in burrowing bivalves. Journal of Experimental Marine Biology and Ecology 399 (1):1–7. doi:10.1016/j.jembe.2011.01.003.
- Witte, W. 2017. Abalone stock enhancement at Cape Recife, Eastern Cape province of South Africa. MSc Thesis., Rhodes University. 1–168. http://hdl.handle.net/10962/45695
- Yu, X., Y. Yan, and H. Li. 2010. The effects of chemical cues on larval settlement of the abalone, haliotis diversicolor supertexta. Journal of the World Aquaculture Society 41 (4):625 ̶632. doi:10.1111/j.1749-7345.2010.00403.x.