ABSTRACT
This study aimed to determine the optimal concentration of sodium bicarbonate (NaHCO3) and its effect on the Hematological parameters of three-spotted tilapia (Oreochromis andersonii) broodstock. Using a Completely Randomized Design, 150 broodstock (weight: 180 ± 10 g) were immersed in nine NaHCO3 concentrations (0–50 g/L) in triplicate. Induction and recovery times were recorded, and Red Blood Cells (RBC), White Blood Cells (WBC), Packed Cell Volume (PCV), and Hemoglobin (Hb) were measured. Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC) were calculated. Induction time decreased and recovery time increased with higher NaHCO3 concentrations, without any mortality. A 10 g/L concentration only significantly affected RBC and PCV. At 45 and 50 g/L there were significant changes to MCH, while significant increase was observed at 10 and 15 g/L in Hb and WBC, respectively. The optimal concentrations established for routine handling in hatcheries were 30 and 35 g/L.
Introduction
Three Spotted Tilapia (Oreochromis andersonii) holds significant importance within Zambia’s aquaculture sector and is farmed in most provinces excluding the northern regions (Hasimuna et al. Citation2021; Maulu et al. Citation2019; Mphande et al. Citation2023; Siavwapa et al. Citation2022). Recognizing its pivotal role, the Department of Fisheries has adopted this fish as a leading species for aquaculture development and is currently undergoing a genetic improvement program through selective breeding to enhance its growth performance (Siavwapa et al. Citation2022), thus potentially escalating the demand for broodstock within and beyond Zambia (Siavwapa et al. Citation2022). However, ensuring sustainable production of high-quality fingerlings hinges greatly on the mitigation of stress during routine operations by fish farmers and hatchery proprietors (Mphande et al. Citation2023; Siavwapa et al. Citation2022), given that stress significantly predisposes fish to disease outbreaks (Maulu et al. Citation2021). In contemporary aquaculture and hatchery management, stress is minimized by using anaesthetics during activities like artificial reproduction, weighing, injecting, marking, blood sampling, tagging, experimental surgery, and veterinary procedures (Gabriel, Erasmus, and Namwoonde Citation2020; Githukia, Kembenya, and Opiyo Citation2016; Hasimuna et al. Citation2021; Mphande and Chama Citation2015; Opiyo, Ogello, and Charo-Karisa Citation2013; Siavwapa et al. Citation2022; Simfukwe, Limuwa, and Njaya Citation2022). Anaesthetics induce reversible, controlled drug-induced sedation of the central nervous system (CNS), effectively preventing the perception or recollection of noxious stimuli (Donohue, Hobson, and Stephens Citation2013). Essential attributes for anaesthetic agents in aquaculture include effectiveness, safety, affordability, and accessibility (Hasimuna et al. Citation2020; Mphande et al. Citation2023; Velisek et al. Citation2007). In the aquaculture industry, the commonly used anaesthetics are Tricaine methanesulfonate (MS-222) (Burka et al. Citation1997), benzocaine (Bolasina, de Azevedo, and Petry Citation2017) quinaldine sulfate (Massee et al. Citation1995; Rust, Hardy, and Stickney Citation1993), clove oil and powder (Weber et al. Citation2009), eugenol, 2-phenoxyethanol, metomidate (King et al. Citation2005; Palić et al. Citation2006), and carbon dioxide (CO2) (Fish Citation1943; Hasimuna et al. Citation2020; Mbewe et al. Citation2024; Rairat et al. Citation2021; Siavwapa et al. Citation2022). This is even more pivotal in broodstock as it is a major factor that compromises egg and sperm quality, hatchability, fry survival rate, and reproductive performance (Mphande et al. Citation2023; Siavwapa et al. Citation2022). However, there is a call to use ecologically friendly anaesthetics which do not comprise the welling of the fish, are cheaper, readily available, and safe for the user and the consumer (Gabriel, Erasmus, and Namwoonde Citation2020; Hasimuna et al. Citation2020; Mphande et al. Citation2023; Siavwapa et al. Citation2022). In the pursuit of ecologically friendly and cost-effective anaesthetics, research endeavors in the aquaculture sector persist in exploring alternatives to conventional agents, especially in resource-limited settings (Gabriel, Erasmus, and Namwoonde Citation2020; Hasimuna et al. Citation2021). Sodium bicarbonate (NaHCO3), commonly known as baking soda, has emerged as a promising anaesthetic due to its nontoxic nature to fish, humans, and the environment. It can be administered directly through air-stone diffusion or indirectly by adding NaHCO3 to water, releasing CO2 (Fish Citation1943). Numerous studies have investigated NaHCO3 as an anaesthetic for various fish species, including Oreochromis niloticus, Clarias gariepinus, Oreochromis mossambicus, Oreochromis andersonii, Oreochromis macrochir, and Cyprinus carpio, affirming its effectiveness and recommending dosage adjustments based on species, size, and sex (Akinrotimi, Gabriel, and Deekae Citation2014; Gabriel, Erasmus, and Namwoonde Citation2020; Gajutos and Gajutos Citation2023; Hasimuna et al. Citation2020; Opiyo, Ogello, and Charo-Karisa Citation2013; Siavwapa et al. Citation2022).
However, it’s imperative to not solely rely on behavioral assessments but also evaluate the impact of anaesthetics on Hematological parameters when determining appropriate dosages (Aydın and Barbas Citation2020; Mirghaed et al. Citation2018; Yousefi et al. Citation2018). This is crucial as certain anaesthetic agents, such as essential oils, have been observed to elicit undesirable effects, such as increased cortisolemia, in fish species (Cárdenas et al. Citation2016; Mazandarani, Hoseini, and Dehghani Ghomshani Citation2017). Hematological and biochemical analyses of fish blood serve as vital indicators of physiological status and welfare, facilitating the detection of environmental stressors and toxicants (Kanu, Okoboshi, and Otitoloju Citation2023). Consequently, they play a fundamental role in both ecotoxicological and pharmacotoxicological studies by elucidating the structural and functional implications of exposure to contaminants (Fazio et al. Citation2019) and serve as early warning indicators of environmental threats (Brosset et al. Citation2021)
Despite the need for a comprehensive understanding of both behavioral and physiological response of fish species to anaesthetic substances when determining the ideal concentration to use for various routine operations in aquaculture and hatcheries in particular, to the best of our knowledge there are no studies that have been done to use behavioral and physiological response of O. andersonii broodstock to NaHCO3 and it the effect on Hematological parameters.
For this reason, this study aimed to determine the ideal (appropriate) concentration level (s) of sodium bicarbonate (NaHCO3) and its effect on the Hematological parameters of three spotted tilapia (Oreochromis andersonii) broodstock.
Materials and methods
Experimental site
This study was carried out 20 kilometers North-East of Lusaka at Kalimba Farm () which is adjacent to Ngwerere River.
Ethical consideration
Ethical approval for this was granted by the Tropical Diseases Research Centre (TDRC) Ethics Research Committee (ERC) number 00003729 at Ndola Teaching Hospital subsequent to the comprehensive review of protocols governing fish experimentation. Furthermore, all experimental fish were handled according to the Canadian Council’s guide on the care and use of experimental animals (Olfert, Cross, and McWilliam Citation1993).
Source of sodium bicarbonate
Sodium bicarbonate (NaHCO3) commonly referred to as baking soda or Chapa Mandashi locally, was sourced from a local super-market in Matero township in Lusaka, Zambia.
Experimental fish and conditioning
One hundred and fifty (150) broodstocks (weight: 180 ± 10 g) were obtained from an outdoor earthen pond of an approximate size of 20 m × 30 m using a seine net. The fish were placed in four black plastic troughs of about 2 m × 2 m × 6 m each having about 50 fish for conditioning in one-third (1/3) of borehole water which was aerated the whole night. The fish were starved for 24 h before the experiment to ensure gut evacuation (Hasimuna et al. Citation2020; Siavwapa et al. Citation2022). The period was picked to mimic the real routine handling of fish in hatcheries at fish farms. All the fish were in good health based on external examination (Fazio et al. Citation2017).
Induction and recovery time
After the conditioning, fish underwent exposure to nine different concentration levels of NaHCO3 (0 [Control], 10, 15, 20, 25, 30, 35, 40, 45, and 50 g/L) along with a control through bath immersion in 20 L transparent plastic containers, with each concentration tested in triplicate using a Completely Randomized Design (CRD) to ensure impartiality. Fifteen (15) fish were immersed at each concentration levels, which were selected based on prior research on similar species, such as Cyprinus carpio (Altun, Bilgin, and Danabaş Citation2009) and Oreochromis macrochir (Hasimuna et al. Citation2020). The duration of induction and recovery stages was recorded using a stopwatch following the stages of anaesthesia and description as shown in . Once fully anesthetized, fish were transferred to tanks containing fresh water for recovery, with recovery time also recorded (Hasimuna et al. Citation2021; Sorensen et al. Citation2023).
Table 1. Behavioral stages of anaesthesia and recovery.
Blood sampling and analysis
Once the fish had reached stage three of induction, three fish per concentration (1 per replicate) were obtained for blood sampling. About 2 mL of blood was sampled within a minute via puncturing the caudal vein using a 20 G 1½ syringe and collected in microtubes containing Ethylene Diamine Tetraacetic Acid (EDTA) as the anticoagulant agent (Fazio et al. Citation2012). All 30 blood samples were transported on dry ice to the University Teaching Hospital (UTH) for Hematological analysis.
Hematological analysis
The protocols outlined by Fazio et al. (Citation2013) and Shah and Altindağ (Citation2005) were employed to analyze Red Blood Cells (RBC), White Blood Cells (WBC), Packed Cell Volume (PCV), and Hemoglobin (Hb). RBC counts were manually determined using a Neubauer hemocytometer, while PCV and Hb levels were assessed through microhematocrit centrifugation and cyanmethemoglobin, respectively. In brief, each 20 μl of whole blood was accurately diluted with 0.98 ml of Dacie’s fluid, containing 40% of formaldehyde, 3.13 g trisodium citrate, and 0.1 g brilliant cresyl blue dissolved in 100 ml of distilled water. The prepared mixture was then stirred to dissolve the blood cells then this was followed by drawing the solution using a disposable plastic pipette and a few drops discarded and only a single touch drop placed between the counting chamber of Neubauer hemocytometer and a cover slip. After which, RBCs were counted under a microscope in five secondary squares at a magnification of 640X. As of the WBC count, 20 μl of blood was diluted with Turk’s diluting fluid, which is a solution of 1% glacial acetic acid and 0.3% Gentian violet dissolved in distilled water. After which, four large (1 square millimeter) corner squares of the hemocytometer were then counted under a microscope at 640X magnification to obtain the WBC. In addition, PCVs were determined through microhematocrit centrifugation. A microcapillary tube was filled with blood mixture then plugged with clay and then subjected to centrifugation at 19,000 g 5 min. There after, the length of column containing packed red cells and packed red cells plus the supernatant were measured, and the hematocrit was calculated as the ratio of packed red cells to packed red cells plus supernatant, expressed as a percentage. Likewise, Hb levels were measured using a test kit utilized the cyanmethemoglobin method.
Calculation of erythrocyte indices
All the three erythrocyte indices, namely Mean Corpuscular Hemoglobin Concentration (MCHC), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Volume (MCV) were calculated indirectly using the following formulas as prescribed by Fazio et al. (Citation2013).
MCHC = Hb x 100/PCV
MCH = Hb/RBC
MCV = PCV x 10/RBC
Water parameters
Four water quality parameters (temperature, dissolved oxygen, ammonia, and pH) were determined before administering the test chemical using a water test kit (Sunpu Test ZL 2010 2 0,681,385. X – Beijing Sunpu Biochem. Tech. CO., LTD). The values obtained were 23.8 ± 0.5°C, 5.7 ± 0.4 mg/L, ˂ 0.1 mg/L and 7.8 ± 0.2 (Unitless) for temperature, dissolved oxygen, ammonia, and pH, respectively.
Data analysis
Shapiro-Wilk and Levene’s tests were performed to check for normality and homogeneity assumptions of analysis of Variance (ANOVA), respectively. One-way ANOVA was used to analyze the differences in the means since the data met the two assumptions. To establish where differences in the means existed, Turkey’s honestly significant difference test was performed. The differences were considered significant at p < .05 and all tests were executed in Statistical Package for Social Scientists (SPSS) version 22. Additionally, polynomial regression was used to plot the concentration as independent variable against induction and recovery times as dependent variables, including their optimum times.
Results
NaHCO3 effect on induction time and recovery time
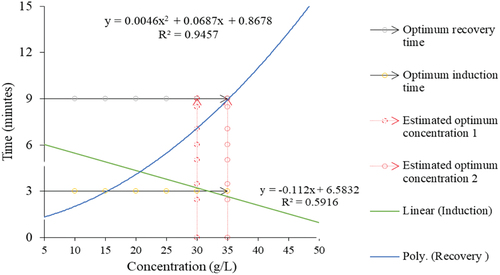
NaHCO3 exhibited concentration-dependent effects on both induction and recovery times. Induction time, ranging from 0.11 to 9.55 minutes (), decreased with increasing NaHCO3 concentration. Similarly, recovery time, spanning from 2.45 to 18.06 min (), increased with higher NaHCO3 concentration. In addition, polynomial regression analysis identified optimal concentrations for NaHCO3, suggesting 30 g/L for induction time less than 3 min and 35 g/L for recovery time less than 9 min ().
Table 2. O. andersonii broodstock exposed to various concentrations of NaHCO3 shows effects on induction and recovery time.
NaHCO3 influences the haematological parameters of O. andersonii broodstock
NaHCO3 influenced the measured Hematological parameters of O. andersonii broodstock, demonstrating a statistically significant effect (p < .05), as shown in . Red Blood Cell (RBC) and Packed Cell Volume (PCV) exhibited significant effects at all concentrations, while Mean Corpuscular Volume (MCV) and Mean Corpuscular Hemoglobin (MCH) showed significant effects only at higher concentrations. Additionally, no significant difference was attained at all concentration levels in Mean Corpuscular Hemoglobin Concentration (MCHC), while Hemoglobin (Hb) and White Blood Cell (WBC) counts showed significant difference at concentrations exceeding 10 and 15 g/L, respectively.
Table 3. NaHCO3 influences the Hematological parameters of O. andersonii broodstock.
Discussion
The present study delved into the potential of NaHCO3 as an anaesthetic agent for Oreochromis andersonii broodstock, indicating its efficacy, suitability, effect on Hematological parameters and as an alternative to conventional anaesthetic substances employed in aquaculture operations. In terms of induction time, an inverse relationship was observed between NaHCO3 concentration levels and the time required for induction. This phenomenon can be attributed to the increased release of CO2 from NaHCO3, leading to a reduction in dissolved oxygen levels in the water, which in turn may induce anesthesia. The increase in CO2 from NaHCO3 could have been the cause of a concussion O. andersonii broodstock. This aligns with previous studies (Fish Citation1943; Mbewe et al. Citation2024; Siavwapa et al. Citation2022) suggesting CO2-induced anesthesia in fish species. Conversely, a direct correlation was noted between NaHCO3 concentration and recovery time, likely due to elevated CO2 levels in the fish blood, necessitating extended recovery periods for CO2 elimination. This finding resonates with previous research on Oreochromis species (Avillanosa and Caipang Citation2019; Gabriel, Erasmus, and Namwoonde Citation2020; Hasimuna et al. Citation2020; Siavwapa et al. Citation2022), indicating prolonged recovery times associated with higher NaHCO3 concentration levels. Importantly, no mortality was observed across varying NaHCO3 concentration levels in O. andersonii broodstock, consistent with prior studies (Hasimuna et al. Citation2020; Opiyo, Ogello, and Charo-Karisa Citation2013; Siavwapa et al. Citation2022). This lack of mortality could be attributed to minimal alterations in erythrocyte indices at lower NaHCO3 concentration levels, suggesting the preservation of blood morphology and characteristics, crucial for fish health and survival. The study established optimal NaHCO3 concentration levels of 30 and 35 g/L for effective anesthesia induction and recovery within 3 and 9 minutes, respectively. These concentrations partly agree with the commendation made by Siavwapa et al. (Citation2022) who stated that 30 g/L could be used for male O. andersonii broodstock and disagreed with the recommendation of 25 g/L for female species of the O. andersonii and further aligns with recommendations for routine hatchery operations (Hasimuna et al. Citation2021). This disagreement is ascribed to differences in fish sizes or age investigated (Effati and Bahrekazemi Citation2017). Additionally, our recommendation disagrees with the concentration of 50 g/L for C. gariepinus juveniles reported by Githukia, Kembenya, and Opiyo (Citation2016). The differences in the concentrations commended for use can be attributed to differences in the species. It’s not surprising that C. gariepinus, despite being juveniles, required higher concentrations than broodstock of O. andersonii because C. gariepinus is a very hard species and can tolerate low levels of dissolved oxygen than Oreochromis species like O. andersonii investigated in the present study. These concentration levels demonstrated safety and effectiveness, without significant impact on Hematological parameters or mortality, thereby presenting a viable option for aquaculture handling activities. Regarding Hematological responses, an increase in both RBC and WBC counts was observed with rising NaHCO3 concentration levels, consistent with findings in other fish species exposed to various anaesthetic agents (Gressler et al. Citation2014; Jia et al. Citation2022; Shaluei et al. Citation2012; Yousefi et al. Citation2022; Abd El-Hack et al. Citation2022). This physiological response, characterized by heightened immune activity and oxygen transport, likely aids in fish recovery from anesthesia-induced stress. Furthermore, PCV exhibited a reduction while Hb levels increased with increasing NaHCO3 concentration levels, in agreement with previous studies on different fish species and anaesthetic agents (Bagheri and Imanpour Citation2011; de Oliveira et al. Citation2019; Olufayo and Adeyanju Citation2012; Zahran, Risha, and Rizk Citation2021). These Hematological alterations reflect adaptive responses to anesthesia-induced stress, emphasizing the resilience of O. andersonii broodstock to NaHCO3 exposure. Erythrocyte indices (MCV, MCHC and MCH) remained relatively stable at lower NaHCO3 concentration levels, with significant differences observed only at higher levels. This aligns with findings in other fish species exposed to anaesthetic agents (Adeshina, Adewale, and Yusuf Citation2016; Akinrotimi et al. Citation2014), suggesting a low-cost response to anesthesia-induced stress. This stability in erythrocyte indices further supports the suitability of NaHCO3 as an anaesthetic substance for O. andersonii broodstock.
Conclusion
The anaesthetic effect of sodium bicarbonate and its effect on Hematological parameters of Oreochromis andersonii broodstock has been investigated. Furthermore, the study has demonstrated the efficacy of NaHCO3 as an effective anaesthetic for Oreochromis andersonii broodstock, with concentration levels of 30 and 35 g/L identified as suitable for anesthesia induction. Notably, no mortality was observed across varying NaHCO3 concentration levels, underscoring its safety for fish. Furthermore, NaHCO3 exhibited minimal impact on Hematological parameters and erythrocyte indices, particularly at lower concentration levels.
Based on these findings, NaHCO3 is recommended for anaesthetizing O. andersonii broodstock, and this has the potential to enhance stress management and welfare of broodstock fish in Zambian hatcheries. To provide comprehensive insights into the broader impact of NaHCO3 anesthesia on O. andersonii broodstock physiology and health, further research is necessary that will investigate the effects of NaHCO3 on the biochemical parameters and determine its acute toxicity levels. This will help to formulate appropriate, timely, and practical stress management in hatchery across the country.
Acknowledgments
The authors wish to thank all individuals that assisted in the execution of this study in any form.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available and can be requested from the corresponding author.
Additional information
Funding
References
- Abd El-Hack, M. E., M. T. El-Saadony, M. M. Nader, H. M. Salem, A. M. El-Tahan, S. M. Soliman, and A. F. Khafaga. 2022. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). International Journal of Biometeorology 66 (11):2183–94. doi:10.1007/s00484-022-02347-6.
- Adeshina, I., Y. A. Adewale, and Y. O. Yusuf. 2016. Eugenia cayrophyllata oil as anesthetic in cultured African catfish, (clarias gariepinus, Burchell 1822) juveniles. Nigerian Journal of Fisheries and Aquaculture 4 (2):8–17.
- Akinrotimi, O. A., U. U. Gabriel, and S. N. Deekae. 2014. Anaesthetic efficacy of sodium bicarbonate and its effects on the blood parameters of African catfish, clarias gariepinus ( Burchell, 1822). Journal of Aquatic Sciences 29 (1):233–46.
- Altun, T., R. Bilgin, and D. Danabaş. 2009. Effects of sodium bicarbonate on anaesthesia of common carp (Cyprinus carpio L. 1758) juveniles. Turkish Journal of Fisheries and Aquatic Sciences 9 (1). doi:10.4194/trjfas.2009.005.
- Avillanosa, A. L., and C. M. A. Caipang. 2019. Use of sodium bicarbonate as an inexpensive general anesthetic for juvenile red tilapia hybrids. International Aquatic Research 11 (3):287–94. doi:10.1007/s40071-019-00235-1.
- Aydın, B., and L. A. L. Barbas. 2020. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 520:734999. doi:10.1016/j.aquaculture.2020.734999.
- Bagheri, T., and M. R. Imanpour. 2011. The efficacy, physiological responses and hematology of Persian sturgeon, Acipenser persicus, to clove oil as an anesthetic agent. Turkish Journal of Fisheries and Aquatic Sciences 11 (3). doi:10.4194/1303-2712-v11_3_20.
- Bolasina, S. N., A. de Azevedo, and A. C. Petry. 2017. Comparative efficacy of benzocaine, tricaine methanesulfonate and eugenol as anesthetic agents in the guppy Poecilia vivipara. Aquaculture Reports 6:56–60. doi:10.1016/j.aqrep.2017.04.002.
- Brosset, P., S. J. Cooke, Q. Schull, V. M. Trenkel, P. Soudant, and C. Lebigre. 2021. Physiological biomarkers and fisheries management. Reviews in Fish Biology and Fisheries 31 (4):1–23. doi:10.1007/s11160-021-09677-5.
- Burka, J. F., K. L. Hammell, T. E. Horsberg, G. R. Johnson, D. J. Rainnie, and D. J. Speare. 1997. Drugs in salmonid aquaculture–a review. Journal of Veterinary Pharmacology and Therapeutics 20 (5):333–49. doi:10.1046/j.1365-2885.1997.00094.x.
- Cárdenas, C., C. Toni, J. A. Martos‐Sitcha, S. Cárdenas, V. de Las Heras, B. Baldisserotto, B. M. Heinzmann, R. Vázquez, and J. M. Mancera. 2016. Effects of clove oil, essential oil of Lippia alba and 2‐phe anaesthesia on juvenile meagre, argyrosomus regius (Asso, 1801). Journal of Applied Ichthyology 32 (4):693–700. doi:10.1111/jai.13048.
- de Oliveira, C. P. B., C. H. da Paixão Lemos, L. V. O. Vidal, R. D. Couto, D. S. P. Pereira, and C. E. Copatti. 2019. Anaesthesia with eugenol in hybrid Amazon catfish (pseudoplatystoma reticulatum × leiarius marmoratus) handling: Biochemical and haematological responses. Aquaculture 501:255–59. doi:10.1016/j.aquaculture.2018.11.046.
- Donohue, C., B. Hobson, and R. C. Stephens. 2013. An introduction to anaesthesia. British Journal of Hospital Medicine 74 (5):71–75. doi:10.12968/hmed.2013.74.Sup5.C71.
- Effati, M., and M. Bahrekazemi. 2017. Effects of four anesthetics, clove extract, thyme extract, lidocaine, and sodium bicarbonate on the blood parameters and cortisol amount in grass carp (Ctenopharyngodon idella). Journal of Marine Biology and Aquaculture 4 (1):1–4. doi:10.15436/2381-0750.18.1586.
- Fazio, F., V. Ferrantelli, C. Saoca, G. Giangrosso, and G. Piccione. 2017. Stability of haematological parameters in stored blood samples of rainbow trout Oncorhynchus mykiss (walbaum, 1792). Veterinární Medicína 62 (7):401–05. doi:10.17221/51/2017-VETMED.
- Fazio, F., F. Filiciotto, S. Marafioti, V. Di Stefano, A. Assenza, F. Placenti, G. Buscaino, G. Piccione, and S. Mazzola. 2012. Automatic analysis to assess haematological parameters in farmed gilthead sea bream (sparus aurata Linnaeus, 1758). Marine and Freshwater Behaviour and Physiology 45 (1):63–73. doi:10.1080/10236244.2012.677559.
- Fazio, F., S. Marafioti, A. Torre, M. Sanfilippo, M. Panzera, and C. Faggio. 2013. Haematological and serum protein profiles of mugil cephalus: Effect of two different habitats. Ichthyological Research 60 (1):36–42. doi:10.1007/s10228-012-0303-1.
- Fazio, F., C. Saoca, V. Ferrantelli, G. Cammilleri, G. Capillo, and G. Piccione. 2019. Relationship between arsenic accumulation in tissues and Hematological parameters in mullet caught in Faro Lake: A preliminary study. Environmental Science and Pollution Research 26 (9):8821–27. doi:10.1007/s11356-019-04343-7.
- Fish, F. F. 1943. The anaesthesia of fish by high carbon-dioxide concentrations. Transactions of the American Fisheries Society 72 (1):25–29. doi:10.1577/1548-8659(1942)72[25:TAOFBH]2.0.CO;2.
- Gabriel, N. N., V. N. Erasmus, and A. Namwoonde. 2020. Effects of different fish sizes, temperatures and concentration levels of sodium bicarbonate on anaesthesia in Mozambique tilapia (Oreochromis mossambicus). Aquaculture 529:735716. doi:10.1016/j.aquaculture.2020.735716.
- Gajutos, L. J. B., and A. B. Gajutos. 2023. Anaesthetic effects of different concentrations of sodium bicarbonate on common carp (Cyprinus carpio). Journal of Fisheries 11 (1):111206. doi:10.17017/j.fish.425.
- Githukia, C. M., E. M. Kembenya, and M. A. Opiyo. 2016. Anaesthetic effects of sodium bicarbonate at different concentrations on African Catfish (Clarias gariepinus) juveniles. Journal of Aquaculture Engineering and Fisheries Research 2 (3):151–58. doi:10.3153/JAEFR16017.
- Gressler, L. T., A. P. K. Riffel, T. V. Parodi, E. M. H. Saccol, G. Koakoski, S. T. da Costa, M. A. Pavanato, B. M. Heinzmann, B. Caron, D. Schmidt, et al. 2014. Silver catfish rhamdia quelen immersion anaesthesia with essential oil of Aloysia triphylla (L’hérit) Britton or tricaine methanesulfonate: Effect on stress response and antioxidant status. Aquaculture Research 45 (6):1061–72. doi:10.1111/are.12043.
- Hasimuna, O. J., C. Monde, I. Bbole, S. Maulu, and M. Chibesa. 2021. The efficacy of sodium bicarbonate as an anaesthetic agent in Oreochromis macrochir juveniles. Scientific African 11:e00668. doi:10.1016/j.sciaf.2020.e00668.
- Hasimuna, O. J., C. Monde, M. Mweemba, and A. Nsonga. 2020. The anaesthetic effects of sodium bicarbonate (baking soda) on greenhead tilapia (Oreochromis macrochir, Boulenger 1912) broodstock. The Egyptian Journal of Aquatic Research 46 (2):195–99. doi:10.1016/j.ejar.2019.12.004.
- Jia, Y., T. Xie, Y. Gao, H. Qin, and C. Guan. 2022. Anesthetics efficacy and physiological response of MS222 and clove oil in spotted knifejaw oplegnathus punctatus. Aquaculture Reports 25:101201. doi:10.1016/j.aqrep.2022.101201.
- Kanu, K. C., A. C. Okoboshi, and A. A. Otitoloju. 2023. Haematological and biochemical toxicity in freshwater fish clarias gariepinus and Oreochromis niloticus following pulse exposure to atrazine, mancozeb, chlorpyrifos, lambda-cyhalothrin, and their combination. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 270:109643. doi:10.1016/j.cbpc.2023.109643.
- King, W., B. Hooper, S. Hillsgrove, C. Benton, and D. L. Berlinsky. 2005. The use of clove oil, metomidate, tricaine methanesulphonate and 2‐phenoxyethanol for inducing anaesthesia and their effect on the cortisol stress response in black sea bass (centropristis striata L). Aquaculture Research 36 (14):1442–49. doi:10.1111/j.1365-2109.2005.01365.x.
- Massee, K. C., M. B. Rust, R. W. Hardy, and R. R. Stickney. 1995. The effectiveness of tricaine, quinaldine sulfate and metomidate as anesthetics for larval fish. Aquaculture 134 (3–4):351–59. doi:10.1016/0044-8486(95)00057-9.
- Maulu, S., O. J. Hasimuna, J. Mphande, and H. M. Munang’andu. 2021. Prevention and control of streptococcosis in tilapia culture: A systematic review. Journal of Aquatic Animal Health 33 (3):162–77. doi:10.1002/aah.10132.
- Maulu, S., B. P. Munganga, O. J. Hasimuna, L. H. Haambiya, and B. Seemani. 2019. A review of the science and technology developments in Zambia’s aquaculture industry. Journal of Aquaculture Research and Development 10 (567):2.
- Mazandarani, M., S. M. Hoseini, and M. Dehghani Ghomshani. 2017. Effects of linalool on physiological responses of Cyprinus carpio (Linnaeus, 1758) and water physico‐chemical parameters during transportation. Aquaculture Research 48 (12):5775–81. doi:10.1111/are.13400.
- Mbewe, G., K. Nawanzi, M. Chibesa, J. Mphande, I. Mumbula, H. Bwalya, R. C. Chibiya, J. Mbewe, M. Mweemba, O. S. Mwale, et al. 2024. Efficacy of sodium bicarbonate (baking soda) and clove powder (Syzygium aromaticum) as anaesthetic agents for Nile tilapia (Oreochromis niloticus, Linnaeus 1758) juveniles. Journal of Applied Animal Research 52 (1):2329567. doi:10.1080/09712119.2024.2329567.
- Mirghaed, A. T., M. Yasari, S. S. Mirzargar, and S. M. Hoseini. 2018. Rainbow trout (Oncorhynchus mykiss) anesthesia with myrcene: Efficacy and physiological responses in comparison with eugenol. Fish Physiology and Biochemistry 44 (3):919–26. doi:10.1007/s10695-018-0481-5.
- Mphande, J., and L. Chama. 2015. Preservation methods and storage period affect the mineral and moisture composition of freshwater fish species. International Journal of Food Science & Nutrition Engineering 5 (3):147–53. doi:10.5923/j.food.20150503.06.
- Mphande, J., O. J. Hasimuna, E. Kikamba, S. Maulu, K. Nawanzi, D. Phiri, M. Chibesa, E. Siankwilimba, C. J. Phiri, B. M. Hampuwo, et al. 2023. Application of anaesthetics in fish hatcheries to promote broodstock and fish seed welfare in Zambia. Cogent Food & Agriculture 9 (1):1, 2211845. doi:10.1080/23311932.2023.2211845.
- Olfert, E. D., B. M. Cross, and A. A. McWilliam, Eds. 1993. Guide to the care and use of experimental animals, 1 (2). Ottawa: Canadian Council on Animal Care.
- Olufayo, M. O., and A. A. Adeyanju. 2012. Haematological effect of sub-lethal concentration of neem leaves (Azadirachta indica) on heterobranchus bidorsalis. Forest Product Journal 5:37–41.
- Opiyo, M. A., E. O. Ogello, and H. Charo-Karisa. 2013. Effectiveness of sodium bicarbonate as an anaesthetic for different sizes of Nile tilapia (Oreochromis niloticus L. 1758) juveniles. International Journal of Aquatic Science 4 (2):14–22.
- Palić, D., D. M. Herolt, C. B. Andreasen, B. W. Menzel, and J. A. Roth. 2006. Anesthetic efficacy of tricaine methanesulfonate, metomidate and eugenol: Effects on plasma cortisol concentration and neutrophil function in fathead minnows (Pimephales promelas Rafinesque, 1820) ( Pimephales promelas Rafinesque, 1820). Aquaculture 254 (1–4):675–85. doi:10.1016/j.aquaculture.2005.11.004.
- Rairat, T., Y. Chi, S. K. Chang, C. Y. Hsieh, N. Chuchird, and C. C. Chou. 2021. Differential effects of aquatic anaesthetics on the pharmacokinetics of antibiotics: Examples using florfenicol in Nile tilapia (Oreochromis niloticus). Journal of Fish Diseases 44 (10):1579–86. doi:10.1111/jfd.13480.
- Rust, M. B., R. W. Hardy, and R. R. Stickney. 1993. A new method for force-feeding larval fish. Aquaculture 116 (4):341–52. doi:10.1016/0044-8486(93)90418-X.
- Shah, S. L., and A. Altindağ. 2005. Alterations in the immunological parameters of Tench (Tinca tinca L. 1758) after acute and chronic exposure to lethal and sublethal treatments with mercury, cadmium and lead. Turkish Journal of Veterinary & Animal Sciences 29 (5):1163–68.
- Shaluei, F., A. Hedayati, A. Jahanbakhshi, and M. Baghfalaki. 2012. Physiological responses of great sturgeon (Huso huso) to different concentrations of 2-phenoxyethanol as an anesthetic. Fish Physiology and Biochemistry 38 (6):1627–34. doi:10.1007/s10695-012-9659-4.
- Siavwapa, S., O. J. Hasimuna, S. Maulu, and C. Monde. 2022. A comparative analysis of the anaesthetic effect of sodium bicarbonate (NaHCO3) on male and female three spotted tilapia (Oreochromis andersonii). Journal of Applied Animal Research 50 (1):269–74. doi:10.1080/09712119.2022.2064478.
- Simfukwe, K., M. M. Limuwa, and F. Njaya. 2022. Are chilimira fishers of engraulicypris sardella (günther, 1868) in Lake Malawi productive? The case of Nkhotakota District. Sustainability 14 (23):16018. doi:10.3390/su142316018.
- Sorensen, K., S. R. Craig, A. Cnaani, and E. McLean. 2023. Hematological response of juvenile cobia to three anesthetics. Fishes 8 (1):31. doi:10.3390/fishes8010031.
- Velisek, J., T. Wlasow, P. Gomulka, Z. Svobodova, and L. Novotny. 2007. Effects of 2-phenoxyethanol anaesthesia on sheatfish (silurus glanis L.). Veterinarni medicina 52 (3):103. doi:10.17221/2011-VETMED.
- Weber, R. A., J. B. Peleteiro, L. G. Martín, and M. Aldegunde. 2009. The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (solea senegalensis kaup 1858). Aquaculture 288 (1–2):147–50. doi:10.1016/j.aquaculture.2008.11.024.
- Yousefi, M., S. M. Hoseini, B. Aydın, A. T. Mirghaed, E. V. Kulikov, S. G. Drukovsky, S. B. Seleznev, P. A. Rudenko, S. H. Hoseinifar, and H. Van Doan. 2022. Anesthetic efficacy and hemato-biochemical effects of thymol on juvenile Nile tilapia, (Oreochromis niloticus). Aquaculture 547:737540. doi:10.1016/j.aquaculture.2021.737540.
- Yousefi, M., S. H. Hoseinifar, M. Ghelichpour, and S. M. Hoseini. 2018. Anesthetic efficacy and biochemical effects of citronellal and linalool in common carp (cyprinus carpio Linnaeus, 1758) juveniles. Aquaculture 493:107–12. doi:10.1016/j.aquaculture.2018.04.054.
- Zahran, E., E. Risha, and A. Rizk. 2021. Comparison of propofol and eugenol anesthetics efficacy and effects on general health in Nile Tilapia. Aquaculture 534:736251. doi:10.1016/j.aquaculture.2020.736251.