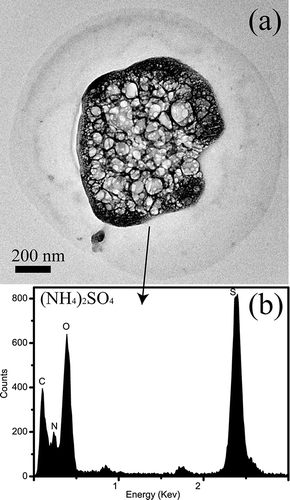
ABSTRACT
Direct observation of the mixing state of aerosol particles in a coastal urban city is critical to understand atmospheric processing and hygroscopic growth in humid air. Morphology, composition, and mixing state of individual aerosol particles from Macao, located south of the Pearl River Delta (PRD) and 100 km west of Hong Kong, were investigated using scanning electron microscopy (SEM) and transmission electron microscopy coupled with energy-dispersive X-ray spectrometry (TEM/EDX). SEM images show that soot and roughly spherical particles are prevalent in the samples. Based on the compositions of individual aerosol particles, aerosol particles with roughly spherical shape are classified into coarse Na-rich and fine S-rich particles. TEM/EDX indicates that each Na-rich particle consists of a Na-S core and NaNO3 shell. Even in the absence of heavy pollution, the marine sea salt particles were completely depleted in chloride, and Na-related sulfates and nitrates were enriched in Macao air. The reason could be that SO2 from the polluted PRD and ships in the South China Sea and NO2 from vehicles in the city sped up the chlorine depletion in sea salt through heterogeneous reactions. Fresh soot particles from vehicular emissions mainly occur near curbside. However, there are many aged soot particles in the sampling site surrounded by main roads 200 to 400 m away, suggesting that the fresh soot likely underwent a quick aging. Overall, secondary nitrates and sulfates internally mixed with soot and sea salt particles can totally change their surface hygroscopicity in coastal cities.
This study of compositional changes in mixed marine aerosols and anthropogenic pollutants underscores the importance of aerosol mixing states with regard to their role in visibility, climate, as well as health inhalation. Almost all sea salt particles in Macao air were depleted in chloride but enriched in Na-related sulfates and nitrates through heterogeneous atmospheric reactions. Most soot particles are aggregated with or encapsulated by S-rich particles, suggesting that the fresh soot particles likely underwent a quick aging. Future climate model should consider the mixing states of sea salt and soot particles in the atmosphere of the China coast.
INTRODUCTION
Aerosols in the atmosphere can undergo heterogeneous reactions and alter trace gas-phase species. Aerosol particles from different sources become internal mixtures of both water-soluble and insoluble components due to condensation and coagulation. As a result of the atmospheric processing, aerosols show diverse changes in their chemical and physical properties, such as chemical composition, size, morphology, and hygroscopicity.Citation1–3 These changes can affect the role of aerosols in the regional climate through the scattering and absorption of solar radiation (direct radiative forcing) or by acting as cloud condensation nuclei (CCN) (indirect radiative forcing).Citation4–6 In order to accurately account for the effects of aerosols on these processes, their detailed species and corresponding chemical and physical properties must be investigated. It is well known that concentrations and properties of aerosols are quite different in different regions corresponding to sources of pollutants, local climate, and geography. In particular, properties of aerosol particles in East Asia, one of the most populated and rapidly developing regions during the last decades, need to be understood.
Physical combinations of organic and secondary aerosols (e.g., sulfate and nitrate) with some insoluble aerosol particles (e.g., metal, soot, and mineral particle) can substantially change the aerosol properties.Citation1,Citation7 In particular, sulfate particles internally mixed with soot should be investigated because the mix can enhance the light absorption of soot particles by at least 30%.Citation8
In addition to physical attachment, heterogeneous reactions of sea salt and mineral dust particles in the atmosphere can affect their properties.Citation2,Citation3 Through laboratory experiments it is becoming increasingly clear that ubiquitous sea salt and alkaline mineral dust can heterogeneously react with acidic gases (e.g., SO2, NO2, HNO3, and organic acids).Citation9,Citation10 Li and ShaoCitation11 observed that mineral dust particles in regional brown hazes over North China are commonly coated by calcium nitrate through heterogeneous reactions with HNO3. However, heterogeneous reactions of sea salt along the approximately 18,000 km coastline of China have not been studied. Our limited knowledge of the interactions between marine sea salt aerosols and anthropogenic pollutants from the continent influences the evaluation of the impacts of aerosols along the China coast as well as many other places on regional climates and human health. This knowledge is also necessary to understand the hygroscopic growth of aged sea salt and their ability to act as CCN in coastal air.Citation12–14
One of the special administrative regions in China, Macao, is a coastal city located south of the Pearl River Delta (PRD) and 100 km west of Hong Kong. With its area of 28.6 km2 and its population of 525,500, it is one of the most densely populated areas in the world. Local air pollution emissions exclude heavy industry but include vehicular traffic, with about 171,000 vehicles registered.Citation15
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are state-of-the-art instruments to observe morphologies, compositions, and mixing states of individual aerosol particles.Citation16–19 In this work, SEM and TEM were used to investigate individual aerosol particles in Macao where the atmosphere during 5 days in July 2008 was mainly influenced by a mixed air mass from the urban area and the South China Sea.
EXPERIMENTS
Climatological Characteristics
Macao is one of the special administrative regions in South China (). The area is subject to typical Asian monsoon climates. It is humid and hot in summer and spring with strong southeastern monsoon winds from the South China Sea, and relatively cool in autumn and winter with northern monsoon winds from China mainland. During the summer monsoon (including June, July, and August), the average wind speed is 3 m sec−1 and the southwesterly wind direction dominates the area. In addition, the monthly precipitation also is highest in a year, up to 340 mm. Although monsoon winds from the South China Sea and frequent precipitation strongly influence Macao air in summer, air pollution was frequently monitored in Macao, with the main pollutants being aerosol particles.
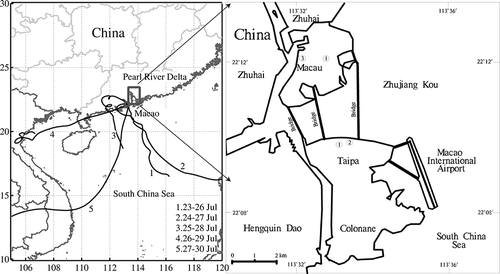
Figure 1. Air mass back trajectory during the sampling period. Five 96-hr back trajectories from 26 to 30 July 2008. Each trajectory arrived at Macao at 500 m height, and arriving time was assigned according to the sampling time (data from http://arl.noaa.gov). Right map shows three different sampling sites in Macao: (1) main street in residential area; (2) University of Macao; (3) Inner Harbour.
Sample Collection
Nine aerosol samples were collected at three sites in Macao in summer, involving the main street in residential area (curbside), the campus of the University of Macao (residential area), and Inner Harbour (curbside) during 26–30 July 2008 ( and ). Five samples were collected on TEM Cu grids coated with carbon or silicon oxide film using an impactor with a 0.5-mm-diameter jet and an airflow of 1.0 L min−1. In this study, the collection efficiency of the impactor was calculated using the results of Ranz and Wong.Citation20 If the density of aerosol particles is 2 g cm−3, the collection efficiency of the impactor is 50% for 0.15-μm particles and 100% for 0.25-μm particles. In addition, four PM10 samples were collected on polycarbonate filters using a MiniVol (Ametrica, USA) sampler with a constant air flow of 5 L min−1. The different sampling times were conducted based on the visibility (), so that aerosol particles didn't overlap on the filters. The good diffusion of aerosol particles can reflect the actual mixing states of ambient aerosol particles through electron microscopic observation. A Kestral 4000 instrument was employed to record air temperature (32–38 °C) and humidity (52–81%) for each sampling period ().
Table 1. Meteorological parameter and sample information
Sample Analyses
To study the morphologies of aerosol particles collected in Macao, individual aerosol particles were observed through secondary electron images of the LEO 435VP SEM. Samples on the polycarbonate filters were coated with a 20-nm-thick gold substrate before settling into the SEM chamber.
The composition and mixing state of individual aerosol particles were examined in detail by a Philip CM200 TEM coupled with energy-dispersive X-ray (EDX) microanalysis. This instrument has been used effectively to study the mixing state and elemental compositions of individual aerosol particles.Citation21 For some particles, selected-area electron diffraction (SAED) was also obtained to verify their crystal phases. Volatile and semivolatile particles, such as water, some organic compounds, and NH4NO3, could not be analyzed because they were lost in the vacuum chamber of the electron microscope. In addition to the normal EDX, elemental profiles of individual aerosol particles were obtained through the EDX line-scan function in scanning TEM (STEM). This technology can determine elemental variation along a preset line on the two analyzed particle.Citation7
RESULTS AND DISCUSSION
Pollutant Concentration in Macao
Daily concentrations of pollutants (i.e., O3, PM10 [particulate matter with aerodynamic diameter ≤10 mm], SO2, and NO2) were obtained from continuous instruments at the Macao Meteorological Station. During this 5-day period, 24-hr average PM10 concentrations ranged from 22 to 84 μg m−3; NO2, from 5.73 to 8.70 ppb; O3, from 16.37 to 37.50 ppb; and SO2, from 0.08 to 0.20 ppb (). In addition, the average PM10 concentration during July–August 2008 was 30 μg m−3, ranging from 4 to 172 μg m−3. Because the precipitation frequently occurred in every July and August in PRD region, some extremely low daily PM10 concentrations can be monitored. During the sampling period, there was no precipitation at sampling sites. Compared to monthly PM10 concentration in Macao, the sampling period should be considered as the clean air on 26, 27, and 30 July, and the light polluted air on 28 and 29 July 2008. Four days' back trajectories shown in indicates that Macao was not generally affected by plumes transported from the heavily industrial PRD region. Macao air in the sampling days can be characterized as mixed air masses from the urban area and the South China Sea.
Table 2. Daily mass concentrations of pollutants in Macao air
Particle Classification
Four distinct particle classes with different morphologies were identified from the low-magnification SEM images: round-like (21% of the 44,340 particles), irregular (1%), soot (36%), and unknown fine particles (42%) (particles with diameter smaller than 20 nm cannot be identified by SEM). Therefore, soot and roughly spherical particles are prevalent in Macao air (). We only found a few cubic or elongated particles, although such particles have been commonly detected in the coastal air of Shenzhen.Citation22
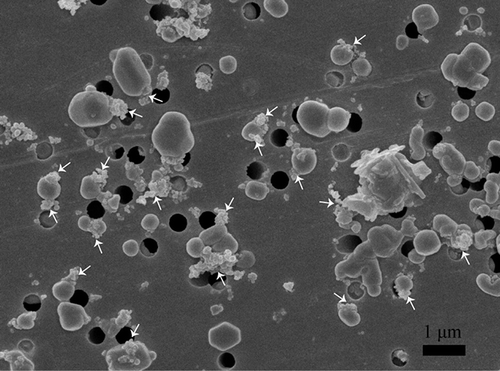
Figure 2. SEM image of aerosol particles collected at the University of Macao. The white arrows point to the internally mixed soot particles.
Roughly Spherical Particles
A cursory inspection of the composition and morphology of individual roughly spherical particles using TEM/EDX revealed two prominent particle groups ():
Figure 3. Low-magnification TEM image of roughly spherical particles collected at the University of Macao. These roughly spherical particles detected by TEM/EDX are coarse Na-rich (indicated by black arrows) and fine S-rich particles.
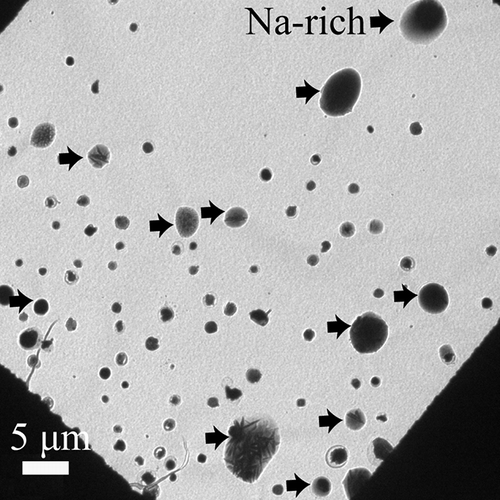
| • | Coarse particles (≥2 µm), mostly sodium, and called “Na-rich” | ||||
| • | Fine particles (<2 µm), mostly sulfur, called “S-rich” | ||||
Na-rich particles: Na-rich particles originating from ocean spray typically occur in coastal cites as cubic NaCl, but these particles are not found in the Macao air. The Na-rich particles in Macao air are rounded and consist of cores and shells ( and ). These Na-rich particles are classified into two different components: (1) a core of Na and Cl has a shell with C, N, O, S, Na, and Cl; and (2) a core of O, Na, S, and Mg has a shell with C, N, O, Na, and Mg. displays a SAED pattern of a particle of the first composition. The core is confirmed to be NaCl phase, whereas its corresponding shell yields an amorphous pattern. Elemental profiles shown in –c clearly display variations of each element in the Na-rich particles along the preset lines. –c shows that the particle core includes mostly O, Na, Mg, and S, but its shell includes C, N, O, Mg, and Na.
Figure 4. TEM image (a) and STEM/EDX elemental profiles (b and c) along a preset line (arrow) of a roughly spherical Na-rich particle of the first composition collected on Cu grid and coated with Si-O film. The Na-rich particle includes a NaCl core and Na-rich shell. Inset in (a) shows the SAED pattern of NaCl.
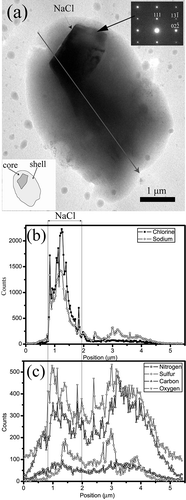
Figure 5. TEM image (a) and STEM/EDX elemental profiles (b and c) along a preset line (arrow) of a roughly spherical Na-rich particle of the second composition collected on a Cu grid and coated with Si-O film. The Na-rich particle has two parts: Na-S core and Na-rich shell.
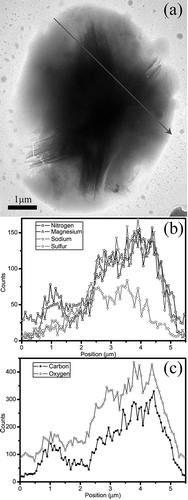
These roughly spherical Na-rich particles that consist of cores and shells are consistent with the aged sea salt particles depleted chloride through atmospheric reactions with nitric and/or sulfuric acids and/or their precursors.Citation23–25 and further indicate that their cores are NaCl and Na-S, whereas the corresponding shells are hydrated NaNO3. Similar aged sea salt particles were observed by Li et al.Citation25 in the Canary Islands when North Atlantic air became mixed with the polluted air of Europe. In addition, Geng et al.Citation24 found that the aged sea salt particles frequently occurred at Ny-Ålesund, Svalbard, near the Arctic polar. Although EDX spectra of cores in the Na-rich particles demonstrated that they consisted of Na and S, we did not obtain any clear SAED patterns to confirm their phases. Hopkins et al.Citation23 recently indicated that the Na-S component in reacted sea salt particles could be Na2SO4, CH3SO4Na, and/or HO-CH2SO3Na because of substantial heterogeneous replacement of chloride by methanesulfonate (CH3SO3 −) and by non-sea-salt sulfate (nss-SO4 2−). However, these authors only observed chemical modification on the surfaces of sea salt particles. In this study, we believe that sulfuric acid produced from the methanesulfonate can react with the sea salt particles but cannot become the aged particles like those shown in and . Therefore, most sulfates in the Na-rich particles should be non-sea-salt sulfates. In addition, the abundant C in the shells of aged sea salt particles collected on TEM grids with silicon oxide films ( and ) is expected to be from atmospheric organic components. Such organic compounds commonly occur on surfaces of inorganic particles in urban areas.Citation7
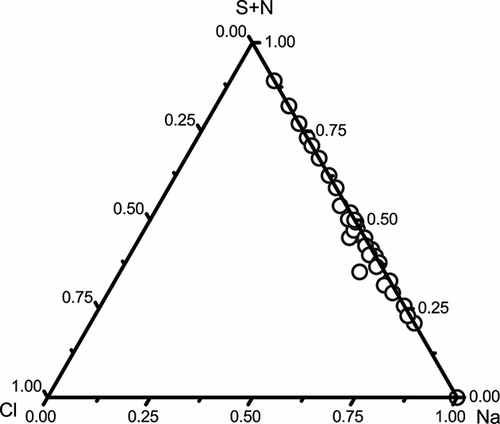
Most of the sea salt particles are aged and contain Na-S and NaNO3 (). Heterogeneous reactions between NO2 from vehicular emissions and sea salt particles can explain NaNO3 formation. Given the lack of nearby heavy industry, we did not expect to see many Na-S cores in aged sea salt particles. In fact, the concentration of SO2 in Macao air was too low to account for much of the S. Back trajectories 3 and 4 shown in indicate that air masses from the open South China Sea traveled inland and came back to Macao area. It could be explained that the air masses brought large amounts of NaCl had chemical interactions with the pollutants over PRD. Back trajectories 1, 2, and 5 indicate that the air masses only traveled over South China Sea. During the sampling period, we also noticed that the busy shipping lanes in the South China Sea are not far from the Macao coast. Many of these ships burn high-sulfur fuel oil that can emit massive quantities of SO2.26 The back trajectories of air mass during the sampling period also generally came from South China Sea or its coastal zone (). Therefore, we could not exclude the possibility that sea salt particles from the South China Sea reacted with H2SO4 or SO2 emitted by the ships before arriving at Macao.
Figure 6. Ternary diagrams of Cl-Na-(S and N) in 33 Na-rich particles. Almost all the chlorine in all sea salt particles was depleted.
S-rich particles: Fine S-rich particles display a roughly spherical shape. Most S-rich particles are mixed with soot particles (). Based on EDX analysis, the S-rich particles should be ammoniated sulfate. The ammoniated sulfate particles are extremely beam-sensitive in TEM. Even under weak electron beams, they suffer damage and bubbled readily, leaving residues on the TEM grids (). shows that the S-rich particles on the TEM grids are frequently enclosed by a prominent circle, suggesting that these particles probably held water in ambient atmosphere.
Soot
Soot, a ubiquitous by-product of all fuel combustion, is a chain-like aggregate of carbon-bearing spheres (). One soot aggregate consists of from tens to hundreds of linked carbon spheres with typical diameters of 10–100 nm.
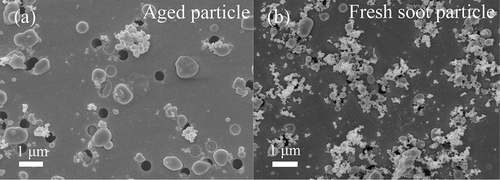
The soot particles collected at the University of Macao, where main roads are 200–400 m away, are mixed with S-rich particles (), suggesting that the soot particles from vehicular emissions underwent aging processes during transport. In contrast, soot particles collected near main roads are fresh (), suggesting that soot particles age quickly at the high relative humidity in coastal cities.Citation27 On the other hand, photochemistry can also promote transformation of fresh soot into an internal mixture of soot, sulfate, organics, nitrate, ammonium, and water.Citation27 Note that time scale of the aging processes in the coastal cities could be shorter than 8 hr close to the sources from the modeling calculation by Riemer et al.Citation28 The further study should be conducted for understanding the soot aging time scale in the coastal cities.
Hygroscopic Properties of Aged Aerosol Particles
As previously noted, coarse aged sea salt particles and fine secondary sulfate and soot particles both are prevalent in Macao air. Direct observation of the internally mixed particles allows us to consider their hygroscopic properties. The deliquescence relative humidities (DRHs) of NaCl and Na2SO4 are 76% and 84%, respectively,Citation12,Citation29 and hydrated NaNO3 cannot display a distinct DRH.Citation14 In this study, the hydrated NaNO3 shell on NaCl and Na2SO4 particles ( and ) probably determines their surface hygroscopic properties. The work of Wise et al.Citation12 indicates that aged sea salt particles, but not fresh ones, can grow to some extent at RH between 40% and 60%. As a result, the RH from 52% to 81% in Macao air in summer probably can cause the aged sea salt particles to enlarge because of water uptake.
Although fresh soot particles are hydrophobic, they acquire a shell of secondary sulfates and organic particles through condensation and coagulation ( and ), which causes them to become hydrophilic.Citation2,Citation19,Citation30 The humid coastal air, therefore, promotes water uptake on the aged aerosol particles. The Na-rich and ammoniated sulfate particles were assumed to deliquesce in the early morning with RH of 80–90%. After sunrise, RH dropped but still remained higher than 40% during the daytime. The efflorescence relative humidities (ERHs) of NaCl, Na2SO4, and NH4(SO4)2 are 43%, 57%, and 37%, respectively.30 Comparing the ERHs of the aerosol particles and the ambient RH shown in , ammoniated sulfate and hydrated NaNO3 in these deliquesced particles probably retained their aqueous phase in air. Their retention of the aqueous phase may explain why the TEM images always displayed some small particles but usually had large circles around the Na-rich ( and ) and S-rich () particles.
Water uptake by inorganic aerosol particles and their subsequent hygroscopic growth can enhance light scattering.Citation31 Zhang et al.Citation30 also showed that subsequent hygroscopic growth of aged soot particles enhances their scattering and absorption by several fold at 80% RH, compared with fresh particles. As a result, these aged aerosol particles could dramatically decrease local visibility, explaining why low visibility prevailed during the sampling period in Macao even though it is not affected by the polluted air from the Pearl River Delta. Therefore, subsequent hygroscopic growth of the aged aerosol particles may impair local visibility and impact climate in coastal areas of China.
CONCLUSIONS
TEM/EDX and SEM were used to investigate individual aerosol particles from the coastal city. The roughly spherical particles mainly consist of coarse Na-rich and fine S-rich particles. Na-rich particles consist of Na-S/NaCl cores and NaNO3 shell. During the sampling period, almost all sea salt particles in Macao air were depleted in chloride but enriched in Na-related sulfates and nitrates through heterogeneous atmospheric reactions. Most secondary sulfates are roughly spherical (NH4)2SO4 particles and each particle is enclosed by a prominent circle on the TEM grids, suggesting these particles held water in ambient atmosphere. In addition, fresh soot particles from vehicular emissions were mainly found close to roads. Many soot particles collected at the University of Macao, with main roads from 200 to 400 m away, are coalesced with or encapsulated by S-rich particles, suggesting that they underwent rapid aging.
This study provides some insights into the interaction between marine particles and continental polluted air. When the winds from the west and south bring an abundance of sea salt particles into the air along the coastline of China, the chemical interactions of these sea salt particles with anthropogenic gases or particles likely alter the chemistry of coastal air.
ACKNOWLEDGMENTS
The authors are grateful to Prof. Buseck for sponsoring the visit of W.J.L. to Arizona State University. The authors acknowledge the use of TEM in the LeRoy Eyring Center for Solid State Science at Arizona State University. We thank Dr. Pamela Holt for proofreading the manuscript. The authors wish to thank the Macao Meteorological and Geophysical Bureau for providing the monitoring data during the specified sampling periods. Financial support was provided by the National Natural Science Foundation of China (No. 41105088), Natural Science Foundation of Shandong Province (ZR2011DQ001), National Basic Research Program of China (2011CB403401), Macao Foundation for Development of Science and Technology (No. 023/2004/A), State Key Laboratory of Coal Resources and Safe Mining (SKLCRSM09KFB04).
REFERENCES
- Gibson , E.R. , Gierlus , K.M. , Hudson , P.K. and Grassian , V.H. 2007 . Generation of Internally Mixed Insoluble and Soluble Aerosol Particles to Investigate the Impact of Atmospheric Aging and Heterogeneous Processing on the CCN Activity of Mineral Dust Aerosol . Aerosol. Sci. Technol , 41 : 914 – 924 .
- Li , W.J. and Shao , L.Y. 2009 . Transmission Electron Microscopy Study of Aerosol Particles from the Brown Hazes in Northern China . J. Geophys. Res. , 114 : 1 – 10 . doi: 10.1029/2008JD011285
- Murphy , D.M. , Anderson , J.R. , Quinn , P.K. , McInnes , L.M. , Brechtel , F.J. , Kreidenweis , S.M. , Middlebrook , A.M. , Posfai , M. , Thomson , D.S. and Buseck , P.R. 1998 . Influence of Sea-Salt on Aerosol Radiative Properties in the Southern Ocean Marine Boundary Layer . Nature , 392 : 62 – 65 .
- Andreae , M.O. 2009 . Correlation between Cloud Condensation Nuclei Concentration and Aerosol Optical Thickness in Remote and Polluted Regions . Atmos. Chem. Phys. , 9 : 543 – 556 .
- Eck , T.F. , Holben , B.N. , Dubovik , O. , Smirnov , A. , Goloub , P. , Chen , H.B. , Chatenet , B. , Gomes , L. , Zhang , X.Y. , Tsay , S.C. , Ji , Q. , Giles , D. and Slutsker , I. 2005 . Columnar Aerosol Optical Properties at AERONET Sites in Central Eastern Asia and Aerosol Transport to the Tropical Mid-Pacific . J. Geophys. Res. , 110 : 1 – 18 . doi: 10.1029/2004JD005274
- Dusek , U. , Frank , G.P. , Hildebrandt , L. , Curtius , J. , Schneider , J. , Walter , S. , Chand , D. , Drewnick , F. , Hings , S. , Jung , D. , Borrmann , S. and Andreae , M.O. 2006 . Size Matters More Than Chemistry for Cloud-Nucleating Ability of Aerosol Particles . Science , 312 : 1375 – 1378 .
- Li , W.J. and Shao , L.Y. 2010 . Mixing and Water-Soluble Characteristics of Particulate Organic Compounds in Individual Urban Aerosol Particles . J. Geophys. Res. , 115 : 1 – 9 . doi: 10.1029/2009JD012575
- Schwarz , J.P. , Spackman , J.R. , Fahey , D.W. , Gao , R.S. , Lohmann , U. , Stier , P. , Watts , L.A. , Thomson , D.S. , Lack , D.A. , Pfister , L. , Mahoney , M.J. , Baumgardner , D. , Wilson , J.C. and Reeves , J.M. 2008 . Coatings and Their Enhancement of Black Carbon Light Absorption in the Tropical Atmosphere . J. Geophys. Res. , 113 : 1 – 10 . doi: 10.1029/2007JD009042
- Prince , A.P. , Kleiber , P.D. , Grassian , V.H. and Young , M.A. 2008 . Reactive Uptake of Acetic Acid on Calcite and Nitric Acid Reacted Calcite Aerosol in an Environmental Reaction Chamber . Phys. Chem. Chem. Phys. , 10 : 142 – 152 .
- Laskin , A. , Gaspar , D.J. , Wang , W.H. , Hunt , S.W. , Cowin , J.P. , Colson , S.D. and Finlayson-Pitts , B.J. 2003 . Reactions at Interfaces as a Source of Sulfate Formation in Sea-Salt Particles . Science , 301 : 340 – 344 .
- Li , W.J. and Shao , L.Y. 2009 . Observation of Nitrate Coatings on Atmospheric Mineral Dust Particles . Atmos. Chem. Phys. , 9 : 1863 – 1871 .
- Wise , M.E. , Semeniuk , T.A. , Bruintjes , R. , Martin , S.T. and Russell , L.M. 2007 . Buseck, P.R.Hygroscopic Behavior of NaCl-Bearing Natural Aerosol Particles Using Environmental Transmission Electron Microscopy . J. Geophys. Res. , 112 : 1 – 12 . doi: 10.1029/2006JD007678
- Ebert , M. , Inerle-Hof , M. and Weinbruch , S. 2002 . Environmental Scanning Electron Microscopy as a New Technique to Determine the Hygroscopic Behaviour of Individual Aerosol Particles . Atmos. Environ. , 36 : 5909 – 5916 .
- Liu , Y. , Yang , Z. , Desyaterik , Y. , Gassman , P.L. , Wang , H. and Laskin , A. 2008 . Hygroscopic Behavior of Substrate-Deposited Particles Studied by Micro-FT-IR Spectroscopy and Complementary Methods of Particle Analysis . Anal. Chem. , 80 : 633 – 642 .
- Li , W.J. , Shao , L.Y. , Shen , R. , Wang , Z. , Yang , S. and Tang , U. 2010 . Size, Composition and Mixing State of Individual Aerosol Particles in a South China Coastal City . J. Environ. Sci. , 22 : 561 – 569 .
- Buseck , P.R. and Posfai , M. 1999 . Airborne Minerals and Related Aerosol Particles: Effects on Climate and the Environment . Proc. Natl. Acad. Sci. U.S.A. , 96 : 3372 – 3379 .
- Chen , Y. , Shah , N. , Huggins , F.E. and Huffman , G.P. 2006 . Microanalysis of Ambient Particles from Lexington, KY, by Electron Microscopy . Atmos. Environ. , 40 : 651 – 663 .
- Geng , H. , Jung , H.-J. , Park , Y. , Hwang , H. , Kim , H. , Kim , Y.J. , Sunwoo , Y. and Ro , C.-U. 2009 . Morphological and Chemical Composition Characteristics of Summertime Atmospheric Particles Collected at Tokchok Island, Korea . Atmos. Environ. , 43 : 3364 – 3373 .
- Shi , Z.B. , Zhang , D.Z. , Ji , H.Z. , Hasegawa , S. and Hayashi , M. 2008 . Modification of Soot by Volatile Species in an Urban Atmosphere . Sci. Total Environ. , 389 : 195 – 201 .
- Ranz , W.E. and Wong , J.B. 1952 . Impaction of Dust and Smoke Particles on Surface and Body Collectors . Ind. Eng. Chem. , 44 : 1371 – 1381 .
- Li , W.J. and Shao , L.Y. 2010 . Characterization of Mineral Particles in Winter Fog of Beijing Analyzed by TEM and SEM . Environ. Monit. Assess. , 161 : 565 – 573 .
- Shi , Z. , He , K. , Xue , Z. , Yang , F. , Chen , Y. , Ma , Y. and Luo , J. 2009 . Properties of Individual Aerosol Particles and Their Relation to Air Mass Origins in a South China Coastal City . J. Geophys. Res. , 114 : 1 – 11 . doi: 10.1029/2008JD011221
- Hopkins , R.J. , Desyaterik , Y. , Tivanski , A.V. , Zaveri , R.A. , Berkowitz , C.M. , Tyliszczak , T. , Gilles , M.K. and Laskin , A. 2008 . Chemical Speciation of Sulfur in Marine Cloud Droplets and Particles: Analysis of Individual Particles from the Marine Boundary Layer over the California Current . J. Geophys. Res. , 113 : 1 – 15 . doi: 10.1029/2007JD008954
- Geng , H. , Ryu , J. , Jung , H.-J. , Chung , H. , Ahn , K.-H. and Ro , C.-U. 2010 . Single-Particle Characterization of Summertime Arctic Aerosols Collected at Ny-Alesund, Svalbard . Environ. Sci. Technol. , 44 : 2348 – 2353 .
- Li , J. , Anderson , J.R. and Buseck , P.R. 2003 . TEM Study of Aerosol Particles from Clean and Polluted Marine Boundary Layers over the North Atlantic . J. Geophys. Res. , 108 ACH8-1-ACH8-11 doi: 10.1029/2002JD002106
- Streets , D.G. , Guttikunda , S.K. and Carmichael , G.R. 2000 . The Growing Contribution of Sulfur Emissions from Ships in Asian Waters, 1988–1995 . Atmos. Environ. , 34 : 4439
- Moffet , R.C. and Prather , K.A. 2009 . In-Situ Measurements of the Mixing State and Optical Properties of Soot with Implications for Radiative Forcing Estimates . P.Natl. Acad. Sci. U.S.A. , 106 : 11872 – 11877 .
- Riemer , N. , Vogel , H. and Vogel , B. 2004 . Soot Aging Time Scales in Polluted Regions During Day and Night . Atmos. Chem. Phys. , 4 : 1885 – 1893 .
- Tang , I.N. and Munkelwitz , H.R. 1994 . Water Activities, Densities, and Refractive Indices of Aqueous Sulfates and Sodium Nitrate Droplets of Atmospheric Importance . J. Geophys. Res. , 99 : 18801 – 18808 .
- Zhang , R.Y. , Khalizov , A.F. , Pagels , J. , Zhang , D. , Xue , H.X. and McMurry , P.H. 2008 . Variability in Morphology, Hygroscopicity, and Optical Properties of Soot Aerosols during Atmospheric Processing . Proc. Natl. Acad. Sci. U.S.A. , 105 : 10291 – 10296 .
- Martin , S.T. 2000 . Phase Transitions of Aqueous Atmospheric Particles . Chem. Rev. , 100 : 3403 – 3453 .