ABSTRACT
Deokjeok Island is located off the west coast of the Korean Peninsula and is a suitable place to monitor the long-range transport of air pollutants from the Asian continent. In addition to pollutants, Asian dust particles are also transported to the island during long-range transport events. Episodic transport of dust and secondary particles was observed during intensive measurements in the spring (March 31–April 11) and fall (October 13–26) of 2009. In this study, the chemical characteristics of long-range-transported particles were investigated based on highly time-resolved ionic measurements with a particle-into-liquid system coupled with an online ion chromatograph (PILS-IC) that simultaneously measures concentrations of cations (Li+, Na+, NH4 +, K+, Ca2+, Mg2+) and anions (F−, Cl−, NO3 −, SO4 2−). The aerosol optical thickness (AOT) distribution retrieved by the modified Bremen Aerosol Retrieval (M-BAER) algorithm from moderate resolution imaging spectroradiometer (MODIS) satellite data confirmed the presence of a thick aerosol plume coming from the Asian continent towards the Korean peninsula. Seven distinctive events involving the long-range transport (LRT) of aerosols were identified and studied, the chemical components of which were strongly related to sector sources. Enrichment of acidic secondary aerosols on mineral dust particles, and even of sea-salt components, during transport was observed in this study. Backward trajectory, chemical analyses, and satellite aerosol retrievals identified two distinct events: a distinctively high [Ca2++Mg2+]/[Na+] ratio (>2.0), which was indicative of a preprocessed mineral dust transport event, and a low [Ca2++Mg2+]/[Na+] ratio (<2.0), which was indicative of severe aging of sea-salt components on the processed dust particles. Particulate Cl− was depleted by up to 85% in spring and 50% in the fall. A consistent fraction of carbonate replacement (FCR) averaged 0.53 in spring and 0.55 in the fall. Supporting evidences of Cl− enrichment on the marine boundary layer prior to a dust front were also found.
Supplemental materials are available for this article. Go to the publisher's online edition of the Journal of the Air & Waste Management Association for sector and air mass classifications of clean and LRT cases.
The chemical characteristics of aerosol particles evolve as they undergo long-range transport (LRT) in the atmosphere from the source region. Aside from meteorological conditions, the signature of the source region influences the loading and chemical characteristics of LRT aerosols.
INTRODUCTION
Asian continental outflow transports large quantities of primary and secondary aerosols over the Korean peninsula during the spring and fall seasons, mostly influenced by the passage of cold fronts.Citation1 Observations during the spring have been extensively studied during the Asian Pacific Regional Aerosol Characterization Experiment (ACE-Asia)Citation2–5 and other field campaigns (Joint Research on Long-Range Transboundary Air Pollutions in Northeast Asia [LTP],Citation6 Transport and Chemical Evolution over the Pacific (TRACE-P)Citation7,Citation8) These aerosols are either emitted directly from multiple sources or produced through various atmospheric processes. Furthermore, atmospheric aerosols engage in various processes during transport that modify their chemical composition, mixing state, and reactivity.Citation9–12
Particularly in the northeast region (China, Mongolia, Siberia, Korea, Japan) of the Asian continent, source regions of mineral dusts include loess soils from the arid Gobi desert of northern China and Mongolia, and the Taklimakan desert of western China.Citation13 Mineral dust particles are considered the largest single contributor to transported particles in the atmosphereCitation14 and are susceptible to processing and chemical transformation during transport. Mineral dust particles can also be coated with sulfate and nitrate,Citation12,Citation15,Citation16 as well as a carbonaceousCitation10 or sea-saltCitation17 component via heterogeneous processes during transport. These in turn may be removed from their surface, making them more available for processing and the production of secondary aerosols.Citation9 Such processes would determine the chemical characteristics of the resulting aerosol. The long-range transport of mineral dust associated with urban pollution has therefore become one of the major challenges in Northeast Asia.
Long-range-transported aerosol particles can impact an environment a considerable distance from its source. However, limitations concerning the availability of long-term monitoring data have made it difficult to achieve scientific and political consensus regarding the relative impacts of long-range-transported aerosol particles. The Northeast Asian countries have undertaken a wide range of collaborative activities in order to build a scientific consensus on the basis of coordinated multilateral research activities. The Joint Research on Long-Range Transboundary Air Pollutions in Northeast Asia (LTP),Citation6,Citation18 the so-called “LTP Project”, was established to characterize the transport and transformation of atmospheric aerosols over Northeast Asia, and to identify their sources based on continuous, intensive, and remote sensing measurements and receptor models. Long-term (continuous) and intensive (seasonal) measurement campaigns were implemented at multiple surface sites in Northeast Asia. The 2009 LTP Project initialized the use of highly time-resolved aerosol chemistry measurements to study the transport of pollutants to a background site in Korea.
This study reports the results from the 2009 LTP intensive measurement campaigns at a background marine site in Korea. The aim of the intensive measurement was to investigate the chemical characteristics of local and long-range-transported Asian aerosols under different synoptic conditions from the unique Northeast Asian outflow. In addition, satellite-retrieved aerosol optical thickness and air mass back trajectory analyses were used to identify the spatial distribution and transport of aerosols. Furthermore, detailed analysis was performed to characterize the degree of processing of long-range-transported mineral dust and secondary species.
EXPERIMENTAL METHODS
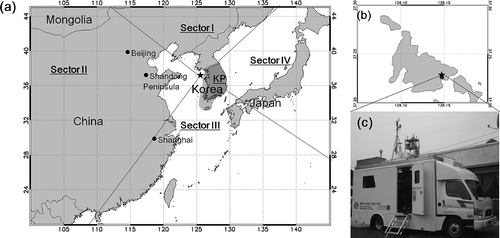
The LTP ground measurement site (Deokjeok Island, 37°13′33″N, 126° 8′51″E, 50 m above sea level [ASL]) is located off the west coast of the Korean peninsula in the effective downwind area of the Asian continent (). Incheon Metropolitan City is located just 40 km east, whereas the capital city, Seoul, is located 75 km east of the measurement site.
Figure 1. Sampling locations (clockwise from left): (a) Northeast Asian region (including the Asian Continent, mainly China and Mongolia, Korea, and Japan), divided by dashed lines into sector sources (Sectors I–IV, KP). (b) Deokjeok Island off the west coast of the Korean peninsula. The island is a background marine site. (c) GIST mobile laboratory, situated on Deokjeok Island, equipped with ambient air quality measurement instruments.
Measurements were taken during the spring (March 31 to April 11) and fall (October 14 to October 26) of 2009. A particle-into-liquid system (PILS; BMI, Hayward, CA, USACitation)19 coupled with an online ion chromatograph (IC) that simultaneously measures cations (Li+, Na+, NH4 +, K+, Ca2+, Mg2+) and anions (F−, Cl−, NO3 −, SO4 2−) was installed inside a mobile laboratory at the Deokjeok Island monitoring site. Ambient aerosols pass through a PM2.5 (particulate matter with an aerodynamic diameter ≤2.5 μm) size cut impactor, and then through an annular and carbon denuder for removal of reactive gaseous components. The overall PILS sampling flow rate was maintained at 12.8 L/min by a critical orifice. The remaining particles were mixed with high-purity steam (18 MΩ,) injected coaxially, and maintained at a flow rate of 1.5 mL/min and a steam temperature of 98 ± 5 °C. The grown particles, now droplets, were collected by an impactor. An internal standard (LiF) of known flow rate and concentration was used to collect and dissolve the droplets. These droplets are then subsequently injected into the IC (Metrohm, model 850, Herisau) for ionic analysis in a 30-min interval. Recovery of LiF was equal to 75% ± 17% for Li+ and 85% ± 10% for F−. Particulate matter (PM2.5) mass concentration was also measured in a 30-min interval using an optical particle counter (OPC; Grimm, model 1.107, Ainring, Germany) 120-Hr back trajectories ending at 200 m ASL at the Deokjeok Island site were analyzed using Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model 4.Citation20 Input data for HYSPLIT utilized the meteorological data from the FNL (Final Run) framework that are archived from the GDAS (Global Data Assimilation System) of NCEP (National Centers for Environmental Protection).Citation20 The computation period from March 31 to April 11, 2009, included all possible in situ monitoring every 6 hrs, ending at 12:00 a.m. Coordinated Universal Time (UTC) (09:00 a.m. Korean Standard Time [KST]), 06:00 a.m. UTC (3:00 p.m. KST), 12:00 p.m. UTC (9:00 p.m. KST), and 6:00 p.m. UTC (03:00 a.m. KST). The aerosol optical thickness (AOT) over the measurement region was retrieved using the MODIS Terra and Aqua hierarchical data format (hdf) satellite data utilizing the modified Bremen Aerosol Retrieval (M-BAER) algorithm.Citation21–23 The study domain covered approximately the Northeast Asian region from 100°E to 145°E and 20°N to 45°N, which includes all of Korea, Japan, most of eastern China, and parts of Mongolia and Russia. Aerosol optical thickness images by M-BAER are integrated over the entire atmospheric column and at a fixed time frame. Meteorological parameters, including wind speed, wind direction, precipitation, temperature, and humidity, were also measured by an automated weather station (AWS). Highly time-resolved ionic chemical composition, PM mass concentration from OPC, and meteorological parameters were averaged to hourly data.
RESULTS AND DISCUSSION
Air Mass History and Temporal Evolution of Secondary Species
The synoptic events and source regions are summarized in . Descriptive statistics and the contribution of each chemical component to the PM2.5 mass are presented in . Aerosol optical thickness (AOT) retrieved from satellite data using the M-BAERCitation19 algorithm combined with HYSPLITCitation18 back trajectory analyses were used to examine how the aerosol was transported spatially. The air mass pathways arriving in Deokjeok Island are categorized into five sectors. Those originating from Siberia, followed by Northeast China and the Yellow Sea, are categorized as Sector I. Those coming from the arid regions of Mongolia and Central China, through the Shandong peninsula, and passing over the Yellow Sea are categorized as Sector II. Those coming from the east coast of China (through the Yellow Sea) in a southern direction are categorized as Sector III. Those originating from Russia, northeastern China, and entering the Korean peninsula through the East Sea towards Deokjeok Island are categorized as Sector IV. Lastly, stagnant air mass pathways circulating over the Korean peninsula are categorized as KP. The sector regions are illustrated together in .
Table 1. Summary of event classifications and source regions
Table 2. Descriptive statistics of PM2.5 and its chemical components.Footnote a
Long-range transport events of secondary aerosol species and mineral dust can thus be verified over the Northeast Asian grid from satellite-based AOT distribution and pathway directions (see Supplementary Materials; available in the publisher's online edition). In addition, the differences in chemical composition can be associated with air mass sources ( and b). Generally, changes in ionic composition observed on the ground are strongly associated with changes in air mass origins. The chemical characteristics of each event are summarized in .
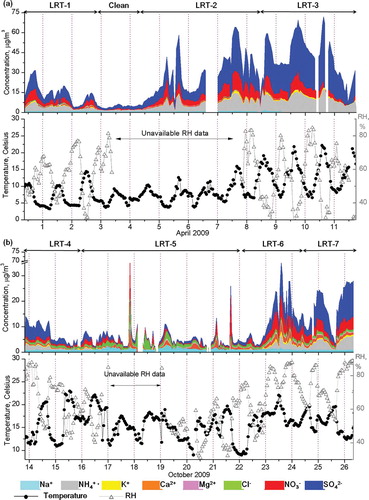
Figure 2. The temporal evolution of ground-based chemical composition during clean and long-range transport events. The top arrow range describes the time periods corresponding to different air mass source regions. The x-axes are scaled to 1 day, with grid lines corresponding to 0000 KST. (a) Spring and (b) fall intensive periods.
On the basis of local wind patterns (), most LTP cases influenced by westerly wind experienced an increase in the distribution of ionic components (, c, e) and dust components (, g). When Kim et al. conducted a 24-hr sampling and measurements of aerosol components in Deokjeok and Gosan from about 2005 to 2007, they observed a 40% increase in secondary aerosols, and most wind cases appeared to be westerly in origin, indicating the influence of long-range-transported (LRT) pollutants from eastern China.Citation6
Figure 3. Wind rose plots of episodic events during spring and fall intensive periods. Top row, from left to right: (a) Clean, northwest wind; (b) LRT-1, west and northwest; (c) LRT-2, west; and (d) LRT-3, southeast. Bottom row, from left to right: (e) LRT-4, northwest; (f) LRT-5, west; (g) LRT-6, north and southwest; and (h) LRT-7, southeast.
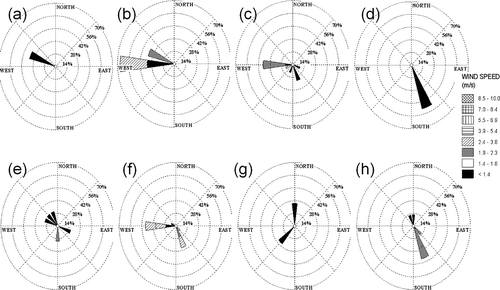
It is apparent from that ground-based concentrations of chemical components of atmospheric aerosols were minimal for the period April 2 11:00 p.m. to April 4 09:00 a.m. KST. Although the local wind direction indicated it was predominantly northwest of the island (), the measurement site was mostly impacted by clean marine air from the Yellow Sea. Moreover, the average PM2.5 mass concentration was also found to be minimal (14.9 ± 2.7 µg/m3). Thus, this period has been classified as “Clean”.
The spring intensive measurement period is characterized by air mass coming from the northeast and eastern regions of China. The fall intensive measurement period is characterized by a 120-hr air mass originating from the arid regions of Mongolia and China, as well as LRT dust transport events. The dust front that passed over the Korean peninsula (KP; ) also brought in elevated levels of secondary aerosol particles. The LRT-3 event during spring and the LRT-7 event during fall resulted in the highest average PM2.5 masses of 74.1 ± 15.1 and 37.1 ± 9.6 µg/m3, respectively. A common factor between the two events is the possible accumulation of aerosol particles while the air mass lingers slowly around the KP ( and h). In addition, the relatively warm (17 to 22 °C) and humid (relative humidity [RH] >80%) air favored the formation of secondary aerosol such as (NH4)2SO4. Both events also accumulated the highest concentration of NH4 + (10.3 ± 3.0 and 2.9 ± 1.6 µg/m3 during LRT-3 and -7, respectively) and SO4 2− (25.4 ± 9.9 and 8.4 ± 4.3 µg/m3 during LRT-3 and -7, respectively).
The air mass originating from Sector I during the LRT-1 event in spring exhibited the lowest concentration of secondary acids: 3.3 ± 2.1 µg/m3 for NO3 − and 5.6 ± 3.4 µg/m3 for SO4 2−. Almost the same sector source during the LRT-4 event in the fall marked the lowest NO3 − concentration of 0.9 ± 0.4 µg/m3, but not of SO4 2−. Sector II air mass can be seen to contribute significantly to the concentration of secondary species in PM2.5, the highest being 22.4% for SO4 2− during the LRT-2 event in spring and 19.8% for SO4 2− during the LRT-7 event in fall. also suggests the arrival of a marine and dust front between the periods LRT-5 and -6. The increase in sea-salt components was first observed in LRT-5, with contributions reaching as high as 7% for Na+ and 8% for Cl−. The event was succeeded by elevated levels of dust components during LRT-6, having a Ca2+ concentration of 1.1 ± 0.8 µg/m3 and Mg2+ concentration of 0.3 ± 0.2 µg/m3. Mineral dust sampled in its preprocessing form was commonly detected during the dust transport event from October 16 12:00 a.m. to October 22 12:00 a.m. Dust in combination with secondary aerosols coming from the KP was also sampled between October 24 09:00 a.m. and October 26 12:00 p.m. In general, spring LRT events contained aerosols that were already processed during transport, and thus represented more aged aerosols. LRT events during fall measurements contain mineral dust and sea-salt particles in their preprocessing form. The chemical composition can be influenced by the enrichment of acidic secondary aerosols on mineral dust particles, and even of sea-salt components. These observations and their processes will be discussed in the succeeding sections.
Chemical Characteristics of Sea-Salt Components in Long-Range-Transported Particles
On a single-particle basis, Sullivan et al. established the least reported occurrence of the presence of chloride on mineral dust.Citation17 This could happen when the NaCl in the marine boundary layer has been converted into HCl(g) via the heterogeneous reaction with SO4 2−, CH3SO3 −, NO3 −, and their precursors,Citation24 prior to the dust front. The released HCl(g) would then enrich the mineral dust by exchanging with more volatile CO3 2− (ibid.). Sea-salt particles are important tracers for understanding the heterogeneous reaction with reactive gases during transport. If the sea-salt particles were freshly injected into the measured aerosols, it would be expected that the measured particles would follow the seawater ratio (SWR) proposed by WilsonCitation25 based on the equivalent concentrations of Na and Cl ([Na+]:[Cl−] = 1:1.18). However, it can be seen from that there were distinct characteristics of transported sea-salt components for each seasonal measurement period, aside from the noticeable deviation from the SWR line. In spring, there is almost no correlation (r 2 = 0.06) between the major sea-salt components [Na+] and [Cl−]. On the other hand, sea-salt aerosol components during the fall intensive period are moderately correlated (r 2 = 0.74). The deviations from the SWR line indicate that the sea-salt aerosols have already been processed and have undergone depletion. Furthermore, the correlations show that the spring sea-salt aerosol components have undergone more extensive processing compared with the fall intensive sea-salt aerosol components. Thus, a formula for the calculation of Cl− depletion (% Cl− depleted) in the marine background site is proposed as follows:
Figure 4. Scatter plot of the major sea-salt components Na+ and Cl−, expressed in µEq/m3. Dotted line corresponds to the sea water ratio. (a) Spring and (b) fall.
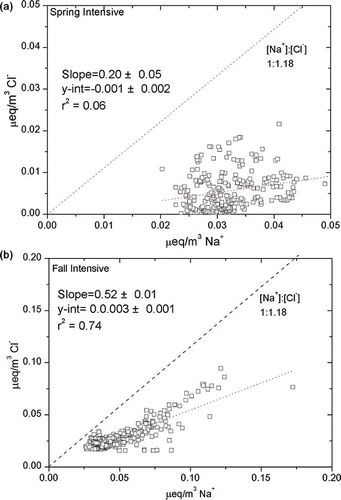
based on the assumption that freshly released sea-salt aerosols have an SWR of 1.18. More processed sea-salt aerosol component in spring was depleted (% Cl depleted = 85% ± 12%) compared to the fall sea-salt aerosol component (% Cl depleted = 50% ± 9%).
Specifically, we observed remarkable concentrations of Na+ (LRT-5: 1.2 ± 0.4 µg/m3, LRT-6: 1.0 ± 0.2 µg/m3) and Cl− (LRT-5: 1.3 ± 1.0 µg/m3, LRT-6: 0.9 ± 0.2 µg/m3) during the arrival of marine and dust fronts in the fall, as seen in . This could have occurred after Cl− liberation24 prior to the transport of mineral dust, followed by Cl− enrichment in the mineral dust. Thus, HCl(g) was most likely elevated in the boundary layer before the arrival of the dust at Deokjeok Island. The HCl(g) produced by an acidic gas displacement reaction with NaCl was then mixed with incoming dusts, and the liberated Cl− could then enrich the mineral dust making the particles susceptible to a CO3 2− and Cl− heterogeneous reaction. On the other hand, prior to reaching the measurement site, the LRT-7 air mass passed over dust-laden Sector II of Northeast Asia, followed by the reactive-species-rich (NO3 − and SO4 2−) Korean peninsula (see Supplementary ; ). Note that this event resulted in NO3 − and SO4 2− enrichment, which could have displaced existing Cl− in the transported mineral dust, thus producing NO3 −- and SO4 2−-enriched aerosols in the LRT-7 case. The sea-salt component has been actively modified in the marine boundary layer, prior to its association with dust components and later associations with secondary acids.
Chemical Characteristics of Long-Range-Transported Mineral Dust and Secondary Aerosols
The atmospheric chemistry, processing, uptake and formation of NO3 − and SO4 2− on mineral dust particles during long-range transport has been studied at field sites in Northeast Asia,Citation2,Citation15,Citation16,Citation26,Citation27 as well as in laboratory studies15 and with aerosol interface modeling.1 Previously, it was shown that the sea-salt particles sampled with the transported mineral dust had already undergone depletion and processing. The mineral dust components associated with these sea salts had undergone aging to a similar extent. It is known that the reactive constituents of dust particles in East Asia are CaCO3 (calcite), MgCO3 (magnetite), and CaMg(CO3)2 (dolomite), among which CaCO3 is dominant and accounts for ∼90% of the total dust component.Citation28 Other major components of mineral dust such as Al2O3, SiO2, and Fe2O3 can be considered unreactive,Citation29 whereas the volatile CO3 2− is expected to be reactive. Our method is unable to measure CO3 2− directly due to its interference in the measured anionic conductivity. Assuming the particle is neutrally charged, it was assumed that CO3 2− is the only anionic species not measured by the PILS-IC instrument, and thus the difference between cations and anions measured is designated as [CO3 2−]estimated 4:
where n and m are the total number of cations and anions measured by the IC, respectively.
Considering the major cationic and anionic species, and the assumption that the only unmeasured anionic species is CO3 2−, we maintain that the association of Ca2+ and Mg2+ with carbonate represents preprocessed dust. The fraction of carbonate replacement (FCR; Equationeq 3) method proposed by Song et al.Citation29 was used to investigate the degree of aging of mineral dust aerosols.
where [CO3 2−]replaced is the amount of CO3 2− that remained in the aerosol after deducting the estimated CO3 2− (in equivalent concentrations), that is, [CO3 2−]original − [CO3 2−]estimated. [CO3 2− original], on the other hand, is estimated from the sum of Ca2+ and Mg2+, assuming that all CO3 2− is the product of the dissociation of CaCO3 (calcite), MgCO3 (magnetite), and CaMg(CO3)2 (dolomite) along its transport into the atmosphere.
Results from FCR estimations are presented in , as well as comparable results from previous studies in the Northeast Asia region. FCR data were collected from aircraft measurements of aerosols in the marine boundary layer, whereas this study dealt with ground-based measurements. As such, the differences in the levels of reactive acidic gases and the interaction time with gas-phase precursors could have caused the differences between the independent FCR measurements.29
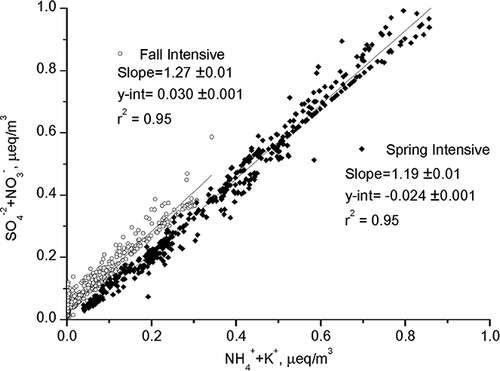
Table 3. Degree of dust processing in Northeast Asia using the FCR method
Despite the direct observation that Ca2+ and Mg2+ do not always show a good correlation with CO3 2−, which can be interpreted as processed or aged mineral dust, correlation analysis () between the same charged ions still suggests that (SO4 2− + NO3 −) and (NH4 + + K+) show a slope, s, of >1 (1.1 < s < 1.3), which means that the acidic components are in excess. Moreover, a strong correlation coefficient of r 2 = 0.95 suggests that these components have been emitted from the same source or have resulted from the same aerosol processes. The range in concentration of SO4 2− (spring: 1.4∼48.6 µg/m3; fall: 0.25∼15.9 µg/m3) and NO3 − (spring: 0.1∼14.3 µg/m3; fall: 0.3∼23.3 µg/m3) during the spring intensive campaign is higher than that of the fall campaign. These major components are indicators of dust aerosol processing because they are secondary in origin.12 Results of atmospheric chemical processing show dust processing on pre-emitted mineral dust transported to Deokjeok Island. Secondary acids that have accumulated in mineral dust can be a sink for NH3, through heterogeneous nucleationCitation30 or direct uptake from gas-phase coagulation with nitrate or sulfate rich ammonium particles.Citation31 Looking at the magnitude of the components, in which the spring transported aerosol event is higher than the fall event, it can be said that the spring event produced more secondary aerosols during the processing. High RH during the spring intensive period may have contributed more to the fine-particle formation induced by a damp atmosphere and abundant acidic sources, as explained previously.
Figure 5. Equivalent concentrations of major anion components (SO4 2− and NO3 −) and major cation components (NH4 + + K+) for the intensive measurements during the spring and fall seasons. The magnitude of the spring intensive measurement data is higher than that of the fall intensive measurement period. Their slopes lie between 1.1 < s < 1.3, and for both periods r 2 = 0.95.
In this study, the presence of Ca2+ and Mg2+ in varying amounts is thought to characterize the long-range-transported mineral dust. When the mineral dust is preprocessed, we would expect it to be in a carbonate form, and with a strong association with depleted sea-salt particles. However, when mineral dusts are chemically modified during transport, the volatile CO3 2− is replaced by less volatile and non-volatile NO3 − and SO4 2−, respectively.29 Meanwhile, after subtracting the contribution of the equivalent [NH4 +] from the combined equivalent [SO4 2−] and [NO3 −], the difference is believed to represent excess acids, which would process the transported dust. Thus, strong associations of Ca2+ and Mg2+ with excess acids are regarded as indicators of processed mineral dust.
To complement the FCR of Song et al.,Citation29 we propose a simple indicator of mineral dust aging and suggest a range for the degree of associations. The triplot in shows the associations in normalized equivalent concentrations of excess acids with mineral dust components and [CO3 2−]estimated. In the triplot, a sample containing primarily Ca2+ and Mg2+ would appear at the top vortex, primarily [CO3 2−]estimated at the right vortex, and primarily excess acids at the left vortex. Using this tool, we can see the extent to which the transported mineral dust has been processed. From both measurements, it can be seen that Ca2+ + Mg2+ are present in a smaller percentage compared with the excess acids. However, the range of the fraction of mineral dust components and excess acids measured during the spring intensive period are less varied, mostly between about 0.25 and 0.4 for Ca2+ + Mg2+, and about 0.6 and 0.75 for the excess acids. In contrast, data for the fall intensive measurement period show that the range of the fraction of mineral dust components and excess acids is wider, between about 0.1 and 0.5 for Ca2+ + Mg2+ and about 0.5 and 0.8 for the excess acids. In addition, there are more fractions of mineral dust components that are associated with excess acids.
Figure 6. Ternary plots of the associated chemical components. Plotted data are normalized such that equivalent concentrations of excess acids, Ca2+ + Mg2+ and CO3 2− are summed to unity. Most of the mineral dust has already undergone complete aging, although not all because levels of [CO3 2−] were estimated in 10% of the samples in spring and in 80% of the aerosol measured in the fall. Symbols correspond to the episodic events described in each intensive measurement period. (a) Spring and (b) fall intensive periods.
![Figure 6. Ternary plots of the associated chemical components. Plotted data are normalized such that equivalent concentrations of excess acids, Ca2+ + Mg2+ and CO3 2− are summed to unity. Most of the mineral dust has already undergone complete aging, although not all because levels of [CO3 2−] were estimated in 10% of the samples in spring and in 80% of the aerosol measured in the fall. Symbols correspond to the episodic events described in each intensive measurement period. (a) Spring and (b) fall intensive periods.](/cms/asset/bbde5f52-c4db-4563-9bff-fee2cc2b2f81/uawm_a_604001_o_f0006g.gif)
The spring intensive data () show the equivalent ratio of Ca2+ + Mg2+ in the range of 25∼50%. When the proportion of CO3 2− is 40∼70%, the mineral dust component is present in the preprocessed form, as in the case of LRT-1. Mineral dust during local events does not show evidence of associations with secondary acids. However, mineral dust components during the LRT-2 period are associated at a constant amount with CO3 2− and excess acids, which are in inverse proportions. This indicates the exchange in the anionic component of the transported mineral dust processed by reactive acids such as NO3 − and SO4 2−. The fall intensive data show unique results. Around 65∼90% of excess acids are in strong association with 10∼25% of mineral dust components in the LRT-4 event. The poor association of mineral dust with the secondary acid component in the case of LRT-7 only confirmed that most of the aerosols sampled are in the processed form, and mostly comprise secondary acids.
One remarkable feature of the fall intensive data is that the degree of acidity of associated secondary acids in mineral dust can be seen by just looking at the percentage of associations with excess acids (). For example, by looking at the left-most panel of (excess acids), the LRT events are shown to be grouped in increasing order of acidity: LRT-4 aerosols have an association of 75∼87%, LRT-6 aerosols possess an association of 62∼75%, and LRT-7 aerosols display an association of 50∼60%. Most of the sampled aerosols during LRT-5 were associated with CO3 2− (ranging from about 15% to 70%), owing to the arrival of a marine and dust front during this period. Thus, the order of acidity of the LRT events is represented by the relationship LRT-4 > LRT-6 > LRT-7.
It is therefore apparent that transported mineral dust particles can process and accumulate reactive acids. A laboratory-scale experiment has shown that nitrate products after calcite mineral processing are still intact even after significant water uptake,Citation32 whereas reaction with SO2 is relatively slow.Citation33 Competitive reactions between SO2 and HNO3 show no apparent effect on the reactivity of both gases (ibid.). On the basis of single-particle measurements, Zhang et al.Citation34 observed that the degree of SO4 2− formation may have been dependent on the oxidation of SO2, but would later be neutralized by surface-adsorbed or gas-phase NH3 present in the troposphere at the time of the measurements. Observations of Fairlie et al.Citation35 have shown that particulate nitrate is primarily associated with dust, sulfate is primarily associated with ammonium, and mineral dust remained alkaline across their observational grid over the Pacific. Their results were even reproduced using models.
There are several studies in the Northeast Asian region that also utilized the ratio method for indicators of dust episodes, some of which are tabulated in . Most of the values were obtained by analyzing filter-based samples for chemical composition, followed by the ratio method of mineral dust components using Ca as the common chemical indicator.Citation36–40 However, most of the studies were conducted at continental sites (Beijing, Mongolia, China), such that the partner species in their ratio method was either Al or Si. Because our study site was conducted at a background marine site, we employed the ratio method using Ca and Mg in partnership with the sea-salt component Na. As shown in , the degree of associations of mineral dust with reactive acids was explored. The extent of processing was based on the magnitude of reactive acids, utilizing the scatter plot of NO3 − and SO4 2− in relation to Ca2+. The sea-salt and dust components were reconciled using the ratio method between mineral dust and sea-salt indicators, namely, [Ca2+ + Mg2+] and [Na+], respectively. To reconcile the associations of reactive acids with transported mineral dust, the ratio of NO3 − and SO4 2− individually to Ca2+ was used. The color bar indicates the degree of association of mineral dust to sea-salt components. The range of the [Ca2+ + Mg2+]/[Na+] ratio itself reflects the extent of the difference in aerosol events. A higher [Ca2+ + Mg2+]/[Na+] (ratio >2) indicates that major dust events dominate during that period, which decreases the possibility of dust processing. A lower [Ca2+ + Mg2+]/[Na+] (ratio <2) ratio suggests that sea-salt aging has already occurred on the dust particles. The equivalent ratio of SO4 2−/Ca2+ and NO3 −/Ca2+ shows the degree of association of the acidic component on dust particles. Observed higher magnitudes for the SO4 2−/Ca2+ or NO3 −/Ca2+ ratio (>10) suggest that the ground aerosols have already been processed by the reactive gaseous acids, whereas a lower ratio (<10) suggests that the dust has been processed to a lesser extent with estimated amounts of CO3 2−.
Table 4. Ratio method for mineral dust and soil indicators at different sites in Northeast Asia
Figure 7. Ratio method of major reactive acids to Ca2+ to describe the extent of LRT aerosol processing. For both intensive periods, aerosol particles with aged sea salt (i.e., [Ca2++Mg2+]/[Na+] ratio <2) are shown to have higher magnitudes of acidic components. (a) The Ca2+-normalized equivalent ratio of NO3 − and SO4 2− during the spring intensive measurement period occurs mostly between the 1:2∼1:6 line, and (b) between the 1:1∼1:6 line for the fall intensive measurement data. (Inset) Larger x-y range to show the acid/[Ca2+] ratio >10.
![Figure 7. Ratio method of major reactive acids to Ca2+ to describe the extent of LRT aerosol processing. For both intensive periods, aerosol particles with aged sea salt (i.e., [Ca2++Mg2+]/[Na+] ratio <2) are shown to have higher magnitudes of acidic components. (a) The Ca2+-normalized equivalent ratio of NO3 − and SO4 2− during the spring intensive measurement period occurs mostly between the 1:2∼1:6 line, and (b) between the 1:1∼1:6 line for the fall intensive measurement data. (Inset) Larger x-y range to show the acid/[Ca2+] ratio >10.](/cms/asset/d0437b57-24a9-4508-b61f-4ef8897354dd/uawm_a_604001_o_f0007g.jpg)
SUMMARY AND CONCLUSION
The chemical characteristics of long-range-transported aerosols downwind of the Asian continent were investigated to identify the levels of mineral dust transport and processing using ground-based measurements. Two mineral dust transport events and details of the degrees of associations of their components have been identified. Evidence was found for the preprocessing of mineral dust by Cl− and reprocessing by reactive acids (NO3 − and SO4 2−). Sea-salt components were aged to the same extent as the mineral dust. The prior reaction of HCl(g) with mineral dust caused the displacements of various amounts of CO3 2−, and activated the mineral dust for further reaction with nitrates and sulfates. The spring observations indicate that CO3 2− was almost fully depleted by excess NO3 − and SO4 2−, whereas the fall observations show that fine-particle mode dust particles were not completely depleted by these excess acids. Additionally, the estimated [CO3 2−] was strongly associated with [Ca2+] and [Mg2+] in the fall. The analysis of measurement data suggests that the fraction of carbonate replacement (FCR), which is a measure of the degree of chemical interaction between mineral dust and reactive gases, was comparable with that obtained from independent ground-based measurements. In addition, our triplot analysis and ratio approach supported the degree of association between CO3 2−, excess acids, and mineral dust components. This study is important for an understanding of the chemical evolution of dust particles in Northeast Asia, and clarifies the chemical processes of aerosols from anthropogenic and natural sources. It was possible to estimate CO3 2− from the difference of the summed anions and excess cations, and the degree of replacement as shown by the FCR method, utilizing time-resolved ion data. Our observations on the processing of long-range-transported mineral dust and associated sea-salt components suggest important evidence on the temporal evolution of naturally occurring atmospheric particles and the subsequent anthropogenic reactive components. Generally, source regions influence the loading and chemical characteristics of LRT aerosols.
uawm_a_604001_sup_22237539.pdf
Download PDF (4.7 MB)ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (Project No. 2008-0060618) and by the 2009 LTP Project supported by the National Institute of Environmental Research (NIER). It was also funded by the Korea Meteorological Administration Research and Development Program under grant RACS_2010-1002 through the Advanced Environmental Monitoring Research Center at Gwangju Institute of Science and Technology. The authors also gratefully acknowledge the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY Web site (http://www.arl.noaa.gov/ready.php) used in this publication. Lastly, the authors would like to acknowledge the assistance of local residents from Deokjeok Island.
REFERENCES
- Tang , Y. , Carmichael , G.R. , Seinfeld , J.H. , Dabdub , D. , Weber , R.J. , Huebert , B. , Clarke , A.D. , Guazzotti , S.A. , Sodeman , D.A. , Prather , K.A. , Uno , I. , Woo , J.-H. , Yienger , J.J. , Streets , D.G. , Quinn , P.K. , Johnson , J.E. , Song , C.-H. , Grassian , V.H. , Sandu , A. , Talbot , R.W. and Dibb , J.E. 2004 . Three-dimensional Simulations of Inorganic Aerosol Distributions in East Asia During Spring 2001 . J. Geophys. Res. , 109 : 1 – 32 .
- Bates , T.S. , Quinn , P.K. , Coffman , D.J. , Covert , D.S. , Miller , T.L. , Johnson , J.E. , Carmichael , G.R. , Uno , I. , Guazzotti , S.A. , Sodeman , D.A. , Prather , K.A. , Rivera , M. , Russell , L.M. and Merrill , J.T. 2004 . Marine Boundary Layer Dust and Pollutant Transport Associated with the Passage of a Frontal System Over Eastern Asia . J. Geophys. Res. , 109 : D19S19
- Park , S.S. , Kim , Y.J. , Cho , S.Y. and Kim , S.J. 2007 . Characterization of PM2.5 Aerosols Dominated by Local Pollution and Asian Dust Observed at an Urban Site in Korea during Aerosol Characterization Experiments (ACE) Asia Project . J. Air Waste Manage. Assoc. , 57 : 434 – 443 . doi: 10.3155/1047-3289.57.4.434.
- Song , C.H. , Maxwell-Meier , K. , Weber , R.J. , Kapustin , V. and Clarke , A. 2005 . Dust Composition and Mixing State Inferred from Airborne Composition Measurements During ACE-Asia C130 Flight #6 . Atmos. Environ. , 39 : 359 – 369 .
- Arimoto , R. , Kim , Y.J. , Kim , Y.P. , Quinn , P.K. , Bates , T.S. , Anderson , T.L. , Gong , S. , Uno , I. , Chin , M. , Huebert , B.J. , Clarke , A.D. , Shinozuka , Y. , Weber , R.J. , Anderson , J.R. , Guazzotti , S.A. , Sullivan , R.C. , Sodeman , D.A. , Prather , K.A. and Sokolik , I.N. 2006 . Characterization of Asian Dust during ACE-Asia . Global Planet. Change , 52 : 23 – 56 .
- Kim , Y.J. , Woo , J.-H. , Ma , Y.-I. , Kim , S. , Nam , J.S. , Sung , H. , Choi , K.-C. , Seo , J. , Kim , J.S. , Kang , C.-H. , Lee , G. , Ro , C.-U. , Chang , D. and Sunwoo , Y. 2009 . Chemical Characteristics of Long-Range Transport Aerosol at Background Sites in Korea . Atmos. Environ. , 43 : 5556 – 5566 .
- Jordan , C.E. , Anderson , B.E. , Talbot , R.W. , Dibb , J.E. , Fuelberg , H.E. , Hudgins , C.H. , Kiley , C.M. , Russo , R. , Scheuer , E. , Seid , G. , Thornhill , K.L. and Winstead , E. 2003 . Chemical and Physical Properties of Bulk Aerosols within Four Sectors Observed During TRACE-P . J. Geophys. Res. , 108 : 8813 – 8832 .
- Weber , R.J. , Lee , S. , Chen , G. , Wang , B. , Kapustin , V. , Moore , K. , Clarke , A.D. , Mauldin , L. , Kosciuch , E. , Cantrell , C. , Eisele , F. , Thornton , D.C. , Bandy , A.R. , Sachse , G.W. and Fuelberg , H.E. 2003 . New Particle Formation in Anthropogenic Plumes Advecting from Asia Observed During TRACE-P . J. Geophys. Res. , 108 : 8814 – 8827 .
- Song , C.H. , Park , M.E. , Lee , E.J. , Lee , J.H. , Lee , B.K. , Lee , D.S. , Kim , J. , Han , J.S. , Moon , K.J. and Kondo , Y. 2009 . Possible Particulate Nitrite Formation and its Atmospheric Implications Inferred from the Observations in Seoul, Korea . Atmos. Environ. , 43 : 2168 – 2173 .
- Sullivan , R.C. and Prather , K.A. 2007 . Investigations of the Diurnal Cycle and Mixing State of Oxalic Acid in Individual Particles in Asian Aerosol Outflow . Environ. Sci. Technol. , 41 : 8062 – 8069 .
- Trochkine , D. , Iwasaka , Y. , Matsuki , A. , Yamada , M. , Kim , Y.S. , Nagatani , T. , Zhang , D. , Shi , G.Y. and Shen , Z. 2003 . Mineral Aerosol Particles Collected in Dunhuang, China, and Their Comparison with Chemically Modified Particles Collected Over Japan . J. Geophys. Res. , 108 : 8642 – 8653 .
- Sullivan , R.C. , Guazzotti , S.A. , Sodeman , D.A. and Prather , K.A. 2007 . Direct Observations of the Atmospheric Processing of Asian Mineral Dust . Atmos. Chem. Phys. , 7 : 1213 – 1236 .
- Prospero , J.M. , Ginoux , P. , Torres , O. , Nicholson , S.E. and Gill , T.E. 2002 . Environmental Characterization of Global Sources of Atmospheric Soil Dust Identified with the NIMBUS 7 Total Ozone Mapping Spectrometer (TOMS) Absorbing Aerosol Product . Rev. Geophys. , 40 : 1002 – 1033 .
- Solomon , S. , Qin , D. , Manning , M. , Alley , R.B. , Berntsen , T. , Bindoff , N.L. , Chen , Z. , Chidthaisong , A. , Gregory , J.M. , Hegerl , G.C. and Heimann , M. 2007 . Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change , Edited by: Solomon , S. , Qin , D. , Manning , M. , Chen , Z. , Marquis , M. , Averyt , K.B. , Tignor , M. and Miller , H.L. Cambridge, UK and New York, NY , , USA : Cambridge University Press .
- Usher , C.R. , Michel , A.E. and Grassian , V.H. 2003 . Reactions on Mineral Dust . Chem. Rev. , 103 : 4883 – 4940 .
- Jordan , C.E. , Dibb , J.E. , Anderson , B.E. and Fuelberg , H.E. 2003 . Uptake of Nitrate and Sulfate on Dust Aerosols During TRACE-P . J. Geophys. Res. , 108 : 8817 – 8827 .
- Sullivan , R.C. , Guazzotti , S.A. , Sodeman , D.A. , Tang , Y. , Carmichael , G.R. and Prather , K.A. 2007 . Mineral Dust is a Sink for Chlorine in the Marine Boundary Layer . Atmos. Environ. , 41 : 7166 – 7179 .
- The Secretariat of LTP Project . 2009 . The 12th Expert Meeting for Long-Range Transboundary Air Pollutants in Northeast Asia , Edited by: Kim , J.-S. Incheon , , Korea : National Institute of Environmental Research . Number 11-1480523-000551-10, Korea Government Publications Registration
- Orsini , D.A. , Ma , Y. , Sullivan , A. , Sierau , B. , Baumann , K. and Weber , R.J. 2003 . Refinements to the Particle-Into-Liquid Sampler (PILS) for Ground and Airborne Measurements of Water Soluble Aerosol Composition . Atmos. Environ. , 37 : 1243 – 1259 .
- Draxler, R.R.; Rolph, G.D. NOAA ARL READY. 2011. HYSPLIT (Hybrid Single-Particle Lagrangian Integrated Trajectory) http://ready.arl.noaa.gov/HYSPLIT.php (http://ready.arl.noaa.gov/HYSPLIT.php)
- Lee , K.H. , Kim , Y.J. and Kim , M.J. 2006 . Characteristics of Aerosol Observed During Two Severe Haze Events Over Korea in June and October 2004 . Atmos. Environ. , 40 : 5146 – 5155 .
- Lee , K.H. , Kim , J.E. , Kim , Y.J. , Kim , J. and von Hoyningen-Huene , W. 2005 . Impact of the Smoke Aerosol from Russian Forest Fires on the Atmospheric Environment Over Korea during May 2003 . Atmos. Environ. , 39 : 85 – 99 .
- Lee , K.H. , Kim , Y.J. , von Hoyningen-Huene , W. and Burrow , J.P. 2006 . Influence of Land Surface Effects on MODIS Aerosol Retrieval Using the BAER Method Over Korea . Int. J. Remote Sensing , 27 : 2813 – 2830 .
- Hara , K. , Osada , K. , Kido , M. , Hayashi , M. , Matsunaga , K. , Iwasaka , Y. , Yamanouchi , T. , Hashida , G. and Fukatsu , T. 2004 . Chemistry of Sea-Salt Particles and Inorganic Halogen Species in Antarctic Regions: Compositional Differences Between Coastal and Inland Stations . J. Geophys. Res. , 109 : D20208
- Wilson , T.R. 1975 . “ Salinity and the major elements of sea-water ” . In Chemical Oceanography , Edited by: Riley , J. P. and Skirrow , G. 365 – 413 . London , , UK : Acadamic Press .
- Fan , X.-B. , Okada , K. , Niimura , N. , Kai , K. , Arao , K. , Shi , G.-Y. , Qin , Y. and Mitsuta , Y. 1996 . Mineral Particles Collected in China and Japan During the Same Asian Dust-Storm Event . Atmos. Environ. , 30 : 347 – 351 .
- Laskin , A. , Iedema , M.J. and Cowin , J.P. 2002 . Quantitative Time-Resolved Monitoring of Nitrate Formation in Sea Salt Particles Using a CCSEM/EDX Single Particle Analysis . Environ. Sci. Technol. , 36 : 4948 – 4955 .
- Song , C.H. and Carmichael , G.R. 2001 . Gas-Particle Partitioning of Nitric Acid Modulated by Alkaline Aerosol . J. Atmos. Chem. , 40 : 1 – 22 .
- Song , C.H. , Kim , C.M. , Lee , Y.J. , Carmichael , G.R. , Lee , B.K. and Lee , D.S. 2007 . An Evaluation of Reaction Probabilities of Sulfate and Nitrate Precursors Onto East AsianDust Particles . J. Geophys. Res. , 112 : D18206
- Korhonen , H. , Napari , I. , Timmreck , C. , Vehkamäki , H. , Pirjola , L. , Lehtinen , K.E.J. , Lauri , A. and Kulmala , M. 2003 . Heterogeneous Nucleation As a Potential Sulphate-Coating Mechanism of Atmospheric Mineral Dust Particles and Implications of Coated Dust on New Particle Formation . J. Geophys. Res. , 108 : 4546 – 4555 .
- Mori , I. , Nishikawa , M. and Iwasaka , Y. 1998 . Chemical Reaction During the Aoagulation of Ammonium Sulphate and Mineral Particles in the Atmosphere . Sci. Total Environ. , 224 : 87 – 91 .
- Preszler Prince , A. , Grassian , V.H. , Kleiber , P. and Young , M.A. 2007 . Heterogeneous Conversion of Calcite Aerosol by Nitric Acid . J. Phys. Chem. Chem. Phys. , 9 : 622 – 634 .
- Preszler Prince , A. , Kleiber , P. , Grassian , V.H. and Young , M.A. 2007 . Heterogeneous Interactions of Calcite Aerosols with Sulfur Dioxide and Sulfur Dioxide-Nitric Acid Mixtures . J. Phys. Chem. Chem. Phys. , 9 : 3432 – 3439 .
- Zhang , D. , Shi , G.-Y. , Iwasaka , Y. and Hu , M. 2000 . Mixture of Sulfate and Nitrate in Coastal Atmospheric Aerosols: Individual Particle Studies in Qingdao (36°4′N, 120°1′E), China . Atmos. Environ. , 34 : 2669 – 2679 .
- Fairlie , T.D. , Jacob , D.J. , Dibb , J.E. , Alexander , B. , Avery , M.A. , van Donkelaar , A. and Zhang , L. Impact of Mineral Dust on Nitrate, Sulfate, and Ozone in Transpacific Asian Pollution Plumes . Atmos. Chem. Phys. , 10 3999 – 4012 .
- Wang , Y. , Zhuang , G. , Tang , A. , Yuan , H. , Sun , Y. , Chen , S. and Zheng , A. 2005 . The ion Chemistry and the Source of PM2.5 Aerosol in Beijing . Atmos. Environ. , 39 : 3771 – 3784 .
- Xuan , J. 2005 . Emission Inventory of Eight Elements, Fe, Al, K, Mg, Mn, Na, Ca and Ti, in Dust Source Region of East Asia . Atmos. Environ. , 39 : 813 – 821 .
- Song , Y. , Tang , X. , Xie , S. , Zhang , Y. , Wei , Y. , Zhang , M. , Zeng , L. and Lu , S. 2007 . Source Apportionment of PM2.5 in Beijing in 2004 . J. Hazard. Mater. , 146 : 124 – 130 .
- Krueger , B.J. , Grassian , V.H. , Cowin , J.P. and Laskin , A. 2004 . Heterogeneous Chemistry of Individual Mineral Dust Particles from Different Dust Source Regions: The Importance of Particle Mineralogy . Atmos. Enviro. , 38 : 6253 – 6261 .
- Cheng , T. , Lu , D. , Wang , G. and Xu , Y. 2005 . Chemical characteristics of Asian dust aerosol from Hunshan Dake Sandland in Northern China . Atmos. Environ. , 39 : 2903 – 2911 .