ABSTRACT
A quantitative energy-dispersive electron probe X-ray microanalysis (ED-EPMA), namely low-Z (atomic number) particle EPMA, was used to characterize the chemical compositions of the individual aerosol particles collected at the Gosan supersite, Jeju Island, Korea, as a part of the Asian Pacific Regional Aerosol Characterization Experiment (ACE-Asia). On 4–10 April 2001 just before a severe dust storm arrived, seven sets of aerosol samples were obtained by a seven-stage May cascade impactor with a flow rate of 20 L/min. Overall 11,200 particles on stages 1–6 with cutoff diameters of 16, 8, 4, 2, 1, and 0.5 μm, respectively, were examined and classified based on their secondary electron images and X-ray spectra. In general, sea salt particles were the most frequently encountered, followed by mineral dust, organic carbon (OC)-like, (NH4)2SO4/NH4HSO4-containing, elemental carbon (EC)-like, Fe-rich, and K-rich particles. Sea salt and mineral dust particles had a higher relative abundance on stages 1–5, whereas OC-like, (NH4)2SO4/NH4HSO4-containing, Fe-rich, and K-rich particles were relatively abundant on stage 6. The analysis on relative number abundances of various particle types combined with 72-hr backward air mass trajectories indicated that a lot of reacted sea salt and reacted mineral dust (with airborne NOx and SO2 or their acidic products) and OC-like particles were carried by the air masses passing over the Yellow Sea (for sample “10 April”) and many NH4HSO4/(NH4)2SO4-containing particles were carried by the air masses passing over the Sea of Japan and Korea Strait (for samples “4–9 April”). It was concluded that the atmosphere over Jeju Island was influenced by anthropogenic SO2 and NOx, organic compounds, and secondary aerosols when Asian dust was absent.
A quantitative energy-dispersive electron probe X-ray microanalysis, called low-Z particle EPMA, was used to characterize aerosol particles collected at Jeju Island, Korea, on 4–10 April of 2001 during the intensive observation period of the Asian Pacific Regional Aerosol Characterization Experiment (ACE-Asia). The particle size distribution and chemical compositions of individual atmospheric particles over Jeju Island were obtained, and many environmentally important atmospheric particles containing sulfate, nitrate, sea salt, alminosilicate, and carbonaceous species in the size range of 0.5–16 μm were characterized. It was found that anthropogenic SO2 and NOx made great impacts on compositions of aerosol particles. The results helped better understand how air pollutants interacted with aerosol particles in the Asia-Pacific region.
INTRODUCTION
Because of its unique location with no nearby and local industrial sources, the Gosan site on Jeju Island of Korea is considered as an ideal place to monitor the regional background atmosphere in the East Asian region and to study the impact of continental outflow events on aerosol composition.Citation1 Aerosol characteristics observed at Gosan have shown that Jeju Island is frequently affected by Asian dust storms originated from Mongolia and northwestern China. For instance, severe dust storms that blanketed much of eastern Asia occurring in April 2001 were observed at the Gosan supersite on 11–13 April and 25–26 April during the intensive observation period (IOP) of the Asian Pacific Regional Aerosol Characterization Experiment (ACE-Asia) field campaign.Citation2
Up to now, many studies on the chemical and physical properties and climatic effects of mineral dust and other types of aerosol particles (of both anthropogenic and natural origins) during Asian dust episodes at Gosan were conducted.Citation3 However, few papers have been published on single-particle characterization of the springtime aerosols in the eve of severe dust storms. In the present study, a quantitative energy-dispersive electron probe X-ray microanalysis (ED-EPMA) method, namely low-Z (atomic number) particle EPMA,Citation4 was used to determine individual aerosol particles collected at Gosan on 4–10 April of 2001 just before an Asian dust storm came. Low-Z particle EPMA has shown powerful advantages in characterization of environmental and geological single particles because it can give the size, morphology, and quantitative chemical compositions of individual particles without a complicated sample pretreatment process, and many environmentally important atmospheric particles, for example, sulfates, nitrates, ammonium, and carbonaceous particles, can be elucidated based on its measurement result.Citation5–7
The objective of the present study is to characterize size-segregated springtime aerosol particles collected at Gosan using the low-Z particle EPMA technique, and to investigate the effects of air pollutants (such as NOx and SO2) on the components of the atmospheric particles over Jeju Island, a relatively clean site in Korea.
MATERIALS AND METHODS
Sampling
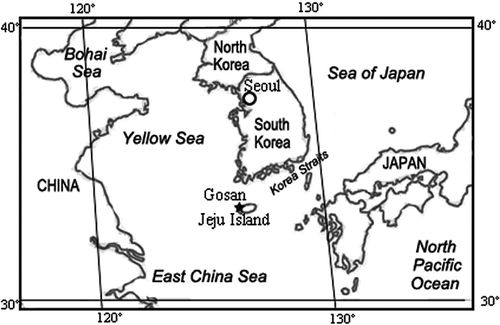
A seven-stage May cascade impactor at a flow rate of 20 L/min was used to collect seven sets of aerosol samples in the period from 4 to 10 April of 2001 at Gosan (126.17˚E, 33.28˚N), which is located on the western edge of Jeju Island, Korea, and has an altitude of ∼60 m above sea level (). The sampling duration varied from several minutes to hours for different stages (stages 1–6) to obtain a good loading of particles without agglomeration. The collected samples were put in plastic carriers, sealed, and stored in desiccators before EPMA measurement.
Measurement and Analysis
Overall 11,200 particles on stages 1–6 with nominal cutoff diameters of 16, 8, 4, 2, 1, and 0.5 μm, respectively, were investigated by using a JEOL JSM-6390 scanning electron microscope (SEM) equipped with an Oxford Link SATW ultrathin window energy-dispersive X-ray (EDX) detector (Japan Electron Optics Laboratory, Co., Ltd.) (100 particles per stage for the samples of stage 1 and 300 particles per stage for the samples of stages 2–6). The resolution of the detector was 133 eV for Mn Kα X-ray. The net X-ray intensities of the chemical elements were fitted by the nonlinear, least squares method using the Analysis of X-ray speetra by iterative least-squares fitting (AXIL) program.Citation8 A Monte Carlo simulation with successive approximation was employed for quantification.Citation9 The “expert system” program that can rapidly and reliably perform chemical speciation was used to determine the formula concentrations and group distributions.Citation10 The basic classification rule for the measured particles was given elsewhere.5,6 In general, particle samples collected on substrates may suffer from some artifacts, which included evaporation of volatile and semivolatile species during the sampling, storage, and measurement. In the vacuum of electron microscope and under the electron beam of EPMA, water, NH4NO3, NH4Cl, and certain volatile and semivolatile organic materials, if present, might be lost. Despite this, the compositions of the particles we measured should reflect the majority of their original compositions before collection. And the lost compositions had no influence on accuracy of the analytical approach of low-Z EPMA. The quantification procedure provided results accurate within 12% relative deviations between the calculated and nominal elemental concentrations when the method was applied to various types of standard particles such as NaCl, Al2O3, CaSO4·2H2O, Fe2O3, CaCO3, and KNO3.Citation11
The speciation of carbonaceous particles was carried out based on the morphologies and the C and O content of particles (in general a particle containing more than 90% of C and O in atomic concentration was regarded as an organic or carbon-rich particle).11 Since the presence of hydrogen failed to be detected in EPMA, elemental carbon (EC) and organic carbon (OC) were defined in a somewhat arbitrary way. To avoid confusion, we used OC-like and EC-like rather than OC and EC in the following description. The EC-like particle, for example, soot aggregate, tar ball, and char or coal dust, was easily identified by its shape and by the ratio of carbon to oxygen content (the atomic concentration of C was usually 3 times larger than that of O in its X-ray spectra), whereas the OC-like particle had an irregular shape and the atomic concentration of C was not so much larger than that of O (often, N signal could be detected in it).
Air Mass Transport History
To investigate the air mass transport pathways and the potential aerosol source regions, 3-day (72-hr) backward air mass trajectories were obtained using the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model from the National Oceanic and Atmospheric Administration (NOAA) Air Resources Laboratory's Web server (http://www.arl.noaa.gov/ready/hysplit4.html). Backward trajectories at the altitude of 60 m were calculated at the end of sampling.
RESULTS AND DISCUSSION
Particle Types
Based on the X-ray spectral data and “expert system” classification, the particle types were identified. They were (1) sea salt group, including (a) genuine sea salt, (b) reacted (or aged) sea salt with sulfur and nitrogen oxides or their acidic products in the air, and (c) the mixture of genuine or reacted sea salt with mineral dust particles; (2) mineral dust group, including (a) genuine mineral dust such as aluminosilicate, quartz (SiO2), and calcite (CaCO3)/dolomite (CaMg(CO3)2), and (b) reacted (or aged) mineral dust particles such as sulfate- and/or nitrate-containing aluminosilicate and CaCO3/CaMg(CO3)2; (3) (NH4)2SO4/NH4HSO4-containing particles; (4) carbonaceous particles, including EC-like and OC-like particles; (5) Fe-rich particles; and (6) K-rich particles. The characteristics of their morphologies and chemical compositions have been described in detail elsewhere.Citation5–7
Particle Size Distribution
Particle size distribution plays an important in our understanding the physical and chemical properties of particles and their reactivity with other matters. Herein, the size distribution of particles at different stages of the May cascade impactor was investigated on the basis of the information provided by EPMA. Our study focused on the particulate matter with aerodynamic diameters (ADs) of 0.5–16 μm on stages 1–6, since smaller particles (on stage 7, AD <0.5 μm) were not fit to be detected by EPMA. In the measured particles, 96.9% of them were in the size range of 0.5–16 μm (). Probably, some size misclassification might have occurred owing to particle bounce-off during sampling.Citation12 For stages 1 and 2, most of the measured particles had the equivalent diameter of 4–16 μm (87.6% for stage 1 and 92.2% for stage 2, respectively); for stage 3, 83.8% of the measured particles were in the size range of 2–8 μm; for stage 4, 84.6% of the measured particles were in the range of 1–2 μm; for stage 5, 83.4% of the measured particles were in the size range of 0.5–2 μm; and for stage 6, 72.2% of the measured particles were in the size range of 0.25–1.0 μm.
Table 1. Size distribution of the analyzed particles collected at Gosan on 4–10 April 2001
Three-Day (72-Hr) Backward Air Mass Trajectories
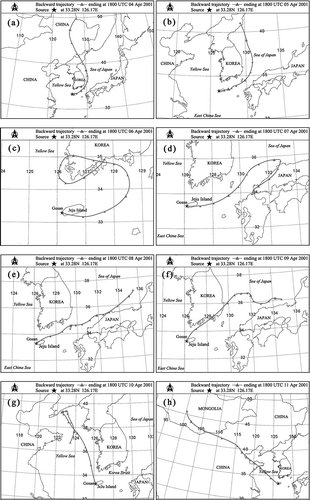
Based on the 72-hr backward air mass trajectories at receptor height of 60 m above the sea level at the sampling site, three categories of air mass transport routes were classified (–g). The first category is for samples collected in the afternoon on 4, 5, and 6 April of 2001 (Coordinated Universal Time [UTC]), in which the air masses originated from northeastern China or Korea, traveled over the Sea of Japan and Korea Straits, and then arrived at the sampling site from the east direction (–c). The second category is for samples collected in the afternoon on 7, 8, and 9 April of 2001 (UTC), in which the air masses originating from near the coast of Japan passed over the Sea of Japan and Korea Strait, and arrived at the sampling site from the east or northeast direction (–f). The third category is for the sample collected in the afternoon on 10 April of 2001 (UTC), in which the air masses originated from and traveled over the Yellow Sea, and arrived at the sampling site from the northwest direction (). In early April of 2001, a severe dust storm occurred.Citation13 The dust clouds originated from the arid deserts of Mongolia (the Gobi Desert) and passed over China and the Yellow Sea. On 11 April, the dust storm front reached Gosan with high wind speed ().
Figure 2. Three-day (72-hr) backward air mass trajectories at sampling height (60 m above the sea level) from 4 to 10 April 2001. The backward air mass trajectories at the sampling site were obtained using the Hybrid Lagrangian Integrated Trajectory (HYSPLIT) model from the NOAA Air Resources Laboratory's web server (http://www.arl.noaa.gov/ready/hysplit4.html). On 11 April 2001, a severe dust storm front arrived at Gosan, so the air mass back trajectory was different from those on 4–10 April. (a) 04 April 2001; (b) 05 April 2001; (c) 06 April 2001; (d) 07 April 2001; (e) 08 April 2001; (f) 09 April 2001; (g) 10 April 2001; (h) 11 April 2001.
Relative Abundances of Various Particle Types
Jeju Island is regarded as an ideal location to monitor the atmospheric chemistry of East Asia, since it is surrounded by China, the Korean peninsula, and the Japanese islands to the west, north, and east, respectively (about 100 km south of the Korea mainland, 250 km west of Kyushu, Japan, 500 km east-northeast of Shanghai, China, and 1000 km north-northeast of Taipei), and is a famous resort with no large industrial sources.Citation14 It is one of the cleanest areas in Korea with very low emissions of primary air pollutants and the atmosphere over it may be affected by long-range transport of air pollutants from outside of the island.14 In the days before air parcels carrying Asian dust arrived at Gosan site (1–10 April 2001), the daily mass concentrations of particulate matters of aerodynamic diameters ≤10 (PM10) and ≤2.5 (PM2.5) μm were approximately 55 and 25 μg/m3, respectively.Citation15 When the dust storm advected off the Chinese mainland and arrived at the Jeju Island, the daily mass concentrations of PM10 and PM2.5 over Gosan were sharply increased to around 140 and 71 μg/m3, respectively (on 13 April 2001).Citation2,Citation15
In the single-particle analysis, the characteristics of aerosol components were reflected by relative abundances of particle types. They were obtained by dividing the number of a specific type of particles by the total number of particles analyzed for a sample ( and ). By comparisons of relative abundances of major particle types at different size levels on different sampling days, we expected to find the major components of aerosols in the atmosphere of Jeju Island and to understand how the air mass histories affected them.
Figure 3. Relative abundances of various particle types in different size ranges for the aerosol samples collected at Gosan, Korea, on 4–10 April 2001.
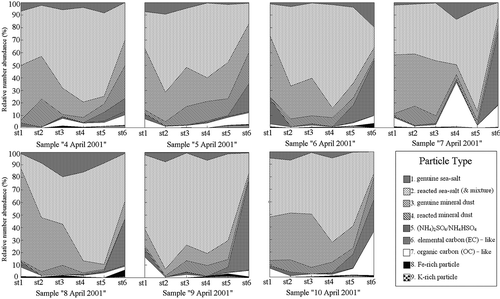
Table 2. Relative abundances of different types of particles in different size ranges (on stages 1–6) for the samples collected at Gosan on 4–10 April 2001
Sea Salt Particles
The genuine sea salt particles, which did not experience chemical reactions after being freshly emitted into the air by the so-called bubble bursting or sea spray process, were identified by the presence of Na and Cl peaks in their X-ray spectra, often with minor C, O, and Mg signals.5–7 The C and O signals might result from organic matter that adhered to sea salt as coatings. Also, the O signal probably came from NaOH shell (an alkaline hygroscopic coating around the NaCl), which was generated at the air-solution interface by the reaction of NaCl with OH– (OH– was produced from photolysis of O3 on water vapor).Citation16 When the genuine sea salt particles reacted with nitrogen and sulfur oxides species in the atmosphere, the reacted (or aged) particles were formed, usually resulting in chlorine loss. Based on their secondary electron image (SEI) and X-ray spectral data, three types of reacted sea salt particles were classified. The first type was for those containing nitrates such as Na(Cl, NO3) and (Na, Mg)(Cl, NO3), which were generated when sea salt reacted with NOx or HNO3, mostly from anthropogenic emission. The second type was for those containing sulfates (SO4 2–) or methanesulfonate (CH3SO3 –) such as (Na, Mg)SO4 and (Na, Mg)CH3SO3, which were generated from the reactions of sea salt with anthropogenic SO2/H2SO4 and/or methylsulfonic acid (MSA) from oxidized dimethylsulfide (DMS).Citation17,Citation18 DMS, the most abundant volatile sulfur compound emitted into the atmosphere from the ocean (usually produced by oceanic phytoplanktons), can be oxidized in the atmosphere to form SO2, MSA, non-sea-salt sulfate (nss-SO4 2–), etc. Hence, SO4 2– and CH3SO3 – were likely to be present simultaneously in the sulfur-containing reacted sea salt particles. It was regarded that nss-SO4 2– were generated when the atomic concentration ratio of [S] to [Na] was >0.06 because the concentration ratio of sea salt sulfate (ss-SO4 2–) to sodium in bulk seawater was 0.06.Citation17 However, since the contribution of biologically produced DMS was not significant for nss-SO4 2– over the North Yellow Sea and the East China Sea (the ratio of the calculated biogenic nss-SO4 2– to the measured total nss-SO4 2– ranged from 1.2% to 11.5% [average 5.8%]),Citation18 the dominant component for atmospheric SO4 2– budget in this region was attributed to anthropogenic sources. So, herein, sulfate aerosols generated from oxidized DMS were neglected. The third type was for those containing both NO3 – and SO4 2–/CH3SO3 –. In addition, some reacted sea salt particles internally mixed with mineral dust species (i.e., CaCO3, Ca(NO3)2, and CaSO4) were classified into the group of “reacted sea salt + mixture”.
The relative abundances of genuine and reacted (aged) sea salt particles were calculated and illustrated in and . They could provide information on whether and how the aerosols at Gosan were influenced by NOx and/or SO2. The fact that the reacted sea salt (+ mixture) particles were abundant on every stage and greatly outnumbered the genuine ones (20.9–66.6% of relative abundance on average vs. 3.1–6.7%) revealed that the sea salt aerosol particles at Gosan were easily affected by anthropogenic NOx and SO2 produced in China and/or Korea. It was consistent with the reports that marine-originated particles such as NaNO3 –- and Na2SO4 –-containing particles were popular over Jeju IslandCitation19 and the aerosol at Jeju Island was enriched in sulfate, nitrate, and ammonium, but deficient in chlorine (relative to seawater).Citation20 The reacted sea salt particles had nearly 2-fold higher abundances on stages 2–5 than on stages 1 and 6 (), indicating that they were neither too large nor too small in size, being frequently encountered in the size range of 1–16 μm. In all the reacted sea salt particles, those containing NO3 – significantly outnumbered the SO4 2–/CH3SO3 – and both-containing ones on stages 1–5, whereas those containing SO4 2–/CH3SO3 – peaked on stage 6 ( and ). It had a strong implication that nitrates tended to form in supermicrometer particles and sulfates tended to be produced in submicrometer ones, which agreed with the observations made by Sullivan et al.Citation21 We also observed that many reacted sea salt particles (with more than 1.0 µm in equivalent diameter) contained nitrates in the samples collected at Tokchok Island (one of the cleanest areas in Korea with few local emissions of air pollutants)5 and on the marine boundary layer of the Yellow Sea,6 indicative of great influences made by NOx/HNO3 on aerosol components over the Yellow Sea.
Figure 4. Relative abundances of reacted (aged) sea salt and mineral dust particles containing nitrates, sulfates, and both. (a) Reacted sea salt particles; (b) reacted mineral dust particles.
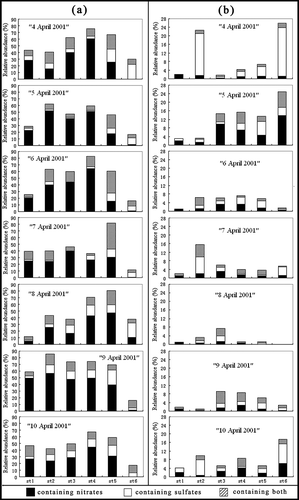
After comparing the average ratios of the relative abundance of the genuine to the reacted sea salt particles among the samples from three categories of air mass sources (13.7%, 10.8%, 6.1% on average for the first, second, and third categories, respectively) based on the 72-hr back trajectories (see ), we speculated that there were more air pollutants for reaction with sea salt particles over the Yellow Sea than over the Sea of Japan and Korea Strait.
Mineral Dust Particles
Mineral dust particles appear irregular and bright on their SEIs.5,6 The typical genuine mineral dust particles include aluminosilicate (with strong X-ray peaks of Al, Si, and O), SiO2, and CaCO3/CaMg(CO3)2. The reacted (or aged) ones mainly include “reacted CaCO3/CaMg(CO3)2” and “aluminosilicate + (N, S)”, where the (N, S) notation represents compounds containing either nitrates, sulfates, or both. They are produced when aluminosilicate (especially Ca2+-containing) and CaCO3/CaMg(CO3)2 particles react with airborne sulfur and nitrogen oxides in the presence of moisture or with “secondary acids” such as H2SO4, HNO3, and HCl, and can be formed from the adsorption of NH4NO3 or (NH4)2SO4/NH4HSO4 on particle surface.Citation21 In the present study, the genuine mineral dust particles had higher abundances than the reacted ones on stages 1–5 (about 11–44% vs. 2–7% on average) ( and ). In particular, on stage 1, the abundance of the genuine particles was nearly 20 times higher than that of the reacted ones. It suggested that mineral dust particles larger than 1.0 µm in diameter did not easily react with SO2 and NOx as the sea salt particles did. On stage 6, the genuine and reacted particles had similar distribution in abundance (both around 11% on average), implying that the smaller mineral dust (less than 1.0 µm in diameter) were prone to reacting with air pollutants. Because of larger surface to volume ratio, the particles smaller in size might have an increased reactivity with SO2 and NOx adsorbed on dust surface compared to the bigger ones. Alternatively, these smaller reacted mineral dust (or soil-derived) particles likely came from remote sources, so they had more reacting chances with SO2 and/or NOx in the process of their long-range transport.
By calculating the abundance ratio of the reacted to genuine mineral dust particles on stage 6 according to the air mass histories, we found that the ratio for the third category of air mass back trajectories was markedly higher than that for the first and second categories (5.6 vs. 0.9 and 0.4), indicating that the air masses passing over the Yellow Sea carried more reacted mineral dust particles than those passing over the Sea of Japan and Korea Strait. Possibly, it was attributed to the higher mass concentrations of airborne SO2 and NOx over the Yellow Sea due to its being adjacent to the mainland of China. Although the mineral dust particles carried by the air masses passing over the Sea of Japan and Korea Strait ( and f) might float for longer time than those found in the trajectories coming directly from the mainland, it didn't mean that these “older” particles must become the reacted ones. Many factors such as meteorological condition, mass concentrations of ambient SO2 and NOx, and saturation effects of uptake of H2SO4 and/or HNO3 on dust surface influenced the formation of reacted (aged) mineral dust.
Similar to the reacted sea salt aerosols, the reacted mineral dust particles were also classified into three types. The first type was for nitrate-containing species, which mostly include Ca(NO3)2, the mixture of Ca(NO3)2 and CaCO3, and “AlSi + (N)”. The (N) notation represents compounds containing nitrates. The second type was for sulfate-containing species, which mostly include CaSO4, the mixture of CaSO4 and CaCO3, and “AlSi + (S)”. The (S) notation represents compounds containing sulfates. The third type was for those containing both nitrates and sulfates, which include Ca(NO3, SO4) and “AlSi + (N, S)”. As shown in , for particles on stages 2, 5, and 6, the sulfate-containing species outnumber nitrate- and nitrate + sulfate-containing ones (especially for samples “4 April”, “7 April”, and “10 April”), whereas for particles on stages 1, 3, and 4, the nitrate-containing species outnumber sulfate- and nitrate + sulfate-containing ones. Hence, it was difficult to judge which type of reacted mineral dust particles was predominant. However, during 30 April to 1 May of 2006 when an Asian dust storm originating from Mongolia passed over the Yellow Sea, it was the nitrate-containing soil-derived particles that were significantly increased, suggesting that Asian dust aerosols in springtime were an important carrier of gaseous inorganic nitrogen species.6
Carbonaceous Particles
Carbonaceous aerosol particles are generally differentiated into two groups: elemental carbon (EC) and organic carbon (OC), which have large but not absolutely distinct difference in their chemical properties.3 Herein, EC-like (also termed “carbon-rich particles”) and OC-like particles were identified using the ED-EPMA. EC-like particles mainly result from complete or incomplete combustion of liquid or solid fuels.Citation22 In this work, they were in lower abundances on stages 2–6 (less than 0.8% on average) compared to on stage 1 (6.0% on average) in all samples (). In the sample “6 April”, their relative abundance on stage 1 was even up to 20.4%. It suggested that the EC-like particles were very big (at least more than 8 µm in diameter). Probably, they were char or coal dust particles. But it was not clear where they came from.
OC-like particles were dominant in the carbonaceous aerosols based on the observation that the abundance of OC-like particles outweighed that of EC-like particles on every stage (). Especially on stage 6, it was about 15 times higher than the abundance of EC-like particles (11.99% vs. 0.79%) (), implying that the OC-like particles on stage 6 were abundant. In the three categories of air mass back trajectories, the air masses passed over the Yellow Sea (the third category) carried more OC-like particles than those passed over the Sea of Japan and Korea Strait, as the abundance for the sample “10 April” was up to 35.1%, around 4 times higher than that for the samples “4–9 April”. These OC-like particles were believed to mainly result from anthropogenic emissions and atmospheric transport and/or transformation of anthropogenic organic species from outside of Jeju Island.Citation14 However, a part of them might be from biogenic emissions,Citation19 since the Jeju Island are covered by plenty of forest and closed by seawater.
(NH4)2SO4/NH4HSO4-Containing Particles
The (NH4)2SO4/NH4HSO4-containing particle is denoted as (C, N, O, S)-containing particle because of the peaks of C, O, S, and N in their EDX spectra.5 It is a secondary species produced from the reactions of ambient sulfate or sulfuric acid (formed by the oxidation of SO2 from anthropogenic sources) with ammonia (largely emitted from regions of high agricultural activity and livestock farming), often internally mixed with soot and organic matter.Citation23,Citation24 Here, the (NH4)2SO4/NH4HSO4-containing particles were in higher abundance on stages 5 and 6 (5.3% and 38.4% on average, respectively) than on stages 1–4 (0%, 0%, 0.2%, and 0.3% respectively), indicating that they were small in size. Hayami and CarmichaelCitation25 reported that over Jeju Island NH4 + was most strongly associated with nss-SO4 2–, and peak values of NH4 + and SO4 2– were in the fine mode, whereas Na+, Ca2+, Mg2+, NO3 –, and Cl– peaks were in the coarse mode. On stage 6, there were higher abundances of (NH4)2SO4/NH4HSO4-containing particles in the samples “6–9 April” (44.3%, 58.1%, 37.1%, and 71.6%, respectively), revealing that a large number of (NH4)2SO4 or NH4HSO4 could be formed over the Sea of Japan and Korea Strait.
Fe-Rich and K-Rich Particles
The Fe-rich particles looked bright on their SEIs and usually contained obvious Fe and O peaks in their X-ray spectra (often with minor C, Si, and Al). They were generally in the form of iron ((oxy)hydr)oxides, being interpreted as goethite, hematite, or magnetite in atmospheric aerosols. Human activities such as mining, steel production, metallurgical industries, the abrasion of brake lining, and erosion of asphalted road might lead to distinctly higher loads of Fe/FeOx.Citation26 The K-rich particles, in which K2SO4 was the most abundant species in the study, were regarded to mostly originate from biomass burning.Citation27 In the present study, Fe-rich and K-rich particles on stages 1–6 for all the samples had lower abundances (most of them were less than 2%), of which the abundances of particles on stages 5 and 6 outweighed those on stages 1–4 (especially for samples “5 April”, “6 April”, and “8 April”).
CONCLUSIONS
This study presented the single-particle observations of chemical compositions of aerosols at different size level (stages 1–6) at Gosan, Jeju Island, Korea, in the eve of a typical dust storm during the ACE-Asia 2001 field campaign. Seven sets of particle samples were collected on 4–10 April of 2001 using a seven-stage May cascade impactor, and 11,200 particles on stages 1–6 with cutoff diameters of 16, 8, 4, 2, 1, and 0.5 μm were analyzed by a quantitative ED-EPMA technique. The measurement on the equivalent diameter of every particle indicated that 96.9% of the analyzed particles were in the size range of 0.5–16 μm. Based on secondary electron images and X-ray spectra of particles, particle types were identified and their relative number abundances were calculated. Results showed that
| a. | Particles of sea origin were the most abundant, followed by mineral dust, carbonaceous, NH4HSO4/(NH4)2SO4-containing, Fe-rich, and K-rich particles. | ||||
| b. | In sea salt particles, the reacted ones with SO2 and NOx (or their acidic products) were predominant, greatly outnumbering the genuine ones. The nitrate-containing reacted sea salt particles were frequently encountered on stages 1–5, whereas the sulfate-containing ones were frequently encountered on stage 6. In mineral dust particles, the genuine ones greatly outnumbered the reacted (aged) ones. A lot of reacted sea salt and reacted mineral dust particles were carried by the air masses passing over the Yellow Sea. | ||||
| c. | In the carbonaceous aerosols, OC-like particles were dominant compared to EC-like particles. They were frequently encountered on stage 6 and largely present in the air masses passed over the Yellow Sea. | ||||
| d. | (NH4)2SO4/NH4HSO4-containing particles had higher abundance on stages 5 and 6 (5.3% and 38.4% on average, respectively) than on stages 1–4. The 72-hr air mass back trajectories showed that many of them came from the marine boundary layer of the Sea of Japan and Korea Strait. | ||||
| e. | Fe-rich and K-rich particles had low abundances. They were rarely encountered on stages 1–4. | ||||
In conclusion, the single-particle characterization of chemical compositions of aerosols at Gosan indicated the reacted (aged) sea salt, organic compounds, and secondary aerosols such as (NH4)2SO4 greatly influenced the atmosphere over Jeju Island before a typical dust storm originating from Mongolia arrived.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0018881) and by the fund provided from the Research Project supported by Shanxi Scholarship Council of China (2010) OS and the Basic Science Research Program of Shanxi Natural Science Foundation (2011011009-2).
REFERENCES
- Kim , J. , Yoon , S.-C. , Jefferson , A. , Zahorowski , W. and Kang , C.-H. 2005 . Air Mass Characterization and Source Region Analysis for the Gosan Super-Site, Korea, during the ACE-Asia 2001 Field Campaign . Atmos. Environ , 39 : 6513 – 6523 .
- Huebert , B.J. , Bates , T. , Russell , P.B. , Shi , G. , Kim , Y.J. , Kawamura , K. , Carmichael , G. and Nakajima , T. 2003 . An Overview of ACE-Asia: Strategies for Quantifying the Relationships between Asian Aerosols and Their Climatic Impacts . J. Geophys. Res , 108 : D23 – 8633 . doi: 10.1029/2003JD003550
- Arimoto , R. , Kim , Y.J. , Kim , Y.P. , Quinn , P.K. , Bates , T.S. , Anderson , T.L. , Gong , S. , Uno , I. , Chin , M. , Huebert , B.J. , Clarke , A.D. , Shinozuka , Y. , Weber , R.J. , Anderson , J.R. , Guazzotti , S.A. , Sullivan , R.C. , Sodeman , D.A. , Prather , K.A. and Sokolik , I.N. 2006 . Characterization of Asian Dust during ACE-Asia . Global Planet. Change , 52 : 23 – 56 .
- Kang , S. , Hwang , H. , Kang , S. , Park , Y. , Kim , H. and Ro , C.-U. 2009 . Quantitative ED-EPMA Combined with Morphological Information for the Characterization of Individual Aerosol Particles Collected in Incheon, Korea . Atmos. Environ , 43 : 3445 – 3453 .
- Geng , H. , Jung , H.-J. , Park , Y. , Hwang , H. , Kim , H. , Kim , Y.J. , Sunwoo , Y. and Ro , C.-U. 2009 . Morphological and Chemical Composition Characteristics of Summertime Atmospheric Particles Collected at Tokchok Island, Korea . Atmos. Environ , 43 : 3364 – 3373 .
- Geng , H. , Park , Y. , Hwang , H. , Kang , S. and Ro , C.-U. 2009 . Elevated Nitrogen-Containing Particles Observed in Asian Dust Aerosol Samples Collected at the Marine Boundary Layer of the Bohai Sea and the Yellow Sea . Atmos. Chem. Phys , 9 : 6933 – 6947 .
- Geng , H. , Ryu , J. , Jung , H.-J. , Chung , H. , Ahn , K.H. and Ro , C.-U. 2010 . Single-Particle Characterization of Summertime Arctic Aerosols Collected at Ny-Ålesund, Svalbard . Environ. Sci. Technol , 44 : 2348 – 2353 .
- Vekemans , B. , Janssens , K. , Vincze , L. , Adams , F. and Van Espen , P. 1994 . Analysis of X-ray Spectra by Iterative Least Squares (AXIL): New Developments . X-ray Spectrom , 23 : 278 – 285 .
- Ro , C.-U. , Osan , J. , Szaloki , I. , de Hoog , J. , Worobiec , A. and Van Grieken , R. 2003 . A Monte Carlo Program for Quantitative Electron-Induced X-ray Analysis of Individual Particles . Anal. Chem , 75 : 851 – 859 .
- Ro , C.-U. , Kim , H. and Van Grieken , R. 2004 . An Expert System for Chemical Speciation of Individual Particles Using Low-Z Particle Electron Probe X-ray Microanalysis Data . Anal. Chem , 76 : 1322 – 1327 .
- Ro , C.-U. , Hwang , H. , Kim , H. , Chun , Y. and Van Grieken , R. 2005 . Single-Particle Characterization of Four “Asian Dust” Samples Collected in Korea, Using Low-Z Particle Electron Probe X-ray Microanalysis . Environ. Sci. Technol , 39 : 1409 – 1419 .
- Arimoto , R. , Zhang , X.Y. , Huebert , B. , Kang , C.H. , Savoie , D.L. , Prospero , J.M. , Sage , S.K. , Schloesslin , C.A. , Khaing , H.M. and Oh , S.N. 2004 . Chemical Composition of Atmospheric Aerosols from Zhenbeitai, China, and Gosan, South Korea, during ACE-Asia . J. Geophys. Res , 109 : D19S04 doi: 10.1029/2003JD004323
- Wang , K.-Y. 2001 . Long Range Transport of the April 2001 Dust Clouds Over the Subtropical East Asia and the North Pacific and its Impacts on Ground-level Air Pollution: A Lagrangian Simulation . J. Geophys. Res , 112 D09203
- Kim , Y.P. , Moon , K.-C. , Lee , J.H. and Baik , N.J. 1999 . Concentrations of Carbonaceous Species in Particles at Seoul and Cheju in Korea . Atmos. Environ , 33 : 2751 – 2758 .
- Cohen , D.D. , Garton , D. , Stelcer , E. , Hawas , Q. , Wang , T. , Poon , S. , Kim , J. , Choi , B.C. , Oh , S.N. , Shin , H.-J. , Ko , M.Y. and Uematsu , M. 2004 . Multielemental Analysis and Characterization of Fine Aerosols at Several Key ACE-Asia Sites . J. Geophys. Res , 109 : D19512
- Laskin , A. , Gaspar , D.J. , Wang , W. , Hunt , S.W. , Cowin , J.P. , Colson , S.D. and Finlayson-Pitts , B.J. 2003 . Reactions at Interfaces as a Source of Sulfate Formation in Sea-Salt Particles . Science , 301 : 340 – 344 .
- Hopkins , R.J. , Desyaterik , Y. , Tivanski , A.V. , Zaveri , R.A. , Berkowitz , C.M. , Tyliszczak , T. , Gilles , M.K. and Laskin , A. 2008 . Chemical Speciation of Sulfur in Marine Cloud Droplets and Particles: Analysis of Individual Particles from the Marine Boundary Layer over the California Current . J. Geophys. Res , 113 : D04209 doi: 10.1029/2007JD008954
- Yang , G.-P. , Zhang , H.-H. , Su , L.-P. and Zhou , L.-M. 2009 . Biogenic Emission of Dimethylsulfide (DMS) from the North Yellow Sea, China and Its Contribution to Sulfate in Aerosol During Summer . Atmos. Environ , 43 : 2196 – 2203 .
- Ro , C.-U. , Oh , K.-Y. , Kim , H. , Kim , Y.P. , Lee , C.B. , Kim , K.-H. , Kang , C.H. , Osan , J. , De Hoog , J. , Worbiec , A. and Van Grieken , R. 2001 . Single-Particle Analysis of Aerosols at Cheju Island, Korea, Using Low-Z Electron Probe X-ray Microanalysis: A Direct Proof of Nitrate Formation from Sea Salts . Environ. Sci. Technol , 35 : 4487 – 4494 .
- Carmichael , G.R. , Zhang , Y. , Chen , L.-L. , Hong , M.-S. and Ueda , H. 1996 . Seasonal Variation of Aerosol Composition at Cheju Island, Korea . Atmos. Environ , 30 : 2407 – 2416 .
- Sullivan , R.C. , Guazzotti , S.A. , Sodeman , D.A. and Prather , K.A. 2007 . Direct Observations of the Atmospheric Processing of Asian Mineral Dust . Atmos. Chem. Phys , 7 : 1213 – 1236 .
- Geng , H. , Kang , S. , Jung , H.-J. , Choël , M. , Kim , H. and Ro , C.-U. 2010 . Characterization of Individual Submicrometer Aerosol Particles Collected in Incheon, Korea, by Quantitative Transmission Electron Microscopy Energy-Dispersive X-ray Spectrometry . J. Geophys. Res , 115 : D15306 doi: 10.1029/2009JD013486
- Adachi , K. and Buseck , P.R. 2008 . Internally Mixed Soot, Sulfates, and Organic Matter in Aerosol Particles from Mexico City . Atmos. Chem. Phys , 8 : 6469 – 6481 . doi: 10.5194/acp-8-6469-2008
- Johnson , K.S. , Zuberi , B.L. , Molina , T. , Molina , M.J. , Iedema , M.J. , Cowin , J.P. , Gaspar , D.J. , Wang , C. and Laskin , A. 2005 . Processing of Soot in an Urban Environment: Case Study from the Mexico City Metropolitan Area . Atmos. Chem. Phys , 5 : 3033 – 3043 . doi: 10.5194/acp-5-3033-2005
- Hayami , H. and Carmichael , G.R. 1997 . Analysis of Aerosol Composition at Cheju Island, Korea, Using a Two-Bin Gas-Aerosol Equilibrium Model . Atmos. Environ , 31 : 3429 – 3439 .
- Flament , P. , Mattielli , N. , Aimoz , L. , Choël , M. , Deboudt , K. , de Jong , J. , Rimetz-Planchon , J. and Weis , D. 2008 . Iron Isotopic Fractionation in Industrial Emissions and Urban Aerosols . Chemosphere , 73 : 1793 – 1798 .
- Zhang , X. , Hecobian , A. , Zheng , M. , Frank , N.H. and Weber , R.J. 2010 . Biomass Burning Impact on PM2.5 over the Southeastern US during 2007: Integrating Chemically Speciated FRM Filter Measurements, MODIS Fire Counts and PMF Analysis . Atmos. Chem. Phys , 10 : 6839 – 6853 . doi: 10.5194/acp-10-6839-2010