ABSTRACT
A simple and highly effective stabilization/solidification (S/S) technology of elemental mercury using only sulfur with paraffin is introduced. First, elemental mercury is mixed with an excess of sulfur powder and heated to 60 °C for 30 min until elemental mercury is converted into mercuric sulfide (HgS black, metacinnabar) (Step 1). Then, metacinnabar with additional sulfur is poured into liquid paraffin (Step 2). Finally, the mixture is melted at 140 °C and settles to the bottom of the vessel where it cools and solidifies under the layer of liquid paraffin (Step 3). The proposed S/S method with sodium sulfide nonahydrate (Na2S·9H2O) as an additive is also tested for comparison. The average toxicity characteristic leaching procedure test values are 6.72 μg/L (no additive) and 3.18 μg/L (with additive). Theses concentrations are well below the Universal Treatment Standard (25 μg/L). Effective diffusion coefficient evaluated from accelerated leach test and average headspace concentration of Hg vapor after 18 hr are 3.62 × 10−15 cm2/sec, 0.55 mg/m3 (no additive) and 5.86 × 10−13 cm2/sec, 0.25 mg/m3 (with additive).
The simple treatment method for elemental mercury by stabilization/solidification introduced in this study will benefit the United Nations Environmental Programme (UNEP) Mercury Programme initiating a project to reduce the global mercury supply and address the safe and long-term storage of mercury. In addition, it will also provide an option and possible solution to the compliance of the recently passed law individually by the European Union and the United States banning the sale of toxic mercury abroad in 2011 and 2013, respectively.
INTRODUCTION
On October 15, 2008, a bipartisan bill introduced by U.S. Senator Barack Obama that will ban the sale of toxic mercury abroad in 2013 was signed into law by President George W. Bush. It also directs the U.S. Department of Energy to begin operating a long-term storage and management facility for excess mercury.
In the United States, the treatment and disposal of mercury contaminated waste is currently controlled by the Land Disposal Restrictions program of U.S. Environmental Protection Agency (EPA). In general, mercury wastes must be treated so that they have a toxicity characteristic leaching procedure (TCLP) value of less than 25 μg/L, which is the Universal Treatment Standard (UTS).Citation1
As for the Republic of Korea, currently there is no regulation on the proper disposal and management of mercury waste. However, the Korea government recently announced that the Comprehensive Management Plan for Mercury (years 2011–2015) were under way. In addition, a nationwide taskforce team has been organized and in activity to prepare for both the United Nations Environmental Programme (UNEP) Mercury Programme and the upcoming 2013 International Mercury Treaty.
The second session of the Intergovernmental Negotiating Committee to prepare a global legally binding instrument on mercury (mercury treaty), expected to be signed in 2013, was held in Chiba, Japan, from 24 to 28 January 2011. The UNEP Governing Council agreed, in paragraph 27 of the decision, that the task of the intergovernmental negotiating committee was to develop a comprehensive and suitable approach to mercury that included provisions (1) to specify the objectives of the instrument; (2) to reduce the supply of mercury and enhance the capacity for its environmentally sound storage; (3) to reduce the demand for mercury in products and processes; (4) to reduce international trade in mercury; (5) to reduce atmospheric emissions of mercury; (6) to address mercury-containing waste and remediation of contaminated sites; (7) to increase knowledge through awareness-raising and scientific information exchange; (8) to specify arrangements for capacity-building and technical and financial assistance, recognizing that the ability of developing countries and countries with economies in transition to implement some legal obligations effectively under a legally binding instrument is dependent on the availability of capacity-building and technical and adequate financial assistance; and (9) to address compliance.Citation2
The purpose of S/S technology is to immobilize liquid waste by converting it into a solid form, which is done most commonly by using Portland cement for solidification and stabilization agents such as ashes of different origin, lime, silicates, activation carbon, etc.Citation3,Citation4 This, however, is not desirable for the treatment of mercury waste, since it is difficult to produce a solid form of mercury due to its low solubility and high volatility.Citation5,Citation6 Especially, mercury wastes originated from the combustion of wood, sewage sludge, subbituminous coal, and a subbituminous coal-petroleum coke mixture are difficult to deal with due to their potential for release mercury as well as arsenic, and selenium through volatilization and/or leaching when placed in landfill or put into new product.Citation7,Citation8 Meanwhile, mercury-containing consumer products such as thermometers, manometers, and hemadynamometer are relatively easier to treat by stabilization/solidification (S/S), since most of the mercury can be recovered as liquid metallic form.
Several alternative S/S technologies have been reported for the treatment of mercury wastes.Citation9–17 The technology that converts mercury into mercury sulfide by reacting it with a sulfur compound is one of the most widely used methods for safe disposal of mercury.Citation9,Citation10,Citation13,Citation14,Citation16,Citation17 This product is more stable and environmentally benign than elemental mercury due to its high insolubility and low vapor pressure. However, since the final product is usually in the form of fine powder, once stabilized, it needs to be further solidified for safe disposal. Solidified forms are disposable because they are nondispersive. Although cement can easily be used to solidify wastes in either paste or powder forms, this process results in a large volume of the final product. Thus, it is desirable to perform both stabilization and solidification using a single material. One of the promising technologies that use a single material for the S/S process is sulfur polymer stabilization/solidification.Citation9,Citation10 It utilizes sulfur polymer cement (SPC) with additives (sodium sulfide, triisobutyl phosphine sulfide) to convert elemental mercury into mercuric sulfide, which is highly insoluble. After additional amount of SPC is added to the waste, the mixture is heated to form a molten liquid, which is then cooled in a mold to form a monolithic waste product. Although patented, licensing of chemically bonded phosphate ceramic and SPC technologies has generally been limited to one or two companies and the industrial-scale applications are limited.Citation5
In this work, a simple and highly effective S/S technology for elemental mercury using sulfur with paraffin is proposed and validated. The proposed S/S process is believed to be economical because (1) sulfur and paraffin are relatively inexpensive; (2) paraffin can be recovered and reused; and (3) no additional encasing materials are necessary. In addition, the use of the proposed S/S technology with sodium sulfide nonahydrate (Na2S·9H2O) as an additive is examined. The additive aids in the treatment of mixed-mercury wastes, which contain compounds such as metallic mercury, mercury oxides, and other leachable mercury salts.Citation9 The analytical results of X-ray diffraction (XRD), TCLP, accelerated leach test (ALT), and the headspace Hg vapor concentration measurement are discussed.
EXPERIMENTAL
Stabilization and Solidification
Twenty grams of elemental mercury (>99.99%; Sigma-Aldrich, Yongin, Korea) was mixed with sulfur powder (Miwon, Anyang, Korea) at a 1:1 weight ratio (a 6-fold excess of S to Hg0 in molar ratio) in the Teflon reaction vessel (4 cm in diameter and 8.7 cm in height). The mixture was then heated to 60 °C for 30 min until Hg0 was converted into mercuric sulfide (HgS black, metacinnabar) (Step 1). Then, additional 20.00 g of sulfur powder was mixed with 40.00 g of mercuric sulfide (i.e., the total waste loading of 33.33 wt.% mercury). The mixture was poured into 40.00 g of liquid paraffin (Duksan, Ansan, Korea) contained in 300-mL stainless steel can (5 cm in diameter and 10 cm in height). Finally, the temperature of the mixture was raised to 140 °C. The mixture of mercuric sulfide and sulfur melted and settled to the bottom of the vessel, and then it was cooled and formed a solid—all under the layer of liquid paraffin (Step 2). While in a liquid form, the paraffin can be separated and recovered for reuse.
The effect of an additive on proposed S/S method is also investigated. Sodium sulfide nonahydrate (Na2S·9H2O; Sigma-Aldrich) was selected because it has been successfully used to limit the leaching of heavy metal wastes encapsulated in sulfur polymer cement.18 Upon addition of Na2S·9H2O (1.5 g) to the product mixture of Step 1 and after heating to 90 °C for 1 hr, metacinnabar was converted into cinnabar (HgS red). The rest of the procedure remained the same. All procedures described above were conducted in a fume hood. The mercury vapor generated during the process was captured by activated carbon before going out through the hood.
Test Methods
First, the TCLP, as defined by EPA method 1311, was performed to measure the leachable mercury contents from the treated mercury wastes.Citation19 TCLP extraction fluid I (acetic acid and sodium hydroxide at pH = 4.93) was used for all TCLP tests. Instead of the recommended 100 g, 50 g of sample was used to reduce the volume of waste generated in this study. However, the same relative reagent quantities required for the standard TCLP test were maintained.Citation9,Citation19
Next, the ALT, as defined by American Society for Testing and Materials (ASTM) C-1308, was used to investigate the longer-term leaching behavior.Citation20 A cylinder of 5 cm in diameter and 1 cm in length was used for casting treated waste. It was then immersed in distilled water (5.5 L) for the selected time of duration (2, 5, 17, and 24 hr) followed by further analysis of water.
The effective diffusion coefficients were determined using the semi-infinite media model, which was based on Fick's diffusion theory.Citation21 This model can be used to determine cumulative fractions leached over time.
For TCLP and ALT solutions, Hg concentrations were measured using EPA method 7470ACitation22 with the cold vapor atomic absorption (CVAA) Hg analyzer (RA-915+/ RP-91; Lumex Ltd., St. Petersburg, Russia), whereas mercury mass in the solid samples (0.5 g each) was calculated from measurements using EPA method 7471BCitation23 with the CVAA Hg analyzer (RA-915+/ RP-91; Lumex).
To determine how much mercury vapor was generated from the treated wastes, the equilibrium static headspace test was used.Citation9 First, a 250-mL plastic bottle (Daejin Plastic, Korea) containing 7 g of the treated wastes was tightly sealed and left for 18 h for Hg vapor to reach equilibrium at 20 °C. Vapor samples from the headspace of these sealed bottles were taken with a 10-mL gas-tight syringe (Korea Vaccine, Korea) and injected into a 1-L, septum-capped tedler bag filled with air. The total Hg vapor concentration was measured by a continuous mercury emission monitor (SM-3; Mercury Instruments, Karlsfeld, Germany). In addition, the Hg concentration of the headspace vapor equilibrated with the treated wastes was measured after 18, 24, 48, 72, 96, 120, 144, 168, 192, 216, 240, and 264 hr. Finally, the treated wastes were analyzed using powder XRD (MiniFlex; Rigaku Co., Japan).
Mass Balance
For mass balance calculation, a higher waste loading sample (33.33 wt.% mercury) was used. An excess amount of sulfur (40.44 g) was mixed with elemental mercury (20.51 g) to form metacinnabar. The measured total mass of the treated waste was 57.85 g, whereas the expected value (sum of sulfur and mercury used) was 60.95 g. This discrepancy was probably due to the loss of mercury by volatilization and transferring and/or the loss of unmelted sulfur powder during the separation of liquid paraffin.
The mercury content of the treated waste was 0.331 g/g (33.1 wt.%), which equals 19.15 g of mercury in the 57.85 g of the treated waste. The amount of mercury inside the recovered paraffin of 35.79 g was 0.609 μg (0.017 μg/g). The difference between mass of original mercury (20.51 g) and mercury retained in the treated waste (19.15 g + 0.609 μg) may come from the volatilization and transferring loss.
RESULTS AND DISCUSSION
Characterization
There are two stable forms of mercuric sulfide, the cubic and orthorhombic phases. A cubic phase is metacinnabar (HgS black) and the orthorhombic phase is cinnabar (HgS red).
The XRD patterns of the treated wastes (with and without the additive) and reagent-grade mercuric sulfides (HgS black, HgS red) are shown in . These results confirm the presence of a metacinnabar in the treated waste with no additive and cinnabar in the treated waste with additive. The treated waste with no additive is dark gray and the treated waste with additive is dark red.
Figure 1. XRD patterns: (a) reagent-grade HgS black; (b) treated waste with no additive; (c) reagent-grade HgS red; (d) treated waste with additive.
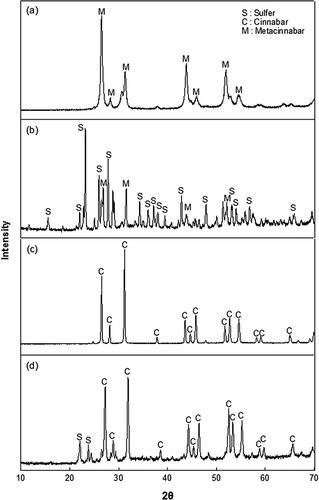
The melting point of sulfur (115.21 °C) is between the melting point of paraffin (46.6 °C) and boiling point of paraffin (370 °C). This enables sulfur to be melted while surrounded by liquid paraffin inside the heated container on an oil bath. Also, since sulfur and paraffin are both nonpolar, they can easily mix with each other. The paraffin prevents Hg vapor from escaping. In addition, air cannot reach the mercuric compound in the paraffin, which minimizes the production of mercuric oxides. The paraffin used in this study is not a solidifying or waterproof agent, as is sometimes used in hazardous waste treatment.Citation18 Although the paraffin is recovered after the solidification process for reuse, the recovered paraffin is still permissible upon demand for immediate disposal due to the TCLP value of 0.36 μg/L, which is well below the UTS (25 μg/L). The proposed S/S process is therefore believed to be economical because (1) sulfur and paraffin are relatively inexpensive; (2) paraffin can be recovered and reused; and (3) no additional encasing materials are necessary.
Toxicity Characteristic Leaching Procedure
The TCLP test results are shown in . The treated waste with no additive (TCLP test IV) has the average TCLP concentration of 6.72 μg/L, which is well below the UTS. The result of TCLP test V (with additive sodium sulfide nonahydrate) also meets the UTS easily with the average TCLP concentration of 3.18 μg/L. The use of additive improves the TCLP test result. This is probably due to the ability of sodium sulfide nonahydrate to react with any nonmetallic mercury (mercury oxides or other leachable mercury salts) that may have formed during the S/S process.
Table 1. Hg concentration of the leachate from the TCLP test
Accelerated Leach Test
As shown in , a curve fit was carried out for the cumulative fraction of Hg leached using the following equationCitation21:
Figure 2. Curves of the accelerated leach test: (a) treated waste with no additive; (b) treated waste with additive.
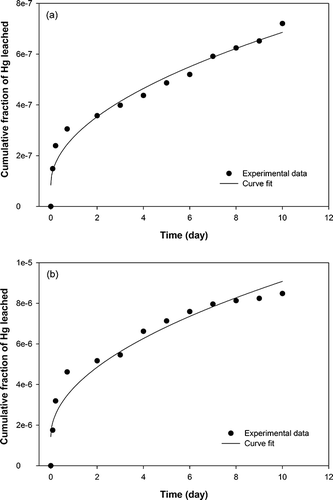
where, f Hg is the fraction of Hg leached in t days and m is the slope obtained from the f Hg versus t 1/2 plot. The resulting equation obtained by curve fit of eq 1 for the treated waste with no additive in this study is as follows:
For comparison with the previously reported value (0.26% for 200-L-drum-sized waste),Citation9 fraction of Hg leached was estimated up to 106 days. The treated waste with no additive (5 cm in diameter and 1 cm in height) will leach 0.019% of its Hg content. This value was adjusted to be 0.0002% for 200 L (58 cm in diameter and 84 cm in height) using the following equationCitation21:
where S and V are geometric surface area (cm2) and volume (cm3) of the treated waste, respectively. As for the treated waste with additive in this study, eq 1 becomes
After 106 days, the treated waste with additive (5 cm in diameter and 1 cm in height) is estimated to leach 0.242% of its mercury content. For a 200-L-drum-sized waste form, 0.002% of the mercury is estimated to be leached in 106 days.
Once m is determined, then the effective diffusion coefficients were determined using the semi-infinite media model:
The values are found to be 3.62 × 10−15 cm2/sec (no additive) and 5.86 × 10−13 cm2/sec (with additive).
In the ALT, the treated waste with no additive had better results than the treated waste with additive. Some mercury may have been leached along with additive upon contact with water due to the solubility of sodium sulfide nonahydrate.
Equilibrium Static Headspace Test (ESHT)
Investigation of likelihood of mercury vaporization from the treated wastes is as important as investigating the leachability. To determine how much mercury vapor was generated, untreated Hg0, reagent-grade HgS (both red and black forms), and the treated wastes (ESHT samples IV and V in ) were sealed in plastic bottles allowing the vapor to reach equilibrium at 20 °C. The headspace vapor samples were then analyzed (). The Hg vapor concentration of the untreated Hg0 ranged from 9.52 to 10.01 mg/m3, which is similar to the result (9.7–12.7 mg/m3) previously reported by Fuhrmann and co-workers.Citation9 The treated wastes had much lower headspace Hg vapor concentration—typically an order of magnitude lower than that of untreated Hg0. The headspace Hg vapor concentrations of the ESHT samples IV (no additive) and V (with additive) measured after 18 hr ranged from 0.24 to 0.75 mg/m3 and from 0.32 to 1.19 mg/m3, respectively.
Table 2. Hg vapor concentration in bottles (250 mL) from the ESHT test.Footnote a
In addition, the extended monitoring of headspace Hg vapor concentrations for the treated wastes was carried out periodically up to 11 days, showing a negligible change over time for both ESHT samples IV and V. The equilibrium vapor concentrations showed much better results than those from the samples of the other reported technologies such as amalgams produced by Hg + Zn (10–12 mg/m3),Citation24 solid waste produced by Hg + SPC (0.60 mg/m3 on the average), and by Hg + SPC + additive (1.15 mg/m3 on the average).Citation9
CONCLUSIONS
In this study, a simple and highly effective mercury treatment process has been proposed. Elemental mercury was successfully stabilized and solidified using only sulfur with paraffin. The formation of metacinnabar in the treated waste was confirmed by XRD analysis. The TCLP test value was lower than the EPA standard, indicating a very low leachability. The equilibrium static headspace test showed very low equilibrium Hg vapor pressure. The proposed S/S process is believed to be economical because sulfur and paraffin are relatively inexpensive, paraffin can be recovered and reused, and no additional encasing materials are used.
The proposed S/S process was also tested with an additive (sodium sulfide nonahydrate), which improved the TCLP test value. However, in the ALT, the treated waste with no additive showed better results. This is probably because the additive is water-soluble and some mercury may have also been leached along with the additive upon contact with water.
For safe disposal and/or long-term storage, either macroencapsulation by strong materials (e.g., Portland cement, slag cement) or the confinement in a sealed container is still required. However, microencapsulation without any encasing materials during the proposed process significantly reduces the volume and weight of the treated waste. This will require fewer macroencapsulating materials and containers, and a smaller storage area.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (no. 2009-0079977).
REFERENCES
- U.S. Environmental Protection Agency . September 2005 . Introduction to Land Disposal Restrictions; 40 CFR , Vol. Part 268 , Washington , DC : U.S. Government Printing Office .
- United Nations Environmental Programme. Report of the Intergovernmental Negotiating Committee to Prepare a Global Legally Binding Instrument on Mercury on the Work of its Second Session; UNEP(DTIE)/Hg/INC.2/20; United Nations Environment Programme, Nairobi, Kenya, 2011 http://www.unep.org/hazardoussubstances/Mercury/Negotiations/INC2/INC2Report/tabid/3485/Default.aspx (http://www.unep.org/hazardoussubstances/Mercury/Negotiations/INC2/INC2Report/tabid/3485/Default.aspx)
- Malviya , R. and Chaudhary , R. 2006 . Factors Affecting Hazardous Waste Solidification/Stabilization: A Review . J. Hazard. Mater. , B137 : 267 – 276 .
- Coz , A. , Andres , A. , Soriano , S. , Viguri , J.R. , Ruiz , M.C. and Irabien , J.A. 2009 . Influence of Commercial and Residual Sorbents and Silicates as Additives on the Stabilization/Solidification or Organic and Inorganic Industrial Waste . J. Hazard. Mater. , 164 : 755 – 761 .
- Randall , P.M. and Chattopadhyay , S. 2004 . Advances in Encapsulation Technologies for the Management of Mercury-Contaminated Hazardous Wastes . J. Hazard. Mater. , B114 : 211 – 223 .
- Piao , H. and Bishop , P.L. 2006 . Stabilization of Mercury-Containing Waste Using Sulfide . Environ. Pollut. , 139 : 498 – 506 .
- Gustin , M.S. , Ericksen , J. and Fernandez , G.C. 2008 . Determination of the Potential for Release of Mercury from Combustion Product Amended Soils: Part 1—Simulations of Beneficial Use . J. Air Waste Manage. Assoc. , 58 : 673 – 683 . doi: 10.3155/1047-3289.58.5.673
- Gustin , M.S. , Xin , M. and Ericksen , J. 2008 . Determination of the Potential for Release of Mercury from Combustion Product Amended Soils: Part 2—Coal Fly Ash Generated Stabilized Soil and Degradation Products . J. Air Waste Manage. Assoc. , 58 : 1495 – 1508 . doi: 10.3155/1047-3289.58.11.1495
- Fuhrmann , M. , Melamed , D. , Kalb , P.D. , Adams , J.W. and Milian , L.W. 2002 . Sulfur Polymer Solidification/Stabilization of Elemental Mercury Waste . Waste Manage. , 22 : 327 – 333 .
- Kalb , P.D. , Melamed , D. , Patel , B.R. and Fuhrmann , M. 2002 . Treatment of Mercury Containing Wastes; United States Patent, US 6 399 899 Vol. June 4 ,
- Guha , B. , Hills , C.D. , Carey , P.J. and Macleod , C.L. 2006 . Leaching of Hg from Carbonated and Non-Carbonated Cement-Solidified Dredged Sediments . Soil Sediment. Contam. , 15 : 621 – 635 .
- Kot , F.S. , Rapoprt , V.L. and Kharitoneva , G.V. 2007 . Immobilization of Soil Mercury by Colloidal Sulphur in the Laboratory Experiment . Cent. Eur. J. Chem. , 5 : 846 – 857 .
- Liu , Z. , Qian , G. , Zhou , J. , Li , C. , Xu , Y. and Qin , Z. 2008 . Improvement of Ground Granulated Blast Furnace Slag on Stabilization/Solidification of Simulated Mercury-Doped Wastes in Chemically Boned Phosphate Ceramics . J. Hazard. Mater. , 157 : 146 – 153 .
- Svensson , M. and Allard , B. 2008 . Leaching of Mercury-Containing Cement Monoliths Aged for One Year . Waste Manage. , 28 : 597 – 603 .
- Zhang , X.Y. , Wang , Q.C. , Zhang , S.Q. , Sun , X.J. and Zhang , Z.S. 2009 . Stabilization/Solidification (S/S) of Mercury-Contaminated Hazardous Wastes Using Thiol-Functionalized Zeolite and Portland Cement . J. Hazard. Mater , 168 : 1575 – 1580 .
- Sparrevik , M. , Eej , E. and Grini , R.S. 2009 . The Importance of Sulphide Binding for Leaching of Heavy Metals from Contaminated Norwegian Marine Sediments Treated by Stabilization/Solidification . Environ. Technol. , 30 : 831 – 840 .
- Randall , P.M. and Chattopadhyay , S. 2010 . Bench-Scale Evaluation of Chemically Bonded Phosphate Ceramic Technology to Stabilize Mercury Waste Mixtures . J. Environ. Eng.-ASCE , 136 : 265 – 273 .
- Kaye , G.I. and Weber , P.B. 2002 . Methods for Treatment and Disposal of Regulated Medical Waste; United States Patent, US 6 437 211 B2 , August 20
- U.S. Environmental Protection Agency . March 1992; Revision II . Toxicity Characteristic Leaching Procedure (TCLP); Method 1311 , Washington , DC : U.S. Government Printing Office .
- ASTM . 2001 . Standard Test Method for Accelerated Leach Test for Diffusive Releases from Solidified Waste and a Computer Program to Model Diffusive, Fractional Leaching from Cylindrical Waste Forms; ASTM Standards, C1308-95 , West Conshohocken , PA : ASTM Designation; ASTM .
- Anigstein , R. , Thurber , W.C. , Mauro , J.J. , Marschke , S.F. and Behling , U.H. 2001 . Technical Support Document: Potential Recycling of Scrap Metal from Nuclear Facilities—Appendix I: Leaching of Radionuclides from Slags , McLean , VA : prepared for U.S. EPA by S. Cohen & Associates .
- U.S. Environmental Protection Agency . September 1994; Revision I . Mercury in Liquid Waste (Manual Cold-Vapor Technique); Method 7470A , Washington , DC : U.S. Government Printing Office .
- U.S. Environmental Protection Agency . February 2007 . Mercury in Solid or Semisolid Waste (Manual Cold-Vapor Technique); Method 7471B , Washington , DC : U.S. Government Printing Office . Revision II
- Mattus , C.H. 1999 . Measurement of Mercury Released from Amalgams and Sulfide Compounds , Oak Ridge , TN : prepared for U.S. DOE by Oak Ridge National Laboratory .