Abstract
In this study, the authors investigated the influence of the valence state of Mn on the efficacy of selective catalytic reduction using a Mn-based catalyst. The nitrogen oxides (NOx) conversion rate of the catalyst was found to be dependent on the type of TiO2 support employed and on the temperature, as the catalyst showed an excellent conversion of > 80% at a space velocity of 60,000 hr−1 when the temperature was above 200 °C. Brunauer-Emmett-Teller, X-ray diffraction, and X-ray photoelectron spectroscopy analyses confirmed that catalyst displaying the highest activity contained the Mn4+ species and that its valence state was highly dependent on the pH during the catalyst preparation.
Recently, various Mn catalysts have been evaluated as selective catalyst reduction (SCR) catalysts. However, in these previous studies, only the reaction characteristics and catalytic activity on the NH3 SCR over Mn catalysts were evaluated. There have been no studies on the effect of pH during catalyst preparation. Therefore, in this study, the effect of pH during the catalyst preparation process was examined and a new application of the Mn catalysts was proposed based on the current findings.
Introduction
Emission of nitrogen oxides (NOx) produced during the combustion of fossil fuels such as natural gas, oil, and coal can cause diminished visibility, green house effects, and acid rain. Combustion also produces two of the most detrimental environmental pollutants, O3 and peroxyacetyl nitrate (PAN), which are components of photochemical smog that bond with oxygen under ultraviolet (UV) light (CitationBosch and Janssen, 1988). Accordingly, the demand for more eco-friendly and cost-effective technologies to remove NOx from fixed sources of nitrogen oxides is increasing, and selective catalyst reduction (SCR) is the most prevalent method developed to meet these demands in terms of reliability and affordability.
SCR involves the installation of a catalyst coated with reducing agents such as NH3 inside a chimney through which exhaust gases are expelled, which selectively decomposes NOx into harmless substances such as N2 and H2O2. Catalyst development is an essential aspect of this process, since the position of the catalyst within the chimney and the operating temperature affect the catalytic activity. Currently, V2O5/TiO2 catalysts are the most widely used commercial catalysts for SCR, with WO3 or MoO3 frequently added as co-catalysts. In order to maximize SCR activity, the catalyst requires a sufficiently high temperature (above 300 °C) and low space velocity. Because reheating the exhaust gas to maintain sufficient catalytic activity would be enormously expensive (CitationKang et al., 2007; CitationPeña et al., 2004), the development of low-temperature SCR catalysts is essential to addressing the current problems of commercial catalysts and the efficient removal of NOx. An additional consideration is that SCR catalysts are often deactivated by SO2, so it must be utilized after the flue gas desulfurization (FGD) process has removed SO2 from the gas emissions.
Toward this end, various transition metals such as Mn, Cu, Cr, Co, and Fe have been placed onto a variety of supports such as Al2O3, SiO2, and TiO2 to develop improved catalysts for SCR, which have shown excellent activity in the low temperature range. In particular, Mn-based catalysts placed on supports such as TiO2 (CitationQi and Yang, 2003; CitationSmirniotis et al., 2001), Al2O3 (CitationSingoredjo et al., 1992), NaY (CitationBentrup et al., 2001), Ultra Stable Y zeolite (USY) (CitationQi et al., 2003), Activated Carbon (AC) (CitationMarbán and Fuertes, 2001; CitationYosikawa et al., 1998), and natural manganese oxides (CitationKim et al., 2011) have been extensively studied, as they readily undergo the redox cycle and the oxidation state of manganese ions can be easily changed (CitationKim and Hong, 2007).
Sol-gel studies conducted on Mn-based catalysts have shown that structure has the largest effect on activity, which is dependent on the manufacturing method but independent of Mn content (CitationBai et al., 2009; CitationMohamed et al., 2007; CitationSchmpf et al., 2002).
Singoredjo et al. (CitationSingoredjo et al., 1992) observed that Mn-based catalysts displayed the highest activity in the temperature range of 107–297 °C and that the activity was dependent on the oxidation state of manganese. In addition, Peña et al. (CitationPeña et al., 2004) showed that the catalysts containing Mn produced the highest NOx conversion in SCR activity tests. Using X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) analysis, these authors found that Mn4+ was the predominant Mn species on the catalyst surface. In contrast, Li et al. (CitationKim et al., 2011) reported that the superior activity of the catalyst was caused by the high Mn/Ti ratio on the catalyst surface as well as the presence of amorphous Mn2O3 species.
Several studies examining the effects of the valence state of manganese and the morphology of Mn/TiO2 catalysts have been reported (CitationEttireddy et al., 2007; CitationKapteijn et al., 1994; CitationLi et al., 2007; CitationPark et al., 2001; CitationPeña et al., 2004; CitationSingoredjo et al., 1992,; CitationYamashita and Vannice, 1996). However, no previous study has evaluated the nature of the Mn oxidation state, which we addressed in this study along with the relationship between Mn valence and catalytic activity.
Experimental
Catalyst Preparation
The physical characteristics of the eight types of commercial TiO2 used as supports are presented in ; lists their compositions. The solution-impregnation method was used to prepare the Mn/TiO2 catalysts. Manganese(II) nitrate hydrate (Mn(NO3)2·xH2O, 98%) was dissolved in 50 mL of distilled water to produce a bright red solution. TiO2 was mixed in with gentle stirring, maintaining the content of Mn in TiO2 at 10 wt% for all samples. After 1 hr of agitation, the water was evaporated under vacuum at 0.086 atm and 70 °C using a rotary vacuum evaporator. Evaporated samples were dried for 24 hr at 110 °C in a dry oven to remove residual water. The samples were then calcined and prepared in a tubular furnace by increasing the velocity from 10 to 400 °C/min over 4 hr.
Table 1. Physical properties of various TiO2 supports tested
Table 2. The composition of various TiO2 supports tested
To adjust the pH of TiO2, nitric acid (HNO3, 60–62%) or ammonium hydroxide (NH4OH, 25–28%) was added to the TiO2 slurry. The pH of the slurry was continuously monitored during adjustment using a pH meter (Ecoscan pH 6). Manganese(II) nitrate hydrate was then added into the pH-adjusted TiO2 slurry using the same process described above.
Characterization
The specific surface area of TiO2 was measured using an ASAP 2010C and the Brunauer Emmett-Teller (BET) formula. For this analysis, the samples were degassed under vacuum at 110 °C for 3–5 hr prior to analysis.
The structure of the materials was examined by XRD using Cu Kα radiation. Diffraction peaks, which were recorded in a 2θ range of 10° to 90°, were used to identify the structure of the sample.
XPS analysis was performed using an instrument equipped with an Al Kα monochromate (1486.6 eV) excitation source. After the catalysts were completely desiccated by drying at 100 °C for 24 hr, they were analyzed without surface sputtering and etching under vacuum maintained at 10–12 mm Hg. Wide-scan spectra were taken to determine the binding energies and intensities of Mn, Ti, O, and C in the samples.
Inductively coupled plasma (ICP) analysis was performed using a Perkin-Elmer Optima 3000XL (PerkinElmer Inc., USA) with a Radio Frequency (RF) power level of 1300 watts, 15 L/min plasma flow, 0.5 L/min coolant flow, and 0.8 L/min nebulizer flow. In a Teflon bottle, 0.1 g of the sample was decomposed with 2 mL of acid (HF, HNO3, and HClO4, 4:4:1 v/v), then diluted with distilled water prior to ICP analysis. Ion chromatography (IC) was conducted using a DIONEX-120 Automated Dual Column IC (Dionex Co., USA) equipped with a AS4A SC 4-mm column using a 1 mL/min flow rate, 1.8 mM Na2CO3/1.7mM NaHCO3 eluent, and a ASRS-I 4-mm anion self-regenerating suppressor (Dionex Co., USA).
Activity Test
The activity test apparatus consisted of a gas injector, a reactor, and a reaction gas analyzer. The amount of gas supplied to the reactor was regulated using a mass flow controller (MFC; MKS Co., USA) on individual cylinders of N2, O2, NO (1% NO/N2), and NH3 (1% NH3/N2). The reaction conditions were as follows: 200 ppm NO, 200 ppm NH3, 8% O2, 8% H2O, balance N2, and 500 mL/min total flow rate. Water was supplied in conjunction with N2 injection through a bubbler to the reactor. Water at 45 °C was circulated in a double jacket outside of the bubbler to maintain the temperature of the reactor using a circulator (CW-05G; JEIO TECH. Co., Korea). The gas supply line was made of stainless steel and maintained at 180 °C using a heating band wrapped around the line to prevent the formation of salts such as NH4NO3, NH4NO2, and NH4HSO4, which are produced by the reaction between NO and NH3 and condensation in the reaction gases.
The continuous-flow reactor had a diameter of 8 mm and height of 65 cm. A quartz tube was used to fix the catalyst layer. The temperature of the reactor was regulated with a PID temperature controller using a K-type thermocouple installed on the lower part of the fixed layer, and the temperature above and below the catalyst layer was measured by the same type of thermocouple installed on the upper catalyst layer near the gas injector.
The concentration of NO was measured using an Non Dispersive Infrared absorption (NDIR) gas analyzer (ZKJ-2; Fuji Electric Co., Ltd., Japan), and the NO2 (9L: NO2 [0–300 ppm]) and ammonia concentrations (3M: NH3 [0–800 ppm]; 3La: NH3 [0–200 ppm]; 3L: NH3 [0–60 ppm]) were measured using a detector tube (GASTEC Co., Japan), respectively. In addition, water from all gases was removed using a water trap in the chiller, which was a cooling bath maintained at −40 °C (CTB-10; JEIO TECH. Co., Korea) that was positioned before the analyzer and detector tube.
Results and Discussions
Effect of the Support Type on Catalytic Activity
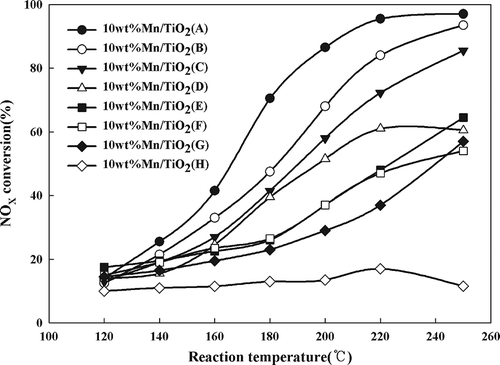
Activity tests of Mn/TiO2 catalysts were carried out at a space velocity of 60,000 hr−1 to investigate the influence of different TiO2 supports. The activity was found to vary depending on the type of TiO2 support employed (). The highest conversion (>80%) was observed for Mn/TiO2 (A) at 200 °C, whereas the Mn/TiO2 (H) displayed the lowest activity (<20%). The order of catalytic activity was as follows: A > B > C > D > F > E > G > H.
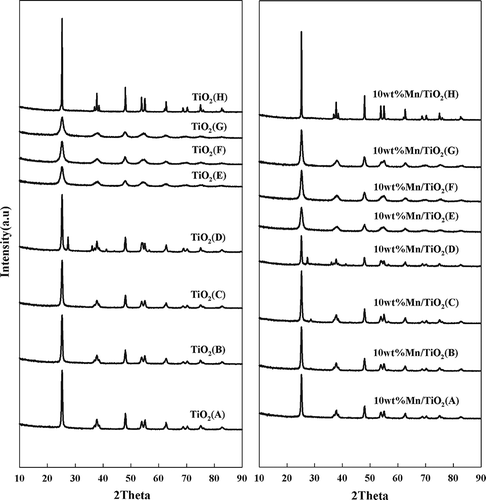
shows the results of the XRD analysis of the TiO2 supports and Mn/TiO2 catalysts. Except for the TiO2 (D) sample, which contained 25% rutile phase, all catalysts were in the anatase phase. The intensity and sharpness of the TiO2 peak did not vary significantly, indicating that the deposited Mn was highly dispersed on the surface of the support and existed in the amorphous phase (CitationPeña et al., 2004).
Effect of Valence State on Catalyst Characteristics
Mn is a multivalent metal that adopts different valence states based on the preparation conditions and temperature, which is relevant because SCR reaction efficiencies are dependent on oxidation state (CitationKapteijn et al., 1994; CitationPark et al., 2001; CitationSingoredjo et al., 1992,). XPS analysis was performed to assess the effect of the oxidation state on the catalytic activity (). The spectra were background-corrected based on the C–C bonding (284.6 eV) of the C 1s peak. shows the wide-scan spectrum of the Mn/TiO2 (A) catalyst, which displayed excellent activity. These peaks were typical for the Mn/TiO2 catalyst and indicated that the sample contained the component atoms C, O, Mn, and Ti. Ti can be identified from the unique bonding energy of the internal electron. The Mn 2p spectrum of Mn/TiO2 (A) is shown in . The peaks corresponding to MnO2 (Mn4+), Mn2O3 (Mn3+), and Mn(NO2)2 revealed bond energies of 642.2 ± 0.4 (CitationPeña et al., 2004; CitationSong et al., 2009), 641.2 ± 0.2 (CitationPeña et al., 2004; CitationSong et al., 2009), and 643.8 (CitationPeña et al., 2004; CitationPark et al., 2001) eV, respectively. When manganese nitrate was used as the Mn precursor in the preparation process and the sample was calcined at a low temperature, it was not completely removed (CitationPeña et al., 2004). Pêna et al. (CitationPeña et al., 2004) performed XPS analysis on a catalyst prepared using manganese nitrate and determined that calcination reduced the intensity of the manganese nitrate peak, in agreement with our results.
Figure 3. (a) Survey spectra measured for 10 wt% Mn/TiO2 (A) catalysts by XPS analysis. (b) Deconvoluted XPS result of Mn 2p for 10 wt% Mn/TiO2 (A).
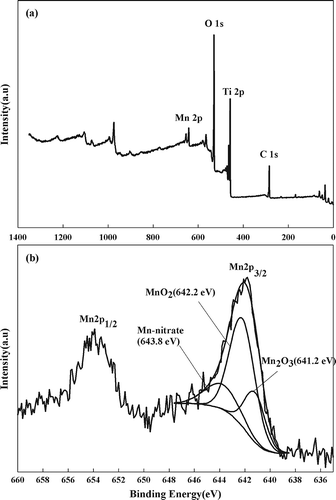
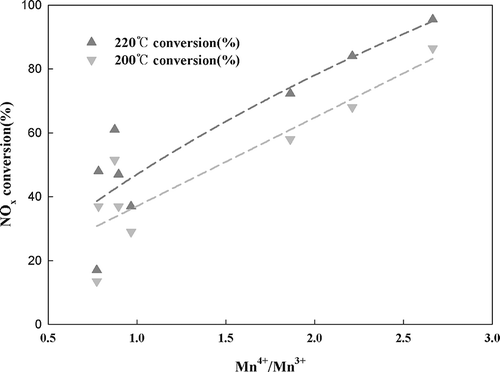
The atomic surface concentrations of Mn/TiO2 catalysts are shown in The Mn/TiO2 (A), Mn/TiO2 (B), and Mn/TiO2 (C), which all displayed excellent activity, had Mn4+/Mn3+ ratios ranging from 1.86 to 2.66, whereas the Mn4+/Mn3+ ratios of the other, poorer catalysts ranged from 0.77 to 0.96. The correlation between the Mn4+/Mn3+ ratio and catalytic activity is shown in Higher Mn4+ content also correlated with higher NOx conversion rates, meaning that catalysts with the Mn4+ species present on the surface displayed excellent SCR activity. Kapteijn et al. (CitationKapteijn et al., 1994), who investigated SCR activity of manganese oxides on γ-Al2O3 supports, reported that MnO2 (Mn4+) was an excellent catalytic metal oxide in terms of its ability to reduce NO. Pêna et al. (CitationPeña et al., 2004) observed similar results, providing further support for our observed result that valence state affects the activity of Mn-based catalysts and that the Mn4+ species provided the highest activity.
Table 3. Atomic surface concentrations of the catalysts determined by XPS
Effect of pH on the Mn Valence State
It has been shown that the pH of the manganese solution used to prepare the catalyst affects the stability of the Mn oxidation state (CitationStone, 1983). Accordingly, we examined the effect of the pH of the TiO2 aqueous slurry during the catalyst preparation process on the Mn valence state. The pH of each TiO2 aqueous slurry is listed in , and the correlation between the pH of the TiO2 aqueous slurry and the rate of NOx conversion is shown in . The latter were shown to be linearly related, with catalysts prepared at low pH values displaying excellent NOx conversion rates. As shown in , the Mn4+/Mn3+ ratio decreased with a decrease in the pH of the slurry.
Table 4. The potential of hydrogen over various TiO2 supports
Figure 5. (a) Correlation between TiO2 pH and NOx conversion over 10 wt% Mn/TiO2. (b) Correlation between TiO2 pH and Mn valence state over 10 wt% Mn/TiO2.
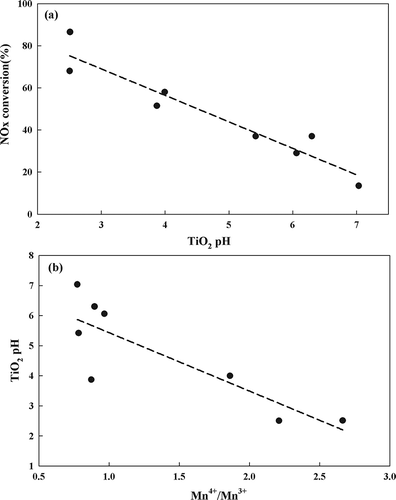
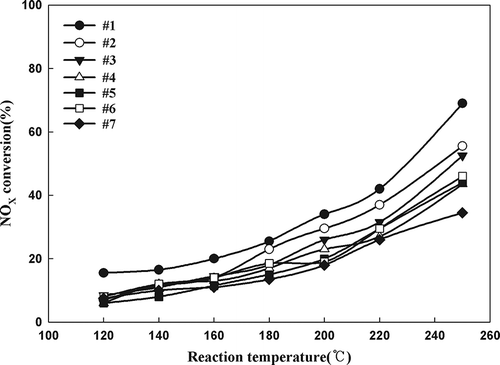
In order to better understand these results, one TiO2 support was selected and 10 wt% Mn/TiO2 catalysts were prepared at 7 different pH values ranging from 1.5 to 9.15. TiO2 (G), which displayed a medium activity level, was selected for these experiments. Before the Mn was impregnated, the pH was adjusted by adding nitric acid or ammonium hydroxide to the TiO2 slurry. shows the pH values used during the preparation of Mn/TiO2 (G); 4 represents the condition used without adjusting the pH, 1–3 represent the addition of nitric acid, and 5–7 represent the addition of ammonium hydroxide. The results of the SCR activity tests of the catalysts prepared under the above conditions are shown in The catalysts prepared at lower pH values displayed higher activities. XPS was used to determine the Mn valence state of catalysts 1–7 prepared with varying pH conditions. The atomic surface concentrations of each peak corresponding to catalysts 1–7 are shown in As shown in , the valence state of Mn was found to be dependent on the pH of the TiO2 aqueous slurry. Thus, the Mn/TiO2 catalytic activity was closely correlated to the oxidation state of Mn and to the pH during the catalyst preparation. These finding clearly demonstrate that the activity can be enhanced by adjusting the pH of the TiO2 aqueous slurry.
Table 5. The potential of hydrogen TiO2(G)
Table 6. Atomic surface concentrations of the catalysts determined by XPS
Conclusions
We found that the catalytic activity of the Mn/TiO2 catalysts studied was dependent on the type of TiO2 support used. XPS and XRD analysis confirmed that high catalytic activity was achieved when the Mn4+ species was predominant on the catalyst surface, and that the Mn valence state was dependent on the pH of the TiO2 aqueous slurry during the catalyst preparation process.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0023963).
References
- Bai , G. , Fan , X. , Wang , H. , Xu , J. , He , F. and Ning , H. 2009 . Catalysis Communications , 10 : 2031 – 2035 .
- Bentrup , U. , Bruckner , A. , Richter , M. and Fricke , R. 2001 . Applied Catalysis B , 32 : 229 – 241 .
- Bosch , H. and Janssen , F. 1988 . Catalysis Today , 2 : 369 – 379 .
- Ettireddy , P.R. , Ettireddy , N. , Mamedov , S. , Boolchand , P. and Smirniotis , P.G. 2007 . Applied Catalysis B , 76 : 123 – 134 .
- Kang , M. , Park , E.D. , Kim , J.M. and Yie , J.E. 2007 . Applied Catalysis A , 327 : 261 – 269 .
- Kapteijn , F. , von Langeveld , A.D. , Moulijn , J.A. , Andreini , A. , Vuurman , M.A. , Turek , M.A. , Jehng , J.M. and Wachs , I.E. 1994 . Journal of Catalysis , 150 : 94 – 104 .
- Kim , S.S. and Hong , S.C. 2007 . Journal of Korean Industry and Engineering Chemistry , 18 : 350 – 355 .
- Kim , S.S. , Lee , S.M. , Park , K.H. , Kwon , D.W. and Hong , S.C. 2011 . Journal of the Air and Waste Management Association , 61 : 552 – 558 .
- Li , J. , Chen , J. , Ke , R. , Luo , C. and Hao , J. 2007 . Catalysis Commumnications , 8 : 1896 – 1900 .
- Marbán , G. and Fuertes , A.B. 2001 . Applied Catalysis B , 34 : 43 – 53 .
- Mohamed , M.M. , Othman , I. and Mohamed , R.M. 2007 . Journal of Photochemisty Photobiolgy A , 191 : 153 – 161 .
- Park , T.S. , Jeong , S.K. , Hong , S.H. and Hong , S.C. 2001 . Industrial and Engineering Chemistry Research , 40 : 4491 – 4495 .
- Peña , D.A. , Uphade , B.S. and Smirniotis , P.G. 2004 . Journal of Catalysis , 221 : 421 – 431 .
- Qi , G. and Yang , R.T. 2003 . Applied Catalysis B , 44 : 217 – 225 .
- Qi , G. , Yang , R.T. and Chang , R. 2003 . Catalysis Letter , 87 : 67 – 71 .
- Schmpf , S. , Lucas , M. , Mohr , C. , Rodemerck , U. , Brukner , A. , Radnik , J. , Hofmeister , H. and Claus , P. 2002 . Catalysis Today , 72 : 63 – 78 .
- Singoredjo , L. , Korver , R. , Kapteijn , F. and Moulijn , J.A. 1992 . Applied Catalysis B , 1 : 297 – 316 .
- Smirniotis , P.G. , Peña , D.A. and Uphade , B.S. 2001 . Chemistry International Education , 40 : 2479 – 2482 .
- Song , K.S. , Bae , J.S. and Lee , G.H. 2009 . Economic and Environment Geology , 42 : 479 – 486 .
- Stone , A.T. 1983 . The reduction and dissolution of manganese (III) and (VI) oxides by organics , Pasadena , CA : Ph.D. dissertation, California Institute of Technology .
- Yamashita , T. and Vannice , A. 1996 . Journal of Catalysis , 163 : 158 – 168 .
- Yosikawa , M. , Yastutake , A. and Mochida , I. 1998 . Applied Catalysis A , 173 : 239