Abstract
Pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), peptidoglycan (PGN), Polyinosinic-polycytidylic acid (poly I:C), and CpG Oligodeoxynucleotides (ODN) are recognized by Toll-like receptors (TLR). This study aimed to investigate the effect of diverse PAMPs on the transcription of TLR signaling pathway genes in goat blood. Whole blood was collected from 3 female BoerXSpanish goats and treated with the following PAMPs: 10 µg/ml LPS, PGN, CpG ODN (2216), CpG ODN (2006), and 12.5 µg/ml Poly I:C. Blood-treated PBS served as a control. The expression of 84 genes in the human TLR signaling pathway RT2 PCR Array (Qiagen) was evaluated using real-time PCR. Treatment with PBS affected the expression of 74 genes, Poly I:C affected the expression of 40 genes, t ODN 2006 affected the expression of 50 genes, ODN 2216 affected the expression of 52 genes, LPS affected the expression of 49 genes, while PGN affected the expression of 49 genes. Our results show that PAMPs modulated and increased the expression of genes in the TLR signaling pathway. These results highlight important insights into how the host responds to different pathogens and may help design adjuvants for therapeutics and vaccines that target different.
Introduction
One of the main host-pathogen defensive mechanisms is the innate immune system. The immune system is triggered when pathogen-associated molecular patterns (PAMPs) expressed by infectious microorganisms interact with receptors present in immune cells. The PAMPs that activate innate immune responses include LPS fraction of Gram-negative bacteria, PGN from Gram-positive bacteria, unmethylated bacterial DNA fragments, glucans, and proteins derived from the fungal cell wall.Citation1,Citation2 Synthetic PAMPS such as bacterial CpG oligodeoxynucleotides (ODNs) and Polyinosinic-polycytidylic acid (Poly (I:C)), a synthetic analog of double-stranded RNA (dsRNA), can serve as adjuvants for vaccines to accelerate and enhance an antigen-specific immune response.Citation3 Pathogen recognition receptors (PRR) detect PAMPS and initiate protective responses.Citation4–6 PRR activates downstream signaling pathways, which induce an innate immune response by producing cytokines, chemokines, and other mediators. As the first line of defense, the innate immune system is initiated by PRR sensing PAMPs or damaged cells.Citation7
One critical PRR that plays a crucial role in innate immunity and forms the first line of defense against infection is the type 1 transmembrane protein called Toll-like receptors (TLR). TLRs can control inflammation and trigger innate immune signaling pathways. Toll-like receptors are innate immune system receptors present in animal cells that recognize microbial markers, namely proteins, carbohydrates, lipids, nucleic acids, and their combinations in an efficient, non-self-reactive manner to initiate a complex signaling cascade and activate a wide variety of transcription factors and inflammatory cytokines.Citation8,Citation9 They are expressed in various cells, including dendritic cells, neutrophil macrophages, natural killer cells, T and B lymphocytes, non-immune cells like epithelial cells, endothelial cells, etc., and fibroblasts.Citation10 Cells transit rapidly to sites of infection, limiting infection and allowing recruitment and activation of other immune cells by releasing inflammatory mediators and antimicrobial products, resulting in pathogen clearance and, ultimately, in the initiation of adaptive response. These interactions have high specificity and involve biochemically different ligands.Citation11 Upon PAMPs and DAMPs recognition, TLRs recruit TIR domain-containing adaptor proteins such as MyD88 and TRIF, which initiate signal transduction pathways that culminate in the activation of NF-κB, IRFs, or MAP kinases to regulate the expression of cytokines, chemokines, and type I IFNs that ultimately protect the host from microbial infection.Citation12
There are at least 10 TLRs expressed in goat blood with the ability to recognize different PAMPs.Citation13 In particular, TLR4 is the receptor for LPS, TLR3 binds dsRNA, including the viral mimic Poly I:C, TLR9 binds to bacterial CpG (ODN) DNA, and TLR2 binds to PGN.Citation8,Citation11,Citation14,Citation15 These receptors are known to differ in location and downstream signaling pathways. Individual TLRs have been shown to use different adaptor proteins. For example, TLR4 signaling involves the adaptor molecules MyD88 and TRIF, the TLR3 pathway involves only the TRIF adaptor, while TLR9 and TLR2 signals through MyD88.Citation16,Citation17
The definition of PAMP binding’s effect may help define how TLR activation guides innate and adaptive immunity and can be exploited to develop more effective immunotherapy strategies to control pathogenic diseases in ruminants.Citation18 This study aimed to investigate the effect of diverse PAMPs on the transcription of TLR signaling pathway genes in goat blood.
Materials and methods
Animals and housing
Three (3) non-pregnant female Boer X Spanish goats from the North Carolina Agricultural and Technical State University Farm were used. The animals were clinically healthy and not under any treatment. All experiments were approved according to the Institutional Animal Care and Use Committee (IACUC ID: 15-006.0).
Preparation of pathogen-associated molecular patterns
Ten (10) µg/ml of Escherichia coli-derived Lipopolysaccharide (LPS) (Sigma-Aldrich St. Louis, Missouri, USA), 10 µg/ml of Staphylococcus aureus-derived Peptidoglycan (PGN) (Sigma-Aldrich St. Louis, Missouri, USA), 12.5 µg/ml of Polyinosinic-polycytidylic acid (poly I:C), a synthetic analog of double-stranded RNA (dsRNA), 10 µg/ml CpG ODN (2216) is a prototype of the class of CpG-A oligodeoxynucleotides (ODN), also known as 'D’-type ODN, and contains a phosphodiester (PO) backbone, and 10 µg/ml CpG ODN (2006) class B: is a prototype 'K'-type ODN, with a full phosphorothioate (PS) backbone were prepared using endotoxin-free PBS.
Blood collection and stimulation with different PAMPs
Whole blood (10 mL) was collected from the jugular vein into tubes containing ACD anticoagulants. Blood samples (106 cells/mL of viable cells) were incubated with 10 µg/mL LPS, PGN (Sigma-Aldrich St. Louis, MO, USA), CpG ODN (2216) class A, CpG ODN (2006) class B, or 12.5 µg/mL of Poly I:C, Phosphate Buffer Saline (PBS) individually to assess the expression of TLR-pathway genes. Samples were incubated at 37 °C, with 85% humidity and 5% CO2, for 30 mins. Samples incubated with PBS served as a negative control. At the end of the incubation period, cells were centrifuged at 1700 × g at 4 °C for 5 minutes.
RNA extraction and cDNA synthesis
The supernatant was collected, and TRIzol was added to cell pellets and stored at −20 °C for RNA isolation. Total RNA was isolated as previously described by Ekwemalor.Citation19 The quantity and quality of RNA were measured using the ND-1000 UV/VIS Nanodrop (NanoDrop Technologies) spectrophotometer (260 and 260/280 nm, respectively). The synthesis of cDNA was performed using the QuantiTect Reverse Transcription Kit (Qiagen), as previously discussed by Adjei-Fremah et al. and Asiamah et al.Citation4,Citation20,Citation21 Total RNA (2 µg) from each treatment group was used for cDNA synthesis. The cDNA products were measured for purity and concentration using the Nanodrop spectrophotometer (NanoDrop Technologies).
Real-time PCR
Real-time PCR was performed using the Human TLR array (Qiagen, Valencia, CA, USA) containing specific primer sets for 84 relevant TLR pathway genes. Each gene tested was done in triplicates. Gene expression was normalized using the housekeeping genes GAPDH, ACTB, HPRT1, TBP, and YWHAZ. Real-time PCR was performed on the CFX96TM detection system (Biorad). Amplification occurred in a 25 µl reaction volume that contained SYBR green master mix under the following conditions.Citation19 The average ct value was taken. Fold change in gene expression was calculated using the 2−ΔΔCt method.Citation22 Fold change was set at a cutoff of 2.
Results
Effect of PAMPs on 84 genes in the TLR signaling pathway
This study evaluated the effect of diverse PAMPs on the transcription of genes in the TLR signaling pathway. Genes on the TLR array are associated with the fungal and parasitic response; TLR signaling, cytokine signaling, and downstream signaling of TLR were expressed in goat blood. Treatment with PBS affected the expression of 74 genes, treatment with Poly I:C affected the expression of 40 genes, treatment with ODN 2006 affected the expression of 50 genes, treatment with ODN 2216 affected the expression of 52 genes, treatment with LPS affected the expression of 49 genes while treatment with PGN affected the expression of 49 genes (). In the PBS group, genes not expressed are listed in .
Figure 1. Comparison of toll-like receptor signaling pathway gene expression in response to divers PAMPS compared to control. LPS: Escherichia coli-derived Lipopolysaccharide; PGN: Staphylococcus aureus-derived peptidoglycan; poly I:C, ODN 2216: CpG ODN (2216) class A, ODN 2006:CpG ODN (2006) class B, and control (phosphate buffered saline).
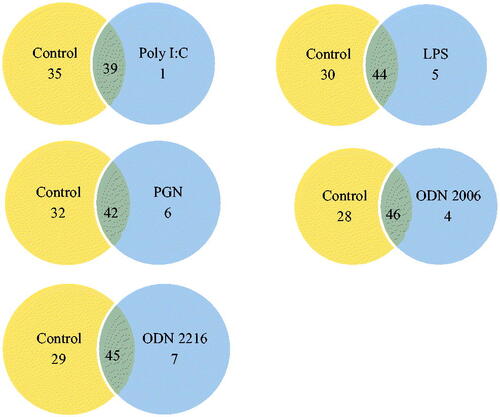
Table 1. Genes that were not expressed as a result of treatment with PBS (control).
A targeted subset of genes was differentially affected by PAMPs. Following Poly I:C treatment, 24 genes were up-regulated (), and 15 were down-regulated (). The gene MAPK8 was induced in response to the poly I:C treatment. Thirty-nine genes were both expressed in the control and poly I:C treatments.
Table 2. Fold change of up-regulated genes due to treatment with Poly I:C.
Table 3. Fold change of down-regulated genes due to treatment with Poly I:C.
Following treatment with LPS, 28 genes were up-regulated (), and 15 were down-regulated (). The genes IRF1, IL2, IL1A, CD80, and CCL2 were induced in response to LPS treatment. Forty-four genes were both expressed in the control group and treatment group.
Table 4. Fold change of up-regulated genes due to treatment with LPS.
Table 5. Fold change of down-regulated genes due to treatment with LPS.
After treatment with PGN, 15 genes were up-regulated (), and 28 were down-regulated (). The gene FADD, IL1B, IRF1, MYD88, RELA, and TLR9 were induced in response to PGN treatment. Forty-three genes were both expressed in the control group and treatment group.
Table 6. Fold change of up-regulated genes due to treatment with ODN 2006.
Table 7. Fold change of down-regulated genes due to treatment with ODN 2006.
Following ODN 2006, 29 genes were up-regulated (), and 17 were down-regulated (). The genes HSPD1, IFNG, MAPK8, and PTGS2 were induced in response to ODN 2006 treatment. Forty-six genes were both expressed in the control group and treatment group.
Table 8. Fold change of up-regulated genes due to treatment with ODN 2216.
Table 9. Fold change of down-regulated genes due to treatment with ODN 2216.
Following ODN 2216, 26 genes were up-regulated (), and 19 were down-regulated (). The genes CLEC4E, HSPD1, IFNG, IRF1, MAPK8, PTGS2, and UBE2V1 were induced in response to ODN 2216 treatment. Forty-five genes were both expressed in the control group and treatment group.
Table 10. Fold change of up-regulated genes due to treatment with PGN.
Table 11. Fold change of down-regulated genes due to treatment with PGN.
Discussion
Toll-like receptors play a critical role in initiating innate immune responses and modulating adaptive immunity by recognizing conserved microbial molecular patterns.Citation23,Citation24 TLR agonist studies as adjuvants are gaining enormous interest because they are prime initiators of inflammation.Citation25–27 Our results show the expression of genes in the TLR signaling pathway in goat blood after treatment with the PAMPs LPS, PGN, ODN2006, ODN2216, and viral RNA Poly I: C. These PAMPs are associated with infectious and metabolic diseases in animals impacting health and production. Previous studies have reported the expression of TLRs 1–10 in goats.Citation13,Citation28 Studies have shown that TLR recognition of microbial products triggers the activation of downstream signaling pathways where MyD88 and TIR domain-containing adaptor-inducing IFN-β (TRIF) leads to activation of NF-κB and subsequent transcription of pro-inflammatory cytokines.Citation29,Citation30
Diverse PAMPS activated the transcription of TLR-signaling pathway genes in goat blood. This study showed that treatment with Poly I:C affected the activation and expression of immune response genes. Poly I:C serves as a PAMP associated with a viral infection. In this study, Poly I:C increased the expression of TLR3 and TLR7 as well as cytokines and other downstream signaling genes, thereby supporting previous by.Citation31–35 Thus, the poly I:C response may be mediated by the TRIF-dependent pathway, which leads to the activation of NF-κB and the production of cytokines such as TNF.Citation8 In our study, treatment with Poly I:C modulated the expression of TICAM1 (TRIF). Our results show the expression and modulation of genes associated with this pathway, thereby suggesting its mediation through this pathway.
The innate immune system modulates the host’s immunological resistance against bacterial infection. Lipopolysaccharide represents a major PAMP from Gram-negative bacteria’s outer membrane.Citation36–38 Lipopolysaccharide is a potent stimulator of the innate immune system that provides the first line of defense against pathogens. The TLR4 belongs to a family of transmembrane receptors initially identified in Drosophila, responsible for recognizing and responding to PAMPs such as LPS.Citation39,Citation40 TLR 4 is mainly expressed on the cytoplasmic membrane of hematopoietic cells such as macrophages, monocytes, and dendritic cells.Citation41 In our study, treatment with LPS increased the expression of TLR4. This result supports previous studies which show that innate immune response to gram-negative bacteria is triggered through TLR4.Citation2,Citation42–44 Our results show an increase in cytokine encoding genes’ expression and modulation, as reported by others.Citation45 Furthermore, it has been shown that the LPS response is mediated by MyD88 and TRIF-dependent signaling pathways.Citation46,Citation47 In our study, treatment with LPS increased the expression and transcription of MYD88 and down-regulated the expression of NFKB1, thus supporting previous research and suggesting their mediation through this pathway.
Peptidoglycan is a component of nearly all bacterial cell walls and has a variety of functions. Previous studies have reported that PGN is recognized by TLR2.Citation11,Citation48,Citation49 We have previously reported that TLR2 is widely expressed in goat blood.Citation50 In this study, PGN increased the expression of TLR2, which supports previous findings.Citation51 Treatment with PGN induced the expression of MyD88, thereby suggesting its mediation through this pathway, as previously reported.Citation17 Our results show the expression of genes, cytokines, and chemokines involved in this signaling pathway. It has been reported that PGN causes excessive production of pro-inflammatory cytokines.Citation52 In our study, treatment with PGN increased the transcription of TNF, IL-10, and other cytokines. It can be said that PGN modulates the transcription of TLR2 and genes involved in downstream signaling.
Both bacterial DNA and synthetic oligodeoxynucleotides containing CpG motifs have been shown to induce or enhance the stimulation of various immune cells. CpG ODN 2006 and 2216 affected the activation and expression of TLR signaling pathway genes. CpG ODNs are short synthetic single-stranded DNA molecules containing unmethylated CpG dinucleotides in particular sequence contexts. In this study, ODN 2006 and 2216 activated and modulated the expression of TLR9. Previous studies have reported that CpG motifs trigger an innate immune response through TLR-9 and induce the secretion of pro-inflammatory cytokines and activation of immune cells.Citation53,Citation54 TLR9 has been identified as a receptor for unmethylated CpG DNA.Citation55 TLR9 signals through the MyD88 signaling pathway.Citation17 In our study, treatment with both ODNs did not affect the expression of MyD88. Previous studiesCitation56 reported an increased level of IL-6. In contrast to this study, both CpG ODN 2006 and 2216 reduced the expression of IL-6.
Conclusion
This study demonstrated that PAMPs affected genes involved in TLR signaling in goat blood. Treatment with different PAMPs modulated the expression of genes involved in the expression of TLR signaling pathway genes. Our results also indicate that the signaling pattern triggered by these distinct receptors is broadly similar but includes reproducible differences. Understanding these crucial processes is vital for understanding how the host responds to different pathogens and may aid in designing adjuvants for therapeutics and vaccines that target different pathways in small ruminants.
Acknowledgments
The authors are grateful to North Carolina Agricultural and Technical State University Small Ruminant Research Unit. Thanks to Gary Summers and Dr. Hamid Ismail for their assistance and to members of the Genomic Diversity and Animal Biotechnology laboratory.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11(5):373–384.
- Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14(1):103–110.
- Schijns V. Immunological concepts of vaccine adjuvant activity. Curr Opin Immunol. 2000;12(4):456–463.
- Adjei-Fremah S, Ekwemalor K, Asiamah EK, Ismail H, Ibrahim S, Worku M. Effect of probiotic supplementation on growth and global gene expression in dairy cows. J Appl Anim Res. 2018;46(1):257–263.
- Tartey S, Takeuchi O. Pathogen recognition and toll-like receptor targeted therapeutics in innate immune cells. Int Rev Immunol. 2017;36(2):57–73.
- Tirumurugaan K, Dhanasekaran S, Raj GD, Raja A, Kumanan K, Ramaswamy V. Differential expression of toll-like receptor mRNA in selected tissues of goat (Capra hircus). Vet Immunol Immunopathol. 2010;133(2–4):296–301.
- Offord V, Coffey TJ, Werling D. LRRfinder: a web application for the identification of leucine-rich repeats and an integrative toll-like receptor database. Dev Comp Immunol. 2010;34(10):1035–1041.
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511.
- Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–1066.
- Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, Bhatta R. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol. 2018;37(1):20–36.
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820.
- Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461.
- Ekwemalor K, Adjei-Fremah S, Asiamah E, Ismail H, Worku M. 0167 Exposure of bovine blood to pathogen associated and non pathogen associated molecular patterns results in transcriptional activation. J Anim Sci. 2016;94(suppl_5):81–81.
- Carl VS, Brown-Steinke K, Nicklin MJ, Smith MF. Toll-like receptor 2 and 4 (TLR2 and TLR4) agonists differentially regulate secretory interleukin-1 receptor antagonist gene expression in macrophages. J Biol Chem. 2002;277(20):17448–17456.
- Zhang Y, Liang X, Bao X, Xiao W, Chen G. Toll-like receptor 4 (TLR4) inhibitors: current research and prospective. Eur J Med Chem. 2022;235:114291.
- Takeda K, Akira S. TLR signaling pathways. Proc. Semin Immunol 2004;16(1):3–9.
- Yang IV, Jiang W, Rutledge HR, et al. Identification of novel innate immune genes by transcriptional profiling of macrophages stimulated with TLR ligands. Mol Immunol. 2011;48(15–16):1886–1895.
- Werling D, Coffey TJ. Pattern recognition receptors in companion and farm animals–the key to unlocking the door to animal disease? Vet J. 2007;174(2):240–251.
- Ekwemalor K. 2018. Detection of galectin expression and its modulation in goat peripheral blood. North Carolina Agricultural and Technical State University. Available from ProQuest Dissertations & Theses Global. (2161761938). Retrieved from http://ncat.idm.oclc.org/login?url=https://www.proquest.com/dissertations-theses/de
- Adjei-Fremah S, Ekwemalor K, Asiamah E, Ismail H, Worku M. Transcriptional profiling of the effect of lipopolysaccharide (LPS) pretreatment in blood from probiotics-treated dairy cows. Genom Data. 2016;10:15–18.
- Asiamah EK, Adjei-Fremah S, Ekwemalor K, Sordillo L, Worku M. Parity and periparturient period affects galectin gene expression in Holstein cow blood. J Appl Biotechn. 2018;6(2):20.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408.
- Kannaki T, Shanmugam M, Verma P. Toll-like receptors and their role in animal reproduction. Anim Reprod Sci. 2011;125(1–4):1–12.
- Tourais-Esteves I, Bernardet N, Lacroix-Lamandé S, Ferret-Bernard S, Laurent F. Neonatal goats display a stronger TH1-type cytokine response to TLR ligands than adults. Dev Comp Immunol. 2008;32(10):1231–1241.
- Dowling JK, Mansell A. Toll‐like receptors: the Swiss army knife of immunity and vaccine development. Clin Transl Immunol. 2016;5(5):e85.
- Goff PH, Hayashi T, Martínez-Gil L, et al. Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses. J Virol. 2015;89(6):3221–3235.
- Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589(1):1–13.
- Ekwemalor K, Adjei-Fremah S, Asiamah E, Worku M. Molecular genetics and genome biology of goats. 2018; https://doi.org/10.5772/intechopen.72414
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801.
- Xia P, Wu Y, Lian S, et al. Research progress on Toll-like receptor signal transduction and its roles in antimicrobial immune responses. Appl Microbiol Biotechnol. 2021;105(13):5341–5355.
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by toll-like receptor 3. Nature. 2001;413(6857):732–738.
- De Waele J, Marcq E, Van Audenaerde J, et al. 2018. PO-419 poly (I: C) prepares glioblastoma cells for anti-PD-L1 therapy via lymphocyte attraction and activation in a TLR3-dependent manner. BMJ Publishing Group Limited. ESMO Open, 3, A394-A395.
- Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322(2):231–238.
- Lopez AG, Bekiaris V, Müller-Luda K, et al. The role of TLR3/TRIF and type I IFN signaling in the migration of intestinal DC subsets in response to poly (I: C). Eur J Immunol. 2018;48(S1):150–150.
- Zhu Q, Egelston C, Vivekanandhan A, et al. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc Natl Acad Sci USA. 2008;105(42):16260–16265.
- Fermino ML, Polli CD, Toledo KA, et al. LPS-induced galectin-3 oligomerization results in enhancement of neutrophil activation. PLOS One. 2011;6(10):e26004.
- Ranf S. Immune sensing of lipopolysaccharide in plants and animals: same but different. PLOS Pathog. 2016;12(6):e1005596.
- Schmitz S, Pfaffl M, Meyer H, Bruckmaier R. Short-term changes of mRNA expression of various inflammatory factors and milk proteins in mammary tissue during LPS-induced mastitis. Domest Anim Endocrinol. 2004;26(2):111–126.
- Cochet F, Peri F. The role of carbohydrates in the lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) signalling. IJMS. 2017;18(11):2318.
- Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20(1):197–216.
- Lim KH, Staudt LM. Toll-like receptor signaling. Cold Spring Harb Perspect Biol. 2013;5(1):a011247.
- Adjei-Fremah S, Asiamah EK, Ekwemalor K, Jackai L, Schimmel K, Worku M. Modulation of bovine Wnt signaling pathway genes by cowpea phenolic extract. JAS. 2016;8(3):21.
- Asiamah EK, Ekwemalor K, Adjei-Fremah S, Osei B, Newman R, Worku M. Natural and synthetic Pathogen associated molecular patterns modulate galectin expression in cow blood. J Anim Sci Technol. 2019;61(5):245–253.
- Peri F, Piazza M, Calabrese V, Damore G, Cighetti R. Exploring the LPS/TLR4 signal pathway with small molecules. Portland Press Limited. Biochem Soc Trans 2010;38(5):1390–1395. https://doi.org/10.1042/BST0381390
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85(2):85–95.
- Bhardwaj N, Gnjatic S, Sawhney NB. TLR agonists: Are they good adjuvants? Cancer J. 2010;16(4):382.
- Gilliet M, Cao W, Liu Y-J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606.
- Takeuchi O, Kawai T, Mühlradt PF, et al. Discrimination of bacterial lipoproteins by toll-like receptor 6. Int Immunol. 2001;13(7):933–940.
- Zhu J, Dong J, Ji L, et al. anti-allergic inflammatory activity of interleukin-37 is mediated by novel signaling cascades in human eosinophils. Front Immunol. 2018;9:1445.
- Ekwemalor K, Asiamah E, Worku M. Effect of a mushroom (Coriolus versicolor) based probiotic on the expression of toll-like receptors and signal transduction in goat neutrophils. JMBR. 2016;6(1):71.
- Ghosh TK, Mickelson DJ, Fink J, et al. Toll-like receptor (TLR) 2–9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006;243(1):48–57.
- Wang F, Li Y, Yang C, et al. Mannan-binding lectin suppresses peptidoglycan-induced TLR2 activation and inflammatory responses. Mediat Inflamm. 2019;2019:1–12.
- Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20(1):709–760.
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146.
- Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451.
- Weeratna RD, Makinen SR, McCluskie MJ, Davis HL. TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848). Vaccine. 2005;23(45):5263–5270.
