Abstract
The study assessed chicken egg storage duration and short periods of incubation during egg storage (SPIDES) on embryo development. Eggs samples from 310 eggs from 72-week-old hybrid layer breeder flocks were divided and stored at 16 °C and 75% RH for 1, 4, 7, 10, 14, 17, and 21 days (D). Some eggs were also divided between 14D and 21D alone and stored. One subgroup received heat application for 6 h during storage resulting in 0-h × 14D (control); 0-h × 21D (control); 6-h × 14D (SPIDES) and 6-h × 21D (SPIDES). Storage durations between 1D and 21D did not influence most egg quality parameters. The interaction of storage duration and SPIDES affected initial, final egg weights and egg weight loss. SPIDES eggs recorded heavier wet embryos than non-SPIDES eggs at embryonic days (ED) 4, ED7, ED11, and dry embryo weight at ED11. SPIDES resulted in longer embryos at ED4 and ED11. In 21D stored eggs hatchability was higher in SPIDES compared to non-SPIDES treatments (66.7 vs. 48.3%). The reverse effect of SPIDES occurred in 14D stored eggs. In conclusion, the study shows positive effect of SPIDES in longer stored eggs and could be due to a positive impact on embryo.
Introduction
The production of day-old chicks is the mainstay of the poultry industry all over the world. As a result, the necessity of poultry hatcheries must be prioritized, and Ghana is no exception when it comes to ensuring food security through poultry production. Poultry production is an area of avian science that involves rearing chicks for eggs and meat (the major products), as well as side products including bird feathers, biodegradable litter, and blood meal.Citation1 In this light, as the cornerstone or core of the poultry business, the production of fertilized eggs and maintenance of quality has garnered more attention in the field of avian science.
In commercial hatcheries, before incubation, chicken eggs are collected, fumigated, and maintained in cold chambers at 15–20 °C and 75–80% relative humidity (RH) for short durations (3–7 days) on the farm and occasionally at the hatchery.Citation2 Meeting large incubator capacity with eggs, increasing demand for uniform day-old chicks, and lowering the cost of transporting small batches of eggs from the farm to the hatchery daily are all reasons for temporary cold egg storage.Citation3 However, where egg collection patterns are irregular and storage is inevitable, commercial hatcheries are more prone to adopt extended storage beyond 7 days. Several studies have found that the drawbacks of long-term egg preservation exceed the benefits. Longer storage times increase albumen deterioration, cell death, and embryonic mortality,Citation2,Citation4–14 reduce hatchability,Citation15–17 increase hatch timeCitation17–19 and increase chicks abnormalities.Citation20
Several optimizations have been proposed to mitigate the deleterious effects of egg storage periods longer than 7 days. Short periods of incubation during egg storage (SPIDES) have been shown to compensate for the negative effects of extended storage periods in broilers.Citation4,Citation16,Citation21,Citation22 Studies show that short-term storage of eggs is less damaging to blastodermal cells and therefore there is little need for cell repair.Citation2,Citation8 Subsequently, SPIDES in eggs stored for a short duration showed less benefit or any additive effect of heat treatment on embryonic performance compared to long-term storage beyond 15 days.Citation23 Additionally, when heat treatment was applied to eggs before storage the expression of genes causing cell death decreased in 14 days stored eggs but remained normal in eggs stored for 4 days.Citation8 Short periods of incubation during egg storage (SPIDES) have been studied thoroughly in primary breeder facilities to develop reliable commercial uses.Citation24 It is thought to mimic the natural conditions that birds experience before hatchingCitation21 and allows embryos to finish the development to hypoblasts, increasing the potential of more eggs to hatch even after 3 weeks of cold storage.Citation24
The effect of SPIDES during eggs storage has been extensively tested using eggs from a grandparent and parent stock flocks of Ross 308 broilers,Citation24,Citation25 with little information on eggs from a hybrid breeder known as ‘Black-tailed’ recently developed in Ghana as a tropical breed to withstand the effects of heat stress for egg production compared to indigenous breeds. Additionally, although egg storage duration and SPIDES have been investigated individually, the influence of the combination of these parameters on incubation parameters is largely unknown. Therefore, using eggs obtained from this hybrid layer grandparent breeder flock, this study evaluated the impacts of storage duration and SPIDES on basic parameters of egg quality, egg weight loss, embryo weight changes, hatching traits, hatchability, and post-hatch juvenile chick quality and haematological indices.
Materials and methods
Study site and the ethical aspects of the study
The study was conducted at the Department of Animal Science, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi. All experimental procedures followed the approved and appropriate ethical Procedure for the Animal Research Ethics Committee (AREC) of the Kwame Nkrumah University of Science and Technology, Kumasi-Ghana, Quality Assurance, and Planning Unit (KNUST POLICY 0016).Citation26
Experimental design and treatment
A total of 310 eggs were collected at random from 72-week-old hybrid breeder flocks called ‘Black-tailed breed’Citation27,Citation28 used for the study. Eggs (n = 70) were separated and stored for 7 different durations (1, 4, 7, 10, 14, 17 and 21 days) at 16 °C and 75% relative humidity (RH) to assess basic egg quality parameters. The eggs were weighed and stored for specific durations and reweighed. Each egg was broken open and the yolk and shell separated from the albumen and weighed. Before weighing the shell was washed under running water and allowed to air dry for 24 h (wet eggshell). The fresh yolk (wet yolk) as placed in a reweighed aluminum foil and weighed. The wet yolk and wet shell were placed in a drying oven set at 60 °C for four days to evaporate moisture before being reweighed to determine the dry weights. The percent egg weight loss was calculated as the difference between egg weight before storage and egg weight after storage all divided by egg weight before egg storage and the quotient multiplied by 100.
The remaining eggs (n = 240) were assessed for the impact of short periods of incubation during egg storage (SPIDES) on incubation parameters for two commonly used long storage durations in industrial settings on hatching eggs (14 days and 21 days). Eggs were selected to be similar in weight making sure that their weight differences were within ±0.5 g. The eggs were weighed and divided into two groups and stored for 14D (n = 120) and 21D (n = 120). Furthermore, each group of eggs was subdivided into two and each subgroup subjected to SPIDES and no-SPIDES as control.
To realize the 21D storage duration treatment for SPIDES and non-SPIDES eggs, the eggs were initially stored for 10 days in 16 °C and 75% RH and then removed placed in a Jamesway P5000 incubator at 37.5 °C for 6 h (1st SPIDES). The eggs were removed from the incubator rand allowed to cool down in ambient temperature between 26 and 28 °C 1 h. The eggs were then placed back in the storage room at 16 °C and 75% RH. Four days after the 1st SPIDES corresponding to 14 days of storage duration, the eggs were removed and the heat treatment repeated as previous (2nd SPIDES). The eggs removed again and placed in the storage room until 21 days of total storage duration (21D SPIDES) (). The control eggs were stored for 21 days continuously without SPIDES application (21D control).
Figure 1. A Graphical representation of the temperature-time chart of egg storage and SPIDES treatment before incubation.
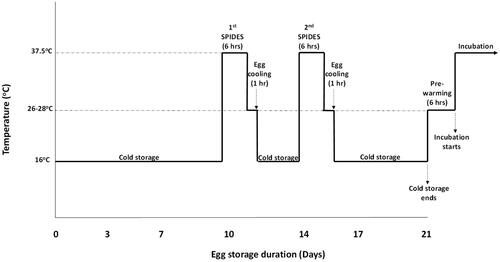
Similarly, the eggs collected and subjected to 14 days storage duration were divided into two; one subgroup was subjected to SPIDES at only 10 days of storage duration (14D SPIDES) and the other half was stored in the storage room continually until incubation (14D control). To ensure all eggs for 21D and 14D were incubated at the same, the egg collection was synchronized such that the one group of 120 eggs were first collected and placed in eggs storage room. After 7 days of egg collection, another group of 120 eggs were collected and placed in the same storage room.
The above treatment application resulted in a 2 × 2 factorial design arranged in a completely randomized design, with two levels of SPIDES treatments (0-h (control) and 6-h (SPIDES)) and two storage durations (14D and 21D) resulting in four treatments; 0-h × 14D (control); 0-h × 21D (control); 6-h × 14D (SPIDES) and 6-h × 21D (SPIDES). To ensure that the eggs were being exposed to the heat treatments evenly, they were turned every 1 h during incubation. After the storage periods, SPIDES and non-SPIDES eggs were both incubated for 21 days on the same day (37.5 °C and 56%). The schematic representation of the SPIDES procedure is shown in .
Incubation of eggs
Following storage durations and SPIDES treatments, all eggs stored for 14 or 21 days were removed from the storage room and subjected to pre-warming for 6 h at room temperature and weighed before setting in an incubator. The difference between initial and final egg weights was expressed as percentage weight loss. The eggs were incubated in 300 egg capacity G.Q.F. M.F.G. CO. incubator (Model GQF1502, Wichita, Kansas) at 37.5 °C incubation temperature and 60% in a completely random design. The machine had three-tier metal trays capable of holding 100 eggs per tray. The eggs were turned manually three times daily until 18 days after which the turning was stopped allowing the embryos to prepare for hatching. The electrical specifications and troubleshooting were done according to a previous study.Citation29
Embryonic development during incubation
On the 4th, 7th, 11th, 14th, and 18th days of incubation, eggs (n = 5) were randomly selected from each treatment and broken to evaluate egg weight loss, embryo weight (wet and dry), and embryo length. Specifically, for embryo weight, the embryos were separated from the yolk, the excess moisture extracted, and then weighed. The embryo was then stretched gently over a laboratory bench for other measurements to be taken.Citation8,Citation29,Citation30 The embryo length was measured by opening a divider over the tip of the beak or the tooth of gently stretched embryo to the tip of its middle toes. The divider was transferred over a laboratory ruler and the length recorded as described previously.Citation29,Citation31 The freshly collected embryos obtained immediately after breaking the eggs were removed from the incubator and were called wet embryos as described previously.Citation8 The wet embryos were placed in a 60 °C drying oven for four days to eliminate moisture. The embryos samples were removed and reweighed and called dry embryo weight. Before and during incubation comparative staging of embryonic development between treatment was carried by following some aspect of procedures of both the Eyal-Giladi and Kochav (EG&K) and Hamburger–Hamilton (HH).Citation32,Citation33 In the EG&K procedure the evidence of development of blastoderm (day zero embryo prior to incubation)Citation2,Citation8 following SPIDES or control treatments in eggs of 14 or 21 d storage duration between stage X to Stage IV before incubation were studied. The HH staging technique was done by matching the stage of embryo development after examination of each treatment to an HH stage and recorded. Following incubation, evidence of development of blood islands at stage 8 between 26 h and 29 h following incubation were expected after 24 h of incubation. Additionally, at 48 h of incubation following incubation, evidence of telencephalon or head arching and advance blood vessel formation, which should form by 45–52 h of incubation and classified as stage 12 and 13 were determined. The hatch chicks after incubation were assessed for chick numbers and chick quality between eggs subjected to Non-SPIDES and SPIDES treatments.
Hatching traits
On the 22nd day of incubation, all hatched chicks were removed from the incubator, which was turned into a hatcher for the last 3 days of incubation and conveyed to the chick room according to the treatments. Five hatched chicks from each treatment were selected randomly to assess chick weight, residual yolk sac, chick length, and shank length according to standard protocols.Citation29,Citation34 The hatchability was calculated to assess the full-scale performance of eggs before and during incubation. The hatchability was equal to the percentage of the total chick hatched divided by the total number of eggs set. The number of chicks hatch were: 21 days storage duration subjected to no-SPIDES = 9 chicks, 14 days storage duration subjected to no-SPIDES = 20 chicks, 21 days storage duration subjected to SPIDES = 12 chicks, and 14 days storage duration subjected to SPIDES = 10 chicks. At hatch, chicks were scored for the presence of a navel button or no navel buttons according to a modified method of the PASCAR score.Citation35 This method is based on the scoring of the navel called Tona score or or PASCAR score. In the PASCAR scoring the following pparameters are assessed to judge th55e quality of a chick: navel strings, navel buttons, opened navel, closed navel, leaky navel, and unhealed navel. If the navel was completely closed and clean, it was scored 1 for navel quality. A score of 2 was given when the navel color was different from the chick’s skin color and had an opening <2 mm and chick navels with discoloration and a navel button more than 2 mm were scored 3 for navel quality. A leaky navel with fluid coming out and unhealed was scored 4 while a navel with string attached was scored 5. In the modified PASCAR score, all chicks with healed and closed navel were scored 1 and all chicks with any of the other 4 categories of navel characteristics were scored as 2. The number of chicks from each scoring were totaled and expressed as percentage of total chicks hatched.
Haematological indices of chicks
The remaining hatched chicks after some chicks were selected for chick quality assessment (0-h × 14D = 16 chicks; 0-h × 21D = 5 chicks); 6-h × 14D = 6 chicks, and 6-h × 21D = 8 chicks) were reared according to their treatments in deep litter pens using standard husbandry practices to be able to collect a second blood sample for analysis. Treatments were replicated twice with at least 2 chicks per replicate as the number of chicks left would permit. The chicks were fed layer starter mash formulated according starter chicks’ nutrient requirements for one week (Crude protein (CP) = 20%, Metabolizable energy (ME) = 2800 kcal/kg). Blood was drawn from two selected chicks per treatment seven days post-hatch. Using sterile needles, five (5) milliliters of blood were drawn from the chicks’ jugular veins and placed in sample bottles containing EDTA (Ethylene Diamine Tetra Acetic Acid). Samples were thereafter taken to the Main Research Laboratory of the Ghana Veterinary Services Directorate, Kumasi-Amakom Division, to determine a total of 10 haematological parameters including leucocyte levels (WBC), erythrocyte levels; red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), thrombocyte; platelet count (PLT), mean platelet volume (MPV), platelet volume distribution width (PDW), and platelet crit (PCT).
Statistical analysis
Data collected from trail 1 were analyzed as one way-ANOVA while data from trail 2 were analyzed as two-way ANOVA using the generalized linear model procedure of SAS version 9.4.Citation36 The lsmeans were separated at p < 0.05 using the Student Newman-Kuels (SNK) test. The statistical model used to analyze data from trial 1 was Yi = µ + αi + εi, where: Yi = effect measured, µ = overall mean, α = main effect of egg storage duration, and ε is the residual error term. The initial egg weight was used as a covariate to remove bias due to the random selection of eggs on all other parameters. The statistical model used to analyze data in trial 2 on all responses included the fixed effect of days of storage at 2 levels and SPIDES at 2 levels. The statistical model used was: Yij = µ + αi + βj + αβij + εij, where: Yij = effect measured, µ = overall mean, α = main effect of egg storage duration, β = the main effect of SPIDES treatment, αβ is the interaction of egg storage duration and SPIDES, and ε is the residual error term. The experimental units were either eggs or chicks depending on the data required.
Results
Basic egg quality parameters
The results showed that the initial egg weights were different between eggs before they were subjected to different storage durations due to the random selection of eggs. The final egg weights after storage were different between storage durations. However, following covariate analysis the final egg weight and all egg quality parameters except the proportion of wet shell weight and shell thickness were not different between storage durations. The wet shell weight was highest in 17 days stored eggs compared to eggs stored for one day. The shell weights were not different between treatments after drying. The shell thickness was highest in eggs stored for days 1, 7, and 10 compared to eggs stored for 4, 14, 17 and 21 days of storage. However, eggs from 17 and 21 days had the lowest eggshell thickness (). There appear not to be any particular trend in these parameters with regard to increasing eggs storage.
Table 1. Effect of cold storage duration on basic egg quality parameters.
Egg moisture during storage
The results showed no significant difference in the initial egg weights between SPIDES treatments and also between 14D and 21D egg storage durations (). However, the final egg weight was higher in 14D stored eggs compared to 21D stored eggs. This shows that the eggs stored for 21D lost a higher amount of moisture compared to 14D stored eggs. There was no difference in the final egg weights between eggs subjected to SPIDES and control. Again, shows a representative profile of initial and final egg weights and percent egg weight loss as a measure of egg quality during egg storage as affected by the length of storage, SPIDES treatment, and their interaction.
Table 2. Effect of short incubation period and different storage durations on egg weight loss of hybrid breeder ‘black-tailed’ eggs.
The result showed that eggs stored for 21D lost greater weight (p < 0.0001) compared to the 14D storage (1.60% vs. 0.71%). There was no difference in percent egg weight loss between the SPIDES and non-SPIDES eggs. For the interaction, both the initial and final egg weights differed significantly. The weight loss of the SPIDES eggs at 21D was significantly higher than non-SPIDES eggs at 21D, which were all higher than the weight loss of both SPIDES and non-SPIDES eggs at 14D. However, the latter two were not different from each other (p = 0.032).
Egg weight loss during incubation
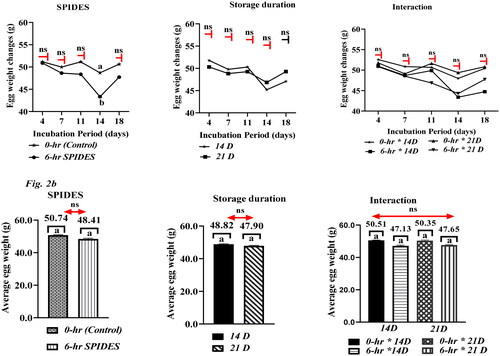
shows representative profiles of egg weight changes (g) across incubation days 4, 7, 11, 14, and 18 as a measure of egg quality as affected by storage duration, SPIDES, and their interaction. The graph () demonstrates a general downward trend in egg weight loss across incubation days for the selected egg breakout days 4, 7, 11, 14, and 18, which is congruent with the observation that chicken eggs lose weight due to water of evaporation during incubation. However, except for incubation day 14 (), where the non-SPIDES eggs had a higher (p = 0.030) egg weight than the SPIDES group (48.69 g vs. 43.33 g), the downward trend in egg weight continued across the incubation period. The average egg weight recorded between SPIDES and non-SPIDES eggs, or between storage durations and SPIDES interactions on day 18 did not differ between the eggs stored for 14 and 21 days ().
Figure 2. Representative profile of the impact of storage duration, SPIDES, and their interaction on egg weight (a) and average egg weight (b) during incubation. Data with bars having similar superscripts showed no significant difference (p > 0.05): ns: not significantly different; 0-h SPIDES – eggs that did not receive short period incubation during (control); 6-h SPIDES: eggs that received 6-h short period incubation during egg storage.
Embryonic development during incubation
summarizes the results of mean embryo weight (g) and length (mm) changes as a measure of embryonic development as affected by storage duration, SPIDES, and their interaction across incubation days 4, 7, 11, 14, and 18. The results showed no variations in embryo weight and length between the two storage durations (14D and 21D) and between the storage duration and SPIDES interactions across the incubation period. Compared to the non-SPIDES eggs, the eggs that received SPIDES recorded a higher (p < 0.05) mean wet embryo weight on incubation days 4 (0.20 vs. 0.06 g), 7 (0.98 vs. 0.52 g), and 11 (5.01 vs. 3.40 g) and a mean dry embryo weight on day 11 (0.74 vs. 0.61 g). A similar observation was made () for the SPIDES eggs in terms of embryo length on incubation days 4 (1.21 vs. 0.88 mm) and 11 (6.75 vs. 5.78 mm). The differential development of embryos subjected to SPIDES versus control is presented in . It is demonstrated that chicks subjected to SPIDES had prominent blastoderm at the start of incubation, for the for initiation of embryonic development. The presence of blood islands was clear at 24 h in the SPIDES embryos while those of control did not show visible signs of initiation of embryonic blood island before the HH stage 8. Additionally, there is increased vascularization and 48 h after incubation in the SPDIDES embryos, and clear initiation of chorioallantois formation compared to control. At hatch over 80% of chicks hatched early in SPIDES compared to control after 480 h of incubation.
Figure 3. Comparative stages of embryonic development and chick quality at hatch for Non-SPIDES & SPIDES treated eggs after prolonged storage according to the procedures of Hamburger–Hamilton (1951) stages. KEY: Plates a, c, e, g, i (orange-arrows transition: Non-SPIDES or control treatments); Plates b, d, f, h, j (blue-arrows transition: SPIDES treatments); (a) Unincubated non–SPIDES egg. Blastoderm under-developed with unclear ‘donut appearance’. (b) Unincubated SPIDES egg. Blastoderm is well-developed with clear ‘donut-appearance’. (c) ‘donut-shape’ enlarged with no appearance of ‘blood islet’ (d) ‘donut-shape’ enlarged greatly with the appearance of ‘blood islets’ (e) ‘donut-shape’ enlarged greatly with only ‘blood islets’ in the outer ring of developing embryo. No appearance of the circulatory system and embryonic appendages at this stage (f) Clear appearance of an embryo with a well-developed circulatory system and embryonic appendage. (g) Protrated terminal hatching with few externally pipped chicks (h) rapid terminal hatching with more hatched and externally pipped chicks. (i) Poor quality chick (light, weak and passive) (j) good quality chick (heavy, thrifty, and active).
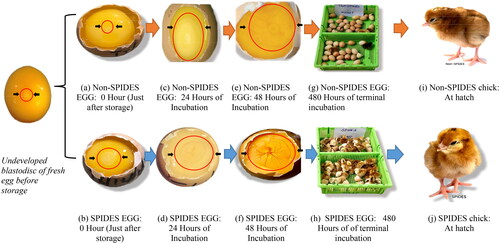
Table 3. Impact of storage duration, SPIDES, and their interaction on mean wet and dry embryo weight changes during incubation.
Table 4. Impact of storage duration, SPIDES, and their interaction on embryo length changes during incubation.
Hatching traits
summarizes the impact of storage duration, SPIDES, and their interaction on hatching traits. The results indicated no difference in any of the hatching parameters measured between the SPIDES and the non-SPIDES eggs, between the two storage durations (14D and 21D), and their interactions () except hatchability (). Generally, the eggs stored for 21 days had a lower (p = 0.030) hatchability compared to eggs stored for 14 days (50.8 vs 76.7%), with a difference of 26%. For the interaction, the eggs stored for 14 days without SPIDES exhibited a hatchability of 83.3% which was 30% higher (p = 0.05) than those that received SPIDES recording a hatchability of 53.3% (). The positive impact of SPIDES resulted in a hatchability of 66% in 21D eggs compared to non-SPIDES eggs stored for the same period. The application of SPIDES had implications on chick quality with regard to navel score. All eggs subjected to SPIDES hatched chicks with no signs of a navel button while 50% of chicks from the control treatment showed signs of navel buttons. However, the degree of the navel button was 25% in both eggs stored for 14D and 21D. This is because 75% of chicks showed cleaned navel buttons from all chicks examined from these treatments. The interactions showed that irrespective of storage durations the degree of clean navel was higher in SPIDES-treated birds compared to the control. However, all signs of the navel buttons disappeared 7 days post-hatch in all treatments ().
Figure 4. Representative profile of the impact of storage duration, SPIDES treatment, and their interaction on hatchability. Data bars with similar superscripts show no significant difference (p > 0.05): 0-h SPIDES: eggs that did not receive short period incubation during egg stage (control); 6-h SPIDES: eggs that received 6-h short period incubation during egg storage; interaction = SPIDES × storage duration interaction.
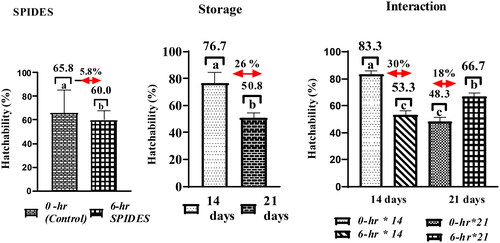
Table 5. Impact of storage duration, SPIDES, and their interaction on hatching traits.
Chick haematological indices
shows the mean haematological variables of chicks examined 7 days after hatching. From , it could be seen that between the SPIDES and the non-SPIDES eggs, as well as the two storage durations (14D and 21D), there were no differences in erythrocyte counts (RBC, HGB, HCT, MCV, MCH, MCHC), and thrombocyte counts (MPV, PDW, PCT, P-LCR). Similarly, no difference existed among the interaction effect of SPIDES and storage duration in the blood parameters assayed. The exception was with MPV, which differed according to the interaction sequence: 6-h*21D ≥ 0-h*14D > 0-h*21D ≥ 6-h*14D.
Table 6. Impact of 6-h short period incubation during egg storage (SPIDES) on hematology of 7-day-old chicks.
Discussion
Egg weight loss occurs over time due to the loss of water, ammonia, carbon dioxide, and nitrogen, as well as hydrogen sulfide. Although there was no significant loss of egg weight between egg storage durations in the current study, there is evidence previously to show that egg weight loss is higher with longer egg storage.Citation4,Citation21 However, with SPIDES or heat treatment of eggs and pooling data together there was higher percentage of egg weight loss recorded for the SPIDES group within the 21D stored eggs. While this could be explained by the extended storage period the head treatment could have played a part. This is coupled with the additional heat treatment performed on days 10 and 14 of storage, which may have raised the eggshell temperature, causing them to dissipate more moisture and other volatile interior contents than the non-SPIDES group stored for the same length of days. The lack of changes in the final egg weights between the two storage periods (14D and 21D) contradicts prior findings that suggested that longer storage duration results in higher weight loss. This weight loss could be carried over during incubation.Citation2,Citation4,Citation21 The downward trend in egg weight loss across the days of incubation is a fact due to embryonic metabolism, and the evaporation of water of metabolism (Hamidu et al. 2007)Citation37. The difference in the wet shell weight observed on 17 days of incubation could be linked to loss or utilization of calcium and other mineral for bone and general embryo development.Citation29 However, the concept of bone mineral utilization and contribution of the eggshell and its relationship with the objectives of the current study should be further investigated to understand if the heat treatment during SPIDES alters eggs shell mineral density.
For the effect of SPIDES on the observed parameters, the current findings support those of Dymond et al.,Citation24 who discovered that exposing eggs to SPIDES promotes embryonic development by minimizing cell attrition, allowing embryos to complete hypoblast formation, which minimizes the tendency to lose weight during incubation. For the effect of storage duration on embryo weight, the current study demonstrates that despite the longer egg storage period of up to 21 days, the embryos kept similar weights and lengths to those that had been stored for 14 days. This finding contradicts the literature, since there is a plethora of well-documented views suggesting that protracted storage is inimical to embryo development throughout incubation.Citation2,Citation4,Citation8,Citation21,Citation38 Furthermore, evidence suggests that when eggs are kept in cool storage for an extended period embryo development slows down resulting in blastodermal cell death including apoptosis (programmed cell death) and necrosis (harmful cell death). These mechanisms of cell death can lower healthy embryonic cell numbers below a critical threshold, resulting in abnormal embryo development and increased embryo mortality.Citation8
The difference in results between this study and previous ones could be explained by a positive additive interaction effect of SPIDES and storage duration especially for 21D which showed a higher hatchability. This may have pushed the embryo to a stage critical for forming metabolic cells that can tolerate the effects of the 21D storage period, thus yielding results similar to those of the 14D storage period. Heat treatment of cold-stored eggs for up to 14D is documented to down-regulate key pro-apoptotic genes resulting in increased metabolic rate and higher weight of embryos during incubation, which are consistent with the results of this study.Citation4,Citation8,Citation39 The positive impact of the heat treatment of longer stored eggs is also reflected in hatchability. Eggs stored for 21D without SPIDES had a decreased hatchability of 48.3% but this was partially rescued by the SPIDES treatment, restoring the hatchability to 66.7%. This confirms the findings of Dymond et al.Citation24 who found that when eggs were stored for only 4 days before being incubated to hatch, the hatchability was 92%, compared to 71% for eggs held for 21 days. However, the finding of the current study implies that when eggs were stored for 14D, the SPIDES application was less effective than when eggs were stored for a longer duration of 21D.
The images illustrated in this study show an advanced development of blastoderm after final egg storage following SPIDES. The images also demonstrate that the time of appearance of the embryo’s primitive streak, the gastrula development, activation of the circulatory system, and the timing of chick hatch are all evidence of the positive benefits of embryo development and chick quality due to the SPIDES treatment. Additionally, the study demonstrates the reduced number of chicks with navel buttons when SPIDES was applied.
Following storage, the ability to resume development to hatching depends on a number of variables, including the quantity of live cellsCitation2,Citation8 and the stage of embryonic development, which is significantly impacted by SPIDES treatment, as shown in the current study. The storage conditions, particularly the duration, temperature, and frequency of SPIDES, have a significant impact on these variables. Therefore, a thorough definition of the starting point—shortly after oviposition—is required to explore the impact of storage conditions on embryonic viability. The analysis of blastomeres from newly laid eggs revealed that the proportion of mitotic index and dying cells at oviposition is 2% and 5%, respectively, while the total nucleus count increases with developmental stage from 60,000 at stage X EG&K to 130,000 at stage XIII EG&K.Citation40 The advance blastoderm development before normal incubation in SPIDES treatment agrees with earlier result that showed that bigger blastoderm size is due to higher number of blastodermal cells and will increase embryo weight during incubation.Citation4
Earlier advice to find and optimize strategies of incubation to increase chick quality beyond the physical classification of chick quality such as chick weight and chick length but rather include the size of residual yolk sac at hatch and navel button examination is aptCitation41,Citation42 (Yeboah et al. 2019). The beneficial effect of SPIDES may include cell repairs especially early apoptotic cells increasing the threshold number of cells needed for proper embryonic development and stronger chicks at hatchCitation2,Citation8,Citation43
The haematological indices measured in the current study were within the normal ranges reported for adult chickens; RBC: 2.5–3.5 × 1012/L, PCV: 22–35%, Hb: 7–13 g/dl, MCV: 90–140 fL, MCH: 33–47 pg/cell and MCHC: 26–35 g/dlCitation44 PLT: 14.3–88.9 × 109/L, MPV: 3.9–7.1 fL, PDW: 15.5–20.5%, PTC: 0.001–0.06%.Citation45 Although the SPIDES eggs were preheated in this study, blood erythrocyte, leukocyte, and thrombocyte levels did not rise above normal, showing that even though the SPIDES treatment exposed the growing embryo to some level of heating during storage, the embryos did not experience thermal stress. Borges et al.Citation46 demonstrated that heat stress could alter the levels of erythrocytes, leukocytes, and thrombocytes in chickens in previous research. Sgavioli et al.Citation47 found that blood cells were lower at a higher incubation temperature of 39 °C than at 37.5 °C.
In conclusion, the study has confirmed that eggs tend to lose more weight when stored in a storage room for an extended period or beyond 7 days. The hatchability of eggs stored for 21 days was found to be 26% lower than those stored for 14 days, demonstrating the negative impact of long-term egg storage on incubation success. However, SPIDES treatment provided a positive additive effect on the hatchability when eggs were stored for 21 days compared to 14 days. The hatchability increased from 48.3% in the non-SPIDES eggs to 66.7% in the SPIDES group, making a difference of 18%. Finally, this study found that combining storage duration with SPIDES treatment has an individual mode of action as well as a positive additive interactive effect. It also reduced the negative impact of long storage periods on egg quality, embryonic development, hatching traits, and haematological variables of chicks. Furthermore, it is observed that the normal industry egg storage practices have challenges when eggs are stored for a long time. However, heat treatment of eggs during longer egg storage duration can help reverse some negative effects of storage. But this heat during egg storage called SPIDES has been demonstrated in the current study to be effective in eggs stored over a longer period, which is 21 days prior to normal incubation. Future studies should be focused on the cellular and molecular mechanisms underpinning the interactive effect of SPIDES on embryonic development, metabolism, and immunology, which will provide renewed insights into manipulating these factors to reduce early embryo mortality, and increase hatchability and chick quality by reducing intermediate factors such as navel buttons, which can cause yolk sac infections.
Authors’ contributions
J.A.H. conceived the idea, designed the study, and supervised the research. S. A. A., E. M. A., M. B., L. N. collected the data. K. A. A., S. A. A., E. M. A., M. B., L. N. completed the analyses on blood profiles, egg quality, and chick quality variables. J. A. H. and P. S. analyzed the data, drafted the manuscript, and in collaboration with all of the rest wrote the article and edited it.
Acknowledgments
The authors would like to express their gratitude to technicians of the Olympio Hatchery, Department of Animal Science, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi-Ghana for their cooperation and genuine aid in providing technical assistance for this study. Laboratory technicians of the Ghana Veterinary Service, Kumasi Division also deserve special mention for their assistance in profiling the haematological parameters in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available and will be provided at the request of the corresponding author.
Additional information
Funding
References
- Millward DJ, Layman DK, Tomé D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr. 2008;87(5):1576S–1581S.
- Hamidu JA, Rieger AM, Fasenko GM, Barreda DR. Dissociation of chicken blastoderm for examination of apoptosis and necrosis by flow cytometry. Poult Sci. 2010;89(5):901–909.
- Gharib H. Effect of pre-storage heating of broiler breeder eggs, stored for long periods, on hatchability and chick quality. Egypt J Anim Prod. 2013;50(3):174–184.
- Addo A, Hamidu JA, Ansah AY, Adomako K. Impact of egg storage duration and temperature on egg quality, fertility, hatchability and chick quality in naked neck chickens. Int J Poult Sci. 2018;17(4):175–183.
- Christensen VL, Wineland MJ, Fasenko GM, Donaldson WE. Egg storage effects on plasma glucose and supply and demand tissue glycogen concentrations of broiler embryos. Poult Sci. 2001;80(12):1729–1735.
- Elibol O, Brake J. Effect of egg position during three and fourteen days of storage and turning frequency during subsequent incubation on hatchability of broiler hatching eggs. Poult Sci. 2008;87(6):1237–1241.
- Fasenko GM. Egg storage and the embryo. Poult Sci. 2007;86(5):1020–1024.
- Hamidu JA, Uddin Z, Li M, Fasenko GM, Guan LL, Barreda DR. Broiler egg storage induces cell death and influences embryo quality. Poult Sci. 2011;90(8):1749–1757.
- Hamidu JA, Adomako K, Adamu S, et al. Impact of layer egg storage conditions on eggs and embryo quality indices prior to incubation. Livest Res Rural Dev. 2018;30:184. http://www.lrrd.org/lrrd30/11/jaham30184.html. Retrieved April 20, 2023.
- Ishaq HM, Akram M, Baber ME, et al. Embryonic mortality in Cobb broiler breeder strain with three egg weight and storage periods at four production phases. J Anim Plant Sci. 2014;24(6):1623–1628.
- Okur N, Eleroğlu H, Türkoğlu M. Impacts of breeder age, storage time, and setter ventilation program on incubation and post-hatch performance of broilers. Rev Bras Cienc Avic. 2018;20(1):27–36.
- Schmidt GS, Figueiredo EAP, Saatkamp MG, Bomm ER. Effect of storage period and egg weight on embryo development and incubation results. Rev Bras Cienc Avic. 2009;11(1):1–5.
- Tona K, Onagbesan O, De Ketelaere B, Decuypere E, Bruggeman V. Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight, and chick post-hatch growth to forty-two days. J Appl Poult Res. 2004;13(1):10–18.
- Uyanga VA, Onagbesan OM, Oke OE, Abiona JA, Egbeyale LT. Influence of age of broiler breeders and storage duration on egg quality and blastoderm of Marshall broiler breeders. J Appl Poult Res. 2020;29(3):535–544.
- Ipek A, Sozcu A. The effects of broiler breeder age on intestinal development during hatch window, chick quality, and first-week broiler performance. J Appl Anim Res. 2015;43(4):402–408.
- Reijrink IAM, Meijerhof R, Kemp B, Graat EAM, Van den Brand H. Influence of pre-storage incubation on embryonic development, hatchability, and chick quality. Poult Sci. 2009;88(12):2649–2660.
- Tona K, Bamelis F, De Ketelaere B, et al. Effects of egg storage time on the spread of hatch, chick quality, and chick juvenile growth. Poult Sci. 2003;82(5):736–741.
- Bergoug H, Burel C, Guinebretiere M, et al. Effect of pre-incubation and incubation conditions on hatchability, hatch time and hatch window, and effect of post-hatch handling on chick quality at placement. World’s Poult Sci J. 2013;69(2):313–334.
- Yildirim I. Effects of breeder age and pre-incubation storage of eggs on hatchability, time of hatch, and relative organ weight of quail chicks at hatch. S Afr J Anim Sci. 2005;35(2):135–142.
- Bloom SE, Muscarella DE, Lee MY, Rachlinski M. Cell death in the avian blastoderm: resistance to stress-induced apoptosis and expression of anti-apoptotic genes. Cell Death Differ. 1998;5(6):529–538.
- Fasenko GM, Christensen VL, Wineland MJ, Petitte JN. Examining the effects of prestorage incubation of turkey breeder eggs on embryonic development and hatchability of eggs stored for four or fourteen days. Poult Sci. 2001;80(2):132–138.
- Gucbilmez M, Özlü S, Shiranjang R, Elibol OKAN, Brake J. Effects of preincubation heating of broiler hatching eggs during storage, flock age, and length of storage period on hatchability. Poult Sci. 2013;92(12):3310–3313.
- Okasha HM, El-Gendi GM, Eid KM. The effect of storage periods and SPIDES on embryonic mortality, hatching characteristics, and quality of newly hatched chicks in broiler eggs. Trop Anim Health Prod. 2023;55(2):133.
- Dymond J, Vinyard B, Nicholson AD, French NA, Bakst MR. Short periods of incubation during egg storage increase hatchability and chick quality in long-stored broiler eggs. Poult Sci. 2013;92(11):2977–2987.
- Nicholson D, French N, Tullett S, van Lierde E, Jun G. Short periods of incubation during egg storage–SPIDES. Lohmann Inform. 2013;48(2):51–61.
- Animal Research Ethics Committee (AREC). Standard operating procedures. https://keep.knust.edu.gh/node/75. 2018. Accessed April 10, 2022.
- Dawson-Amoah HB, Nwachukwu MC, Hamidu JA, Adomako K. Potential of two local commercial hybrid chicken strains: (2) egg quality. Ghana J Anim Sci. 2019;10(1):17–25.
- Nwachukwu M, Dawson-Amoah B, Hamidu JA. Potential of two local commercial hybrid chicken strains for egg production. Ghana J Anim Sci. 2019;10(1):10–16.
- Agyekum G, Okai MA, Tona JK, Donkoh A, Hamidu JA. Impact of incubation temperature profile on chick quality, bone, and immune system during the late period of incubation of Cobb 500 broiler strain. Poult Sci. 2022;101(9):101999.
- Willemsen H, Li Y, Willems E, et al. Intermittent thermal manipulations of broiler embryos during late incubation and their immediate effect on the embryonic development and hatching process. Poult Sci. 2011;90(6):1302–1312.
- Nangsuay A, Ruangpanit Y, Meijerhof R, Attamangkune S. Yolk absorption and embryo development of small and large eggs originating from young and old breeder hens. Poult Sci. 2011;90(11):2648–2655.
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88(1):49–92. https://www.hatchability.com/HenH.pdf
- Eyal-Giladi H, Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol. 1976;49(2):321–337.
- Willemsen H, Everaert N, Witters A, et al. Critical assessment of chick quality measurements as an indicator of post-hatch performance. Poult Sci. 2008;87(11):2358–2366.
- Yeboah PP, Konadu LA, Hamidu JA, et al. Comparative analysis of hatcheries contribution to poor development of day-old chicks based on biological and immunological performance. Vet World. 2019;12(11):1849–1857.
- SAS Institute Inc. SAS/STAT® 9.4 Procedures Guide. Cary, NC: SAS Institute Inc; 2012.
- Hamidu JA, Fasenko GM, Feddes JJR, et al. The effect of broiler breeder genetic strain and parent flock age on eggshell conductance and embryonic metabolism. Poult Sci. 2007;86(11):2420–2432.
- Uddin Z, Hamidu JA. Prolonged egg storage affects broiler breeder embryonic metabolism and chick quality. J Anim Sci Adv. 2014;4(7):973–977.
- Maatjens CM, Reijrink IAM, Molenaar R, Van Der Pol CW, Kemp B, Van Den Brand H. Temperature and CO2 during the hatching phase. I. Effects on chick quality and organ development. Poult Sci. 2014;93(3):645–654.
- Pokhrel N, Ben-Tal Cohen E, Genin O, Sela-Donenfeld D, Cinnamon Y. Cellular and morphological characterization of blastoderms from freshly laid broiler eggs. Poult Sci. 2017;96(12):4399–4408.
- Nasri H, van den Brand H, Najjar T, Bouzouaia M. Egg storage and breeder age impact on egg quality and embryo development. J Anim Physiol Anim Nutr. 2020;104(1):257–268.
- Rocha JSR, Baião NC, Barbosa VM, et al. Negative effects of fertile egg storage on the egg and the embryo and suggested hatchery management to minimise such problems. World’s Poult Sci J. 2013;69(1):35–44.
- Brady K, Talbot CC, Long JA, et al. Transcriptome analysis of blastoderms exposed to prolonged egg storage and short periods of incubation during egg storage. BMC Genomics. 2022;23(1):262.
- Bounous D, Stedman N. Normal avian hematology: chicken and turkey. In: Feldman BF, Zinkl JG, Jain NC, eds. Schalm’s Veterinary Hematology. New York: Wiley; 2000:1147–1154.
- Hong D, Liyun C, Fuwei LI, et al. Effect of age on hematological parameter and reference intervals for commercial Lohmann silver layer. Poult Sci. 2021;100(12):101497.
- Borges SA, Da Silva AVF, Ariki J, Hooge DM, Cummings KR. Dietary electrolyte balance for broiler chickens under moderately high ambient temperatures and relative humidities. Poult Sci. 2003;82(2):301–308.
- Sgavioli S, Domingues CHF, Santos ET, et al. Effect of in-ovo ascorbic acid injection on the bone development of broiler chickens submitted to heat stress during incubation and rearing. Rev Bras Cienc Avic. 2016;18(1):153–162.
