Abstract
Bluetongue virus (BTV), a major peril to the sheep industry, infects a wide range of the cells in the infected animals including mononuclear, dendritic and epithelial cells. However, little is known about its tropism for the secretory epithelial cells of endocrine glands and the pathogenesis it induces. The aim of the study was to assess the BTV load, antigen distribution in the tissue of the pituitary, thyroid as well as adrenal glands and associated histopathological consequences. BTV antigens were localized using immunohistochemistry in the thyroid’s epithelial cells, zona fasciculata and zona reticularis cells and the anterior pituitary epithelial cells. The real-time PCR portrayed the high viral load in adrenals at 7th days postinoculation (DPI) and in thyroid and pituitary glands at 15th DPI. Serum examination revealed variation in the T-3 and T-4 of infected animals in comparison to the control group. Caspase-3 immunolocalization revealed BTV-1 induces apoptosis in the affected cells of endocrine gland of infected animals. Further, this study signifies the tropism of BTV in the novel sites (endocrine glands) of the host that might be one of the reasons for the poor performance of infected animals.
Introduction
Bluetongue (BT), a noncontagious, Culicoides-transmitted viral disease of domestic and wild ruminants, poses enormous socio-economic losses worldwide. The clinical manifestations are frequent in sheep and other wild ruminants, while a subclinical form is seen in cattle, camels and goats. So far, 29 serotypes are known worldwide, of which 23 have been reported from India.Citation1 The double-stranded, segmented RNA genome of bluetongue virus (BTV; Orbivirus genus; Reoviridae family) implicated in BT encodes seven structural (VP1–VP7) and five nonstructural (NS1, NS2, NS3/NS3A, NS4 and NS5) proteins.Citation2,Citation3 Its seg-2 and seg-6 determine the serotypes of the virus. Serotypes 1–6 and 10 are frequently associated with clinical disease with high pathogenic potential.Citation4,Citation5 Previous research data revealed that the seroprevalence of BTV-1 serotype in different geographic regions of India is quite high. Additionally, the potential of BTV-1 to induce transplacental transmission was relatively high in comparison to BTV-8 and pathological consequences induced by BTV-1 was relatively more severe.Citation6,Citation7
The BTV replicates both in Culicoides cells and in the vertebrate host cells. In vertebrate hosts, the virus replicates in leucocytes, lymphoid cells, endothelial cells and other epithelial tissues of the skin, lungs and other organ systems. The secretory epithelial cells of endocrine glands also support BTV replication, but its pathobiology is unclear. The BTV inflicts injuries to the endothelial cells of small blood vessels in various target tissues, causing cytolysis and the release of virus-induced vasoactive mediators. These mediators set the inflammatory process and account for extensive haemorrhages including at the base of the pulmonary artery, formation of ulcers in the digestive tract; necrosis of striated muscles (skeletal and heart); coronitis; oedema of the lungs, hypodermis and facia of various organs; effusions in the body cavities; age-dependent outcomes in foetuses—abortions/stillbirths; cavitating lesions in the central nervous system; viraemic newborn, weak born young ones; reduced fertility rate; meat and fleece losses.Citation8 Besides these direct economic losses, indirect losses also result mainly due to the trade restrictions on animals/animal products; and expenditure for pest control, diagnosis, vaccination as well as treatment of clinically ill animals.Citation9,Citation10 In survivors, squeal may include a loss of body condition, deformities of hooves and abnormal wool growth as well as poor-quality semen.Citation11
BTV may induce alteration in the host immune response via activation of the hypothalamic–pituitary–adrenal axis/stress system, which in turn may cause damage of endocrine gland. Further, viral-mediated modulation of the host endocrine signalling system had also been documented during viral infection. Mumps, Hantana, Herpes simplex virus-1 and -2 and HIV-1 implicated in the direct destruction of the affected endocrine glands.Citation12–15 The autoimmune-mediated destruction of the endocrine glands has been reported in Rubella, Hepatitis-C, Influenza virus, Coxsackie B virus, enterovirus and Epstein Bar Virus infection.Citation16–18 Moreover, viral proteins that alter/affect/inhibit the endocrine signalling of the host has also been described.Citation19–21
Unlike in other viruses, the effect of the BTV on endocrine glands is obscure; therefore, the effect of the BTV-1 infection in the secretory epithelial cells of endocrine glands viz. pituitary, thyroid and adrenal glands with respect to their morphological changes, immunohistochemical antigen localization and virus replication, if any, in experimentally infected Indian pregnant sheep has been delineated in this study.
Materials and methods
Virus
The BTV-1 (BTV-1 SKN-10/India/2007) procured from the Virus Repository of ICAR-Indian Veterinary Research Institute, India was originated from the spleen of a goat aborted foetus at Livestock Farm, Surdarkrushi Nagar, Gujarat.Citation22 Baby hamster kidney (BHK)-21 (vertebrate cell line) passaged BTV-1 inoculum was further cultured in KC cell lines (insect cell line) for a week and then harvested for experimental use in sheep. Viral titration was performed in the BHK-21 cells line by using Reed and Muench method for endpoint titration assay to determine the tissue culture infective dose (TCID50).
Animals
Total of 15 ewes procured from the local animal vendor was screened for BTV antibodies in serum by using c-ELISA (VMRD) and absence of BTV viral RNA in blood by using RT-PCR before inoculation of virus (BTV/SKN-10). Out of 15 BTV-free ewes, 10 were used for experimental inoculation of BTV-1, while the remaining 5 were inoculated with the cell culture media. Prior to the experiment, all the animals were dewormed and housed separately (Control group and infected group) in a well-drained, well-ventilated and insect-proof facility of the Division. The sheep were provided with ad-libitum water and standard feed throughout the experimental period. The sheep were kept for acclimatization for 2 weeks and the behavioural responses were monitored. The present trials were conducted after obtaining approval of the CPCSEA (No. 25/21/2017) EC [No. F.26-1/2015-16/JD (R)]. The animals related experimental procedures have strictly adhered to the Guidelines of the Institutional Animal Ethics Committee and carried out under the guidance of the institutional animal care committee.
Virus inoculation
The mated ewes were tested for pregnancy via real-time ultrasound equipment (Aeroscan-CD-5 Konica Minolta, India) having a convex transducer (C3-A; ). Out of 15 pregnant sheep, 10 ewes at the 60th day of gestation were administrated with BTV-1 virus @ 6 ml (5.5 log10 TCID50/ml) through an intradermal route using a 26-gauge needle at various sites on both right and left sides of prescapular/neck region, followed by 4 ml BTV-1 inoculums (5.5 log10 TCID50/ml) to each ewe intravenously as an infected group. The remaining five pregnant ewes (control group) at the 60th day of gestation were injected intradermally mock cell culture fluid @ 6 ml/sheep at numerous sites in the prescapular/neck region, followed by 4 ml mock cell culture fluid intravenously.
Blood samples’ collection and hormone estimation
Blood samples (∼2ml) were collected from the control and the experimental BTV-infected animals at specified time intervals by jugular vein venepuncture using BD Vacutainer (EDTA coated) for the estimation of hormone concentration. The blood samples were subjected to centrifugation at 1500 × g for 15 min in a hanging rotary centrifuge and sera were pipetted out in polystyrene tubes. Serum samples were stored at −20 °C until further use.
Progesterone, cortisol, and T-3 and T-4 concentrations in the serums were estimated by the ELISA (Biogenix Inc. Pvt. Ltd.) as per the instruction of the manufacturer. The OD value of each sample was calculated by using the ELISA reader (Thermo Scientific, USA) at the prescribed wavelength as recommended with each kit. The OD value was converted in the hormone concentration using the prescribed methods provided with the kit. Viral infection in the animals was confirmed by the real-time PCR at 0th days postinoculation (DPI).
Sacrifice of animals
BTV inoculated ewes (2 animals/interval) and control ewes (1 animal/interval) were sacrificed at the 7th, 15th, 30th, 45th and 60th DPI in the postmortem facility of the Division of Pathology, by injecting an overdose of the thiopental sodium intravenously. The study is conducted for preliminary investigation of the hypothesis. The small sample size was prompted by the first examination of the hypothesis. For this limited number of animals as per the availability of funds was considered for the study.
The pituitary, thyroid and adrenal samples were collected immediately in 10% neutral buffered formalin (NBF) from scarified ewes. The NBF fixed tissue samples were further processed for routine paraffin wax embedding technique, and 4–5 µm thick sections were prepared using a microtome. The tissue sections on the glass slides were stained with the routine haematoxylin–eosin (HE) method as per the standard staining procedure. For the viral quantification, thin-tissue sections were collected in the RNA Later and stored at −20 °C till further use.
Genomic detection of BTV
Total RNA was extracted from 300 μl tissue suspension using Trizol® reagent (Life Technologies, USA) as per the manufacturer’s instruction. The quantity and purity of RNAs were determined by measuring A260/280 using Nanodrop ND-1000 Spectrophotometer (Nanotechnologies, USA). The A260/280 values of all extracted RNAs were in the range of 1.8–2.0. These extracted RNAs were subjected to the genomic detection of BTV using Taqman-based chemistry through one-step RT-PCR (Qiagen, USA), using BTV-NS-3 gene primers and probes for seg-10/NS3 (F:5′-TGGAYAAAGCATGTCAAA-3′; R: 5′-ACRTCATCACGAAACGCTTC-3′ and 6-FAM-ARGCTGCATTCGCATCGTACGC-Tamra-Q.Citation23 Real-time PCR amplified an amplicon of 78 bp in size. The reaction mix of 20 μl was prepared by mixing 5x reaction mix (2.0 μl), dNTP (0.4 μl), 0.5 μl of each primer and probe (10 pM), enzyme mix (0.4 μl), template RNA (1.0 μl) and RNase-free water (4.7 μl). The amplification was carried out in a AriaMx Real-Time PCR System (Agilent, USA). The thermal conditions were: reverse transcription at 50 °C for 30 min, followed by initial PCR activation at 95 °C for 15 min, template denaturation at 94 °C for 30 sec, primer annealing at 56 °C for 30 sec and extension at 72 °C for 30 sec, followed by a final extension at 72 °C for 10 min. During each reaction, no template (negative control) and controls with positive BTV RNA (positive control) were always used in duplicate to rule out contamination in reagents. Further, 5 μl of the amplicons were electrophoresed in agarose gel to confirm the specific product and to document the same using a gel documentation system (Azure Biosystem-C 300, USA). A standard curve was used for the calculation of the copy number of viral genome in tissue. The PCR efficiency (E = 100 × 10(−1/slope) was determined by evaluating the slope of the linear regression connecting the Cq value with the logarithm of the concentration (copies per reaction). The efficiency of real-time PCR was nearly 100%.
Immunohistochemistry
The BTV antigens have been portrayed in endocrine glands via the indirect immune peroxidase technique as described earlier.Citation24 Hyperimmune sera were raised in rabbit by immunizing the BTV-1 core antigen at the Mukteshwar campus of the Indian Veterinary Research Institute (IVRI). Primary antibodies obtained from immunized rabbit were BTV-1 specific and were subsequently used in immunohistochemistry (IHC). The endocrine gland samples from BTV experimentally infected animals and negative control animals were embedded in paraffin and paraffin-embedded blocks were sliced into thin sections (4–5 µm). The tissue section from the uninfected animals serves as a negative control for the IHC. The thin-tissue sections (4–5 µm) on 3-aminopropyltriethoxysilane (Sigma Aldrich, USA) coated slides were deparaffinized and rehydrated through descending grades of alcohol viz., 100%, 90%, 80%, 70%, 60% and 50% and finally kept in the distilled water. For antigen retrieval, the tissue sections were incubated with the proteinase-K (Sigma, USA) for 1 h at 37 °C. Further, tissue sections were washed using 1× PBS (pH 7.4) thrice for 5 min each. Tissue endogenous peroxidase activity was inhibited with freshly prepared 3% hydrogen peroxide (H2O2) in methanol for 30 min in a dark chamber, followed by 3× washing using 1× PBS (pH 7.4).
The tissue sections were further incubated with 10% bovine serum albumin (BSA) for 1 h at RT followed by washing in 1× PBS (thrice). Further, 150 μl of rabbit origin BTV specific serum was added to the sections at 1:20 dilution in 1% BSA and incubated in a humidified chamber at 4 °C for overnight. Sections were thoroughly washed with TBS thrice for 5 min each to remove the unbound antibody and incubated with biotinylated goat anti-rabbit IgG peroxidase conjugate (Sigma, USA) for 1 h at 37 °C, followed by washing thrice as above. The positive immunoreaction was identified by the development of brown colour with freshly prepared 3,3′-diaminobenzidine (DAB, Vector Laboratories, Burlingame, California). The sections were washed and counterstained with Mayer’s haematoxylin (Sigma Aldrich, USA) for 10–20 sec, followed by dehydration in the ascending grades of alcohol and then mounted with DPX mountant (Sigma, USA). Positive signals (brown colour) were recorded under the light microscope.
Immunolocalization of the caspase-3
Like the BTV-1 IHC detection, the IHC staining was carried out for the antigenic demonstration of caspase-3 (Sigma, USA) in the tissue sections of the thyroid, adrenal and pituitary glands.
Statistical analysis
Statistical analyses of data were performed using GraphPad Prism 6.0 software. Data were presented in the form of mean ± SEM. The statistical significance difference between the mean of the infected and control groups was verified by one-tailed, paired T-tests. P value <0.05 was considered as a statistical significance.
Results
Gross changes in endocrine glands of infected group
The noteworthy gross changes in pituitary of experimentally infected ewes were not observed at 7th, 14th, 30th, 45th and 60th DPI; however, mild congestion was observed in the pituitary stalk (infundibulum) at 14th DPI. The gross changes in thyroid glands were also not remarkable. At the 7th DPI, adrenal glands showed slight vascular congestion in the adrenal cortex. At 14th DPI, the vascular congestion became intensified with thickened and oedematous cortex, while the contralateral adrenal cortex, in addition, showed haemorrhages and congestion of the medulla. At subsequent intervals, the gross changes in adrenal glands were not remarkable, when compared with the control.
Histopathological changes in endocrine glands
Pituitary gland (hypophysis)
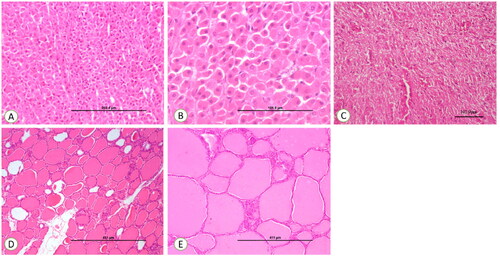
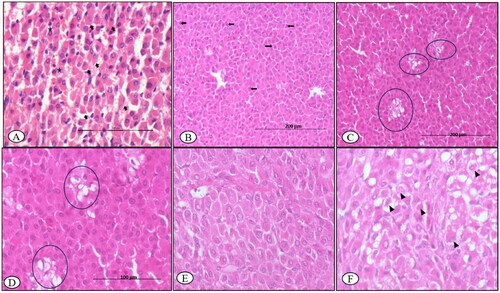
On 7th DPI, the vascular plexuses/sinusoids of the adenohypophysis (pars distalis, pars intermedia, pars nervosa) of BTV-infected ewes were engorged with homogenous, light eosinophilic fluid. The acidophil (α) cells in the anterior pituitary were swollen with pyknotic nuclei in comparison to the sham control (). In addition to microhaemorrhages, oedema, focal lymphocytic infiltration and increased cellularity (pituicytes) of the neurohypophysis (posterior pituitary) on the 14th DPI, vascular plexuses were prominent with engorged blood at the interface of the anterior pituitary and neurohypophysis (). The noteworthy histopathological changes were not observed in 30th DPI. On the 45th DPI, however, there were more pituicytes found among the nonmyelinated axonic fibres of the posterior pituitary (). Further, the adenohypophysis showed mild focal infiltrates of eosinophils in the connective tissue stroma on 60th DPI (). The histopathological changes in the pituitary gland of control sheep were nonsignificant throughout the experimental period.
Figure 2. Bluetongue virus serotype-1 (BTV-1), 6 ml (5.5 log10 TCID50/ml) by I/D and IV routes, pregnant sheep at 60th gestation day. Pituitary sections: (A) at 7th DPI, showing normal capillary plexus (arrow) at C-7 DPI in the infected group, ×100; (B) at 7th DPI, showing dilated venous capillary (asterisk) plexus with blood, ×100; (C) mild infiltration of the lymphocyte (circle) in the pars nervosa of the pituitary observed at 14th DPI, H&E, ×400; (D) increased number of the pituicytes (arrowhead) observed in the pars nervosa of the pituitary, 14th DPI, H&E, ×400; (E) at 30th DPI, showing an increased number of pituicytes (curved arrow) with an enlarged nucleus in the pars nervosa, H&E, ×200 and (F) at 60th DPI, infiltration of the eosinophils (circle) between the glandular epithelium of anterior pituitary, H&E, ×400.
Thyroid gland
On the 7th DPI, the thyroid glands showed lobules with follicles of variable morphology filled with darkly pink colloid and lined with cuboidal follicular epithelial cells (T-3, T-4 hormones) with resorption vacuoles, especially in the peripheral follicles. Furthermore, multi-focal hyperplasia of C-cells (calcitonin-secreting cells) in the vascular-rich stroma was observed in the walls of adjoining follicles. The parathyroid small cells (parathormone-secreting chief cells) showed distinct vacuolation of the cytoplasm ().
Figure 3. BTV-1, 6 ml (5.5 log10 TCID50/ml) inoculated by I/D and IV routes in sheep at 60th gestation day. H&E section, thyroid sections: (A) at 7th DPI, hyperplasia of the parafollicular cells (C-cells) with few undergoing apoptosis (asterisk), ×400; (B) at 30th DPI, enlarged follicles with flattened epithelium (arrowhead) and with dark to lightly stained collide devoid of reabsorption vacuoles, ×200 and (C) at 30th DPI, showing elongated follicle filled (curved arrow) with the colloid with flattened follicular epithelial cells (arrowhead) on 45th DPI, H&E, ×200.
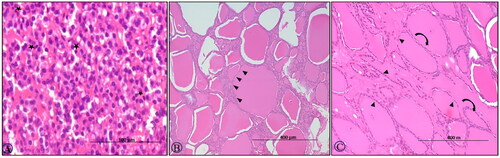
The follicle morphology on the 14th DPI was comparable to that on the 7th DPI. The C-cells and oxyphilic cells, in contrast, did not exhibit any remarkable alterations. On day 30, the thyroid follicles were mostly enlarged in size, filled with darkly stained colloid with no resorption vacuoles and lined with squamous follicular cells. When compared to controls, numerous small sized follicles were found congregated among larger follicles (). On days 45 and 60, the thyroid glands remained similar in histopathological details as on 30th DPI (). The thyroid glands of control ewe were unaffected throughout the experimental period, having normal size follicles filled with colloid and normal tissue stroma.
Adrenal gland
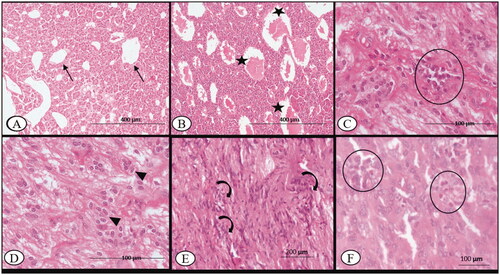
On 15th DPI, HE sections of adrenals showed distorted histoarchitecture with dilated sinusoids containing a sparse number of neutrophils and a few scattered degenerated endocrine cells secreting cortisol as well as cortisone in the zona fasciculata of the adrenal cortex (). The cells in the adrenal cortex were multicentrically dropped out from the surrounding cells and were having condensed eosinophilic cytoplasm and nuclei (apoptotic cells; ). At 30th DPI, clumps of the ruptured vacuolated cells with eccentric nuclei were observed in the zona glomerulosa (). Further, hypertrophy and hyperplasia of cells secreting corticosterone and aldosterone of zona glomerulosa were found at the 45th DPI (). At 60th DPI, adrenal glands showed degenerated cells in the zona fasciculata ().
Figure 4. BTV-1, 6 ml (5.5 log10 TCID50/ml) inoculated by I/D and IV routes in sheep at 60th gestation day. H&E section, adrenal sections: (A) at 15th DPI, distorted histoarchitecture with dilated sinusoids containing sparse neutrophils (asterisk) and a few degenerated cells in the zona fasciculate (arrow), ×200; (B) at 15th DPI, few cells in the glomerulosa have a condensed nucleus, hyper-eosinophilic cytoplasm and distinct boundaries (arrow), ×200; (C) at 30th DPI, clumps of the ruptured cells vacuolated cells with eccentrical nuclei in zona glomerulosa (circle), ×200; (D) a higher magnification of (C), ×400; (E) at 45th DPI, hypertrophy and hyperplasia, cell of zona glomerulosa, H&E, ×400 and (F) at 60th DPI, section showing a degenerated cell (arrowhead) in the zona fasciculata H&E, ×400.
Histology of adrenal, thyroid and posterior pituitary of control ewes showed no significant morphological changes throughout the experimental trial ().
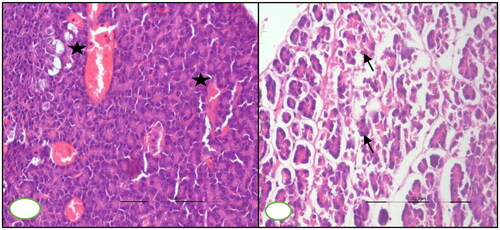
Pancreas
At 7th DPI, congested blood vessels were observed in the pancreas section, microscopically. Pancreatic section revealed atrophy of pancreatic acini and interlobular septa oedema on microscopic examination at 30th DPI ().
Figure 6. BTV-1, 6 ml (5.5 log10 TCID50/ml) inoculated by I/D and IV routes in sheep at 60th gestation day. H&E section, pancreases sections: (A) at 15th DPI, engorged capillaries (asterisk) in the parenchyma, ×200 and (B) at 30th DPI, disorganized atrophic pancreatic lobules characterized degenerated exocrine acini (arrow) having pycnotic nuclei and darkly stained cytoplasm, ×200.
Viral quantification
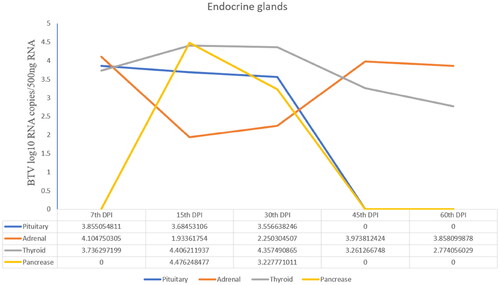
The BTV-1 load in the pituitary gland at 7th DPI and thyroid gland at 15th DPI was highest with the viral copy number (log10 BTV copies/500 ng Tissue RNA) 3.8 and 4.4, respectively. In addition, the adrenals showed the viral load (copy number-4.1) at 7th DPI, which reduced on subsequent days of sacrifice (i.e., 15th, 45th and 60th DPI) as compared to the control. The highest viral copy number of BTV in pancreas tissue was observed at 15th DPI ().
Immunolocalization
At 7th DPI, BTV-1 antigen was localized in the cytoplasm of the follicular epithelial cells of various lobules and parafollicular cells of the stroma in the thyroid glands of infected ewes (). In the adrenal glands, BTV-1 antigen was localized in the degenerated cells of zona fasciculata, and in the radiating cells of zona reticularis at 7th DPI (). Further, cells surrounding the sinusoids had variable (mild-to-moderate) degrees of immunolabeling in pituitary adenohypophysis at 7th DPI (). The control sections of the thyroid glands were devoid of any immunolabeling ().
Figure 8. BTV-1 infected sheep at 65th DPI, ABC-IHC-DAB, immunohistochemically stained section, polyclonal BTV-1 primary antibody. (A) Thyroid section showing positive immunolabeling in the cytoplasm of the follicular epithelial cell (asterisk) at 14th DPI, ×200. (B) Adrenal gland section showing positive immunolabelling in the cytoplasm of the cell of zona fasciculata (arrowhead) at 7th DPI, ×400. (C) Adrenal gland section showing positive immunolabelling in the cytoplasm of the cell of zona reticularis projecting in the medullary region (dotted circle) of the gland at 14th DPI, ×400. (D) Pituitary gland showing positive immunolabeling in the cytoplasm of the cell of the adenohypophysis (curved arrow) at 14th DPI, ×200. (E) Antibody control section devoid of any immunolabeling, IHC-DAB, 14th DPI, ×200.
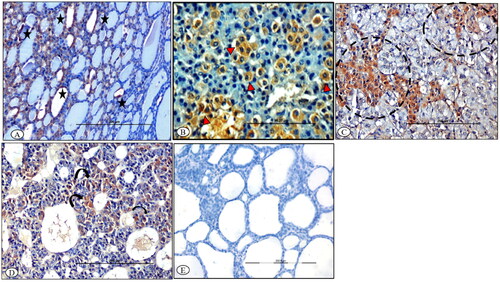
Immunolocalization of caspase-3
In the thyroid gland, C-cells occasionally contained mild-to-moderate cytoplasmic immunoreactivity for caspase-3 at 15th DPI (). Moreover, thyroglobulin synthesizing follicular cells showed mild cytoplasmic immune-reactivity for caspase-3 at 15th and 30th DPI (). The control section was devoid of immunolabeling (). In the adrenal gland, positive immunolabeling in the cells of the zona reticularis adjacent to the chromaffin cells of the medulla was observed at 15th DPI (). The glucocorticoid-producing cells of the zona fasciculata were mildly stained for caspase-3 at 30th DPI. The cells showed a condensed nuclei and cytoplasm, and cells retracted from the adjacent cells at 30th DPI (). The adrenal medulla chromaffin cells were negative for caspase-3 at 7th, 15th and 30th DPI. Control sections were devoid of any immunolabeling at every time point ().
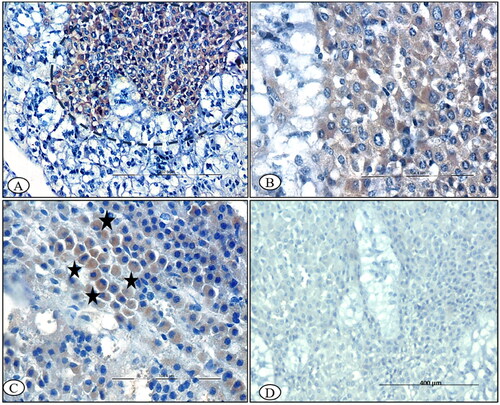
Figure 9. BTV-1 infected sheep at 60th DPI, ABC-IHC-DAB, immunohistochemically stained section, polyclonal caspase-3 primary antibody. (A) Thyroid follicles showing the positive immunostaining for anti caspase-3 in the parafollicular cells of the thyroid as well as in the cytoplasm of the endothelial cell lining the blood vessels (circle). (B) Thyroid follicular epithelial showing the positive immunostaining for anti caspase-3 in the follicular epithelial cells (arrowhead), 30th DPI, DAB-IHC, ×200 (inset: higher magnification, ×400). (C) Positive immunolabeling in the cytoplasm of the infiltrated macrophage (arrowhead), 30th DPI, ×400. (D) No signals were detected in the control group thyroid sections, 30th DPI. (E) No immunostaining was observed in the primary antibody control groups, ×400.
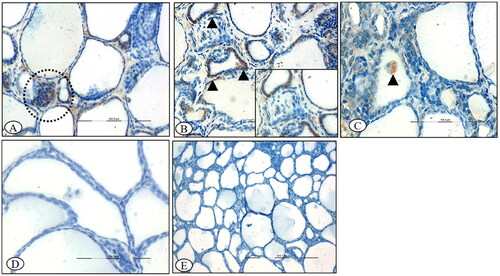
Figure 10. BTV-1 infected sheep at 60th DPI, ABC-IHC-DAB, immunohistochemically stained section, polyclonal caspase-3 primary antibody. (A) Adrenal gland showing the positive immunostaining for anti caspase-3 in the cytoplasm of the cell of zona reticularis cells (circle), ×200. (B) Higher magnification, ×400. (C) Positive immunolabeling in the cytoplasm of zona fasciculata cells (asterisk) that arranged in the linear manner, ×400, 30th DPI. (D) No signals were detected in the control group adrenal sections, 30th DPI, DAB-IHC, ×100.
Serum hormone concentration
Plasma progesterone, cortisol and thyroid hormone (T-3, T-4), concentrations were measured in the BTV-1 and sham infected (control group) ewes. Mean ± standard error of hormone concentration is presented in .
Table 1. Serum hormone concentration (mean ± SE) in the mid-infected group (GrIMP) and control group at different days postinfections.
Kinetic studies of progesterone (P4)
In BTV-1 infected groups, the upsurge in P4 concentration level (43.23 nmol/l) up to 14th DPI, then abruptly drop (27.32 nmol/l) by 21st DPI and later on-again upsurge was observed in subsequent DPI. A similar trend in the mean value of the P4 concentration was observed in the mock infected control group ewes.
Kinetic studies of triiodothyronine (T-3)
BTV-1 infected ewes showed an increasing trend in mean T-3 concentration from 0th (6.8 nmol/l) to 90th (37.14 nmol/l) DPI, when compared with the preinoculated stage. In contrast, the control group ewes showed a decreasing trend in mean T-3 concentration from 0th (23.05 nmol/l) to 90th (7.38 nmol/l) DPI.
Kinetic studies of thyroxine (T-4)
The BTV-1 infected ewes showed the reduction in mean T-4 concentration from 0th (64.69 nmol/l) to 14th (50.23 nmol/l) DPI, which further surge to 90th DPI in comparison to the preinoculation stage.
Kinetic studies of cortisol
The slight increment in mean cortisol serum level from 7th (56.18 g/dl) to 15th DPI (78.84 g/dl) was observed in BTV-1 infected ewes compared to the preinfected stage. However, cortisol upsurging was not statistically significant when compared with the control ewes at equivalent days. The cortisol level in the BTV-1 infected ewes in late phase of infection and control ewes was similar.
Discussion
BT is an endemic and reemerging disease of ruminants, particularly of sheep and other wild ruminants, transmitted by blood-sucking midges. BT control is a major challenging task because of the existence of multiple BTV serotypes, and cropping up of new serotypes from time to time in different geographical regions. The pathogenic potential of these serotypes has not been completely delineated. The tropism of BTV for vascular endothelial cell consequences pathology in the organ where vascular endothelial cells are prominent. The virus-induced inflammatory lesions like coronitis, striated muscle lesions, skin lesions, foetal pathology-cavitating lesions in the brain, abortions/stillbirths and wool defects have been documented. However, the pathogenic potential of BTV serotypes in the secretory epithelial cell of ductless endocrine glands such as pituitary (ectoderm origin), thyroid (endoderm origin) and adrenal glands (intermediate mesoderm and neuro-ectoderm origin) has not been portrayed extensively. Therefore, the BTV-1 serotype-induced endocrine pathology has been portrayed in this study, which is often ignored during the natural outbreaks of BT disease. Herein out of naturally mated 15 pregnant ewes, 10 ewes were experimentally infected with BTV-1 serotype, while the remaining 5 ewes were mock infected with cell culture fluid at the 60th day of their gestation. Both treatment groups were monitored and sacrificed at different time points for the collection of pituitary, thyroid and adrenal glands.
Pituitary gland regulates most of the target endocrines such as thyroid and adrenals. In the BTV-1 infected ewes, pituitaries showed oedematous changes and engorged capillary plexuses/sinusoids grossly as well as microscopically, which might be due to the higher proinflammatory cytokine levels and enhanced permeability as the proinflammatory cytokines and vasoactive mediators accounted for oedematous and haemorrhagic changes in BTV experimentally infected animals.Citation25 Moreover, the microscopically observed congestion in the adenohypophysis and neurohypophysis might be due to enhanced vascular permeability or vascular leakage. The positive immunolocalization of the BTV antigen in secretory epithelial cells of the anterior pituitary and detection of viral RNA in the pituitary glands clearly pointed towards virus mediated pathological consequences. Microscopically, pituicytes hyperplasia might be an adaptive response of the virus-infected gland, which needs further elucidation. The BTV-1 antigen was primarily localized in the cytoplasm of glandular epithelial cells in comparison to other pituitary anatomical regions (intermediate or posterior pituitary cells), indicating a preference of BTV-1 for the anterior pituitary. Thus, the present work ruled out the pituitary cells as a novel site for virus tropism.
The BT viral RNA was detected in adrenal tissue of BTV-1 infected ewes, which is consistent with earlier research.Citation26,Citation27 BTV antigen was localized by the IHC to rule out the possibility that the detection was attributable to the RBC-related virus or infected adrenal cells.
The robust DAB signals in the cells of the zona glomerulosa and the zona fasciculata at 7th and 15th DPI, indicating that BTV-1 infects the cells of the adrenal gland cortex. This observation may be due to the progenitor cells being retained, which makes it more receptive to the BTV as the adrenocortical progenitor cells of the subcapsular cortex, which descend and migrate into the adrenal medulla, maintain cortical homeostasis.Citation28 However, the further detail study is warranted to describe the preferentially tropism of BTV towards progenitor lineage.
BTV-related loss of progenitor neuronal and/or glial cells of the central nervous system has been documented previously.Citation29 The positive caspase-3 signals in the zona reticularis of the adrenal cortex depicted the apoptosis in infected cells. However, the BTV antigen could only be localized in the cortical secretory epithelial cells, not in the endothelial cells. Together, the absence of immunolabeling in arterioles and capillary endothelial cells and positive immunolabeling from zona glomerulosa and zona reticularis at 7th and 15th DPI pointed towards viral-induced and cytokines-mediated direct injury. The effect of BTV-1 on thyroid gland was portrayed via nucleic acid detection and antigen localization in the follicular epithelial cells of the thyroid gland. Vascular alterations in the thyroid gland, such as blood capillary enlargement and endothelial damage, were in line with the previous findings.Citation30 BTV tropism towards the follicular epithelial cells was further confirmed by positive immunolabeling of follicular epithelial cells for BTV antigen. Although morphological indications of apoptosis viz. apoptotic bodies in the flattened follicular epithelial cells were not well evident in the HE sections, however, mild-to-moderate immunosignals against caspase-3 in the cytoplasm of the follicular cells bestowed apoptotic alterations in the thyroid follicular cells. Another possibility for such pathological consequences is that BTV-1 uses the lytic route to exit the follicular epithelial cells (i.e., BTV-1 caused the destruction of the follicular cells). Both apoptosis and proinflammatory cytokines have been proposed as the major events in the lytic release of BTV from mammalian cells.Citation31
BTV-induced apoptosis in bovine and ovine endothelial cells, monocytes and T cells.Citation32–34 VP2 and VP5, the viral outer capsid layer components, cause apoptosis in infected cells by activating NF kappa-β and executioner caspase-3.Citation35 Moreover, apoptosis in mammalian cells needed, simply, the uncoating of the BTV rather than viral multiplication.Citation35
In the BTV-1 infected ewes showed an increased level of the serum T-4 hormone as compared with the control animals. These changes might be due to the reduced conversion of T-4 into T-3. Further, the affected C-cells of the thyroid gland in the infected group may disturb the Ca++ metabolism.
In the later stages of the illness, macrophages frequently act as a professional phagocyte in the thyroid follicular tissue to eliminate apoptotic cells.Citation36 In the current investigation, macrophages were seen in some thyroid follicles but not all of them. The heterogeneity in the lineage of the apoptotic cells responsible for macrophage activation might be the cause of this disparity. Earlier studies have shown that pentraxins and collectins are associated with late apoptotic cell uptake, whereas expression of CD43 is involved in an early stage of apoptotic uptake.Citation37,Citation38 It is worth noting that big, fused, varied-sized thyroid follicles emerge in the later stages of the infection, which may be attributable to the clearing of the epithelial cells and basement membrane between the follicles.
There are certain restrictions on the current investigation. Therefore, a greater number of animals in each group must be included in order to confirm the current study’s findings. Furthermore, it is crucial to check the endocrine glands for the virus during naturally occurring BTV epidemics.
It is crucial to expand the experiment to include nonpregnant animals of all ages to clarify the virus’s effect on endocrine glands. This broader perspective will enable a more thorough comprehension of the virus’s impacts on the endocrine system.
Conclusion
The tropism of the BTV-1/SKN-10 for the secretory epithelial cells of the pituitary, thyroid and adrenal glands has been explored in this study. Immunohistochemical localization of the BTV antigen in the glandular epithelial cells of the pituitary, thyroid and adrenal glands of infected ewes has been shown for the first time, which evidently signifies the role of BTV-1 in the pathology of the endocrine glands. Consequently, growth and production will be affected critically. Based on the present findings, further experimental studies are warranted to underpin a molecular basis of the sequential pathological changes related to BTV infection. Moreover, the present findings will pave the way in better understanding of the pathogenesis in a primary host, which is prime prerequisite for disease control.
Disclosure statement
Karam Pal Singh reports that financial support, administrative support, equipment, drugs or supplies, statistical analysis and writing assistance were provided by Indian Veterinary Research Institute. Karam Pal Singh reports a relationship with Indian Council of Agricultural Research that includes employment and funding grants.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Carpenter S, Groschup MH, Garros C, Felippe-Bauer ML, Purse BV. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100(1):102–113.
- Van Dijk AA, Huismans H. In vitro transcription and translation of bluetongue virus mRNA. J Gen Virol. 1988;69(Pt 3):573–581.
- Ratinier M, Caporale M, Golder M, et al. Identification and characterization of a novel non-structural protein of bluetongue virus. PLoS Pathog. 2011;7(12):e1002477.
- Gerdes GH. South African overview of the virus, vectors, surveillance and unique features of bluetongue. Vet Ital. 2004;40(3):39–42.
- Coetzee P, Stokstad M, Venter EH, Myrmel M, Van Vuuren M. Bluetongue: a historical and epidemiological perspective with the emphasis on South Africa. Virol J. 2012;9(1):198.
- Naresh G, Putty K, Reddy YN, Jyothi YK. Type-specific seroprevalence of bluetongue in India during 2018 and 2019. Vet World. 2020;13(10):2092–2096.
- Van der Sluijs MT, Schroer-Joosten DP, Fid-Fourkour A, et al. Transplacental transmission of bluetongue virus serotype 1 and serotype 8 in sheep: virological and pathological findings. PLoS One. 2013;8(12):e81429.
- MacLachlan NJ, Osburn BI. Impact of bluetongue virus infection on the international movement and trade of ruminants. J Am Vet Med Assoc. 2006;228(9):1346–1349.
- Grewar JD. The economic impact of Bluetongue and other orbiviruses in sub-Saharan Africa with special reference to Southern Africa. Vet Ital. 2016;52(3–4):375–381.
- Gethmann J, Probst C, Conraths FJ. Economic impact of a bluetongue serotype 8 epidemic in Germany. Front Vet Sci. 2020;7:65.
- Muzzle S, Fever MC. Species affected. 2003.
- Adamopoulos DA, Lawrence DM, Vassilopoulos P, Contoyiannis PA, Swyer GI. Pituitary-testicular interrelationships in mumps orchitis and other viral infections. Br Med J. 1978;1(6121):1177–1180.
- Kino T, Mirani M, Alesci S, Chrousos GP. AIDS-related lipodystrophy/insulin resistance syndrome. Horm Metab Res. 2003;35(3):129–136.
- Pekic S, Cvijovic G, Stojanovic M, Kendereski A, Micic D, Popovic V. Hypopituitarism a late complication of hemorrhagic fever. Endocrine. 2005;26(2):79–82.
- Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Semin Pediatr Infect Dis. 2005;16(4):271–281.
- Clarke WL, Shaver KA, Bright GM, Rogol AD, Nance WE. Autoimmunity in congenital rubella syndrome. J Pediatr. 1984;104(3):370–373.
- Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocr Rev. 1993;14(1):107–120.
- Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12(13):1255–1265.
- Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005;115(2):200–208.
- Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14(13):1553–1577.
- Chinnadurai G. CtBP, An unconventional transcriptional corepressor in development and oncogenesis. Mol Cell. 2002;9(2):213–224.
- Chauhan HC, Biswas SK, Chand K, et al. Isolation of bluetongue virus serotype 1 from aborted goat fetuses. Rev Sci Tech. 2014;33(3):803–812.
- Lakshmi IK, Putty K, Raut SS, et al. Standardization and application of real-time polymerase chain reaction for rapid detection of bluetongue virus. Vet World. 2018;11(4):452–458.
- Channappanavar R, Singh KP, Singh R, Umeshappa CS, Ingale SL, Pandey AB. Enhanced proinflammatory cytokine activity during experimental bluetongue virus-1 infection in Indian native sheep. Vet Immunol Immunopathol. 2012;145(1-2):485–492.
- Drew CP, Heller MC, Mayo C, Watson JL, Maclachlan NJ. Bluetongue virus infection activates bovine monocytes-derived macrophages and pulmonary artery endotelial cells. Vet Immunol Immunopathol. 2010;136(3–4):292–296.
- Drolet BS, Reister LM, Rigg TD, et al. Experimental infection of white-tailed deer (Odocoileus virginianus) with Northern European bluetongue virus serotype 8. Vet Microbiol. 2013;166(3–4):347–355.
- Worwa G, Hilbe M, Chaignat V, et al. Virological and pathological findings in bluetongue virus serotype 8 infected sheep. Vet Microbiol. 2010;144(3–4):264–273.
- Finco I, Mohan DR, Hammer GD, Lerario AM. Regulation of stem and progenitor cells in the adrenal cortex. Curr Opin Endocr Metab Res. 2019;8:66–71.
- Maclachlan NJ, Osburn BI. Teratogenic bluetongue and related orbivirus infections in pregnant ruminant livestock, timing and pathogen genetics are critical. Curr Opin Virol. 2017;27:31–35.
- Labadie T, Sullivan E, Roy P. Multiple routes of bluetongue virus egress. Microorganisms. 2020;8(7):965.
- Saminathan M, Singh KP, Khorajiya JH, et al. An updated review on bluetongue virus: epidemiology, pathobiology, and advances in diagnosis and control with special reference to India. Vet Q. 2020;40(1):258–321.
- Barratt-Boyes SM, Rossitto PV, Stott JL, MacLachlan NJ. Flow cytometric analysis of in vitro bluetongue virus infection of bovine blood mononuclear cells. J Gen Virol. 1992;73(Pt 8):1953–1960.
- DeMaula CD, Jutila MA, Wilson DW, MacLachlan NJ. Infection kinetics, prostacyclin release and cytokine-mediated modulation of the mechanism of cell death during bluetongue virus infection of cultured ovine and bovine pulmonary artery and lung microvascular endothelial cells. J Gen Virol. 2001;82(Pt 4):787–794.
- Takamatsu H, Mellor PS, Mertens PPC, Kirkham PA, Burroughs JN, Parkhouse RME. A possible overwintering mechanism for bluetongue virus in the absence of the insect vector. J Gen Virol. 2003;84(Pt 1):227–235.
- Mortola E, Noad R, Roy P. Bluetongue virus outer capsid proteins are sufficient to trigger apoptosis in mammalian cells. J Virol. 2004;78(6):2875–2883.
- Gregory D, Devitt A. The macrophage and the apoptotic cell, an innate immune interaction viewed simplistically. Immunology. 2004;113(1):1–14.
- Bijl M, Horst G, Bijzet J, Bootsma H, Limburg PC, Kallenberg CGM. Serum amyloid P component binds to late apoptotic cells and mediates their uptake by monocyte-derived macrophages. Arthritis Rheum. 2003;48(1):248–254.
- Nauta A, Raaschou-Jensen N, Roos A, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33(10):2853–2863.