Abstract
Satellite cells are an important cellular model for studying muscle growth and development and mammalian locomotion-related molecular mechanisms. In this study, we investigated the effects of voltage, pulse duration, and DNA dosage on horse skeletal muscle satellite cells’ electroporation transfection efficiency using the eukaryotic expression plasmid Td Tomato-C1 (5.5 kb) encoding the red fluorescent protein gene mainly based on fluorescence-positive cell rate and cell survival rate. By comparison of different voltages, pulse durations, and DNA doses, horse skeletal muscle satellite cells have nearly 80% transfection efficiency under the condition of voltage 120 V, DNA dosage 7 µg/ml, and pulse duration 30 ms. This optimized electroporation condition would facilitate the application of horse skeletal muscle satellite cells in genetic studies of muscle function and related diseases.
Introduction
Skeletal muscle satellite cells are adult stem cells located under the basal layer of muscle fibers. They are the main source of muscle-derived precursor cells in postnatal muscles and play an indispensable role in the regeneration and hypertrophy of adult skeletal muscle.Citation1 Satellite cells are important cell models to study the mechanism of muscle growth and development, and molecular mechanisms related to mammalian motion.Citation2 Among different animals, the horse is one of the most suitable and competitive animal models for studying sports.Citation3 Moreover, tendon injury and related diseases are common diseases in this species. Therefore, equine satellite cells can provide basic materials for understanding the functions and diseases of sports-related genes, have great research value and broad application prospects in tissue engineering and regenerative medicine, and may also provide valuable insights for aging and muscle inflammation.Citation4,Citation5
Cell transfection is an important technology in the fields of cell and molecular biology. Transfection is the process of introducing a foreign gene into a mammalian cell to produce a genetically modified cell. The main purpose of transfection is to study the function of genes or gene products by enhancing or inhibiting the expression of a specific gene(s) in cells and to produce recombinant proteins in mammalian cells.Citation6 Currently, the most common transfection methods include virus-mediated infection, calcium phosphate precipitation, lipofection, and electroporation transfection. All these methods have their pros and cons. For example, viral-mediated transfection has high transfection efficiency, insertion induced sustainable transgene expression, but a higher chance of causing inflammatory responses and insertional mutations;Citation7,Citation8 the calcium phosphate precipitation method supports large molecular weight plasmid DNA transfection but is more harmful;Citation9,Citation10 while liposome transfection has no limit to the plasmid size, but is expensive and toxic to some cell types.Citation11,Citation12 Electroporation, the method used in this study is a technique of introducing macromolecules, such as DNA into cells after temporary micropores are formed in the cell membrane in a high-voltage current pulse environmentCitation13,Citation14. At present, electroporation technology is constantly advancing, in addition to the traditional cuvette electroporation, there are micro electroporation and nano electroporation.Citation15 Electroporation is also performed in vivo and has reached clinical trials.Citation16–18 It has the advantages of simplicity, rapidity, good reproducibility, high efficiency, and suitability for cell suspension culture. However, for different types of cells, or even the same type of cells of different species, more stringent parameter settings are required. Therefore, although the successful application of electroporation on satellite cells of other species has been achieved under different parameter settings,Citation19–21 during our research on muscle development, we thought that it is necessary to determine optimized electroporation conditions for horse satellite cells.
Materials and methods
Culture and identification of horse satellite cells
Animal experiments in this study were approved by the Ethics Committee of Inner Mongolia Agricultural University. Semitendinosus muscle samples were collected from 2-year-old healthy colts in a local slaughterhouse. When it arrives laboratory, muscle samples were quickly sterilized with 70% ethanol, and rinsed 3–4 times with 4-fold volume cold Dulbecco’s Phosphate-Buffered Saline (DPBS, Gibco, USA). The whole muscles were excised and cut into small pieces (1–2 mm3 in size) using scissors after visible adipose and connective tissues on the muscle mass were removed with a scalpel. Fractionated enzymatic digestion was performed for 2 × 30 min with trypsin solution (0.25% Trypsin-EDTA, Gibco, USA) and collagenase Type IV (Sigma, USA) solution in a water bath with stirring at 37 °C, and the cell suspension was filtered through 70 and 40 µm cell strainers, successively. Then, filtrates were centrifuged (800 rpm/min for 10 min) to collect the cell pellet. Afterward, the cells pellet was resuspended in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA) with 20% fetal bovine serum (FBS, Gibco, USA), 1% Amphotericin (Gibco, USA), 1% non-essential amino acids (NEAA, Gibco, USA), 2 mmol/L L-glutamine (Sigma, USA). And transferred to a 35 × 10 mm culture dish. To minimize possible fibroblast contamination, 1.5 h preplating was used. Subsequently, the supernatant containing satellite cells was transferred to the culture dish. The medium was changed every 2 days. After obtaining 80% confluence, the cells were passaged using 0.25% trypsin-EDTA (Gibco, USA). After passing to the second generation, some cells were cryopreserved in liquid nitrogen with a cryopreservation solution (10% dimethyl sulfoxide, MP Biomedicals, USA; 90% FBS, Gibco, USA).
The immunofluorescent staining was analyzed using horse satellite cells at the 4th passage. The cells were fixed for 40 min at room temperature with 4% paraformaldehyde (Solarbio, China). After three washes for 10 min each time in washing buffer (the washing buffer was Phosphate Buffer Saline (PBS, Gibco, USA) with 1% Tween 20, 0.1% TritonX-100), the cells were permeabilized with 1% TritonX-100 in PBS for 1 h at room temperature. Afterward, the cells were blocked with 1% bovine serum albumin (BSA, Sigma, USA) in PBS for 1 h at room temperature. The primary antibodies (Pax7, 1:200, AB-528428, DSHBY; Desmin, 1:200, LS-B3122, LSBio) were incubated overnight at 4 °C and then washed three times with washing buffer for 10 min each time. The secondary antibodies (IgG 488 for donkey anti-mouse, 2018296, Invitrogen) were added and treated for 1 h in the dark at room temperature. After three washes for 10 min each time in washing buffer, the cell nuclei were stained with Hoechst 33342 (1 mg/mL, Beyotime, China) for 10 min. Finally, the cells were mounted on glass slides and examined with a fluorescence microscope (ZEISS, Germany). Our fluorescence microscope was equipped with three fluorescent filter cubes: Filter set 49 (G 365, FT 395, BP 445/50), Filter set 10 (BP 450–490, FT 510 BP 515–565), and Filter set 15 (BP 546/12, FT 580, LP 590).
Preparation of electro-competent cells
The fourth generation of horse satellite cells, with 80% confluence were washed twice in DPBS (Gibco, USA) and digested with 0.25% Trypsin-EDTA (Gibco, USA) for 3 min at 37 °C. The cell suspension was transferred into a 15 mL polypropylene centrifuge tube and was centrifuged for 3 min at 1200 rpm/min to collect the cell pellet, and the cell pellet was resuspended with 1 ml OPTI-MEM (31985070, Gibco, USA).
Preparation of plasmid
We selected the mammalian cell red fluorescent overexpression plasmid Td Tomato-C1 for electroporation transfection experiments, which has a neomycin resistance cassette (Neor) that allows G418 screening to obtain stably transfected high-purity eukaryotic cells. Td Tomato-C1 plasmid transfected into DH5a (Qiagen, Germany), incubated in an ice bath for 30 min, heat shocked at 42 °C for 90 s, ice bath for 3 min, added LB medium, and shaken at 37 °C, 140 rpm/min for 1 h. DH5a transfected with Td Tomato-C1 plasmid were evenly spread on LB solid agar medium containing kanamycin, and placed in a constant temperature and humidity incubator at 37 °C overnight. On the next day, a single colony was inoculated into LB medium (kanamycin 100 µg/ml) and cultured overnight at 37 °C. Plasmids were purified using a HiPure plasmid kit (Thermo, USA), as specified by the manufacturer. Plasmid concentration was accurately measured by spectrophotometer. Plasmids were stored at −20 °C for future use.
Design of electroporation transfection experiments
Pulse duration optimization
In this part, we set the voltage at 120 V, DNA dosage at 6 µg, and the pulse duration at different values (20, 25, 30, 35, and 40 ms). The steps were as described below (see ‘For all experiments’ section).
Voltage optimization
In this part, we used 30 ms as the pulse duration value. Electroporation transfection was carried on under the condition of 6 µg DNA dosage, and different voltage values (110, 120, and 130 V). The steps were as described below (see ‘For all experiments’ section).
DNA dosage optimization
Finally, to optimize DNA dosage, the optimized voltage and pulse duration described above were used when the DNA dosage varied between 3, 5, 7, and 9 µg. The steps were as described below (see ‘For all experiments’ section).
For all experiments, the cuvette gap of the electrotransfection cup was 0.2 cm. OPTI-MEM was used as electroporation buffer and cell suspension (100 µl, equivalent to 1 × 105 cells) was mixed well with Td Tomato-C1 plasmid for electrotransfection. Pulsing was performed using the Bio-Rad Gene Pulser Xcell™ (Gene Pulser Xcell™ Electroporation System) with a pulse number of 1 and a pulse interval of 0. After electroporation, cells were inoculated into gelatin-coated 12-well plates with fresh DMEM culture medium containing 20% FBS and no penicillin and Streptomycin. Cells were placed at 37 °C in a 5% CO2 incubator for 24 h, after which transfection efficiency and cell survival rate were measured. Non-transfected cells were used as a negative control.
Obtaining electroporation transfection efficiency and cell survival rate
Transfection efficiency was determined as the percentage of cells with red fluorescence under the fluorescent microscope (ZEISS, Germany). Cell survival rate was determined as the percentage of live non-stained cells after treatment with 0.4% trypan blue (Gibco, USA).
Statistical analysis
We first tested all data for normal distribution and equal variance using GraphPad 8.0.1. The results showed that all data passed the test except for the effect of different DNA doses on transfection efficiency, so we used one-way ANOVA (normality test) for the data that passed the test. p < 0.05 was considered statistically significant for the difference. Secondly, we performed a nonparametric test Kruskal-Wallis for transfection efficiency of different DNA doses that did not pass the test. results are expressed as mean ± standard deviation.
Results
Obtaining purified horse satellite cells
Since fibroblasts adhere faster than satellite cells, we isolated and purified horse satellite cells by the method of enzyme digestion and differential adhesion. Horse satellite cells were predominantly spindle-shaped, uniform in size, with well-defined edges and full morphology, whereas Horse fibroblasts were a polygonal cell type (). Both proliferated vigorously in vitro, lengthened and thinned with a gradual increase in cell density and tended to be arranged in parallel ().
Figure 1. Morphological and characterization of horse skeletal muscle satellite cells. Morphology of newly isolated (A) and proliferated (B) horse skeletal muscle satellite cells and horse fibroblast cells. (C) Immunofluorescence results of Desmin and Pax7 expression in horse fibroblast cells and horse skeletal muscle satellite cells. Secondary antibodies tagged with green fluorescence were used and nuclei were counterstained by DAPI. Scale bars, 20 µm.
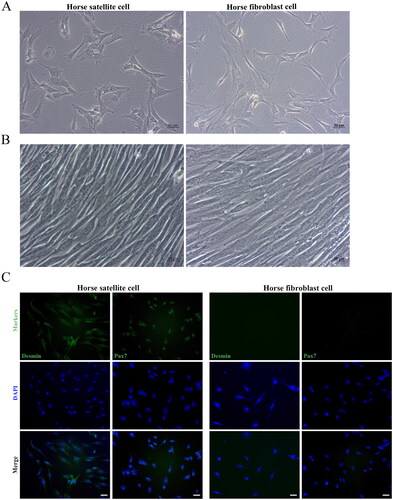
To confirm the obtained cells are horse satellite cells, we conducted an immunofluorescence assay on the cells using the first antibody against satellite cells marker DesminCitation22,Citation23 and Pax7.Citation24 Immunofluorescence assays showed that our purified horse satellite cells are positive for well-proven skeletal muscle satellite cell markers Desmin and Pax7, which are not expressed by horse fibroblast cells ().
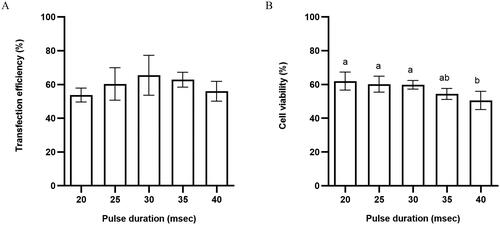
Effect of pulse duration on transfection efficiency and cell viability
First of all, we determined the effect of pulse duration on transfection efficiency and cell viability. We observed cells under the fluorescence microscope after electroporation with different pulse duration. As shown in , the pulse duration had a significant effect on cell survival and the plasmid transfection efficiency increased with increasing pulse duration. The transfection rate was the highest when the pulse duration was 30 ms, while when the pulse duration exceeded 30 ms, transfection efficiency changes less (). Cell viability decreased with higher pulse duration, that pulse duration higher than 35 ms adversely affected the cell viability (). Collectively we determined that 30 ms is the best pulse duration value for horse satellite cells which resulted in higher transfection efficiency [65 ± 8.3 vs. 54 ± 2.9% (20 ms, p > 0.05, ns), 60 ± 6.7% (25 ms, p > 0.05, ns), 63 ± 3.1% (35 ms, p > 0.05, ns), and 56 ± 4.1% (40 ms, p > 0.05, ns)] ( and Table S1) and cell viability [60 ± 2.6 vs. 62 ± 5.3% (20 ms, p > 0.05, ns), 61 ± 4.7% (25 ms, p > 0.05, ns), 54 ± 3.3% (35 ms, p > 0.05, ns), and 51 ± 5.4% (40 ms, p < 0.05, significant)] ( and Table S4).
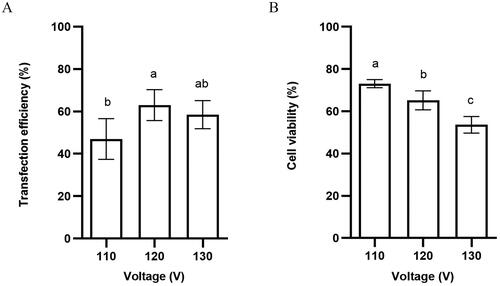
Effect of voltage on transfection efficiency and cell viability
Then we found that, under a fixed pulse duration condition, varying voltages had different influences on transfection efficiency and the survival rate of horse satellite cells. When pulse duration was fixed and voltage was changed from 110 to 120 V, there was a significant difference in transfection efficiency. When voltage exceeded 130 V, transfection efficiency decreased (). Cell death increases as voltage increases, indicating that the high intensity of the electric field is severely harmful to the horse satellite cells (). Collectively, we determined that 120 V is the best voltage value for horse satellite cells and results in significant higher transfection efficiency [63 ± 5.9 vs. 47 ± 7.7% (110 V, p < 0.05, significant), 59 ± 5.3% (130 V, p > 0.05, ns)] ( and Table S2) and cell viability [65 ± 4.5 vs. 73 ± 1.9% (110 V, p < 0.05, significant), 54 ± 3.9% (130 V, p < 0.05, significant)] ( and Table S5).
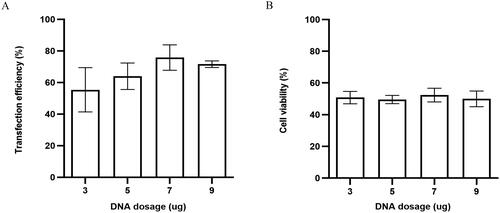
Effect of DNA dosage on transfection efficiency and cell viability
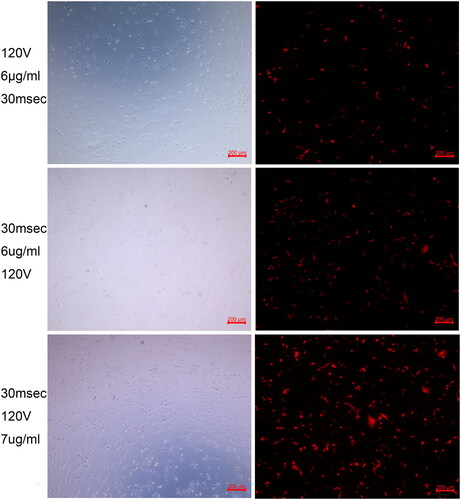
As expected, under the condition of optimal pulse duration and voltage, different DNA dosages would be one of the important parameters affecting the transfection efficiency of Td Tomato-C1 plasmids in horse satellite cells. As shown in , transfection efficiency initially increased along with the DNA dosage increase, but when DNA dosage exceeded 7 µg/ml, the transfection efficiency will not change anymore (). However, we found that there were no significant differences in cell survival rate among groups (). Therefore, collectively, we determined that 7 µg/ml is the best DNA dosage value for horse satellite cells that could result in significantly higher transfection efficiency [76 ± 8 vs. 55 ± 14% (3 µg/ml, p > 0.05, ns), 64 ± 8.4% (5 µg/ml, p > 0.05, ns), 72 ± 2.1% (9 µg/ml, p > 0.05, ns)] ( and Table S3) and cell viability [52 ± 4.3 vs. 51 ± 3.9% (3 µg/ml, p > 0.05, ns), 50 ± 2.6% (5 µg/ml, p > 0.05, ns), 50 ± 4.9% (9 µg/ml, p > 0.05, ns)] ( and Table S6).
Figure 4. (A) Transfection efficiency and (B) cell viability of horse skeletal muscle satellite cells using different DNA dosages.
After all, when utilizing these optimized parameters: voltage at 120 V, DNA dosage at 7 µg/ml, and pulse duration at 30 ms, we could obtain transfection efficiency at 76 ± 8% with cell viability at 52 ± 4.3% and exogenous red Tomato protein high expression in horse satellite cells ().
Discussion
Electroporation transfection is a physical method invented in the mid-1980s to introduce foreign substances into cells.Citation14 Although it is a simple, efficient, and commonly used method, there are differences in transfection efficiency for different types of cells. The conditions for the target cells should be optimized to get a better outcome. The main factors affecting the electroporation transfection efficiency include voltage, pulse duration, and DNA dosage,Citation25 which also became 3 parameters optimized in this study. The length of the pulse time is one of the factors that can directly cause irreversible damage to the cells in addition to the voltage, which leads to an increase in cell mortality. However, too low values for these two parameters fail to change the cell membrane, making it impossible for foreign genes to enter the cell easily.Citation26 In consistence with it, our study also showed that the transfection rate is highest when the pulse duration is 30 ms and the voltage is 120 V, and the cell survival rate decreases with the increase of the pulse duration and voltage. The DNA dosage is the same as the voltage, and the transfection efficiency increases linearly with the increase of its value. After reaching the highest threshold, the transfection efficiency will not change significantly. Interestingly, we found that changes in plasmid concentration had no significant effect on cell survival. It may mainly be because the tunnels opened on cell membranes by electro-treatment are enough for a wide range of DNA dosage.Citation27 Similar trends are also seen in other electroporation transfection practices by others.Citation28
We also compared our study with similar studies in other species. Unfortunately, we only found one published study in rat skeletal muscle satellite cells, and their optimal electroporation conditions, which could yield 35–50% of cell survival rate were pulse time at 20 ms, voltage at 180 V, and DNA at 15 µg.Citation29 Another study was done on human adult myoblasts transfected with an enhanced green fluorescent gene, and their cells expressed green fluorescent protein up to 60–70%.Citation30 Together with our results, it proved again that species is also the main factor affecting the electroporation transfection. In addition, The researchers examined the time course of transgene expression in skeletal muscle and found that the transgene was consistently expressed for more than 3 weeks in regenerating muscle fibers formed after DNA injection and electroporation.Citation31 This demonstrates the feasibility of further understanding of muscle fiber regeneration through electroporation.
Satellite cells are a type of stem cell with strong self-renewal, proliferation, and differentiation capabilities. It can differentiate into muscle cells and fat cells.Citation32 In addition to promoting skeletal muscle hypertrophy, skeletal muscle satellite cells can also repair differentiated skeletal muscle, bringing hope to the treatment of skeletal muscle diseases. There are five types of muscle fibers in horse skeletal muscle. The different contraction characteristics and oxidative potential types of these reactions determine the horse’s speed, strength, and endurance.Citation33 At present, our understanding of satellite cells is still not enough, and more research is needed to discover the molecular and regulatory mechanisms of satellite cells that would benefit our understanding of mechanisms underlying motion performance, muscle injury/disease, and body features. However, although these kinds of studies are needed for the horse industry, unfortunately, we only found a few studies related to horse Skeletal muscle satellite cells. They used the lipofection method to accomplish gene transfer, and the transfection efficiencies have not been mentioned.Citation34 We hope that the present study could facilitate those researchers planning to do some gene regulation on horse satellite cells.
Conclusion
Due to the great significance of horse satellite cells as cell models in studying skeletal muscle development/regulation mechanism, clinical treatment of muscle-related diseases, and the further improvement and promotion of horse motion and meat production performance, in this study, we optimized the electroporation transfection conditions for horse skeletal muscle satellite cells to improve the transfection efficiency and subsequently determined that the parameter combination of voltage 120 V, DNA dosage 7 µg/ml, and pulse duration 30 ms could yield high gene electroporation transfection efficiency with high cell viability in horse satellite cells.
Ethical approval
Inner Mongolia agricultural University Ethics Committee approved the study.
Supplemental Material
Download MS Word (36.1 KB)Acknowledgements
We acknowledge Ms. Zhao YaLi for assistance with collecting Semitendinosus muscle samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004;117(Pt 22):5393–5404.
- DeBoer ML, Martinson KM, Pampusch MS, et al. Cultured equine satellite cells as a model system to assess leucine stimulated protein synthesis in horse muscle. J Anim Sci. 2018;96(1):143–153.
- Lee HG, Choi JY, Park JW, et al. Effects of exercise on myokine gene expression in horse skeletal muscles. Asian-Australas J Anim Sci. 2019;32(3):350–356.
- Reed SA, LaVigne EK, Jones AK, et al. Horse species symposium: the aging horse: effects of inflammation on muscle satellite cells. J Anim Sci. 2015;93(3):862–870.
- Liu Y, Zhou G, Liu Z, et al. Mussel inspired polynorepinephrine functionalized electrospun polycaprolactone microfibers for muscle regeneration. Sci Rep. 2017;7(1):8197.
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393–1398.
- Woods NB, Muessig A, Schmidt M, et al. Lentiviral vector transduction of NOD/SCID repopulating cells results in multiple vector integrations per transduced cell: risk of insertional mutagenesis. Blood. 2003;101(4):1284–1289.
- Ai J, Sun JH, Ma J, et al. Effects of lentivirus-mediated endostatin on endothelial progenitor cells. Oncotarget. 2017;8(55):94431–94439.
- Kingston RE, Chen CA, Okayama H. Calcium phosphate transfection. Curr Protoc Immunol. 2001;Chapter10:Unit 10.13.
- Guo L, Wang L, Yang R, et al. Optimizing conditions for calcium phosphate mediated transient transfection. Saudi J Biol Sci. 2017;24(3):622–629.
- Kawakami S, Higuchi Y, Hashida M. Nonviral approaches for targeted delivery of plasmid DNA and oligonucleotide. J Pharm Sci. 2008;97(2):726–745.
- Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;9(1):E92–104.
- Neumann E, Schaefer-Ridder M, Wang Y, et al. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845.
- Canatella PJ, Karr JF, Petros JA, et al. Quantitative study of electroporation-mediated molecular uptake and cell viability. Biophys J. 2001;80(2):755–764.
- Hyder I, Eghbalsaied S, Kues WA. Systematic optimization of square-wave electroporation conditions for bovine primary fibroblasts. BMC Mol Cell Biol. 2020;21(1):9.
- Lohr F, Lo DY, Zaharoff DA, et al. Effective tumor therapy with plasmid-encoded cytokines combined with in vivo electroporation. Cancer Res. 2001;61(8):3281–3284.
- Guo Y, Zhang Y, Klein R, et al. Irreversible electroporation therapy in the liver: longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer Res. 2010;70(4):1555–1563.
- Mehier-Humbert S, Guy RH. Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Adv Drug Deliv Rev. 2005;57(5):733–753.
- Nowaczyk M, Malcher A, Zimna A, et al. Transient and stable overexpression of extracellular superoxide dismutase is positively associated with the myogenic function of human skeletal muscle-derived stem/progenitor cells. Antioxidants. 2020;9(9):817.
- Blanton JR Jr., Bidwell CA, Sanders DA, et al. Plasmid transfection and retroviral transduction of porcine muscle cells for cell-mediated gene transfer. J Anim Sci. 2000;78(4):909–918.
- Grisolia AB, Curi RA, De Lima VF, et al. Targeted nucleotide exchange in bovine myostatin gene. Anim Biotechnol. 2009;20(1):15–27.
- Miersch C, Stange K, Rontgen M. Separation of functionally divergent muscle precursor cell populations from porcine juvenile muscles by discontinuous Percoll density gradient centrifugation. BMC Cell Biol. 2018;19(1):2.
- Li BJ, Li PH, Huang RH, et al. Isolation, culture and identification of porcine skeletal muscle satellite cells. Asian-Australas J Anim Sci. 2015;28(8):1171–1177.
- Danoviz ME, Yablonka-Reuveni Z. Skeletal muscle satellite cells: background and methods for isolation and analysis in a primary culture system. Methods Mol Biol. 2012;798:21–52.
- Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177(4):437–447.
- Hui SW. Effects of pulse length and strength on electroporation efficiency. Methods Mol Biol. 1995;55:29–40.
- Cheong DE, Lee HI, So JS. Optimization of electrotransformation conditions for Propionibacterium acnes. J Microbiol Methods. 2008;72(1):38–41.
- Guo H, Hao R, Wei Y, et al. Optimization of electrotransfection conditions of mammalian cells with different biological features. J Membr Biol. 2012;245(12):789–795.
- Dong S, Li J, Luo L, et al. Feasibility of transfecting exogenous genes into rat skeletal myoblasts by electroporation. J Clin Rehab Tissue Eng Res. 2008;(12):2206–2210.
- Espinos E, Liu JH, Bader CR, et al. Efficient non-viral DNA-mediated gene transfer to human primary myoblasts using electroporation. Neuromuscul Disord. 2001;11(4):341–349.
- Peng B, Zhao Y, Lu H, et al. In vivo plasmid DNA electroporation resulted in transfection of satellite cells and lasting transgene expression in regenerated muscle fibers. Biochem Biophys Res Commun. 2005;338(3):1490–1498.
- Yada E, Yamanouchi K, Nishihara M. Adipogenic potential of satellite cells from distinct skeletal muscle origins in the rat. J Vet Med Sci. 2006;68(5):479–486.
- Kawai M, Minami Y, Sayama Y, et al. Muscle fiber population and biochemical properties of whole body muscles in Thoroughbred horses. Anat Rec. 2009;292(10):1663–1669.
- Yamanouchi K, Soeta C, Suzuki S, et al. Identification of skeletal muscle satellite cells by transfecting EGFP driven by skeletal alpha-actin promoter. J Vet Med Sci. 2000;62(11):1213–1216.