Abstract
Orychophragmus violaceus (OV) and chicory (Cichorium intybus L., CC) can be used as fresh or dry forage for animals. To determine whether OV and/or CC have beneficial effects on performance and egg quality, a total of 1212 28-wk-old Beijing You Chicken (BYC) laying hens with similar performance were randomly allocated to 4 groups with 3 replicate pens per group, and 101 birds per pen. The birds were fed a basal diet (control), the basal diet + OV (3.507 kg/d/pen), the basal diet + CC (2.525 kg/d/pen), and the basal diet + OV + CC (OVC, 1.7535 kg/d/pen OV + 1.2625 kg/d/pen CC) for 3 wks after one wk of adaptation. The results showed that egg-laying rate was not affected by OV, CC and OVC (p > 0.05), but weekly average egg mass was significantly increased by OV and CC (p < 0.05). The feed egg ratio in the CC group (2.82) was significantly lower than that in the other three groups (p < 0.05). The eggshell thickness (EST), albumen height (AH) and Haugh unit (HU) were decreased by OV and CC (p < 0.05); while yolk color (YC) was increased in the CC and OVC groups (p < 0.05). Egg grade was decreased by OV (p < 0.05). Sensory evaluation showed that there was a trend for increased YC in OV, CC and OVC (p = 0.089). Serum total protein was significantly lower in OV group than those in the control and CC group (p < 0.05); serum albumin content was significantly decreased in OV, CC and OVC groups (p = 0.006). Serum glutathione peroxidase activity in CC and OVC groups was significantly higher than that in the control group (p < 0.05). In conclusion, the present study suggests that CC had a better effect on the performance of the native laying hens than OV. The OV and CC affected egg quality, while YC was increased in CC and OVC groups. The OVC improved YC and serum antioxidative properties of native laying hens without affecting the performance.
Introduction
Free range has attracted a lot of attention in recent years as an animal-friendly production system.Citation1 In free-range conditions hens can have access to sunlight, plenty of space to move around, and the freedom to express their natural behaviors. Free-range eggs are favored by consumers for their dark yolk color and distinctive flavor,Citation2 and often sold at a higher price than those produced by caged hens.Citation3
China is the world’s largest producer and consumer of eggs. The output of egg poultry feed accounts for more than 13% of China’s total output of industrial feed each year. Egg poultry diets are mainly based on corn-soybean meal, but protein feed resources are seriously insufficient and soybean meal is mainly dependent on imports, so many farmers, especially free-range farmers are more inclined to use forages in their effort to improve production performance while reducing the amount of soybean meal used.Citation4
On-range feeding of free-range laying hens is popular, especially common in farms using mobile housing sheds.Citation5 Feed supplements offered to free-range hens included shell grit, limestone, hay, silage, and others such as vegetables, pasture, insects, and harvested grass.Citation5,Citation6 Forage and forage meal can be valuable alternative sources of protein for animals, provided they are easily available and not expensive.Citation7,Citation8 Many forages such as clover, chicory, alfalfa can be used in poultry diets.Citation9,Citation10
Orychophragmus violaceus (OV) is a biennial herb of Brassicaceae, mainly distributed in China. It can be found in plains, mountains, near buildings and other environments, and is commonly used for feed, health care, gardening and other purposes.Citation11,Citation12 It has a strong ability to reproduce itself. Once sown, it will reproduce every year. It contains 9 kinds of amino acids, including threonine, glycine, alanine, isoleucine, phenylalanine, lysine, histidine, valine and leucine, and is also rich in iron, calcium, carotene, vitamin C, etc. The biomass of stems and leaves is large, which can be used as fresh or dry forage.Citation11
Chicory (Cichorium intybus L., CC) is a perennial herb from the Cichorium genus, Asteraceae family, which is cultivated worldwide and has both medicinal and edible functions and is mainly used in animal feed, and in several cases in the food industry.Citation13 It contains a certain amount of proteins, vitamins, minerals and different types of bioactive compounds,Citation14 such as alkaloid, inulin, sesquiterpene lactones, coumarins, flavonoids, saponins, tannins, etc.Citation15 The CC leaves are fresh and tender, with good palatability and high nutritional value, and are very popular among various domestic animals and poultry.Citation4,Citation16 Feeding CC has been shown to stimulate the intestinal absorption of minerals in laying hens.Citation17 Dietary addition of water-soluble extract of CC in laying hens had no adverse effect on production performance and egg quality of laying hens, but it had been shown to reduce the number of harmful microbial flora in the cecum and egg cholesterol content.Citation18
As mentioned above, the two forages are nutritional and available and can be valuable feed additives for animals, but there is a void of information about the effects of OV and CC on animal production, and it is also unclear whether there is a possible synergistic effect between OV and CC. The present study aimed to investigate the effects of OV and/or CC forage on performance, egg quality, sensory evaluation, and serum biochemical indexes in a native chicken- Beijing You Chicken (BYC), which is commonly used for meat and eggs and mostly reared in free-range system in China,Citation19,Citation20 in order to provide some reference for the rearing and management of free-range chickens, also utilizing the forages and developing low soybean meal diets in the future.
Materials and methods
Experimental design and management
The experiment was conducted at a BYC demonstration farm, Shunyi district, Beijing. The farm had 40 identical chicken pens and outdoor areas evenly distributed in the 32.95 acres of aspen trees. Each pen was 4 m wide and 5 m long, usually for 100–120 BYC laying hens, stocking density was 5–6 hen/m2. The feed trough, water nipple line, nest boxes, multi-layer perches and rice hulls as litter were provided in each pen. The outdoor area was 4 m wide and 7.5 m long, enclosed with wire mesh, the ground was covered with sand for the hens to bathe in, and a sunshade net was set up in summer. There was also a feed trough in the outdoor area, outside the wire mesh. The indoor feeding was used in the winter and outdoor feeding was used in other seasons. The hens were free to stay in indoor pen and outdoor area between 8:00 and 18:00 via a pophole. The range area around the pens was planted with chicory. While the chicory was growing, the hens were kept in the pen and outdoor area, when the chicory was ready, the hens were released to the range area freely. For the past two or three years, the OV had grown naturally around the pen and roadside, almost growing bigger than the CC. This experiment was carried out between early April and mid-May when the OV and CC were flourishing on the farm (see ).
A total of 1212 28-wk-old BYC laying hens with similar performance were randomly allocated to 4 groups with 3 replicate pens per group, and 101 birds per pen. The treatment groups included the basal diet (control; ), the basal diet + OV (3.507 kg/d/pen), the basal diet + CC 2.525 kg/d/pen), and the basal diet + OV + CC (OVC, 1.7535 kg/d/pen OV + 1.2625 kg/d/pen CC).
Table 1. Composition and nutrient levels of the basal diet (air dry-basis), %.
The forage grass was provided outdoors every day. At 8:30 a.m., the fresh OV and CC was harvested manually, about 5 cm above the ground. Basic nutrients were measured before the experiment. There were shoots and leaves for CC (containing crude protein 0.93 g, Ca 19.5 mg, P 26.4 mg per 100 g fresh weight); shoots, leaves and flowers for OV (containing crude protein 4.23 g, Ca 166.25 mg, P 35.03 mg per 100 g fresh weight). Hens had access to the grass from 9:00 am to 4:00 pm daily. The grass was weighed before 9:00 am and then after 4:00 pm the unconsumed grass was collected and the amount the hens consumed each day was determined.
The provided forage amount and estimated feed intake was designed to be 1:4 according to Zhu et al.,Citation21 who stated that the best weight gain was achieved when the feed to CC ratio was 4:1. The estimated feed intake was 100 g per hen per day during laying period,Citation22 10.1 kg for 101 hens per pen, then the estimated forage amount was 2.525 kg/d/pen. According to the pretest observation, the edible part of OV accounted for 72% of the whole plant (the stem was a little tough and often left), so the supplement of OV was 3.507 kg/d/pen; chicory was edible as a whole plant, so the supplement of CC was 2.525 kg/d/pen; half of 3.507 kg and 2.525 kg in OVC group, i.e., 1.7535 kg/d/pen OV + 1.2625 kg/d/pen CC.
The experiment lasted 3 wks after one wk of adaptation. The health status and feeding behavior of the laying hens were visually observed and recorded daily.
To keep the same ranging time for the birds, the light regime was 16 L:8D (6:00 ∼ 22:00), the birds were fed a corn-soybean-based diet at 8:00, ranged freely from 8:00 to 17:00. The nutrient levels of diets are based on ‘Technical Code of Practice for Feeding and Management of Beijing-You Chicken’,Citation22 the composition and nutrient levels of the basal diet is shown in , and the nutritive values were calculated according to the Feed Database in China.Citation23
The study was performed in accordance with local ethical guidelines and met the requirement of the institutional animal care and use committee, the ethical agreement number is IHVM11-2203-1.
Measurement and methods
The number of eggs and egg weight of each replicate group were recorded daily, the feed intake was recorded weekly, and weekly average feed intake (AFI), weekly average egg mass (EM), feed egg ratio (FER), egg-laying rate and mortality rate were calculated for 29–32 wks.
Ten fresh eggs were randomly selected from each replicate pen, providing a total of 30 eggs for each group. Egg weight (EW), eggshell strength (ESS), eggshell thickness (EST), eggshell color (ESC), Haugh unit (HU), albumen height (AH), yolk color (YC), egg grade (EG), egg shape index (ESI), relative yolk weight (RYW), relative eggshell weight (RESW), and relative albumen weight (RAW) of these eggs were measured and calculated within 24 h. The method and apparatus were the same as those previously described by our group.Citation19
At the end of the experiment, 15 fresh eggs were collected from each group for sensory evaluation. The eggs were numbered with a pencil on the eggshells. Only the person who marked the eggs knew which group they came from. Added cold water and eggs to a covered saucepan. When the water was boiling, boiled the eggs for 3 min, turned off the heat and simmered for 3 min. Then removed the eggs and soaked them under cold water for 5–10 s before placing them on a plate. Before each set of eggs was distributed to the participants, the eggs were cut into quarters and each participant was given a quarter egg at a time. The tasting and scoring method were explained to the participants before the sensory evaluation. The egg aroma, YC, yolk fineness, albumen tenderness, egg flavor was evaluated according to . The participants were asked to gargle with water after each evaluation. The scoring system consisted of 5-point scale: 1 is the worst, 5 is the best. The two people involved in the egg grouping and cooking were not involved in the evaluation. A total of 16 experienced participants, who were researchers, postgraduate students and farm technicians aged 23–55, took part in the sensory evaluation. A total of 15 evaluation forms were issued and 15 valid evaluation forms were recovered.
Table 2. Indicators of sensory evaluation of eggs.
At the end of 32 wks, blood samples were collected in the morning by venepuncture. 5 ml of blood per hen, four hens per replicate. The blood was transferred into coagulation-promoting tubes by venipuncture and centrifuged at 3000 g for 10 min at 4 °C. The serum was frozen at −20 °C for later analysis of some biochemical parameters, including serum total protein, albumin, glucose, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) content, total cholesterol (TC) content, total antioxidant capacity (T-AOC), peroxidase (POD), glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD) activities. All the kits were purchased from Nanjing Jiancheng Institute of Biological Engineering and determined by a spectrophotometer (Evolution 60, Thermal Fisher Scientific, Shanghai) and ELISA (Multiskan FC, Thermal Fisher Scientific, Shanghai).
Statistical analyses
The data were analyzed statistically using the SPSS 25.0 Software for Windows (SPSS Inc. Chicago, IL). One-way ANOVA was used to analyze the effects of OV and/or CC on performance, egg quality, sensory evaluation, and biochemical parameters. Duncan’s Test was used for multiple comparisons. The percentage was arcsine transformed before the normality test. p < 0.05 was regarded as statistically significant. There was a trend when P was from 0.05 to 0.10. The data are presented as mean and standard error of mean.
Results
Performance
shows that the AFI, egg-laying rate and mortality rate of BYC laying hens were not affected by OV, CC and OVC (p > 0.05), but weekly average EM was significantly increased by OV or CC forage (p < 0.05). There was no significant difference between OVC group and control group. The FER in CC group (2.82) was significantly lower than that in other three groups (p < 0.05).
Table 3. Effects of Orychophragmus violaceus and/or chicory forage on performance of BYC laying hens during 29–32 wks.
Egg quality
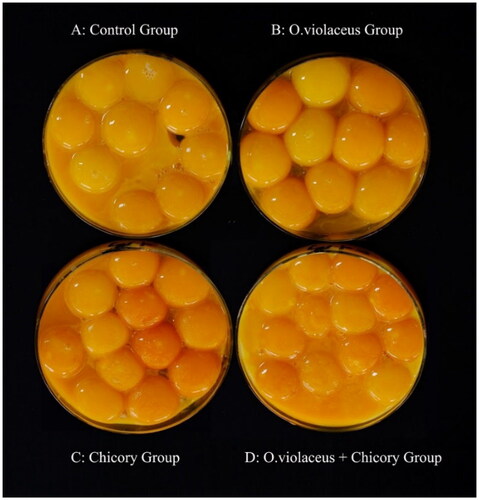
shows that OV, CC and OVC had no effects on ESS, RESW and RAW (p > 0.05), but significantly affected the EST, ESC, ESI, AH, YC, HU and EG (p < 0.05). In detail, the EST was decreased by OV or CC (p < 0.05), but not affected by OVC; The ESC and ESI were increased by CC (p < 0.05); The AH and HU in the control and OVC groups were higher than those in the CC group, and the lowest values were observed in OV group (p < 0.05); The YC was not affected by OV, but significantly increased by CC and OVC (become more reddish in color, p < 0.05), reached the highest in the CC group (the reddest) (see ). The EG was decreased by OV (p < 0.05), but not affected by CC or OVC. There was a trend for decreased EW following OV, CC and OVC supplementation (p = 0.071), and a trend for increased RYW following OV, CC and OVC supplementation (p = 0.099).
Table 4. Effects of Orychophragmus violaceus and/or chicory forage on egg quality of BYC laying hens at 32 wks of age.
Sensory evaluation
shows that there were no significant differences in egg aroma, yolk fineness, albumen tenderness, and egg flavor as an effect of OV, CC and OVC (p > 0.05). There was a trend for increased YC by OV, CC and OVC (p = 0.089). The YC of CC group was the darkest (yellow to red), followed by the OVC group, OV group (see ). Four of the 16 participants complained that the eggs from the control group had a fishy smell of eggs, while those from the forage supplemented groups did not.
Table 5. Sensory evaluation of eggs from Orychophragmus violaceus and chicory forage groups.
Serum biochemical indexes
shows that the serum glucose, HDL-C, LDL-C, TC, TAOC, POD, and T-SOD were not affected by OV, CC and OVC (p > 0.05), but serum TP was significantly lower in OV group than that in the control and CC group (p < 0.05); serum albumin content was significantly decreased in OV, CC and OVC groups (p = 0.006). The GSH-Px activity of OV group was not significantly different to that of the control group, but significantly higher in the CC and OVC groups, compared to the control group (p < 0.05), the GSH-Px activity of CC group was the highest, followed by OVC group.
Table 6. Effects of Orychophragmus violaceus and/or chicory forage on serum antioxidative properties of BYC laying hens.
Discussion
With the improvement of people’s living standards, more and more consumers are paying attention to animal welfare and are willing to pay for animal derived products from animals with high welfare standards.Citation24,Citation25 The range use of hens can be used as an indicator of the impact of a free range system on animal welfare. The high proportion of hens using the range area was associated with the brown genotype, smaller flock size, having roosters in the flock, etc.Citation26 Pettersson et al.Citation27 summarized ranging percentages from 14 studies and showed that the average percentage of hens on the range was 9–38%. In the present study, we observed that more than 80% of the hens grazed outdoors, the reason for which may be due to the small flock size, which is consistent with Bestman and Wagenaar,Citation28 who reported that more than 75% of the hens were observed to be outdoors.
Providing a proper amount of forage can not only provide various nutrients, such as minerals, vitamins, etc., but also reduce feeding cost and improve the performance and economic benefit of poultry. The CC contains many important medicinal compounds such as alkaloids, inulin, sesquiterpene lactones, coumarins, chlorophyll, chlorophyll pigment, unsaturated sterols, flavonoids, saponins, and tannins.Citation15,Citation29 Dietary CC fructan increased the weight gain and feed conversion rate of broilers.Citation30 Dietary supplementation of 1.0% inulin can increase egg production and feed egg ratio of laying hens.Citation31 The water-soluble extract of CC had no adverse effects on performance of laying hens when used in the diet.Citation32 Zheng et al.Citation10 supplemented 0%, 5%, 8% and 10% fresh CC forage in the basal diet of female BYC aged from 16 wks to 29 wks, and found an increase in average feed intake, feed conversion ratio and mortality with increasing supplementation of CC forage in the diet. This present study found that OV or CC can increase the EM, but the FER of CC group was significantly lower than that of OV group, indicating that the effect of CC on performance of laying hens was better than that of the OV, which may be due to different composition of the two forages, and different utilization of nutrients by the native laying hens.
It is interesting to observe that in the pens with OVC, the hens always preferred to eat OV first rather than CC, probably because the OV leaves contain less tannin than CC leaves. Sesquiterpene lactones in CC leaves (about 0.2% of dry matter) contribute to the bitter taste,Citation4 and the purple flower of OV may also be an attraction for hens to peck first.
Exogenous nutrients can change the energy metabolism in poultry, thus affecting the egg quality.Citation33 The ESS and EST are indicators for evaluating eggshells, and the eggshell quality affects the transportation and storage of eggs, as well as the hatching performance of breeder eggs.Citation34 Dietary supplementation of CC has been shown to decrease ESS,Citation10 while in this study, the EST was reduced by OV or CC which was opposite to the previous report that dietary 1.5% inulin and 0.5% CC extract could increase the EST.Citation35 KopBozbay et al.Citation36 showed that the eggshells of the control hens with access to non-vegetated areas were thicker than those of the hens with free access to CC vegetated areas, clover vegetated area, and the mixed CC and clover vegetated areas. The reason may be the lower dietary protein intake of laying hens due to the forage supplement. Iqbal et al.Citation37 reported that access to feed supplements can dilute the formulated nutrient intake significantly, sometimes with severe consequences.
The AH and HU are indicators of the freshness of the eggs, reflecting the albumen quality. The larger the AH and HU, the higher the protein content and viscosity, and the fresher the eggs. Xie et al.Citation38 found that Lonicera confusa (LC) and Astragali radix (AR) extracts (LC-AR) increased HU of eggs, while in the present study, AH and HU were decreased in the OV and CC group, the reason may also be related with the lower dietary protein intake of laying hens, but not decreased in OVC group, which need to be verified in future.
The YC is one of the important indicators of internal egg quality. Though it is not directly related to the nutritional value, most consumers associate a well-colored egg yolk (golden yellow to orange) with healthiness and quality, and the pale or non-uniform colored egg yolks are considered as inferior products from sick and unhealthy hens.Citation39 Carotenoids, which are not endogenously synthesized and must be obtained through the diet, are mainly responsible for the YC. In conventional layer rations, corn gluten, alfalfa or saponified extracts from marigold flower, paprika fruit or synthetic carotenoids are included for egg yolk coloration.Citation39 According to Montefusco et al.,Citation40 the contents of lutein (8.0–30.1 μg/g f.w.), β-carotene (3.3–14.2 μg/g f.w.) varied in cultivated and wild CC cultivars, while the average carotene content was about 16.2 μg/g f.w. in fresh shoots of OV.Citation11 Horsted et al.Citation41 reported that when the laying hens were fed with CC and clover separately for 23 days, the YC of the CC group was darker. Zheng et al.Citation42 reported that when the 16-wk-old BYC was fed a diet supplemented with CC pulp for 60 consecutive days, the YC was improved. In agreement with these studies, the present study indicated that the YC of the CC group was significantly darker than that of control, OV, and OVC groups, and the reason may be that some carotenoids in CC, such as lutein, are more easily transferred to the yolk than those from the OV. The present study only lasted one month and different coloration effects were already observed.
Sensory evaluation is a dynamic field concentrating on using humans to measure sensory perception and/or their effect on food and taste acceptance,Citation43 which is generally a combination of sensory traits including aroma, flavor, off-flavor and overall difference.Citation17 Xie et al.Citation38 used LC and AR extracts in 52-wk-old Lohmann pink-shell hens and showed that the scores of overall acceptability of eggs were lower (p = 0.018) in the LC-AR group than that in the control, LC or AR group, by using a sensory 9-point evaluation scale (where 1 = like extremely, 5 = neither like nor dislike, and 9 = dislike extremely). In this study a 5-point scale was used (1 was the worst, 5 was the best), the results showed that there were no differences in egg aroma, flavor, off-flavor except for YC in OV and/or CC forage groups. The YC of CC group was the darkest, the YC of control group was the lightest, which was consistent with previous egg quality measurement. The OV or CC forage had no significant effects on the improvement of egg sensory quality, which may be also due to lack of professional training of the participants. Hayat et al.Citation17 reported that only trained panelists could detect differences in egg sensory attributes in eggs.
Serum biochemical indicators can reflect the function of various tissues and organs and the nutritional metabolism of the body and indirectly reflect the health status of poultry. The serum total protein and albumin contents reflect the metabolic status of protein in the body to some extent.Citation44 This present study showed that the serum total protein and albumin contents in OV group were significantly lower than that in the control group, and the total protein content in the OVC group was higher than that in the OV group and lower than that in the CC group. The possible reason was that the crude protein intake of laying hens in OV group was lower than that in control group.
T-AOC can reflect the antioxidant capability of the body to a certain extent. The POD, GSH-Px, and T-SOD activities are the main members of the antioxidant defense system, forming a protection system against oxidative damage. Cichoric acid was first identified in chicory leaves and was found in at least 63 genera and species of plants.Citation45 It is the main component of caffeic acid derivatives of CC and echinacea purpurea extracts and possesses antioxidant properties.Citation46 The antioxidant activity of CC has been the subject of investigation in many studies.Citation13 Dalar and KonczakCitation47 studied the antioxidant activity of roots, stems, leaves, flowers, and the whole plant of CC, and found that the leaves contained the highest level of total phenols and exhibited the highest antioxidant capacities. Jurgoński et al.Citation48 reported that CC leaves extract showed higher antioxidant activity (210.1 nmol/g f.w.) compared to the seed extract (505.1 nmol/g f.w.). But there are no reports about antioxidant activity of OV. The present study showed that GSH-Px activity in CC group was higher than those in OV group and control group, and GSH-Px activity in OVC group was higher than that in control group. There was no significant difference between control group and OV group, indicating that the OV has no effect on serum antioxidant capability of laying hens, but CC and OVC can improve the serum antioxidant capability of laying hens, which also provided a possibility for further research on the antioxidant capability of OVC.
Conclusion
The present study indicated that OV and CC forage improved the EM, and CC decreased FCR, indicating a better effect of the CC on the performance of native laying hens than the OV. The OV and CC affected egg quality. The EST, AH and HU were decreased in OV and CC groups, while YC was increased in CC and OVC groups. CC and OVC had higher serum antioxidant capability. The OVC improved YC and serum antioxidative properties of native laying hens without affecting the performance.
Author contributions
Zhao W. Y., Zhang Q. Q. and Zhao Y. F. conducted animal experiment and performance data collection, Zhao W. Y. and Chang C. conducted performance and serum biochemical parameters measurement, Zhao W. Y., Chang C., Wang X. and Geng A. L. performed egg quality measurement, Zhao W. Y. performed statistical analysis, Geng A. L. proposed ideas, wrote and edited papers, and provided financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data sets are available upon request from the corresponding author.
Additional information
Funding
References
- Tactacan GB, Guenter W, Lewis NJ, Rodriguez-Lecompte JC, House JD. Performance and welfare of laying hens in conventional and enriched cages. Poult Sci. 2009;88(4):698–707.
- Wu YJ, Su SG, Li JY, et al. Comparison of egg quality and nutritional components of Huainan ephedra chicken under different feeding modes. China Poult. 2012;34(22):59–61.
- He S, Lin J, Jin Q, et al. The relationship between animal welfare and farm profitability in cage and free-range housing systems for laying hens in China. Animals. 2022;12(16):1–19.
- Yu CL, Peng H, Zhang ZR, et al. Effects of different kinds of pastures on production performance of free-range chicken in the grove. China Poult. 2021;43(1):62–66.
- Ruhnke I, De Koning C, Drake K, Choct M, Singh M. Feed practices in Australian free range egg production. ESPN. 2015;20:250. [Abstract].
- Ruhnke I, Normant C, Campbell D, et al. Impact of on-range choice feeding with black soldier fly larvae (Hermetia illucens) on flock performance, egg quality, and range use of free-range laying hens. Anim Nutr. 2018;4(4):452–460.
- Mourao JL, Pinheiro VM, Prates JAM, et al. Effect of dietary dehydrated pasture and citrus pulp on the performance and meat quality of broiler chickens. Poult Sci. 2008;87(4):733–743.
- Tufarelli V, Ragni M, Laudadio V. Feeding forage in poultry: a promising alternative for the future of production systems. Agriculture. 2018;8(6):81–84.
- Almeida GFD, Hinrichsen LK, Horsted K, Thamsborg SM, Hermansen JE. Feed intake and activity level of two broiler genotypes foraging different types of vegetation in the finishing period. Poult Sci. 2012;91(9):2105–2113.
- Zheng M, Mao P, Tian X, Meng L. Growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken on diets with inclusion of fresh chicory forage. Ital J Anim Sci. 2019;18(1):1310–1320.
- Luo P, Huang BQ, Yin JM, Chen ZL, Chen YH, Lan ZQ. A new forage genetic resource Orychophragmus violaceus (L.). OE Schulz Genetic Resources Crop Evol. 1998;45(6):491–494.
- Weng DB, Huang XF. Evaluation on the protein quality of Orychophragmus violaceus. Acta Boanica BorealiOccidentalia Sinica. 2001;21:673–677.
- Perović J, Tumbas ŠV, Kojić J, et al. Chicory (Cichorium intybus L.) as a food ingredient-nutritional composition, bioactivity, safety, and health claims: a review. Food Chem. 2021;336:127676.
- Sun C, Muhammad S, Abd El Hac ME, et al. Chicory (Cichorium intybus) herb: chemical composition, pharmacology, nutritional and healthical applications. Int J Pharmacol. 2017;13(4):351–360.
- Shad MA, Nawaz H, Rehman T, Ikram N. Determination of some biochemicals, phytochemicals and antioxidant properties of different parts of Cichorium intybus L. a comparative study. J Anim Plant Sci. 2013;23:1060–1066.
- Li GD, Kemp PD. 2005. Forage chicory (Cichorium intybus L.): a review of its agronomy and animal production. In Sparks DL, ed. Advances in Agronomy. San Diego, CA: Elsevier Academic Press Inc.
- Hayat Z, Cherian G, Pasha TN, Khattak FM, Jabbar MA. Sensory evaluation and consumer acceptance of eggs from hens fed flax seed and 2 different antioxidants. Poult Sci. 2010;89(10):2293–2298.
- Liu HY, Ivarsson E, Jonsson L, Holm L, Lundh T, Lindberg JE. Growth performance, digestibility, and gut development of broiler chickens on diets with inclusion of chicory (Cichorium intybus L.). Poult Sci. 2011;90(4):815–823.
- Geng AL, Zhang Y, Zhang J, Wang HH, Chu Q, Liu HG. Effects of lighting pattern and photoperiod on egg production and egg quality of a native chicken under free-range condition. Poult Sci. 2018;97(7):2378–2384.
- Geng AL, Zhang QQ, Chang C, et al. Dietary metabolizable energy and crude protein levels affect the performance, egg quality and biochemical parameters of a dual-purpose chicken. Anim Biotech. 2022. doi:10.1080/10495398.2022.2114001.
- Zhu ZP, Sun XX, Song Z, Gu ZL, Gong DQ. Effects of the different proportion of fresh chicory on the weight of goose at 4-6 weeks of age. Feed Rev. 2013;7:38–40.
- DB11/T 1378-2023. Technical Code of Practice for Feeding and Management of Beijing-You Chicken. Beijing: Beijing Bureau of Quality and Technical Supervision, 2023.
- Feed Database in China. Tables of Feed Composition and Nutritive Values in China. Beijing: Feed Database in China, 2013. http://www.chinafeeddata.org.cn.
- Fredrik C, Peter F, Carl JL. Consumer willingness to pay for farm animal welfare: mobile abattoirs versus transportation to slaughter. Euro Rev Agri Eco. 2007;34(3):321–344.
- Yang YC. Factors affecting consumers’ willingness to pay for animal welfare eggs in Taiwan. Int Food Agribus Manage Assoc. 2018;21(6):1–14.
- Bestman M, Verwer C, van Niekerk T, et al. Factors related to free-range use in commercial laying hens. Appl Anim Beha Sci. 2019;214:57–63.
- Pettersson IC, Freire R, Nicol CJ. Factors affecting ranging behaviour in commercial free-range hens. World’s Poult Sci J. 2016;72(1):137–150.
- Bestman M, Wagenaar JP. Farm level factors associated with feather pecking in organic laying hens. Livest Prod Sci. 2003;80(1–2):133–140.
- Swiatkiewicz S, Koreleski J, Arczewska-Włoek A. 2011. Effect of inulin and oligofructose on performance and bone characteristics of broiler chickens fed on diets with different concentrations of calcium and phosphorus. Br Poult Sci. 2004;52(4):483–491.
- Yusrizal , Chen TC. Effect of adding chicory fructans in feed on broiler growth performance, serum cholesterol and intestinal length. Int J Poult Sci. 2003;3(3):214–219.
- Chen YC, Nakthong C, Chen TC. Improvement of laying hen performance by dietary prebiotic chicory oligofructose and inulin. Int J Poult Sci. 2005;4(2):103–108.
- Shang HM, Hu TM, Lu YJ, Wu HX. Effects of chicory water-soluble extract on egg-yolk cholesterol, gut microflora and performance of laying hens. Chinese J Anim Nutr. 2010;22(4):1037–1045.
- Xia B, Liu Y, Sun D, Liu J, Zhu Y, Lu L. Effects of green tea powder supplementation on egg production and egg quality in laying hens. J Appl Anim Res. 2018;46(1):927–931.
- Stefanello C, Santos TC, Murakami AE, Martins EN, Carneiro TC. Productive performance, eggshell quality, and eggshell ultrastructure of laying hens fed diets supplemented with organic trace minerals. Poult Sci. 2014;93(1):104–113.
- Lu YJ. 2007. Analysis on inulin output of chicory and effect of inulin on layer. Thesis for master degree. Northwest A&F University.
- Kop-Bozbay C, Akdag A, Bozkurt-Kiraz A, Gore M, Kurt O, Ocak N. Laying performance, egg quality characteristics, and egg yolk fatty acids profile in layer hens housed with free access to chicory- and/or white clover-vegetated or non-vegetated areas. Animals. 2021;11(6):1708.
- Iqbal Z, Roberts J, Perez-Maldonado RA, Goodarzi Boroojeni F, Swick RA, Ruhnke I. Pasture, multi-enzymes, benzoic acid and essential oils positively influence performance, intestinal organ weight and egg quality in free-range laying hens. Br Poult Sci. 2018;59(2):180–189.
- Xie T, Bai SP, Zhang KY, et al. Effects of Lonicera confusa and Astragali radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult Sci. 2019;98(10):4838–4847.
- Grashorn M. 2016. Feed additives for influencing chicken meat and egg yolk color. In: Carle R, Schweiggert RM, eds. Handbook on Natural Pigments in Food and Beverages. Cambridge: Woodhead Publishing:283–302.
- Montefusco A, Semitaio G, Marrese PP, et al. Antioxidants in varieties of chicory (Cichorium intybus L.) and wild poppy (Papaver rhoeas L.) of Southern Italy. Hindawi. 2015;2015:1–8.
- Horsted KM, Hammersh J, Hermansen JE. Short-term effects on productivity and egg quality in nutrient-restricted versus non-restricted organic layers with access to different forage crops. Acta Agric Scand Section A Anim Sci. 2006;56(1):42–54.
- Zheng ML, Mao PC, Tian XX, Guo Q, Meng L. Effects of dietary supplementation of chicory on growth performance, carcass traits and egg quality in Beijing-you chickens. J Grassland Forage Sci. 2018;S1:3–5.
- Stone H, Sidel JL. Sensory Evaluation Practices. 3rd ed. New York: Academic Press.
- Zhao YG. Study on the changes of serum protein content of Beijing you chicken during brood period. Beijing Agric. 2014;15:11–13.
- Lee J, Scagel CF. Chicoric acid: chemistry, distribution, and production. Front Chem. 2013;1:40.
- Ma J, Li M, Kalavagunta PK, et al. Protective effects of cichoric acid on H(2)O(2)-induced oxidative injury in hepatocytes and larval zebrafish models. Biomed Pharmacother. 2018;104:679–685.
- Dalar A, Konczak I. Cichorium intybus from Eastern Anatolia: phenolic composition, antioxidant and enzyme inhibitory activities. Ind Crops Prod. 2014;60:79–85.
- Jurgoński A, Milala J, Jusbkiewicz J, Zduńczyk Z, Bogusław K. Composition of chicory root, peel, seed and leaf ethanol extracts and biological properties of their non-inulin fractions. Food Tech Biotech. 2011;49(1):40–47.