?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Scrotal circumference is an important reproductive index of breeding rams, which has a high genetic correlation with ejaculation volume and semen quality. In this study, the scrotal circumference of 1353 male Hu sheep at different stages of development was measured and descriptive statistical analysis was performed. The results showed that the coefficient of variation of scrotal circumference at each stage was greater than 10%, and its heritability were moderately to high, ranging from 0.318 to 0.719. We used PCR amplification and Sanger sequencing to scan the polymorphisms of the IGFALS gene, and performed association analysis with the circumference of the scrotum at different stages. We identified a synonymous mutation g.918 G > C in exon 1 of the IGFALS gene, and this mutation was significantly associated with scrotal circumference at 100, 120, 140, 160 and 180 days (p < 0.05). Therefore, IGFALS gene polymorphism can be used as a molecular marker affecting scrotal circumference of Hu sheep, which can provide a reference for future molecular marker-assisted selection of scrotal circumference in sheep.
Keywords:
Introduction
Sheep (Ovis aries) are one of the earliest domesticated animals in China and play a very important role both economically and culturally. With the continuous improvement of people’s living standards, mutton has become popular because of its unique texture and flavor. As one of the important livestock in China, Hu sheep have the characteristics of high reproductive performance, fast growth and strong adaptability, especially famous for early sexual maturity, year-round estrus, large lambing numbers, and strong lactation ability.Citation1,Citation2 Although the number of sheep in our country is very large, because of the large population base, the number of sheep per capita is very small. Therefore, it is particularly important to speed up molecular breeding and breed new varieties of sheep with high fertility.
Scrotal circumference is an important indicator and reliable method for evaluating the reproductive capacity of rams. The size of the scrotal circumference can directly represent the size of the testicles. In previous studies, it was found that the circumference of the scrotum is closely related to testicular size and sperm production.Citation3–5 Some researchers have found that Scrotal circumference or testicular weight were positively correlated with number of sperm obtained by frequent ejaculation.Citation4,Citation6–9 Scrotal circumference is a highly genetic trait and its age-related changes can be used as a measure of puberty. The relationship between sexual maturity and scrotal circumference has also been reported in male goats.Citation10,Citation11 Menegassi et al.Citation12 found that bulls scrotal circumference was associated with an increase in testicular weight and live weight. Insulin like peptide 3 (INSL3) is considered a specific biomarker of puberty testes,Citation13–16 and in studies of male goats, it was found that the correlation between scrotal circumference and INSL3 concentration is higher than that of testosterone. The average scrotal circumference of male goats also shows significant changes before and after puberty.Citation17 The measurement of scrotal circumference plays an important role in the onset of puberty, total semen volume, semen quality, pathological status of the reproductive system, and reproductive status of breeding bulls.Citation18 A study found that bulls with a scrotal circumference of 27.9 cmCitation19 or 50 million sperm produced at the first ejaculation with at least 10% of forward motilityCitation20 can be considered to have reached puberty. Compared with adolescent bulls, the scrotal circumference and semen parameters of bulls significantly increase after puberty, and the sperm density of bulls after puberty is positively correlated with the scrotal circumference.Citation21 In sheep studies, it has been confirmed that breed and season have a significant impact on scrotal circumference and semen characteristics.Citation22–24 Studies have found that Katahdin and Dubo rams have a higher scrotal circumference than Blackbell and Pelibey rams.Citation25 However, there are few reports on the periodic changes in the circumference of the scrotum in sheep. Scrotum is the external protective layer of the testis, which can regulate the temperature of the cystic cavity and promote the generation and development of sperm. The increase in testicular temperature will reduce sperm motility, concentration, normal morphology, acrosome integrity and chromatin stability.Citation26
The screening of the main genes affecting reproductive ability is very important for sheep breeding. Insulin-like growth factor-binding protein acid-labile subunit (IGFALS) gene was first successfully cloned in a mice.Citation27 The IGFALS gene encodes an acid-labile subunit (ALS) protein that binds to IGF-I and IGFBP-3 to form a ternary complex in the circulation that regulates growth and development and other physiological processes.Citation28 Human ALS deficiency is characterized by slow growth and development, the secretion of GH is the same as or even higher than normal, the levels of IGF-1 and IGFBP-3 are significantly reduced, and they are still low after treatment.Citation29 This is caused by homozygous or compound heterozygous inactivation mutation of IGFALS gene.Citation30 IGFALS gene have been identified in humans,Citation31 mice,Citation27 sheep,Citation32 ratsCitation33 and pigs,Citation34 and plays an important role in the growth and development of mammals. Several studies have shown that polymorphisms in IGFALS are associated with growth traits in Makouei, Ghezel sheep, and cattle.Citation28,Citation35 In the study of S. Li et al.,Citation34 IGFALS was found to be expressed in multiple tissues of the pig testis, ovaries, epididymis, liver, lung, and prostate. However, there are no reports on the association of this gene with the scrotal circumference in sheep. Therefore, the aim of this study was to investigate the relationship between the IGFALS gene SNP and scrotal circumference in Hu sheep.
Materials and methods
Statement of ethics
All experimental procedures in this study were approved by the Animal Care and Use Committee of Biological Research of Gansu Province, China. And the experimental protocol and sample collection were approved by the Ethics Committee of Gansu Agricultural University (permit number for conducting animal experiments: NO. 2012–2-159).
Animal management
A total of 1662 male Hu sheep used in this study were selected from Jinchang Zhongtian Sheep Industry Co. Ltd., Gansu Zhongsheng Huamei Sheep Industry Development Co. Ltd., Gansu Sanyangjinyuan Husbandry Co. Ltd., Shandong Runlin Sheep Industry Co. Ltd, and Wuwei Pukang Sheep Industry Co. Ltd. All lambs were weaned at 56 days of age, and routine immunization before weaning was performed by professional workers. After weaning, all lambs were transferred to Minqin Defu Agriculture Co. Ltd (Gansu, China) for centralized feeding and management. The feeding of experimental animals included a 2-week adaptation period, a 10-day pre-testing period, and a 100-day experimental period. In the adaptation period, the proportion of feed in the diet was gradually reduced, and the proportion of pellet feed was gradually increased by 7.1% until reaching 100%. During the feeding period, all lambs were raised in separate enclosures (0.8 × 1 m) and fed pellet feed (Gansu Runmu Bioengineering Co., Ltb). The experimental animals were in a good ecological environment and perfect management, and were guaranteed to freely eat and drink water.
Data recording, sample collection and DNA extraction
Scrotal circumference was measured and recorded on the morning of each growth stage (the 100th, 120th, 140th, 160th and 180th days) using a clearly graduated tape measure. The greatest SC of each lamb was measured using a clearly graduated tape, after pushing the testes firmly into the scrotum.Citation10 On the last day of the experiment, blood samples (5 mL) were collected from each sheep by jugular vein sampling. DNA was extracted according to the instructions of the EasyPure Blood Genomic DNA Kit (TransGen Biotech, Beijing, China). DNA concentration and purity are measured by ultra-micro spectrophotometer. Qualified DNA was stored at −20° C for subsequent experiments. Finally, the blood DNA of 1353 Hu sheep was successfully extracted.
Polymerase chain reaction and genotyping
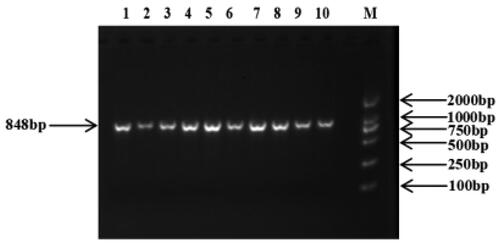
Firstly, we used Oligo 7.0 and Primer 5 software to design a pair of specific primers containing partial exon 1 of the IGFALS gene to amplify an 848 bp fragment by referring to the reference sequence of IGFALS gene (GenBank Accession No. NC_040275.1) in NCBI database. Primer information is shown in . Ten Hu sheep mixed DNA samples were randomly selected from the experimental population for PCR amplification, and then the amplified products were sequenced to detect single nucleotide polymorphisms (SNPs) in IGFALS gene of sheep. PCR amplification was performed in a 35 µl reaction mixture. The reaction mixture is made up of 17.5 µl of 2× TSINGKE Master Mix (TSINGKE Biological Technology, Beijing, China), 14 µl of ddH2O, 1.4 µl of template DNA and 1.12 µl of primers (forward and reverse primers). The PCR thermal cycle is programmed as follows: pre-denaturation at 94 °C for 5 min, deformation at 94 °C for 30 s, annealing at 61.4 °C for 30 s, extension at 72 °C for 30 s and 35 cycles and a final extension at 72 °C for 5 min. SNPs in IGFALS gene were detected by sequencing the target fragment, and genotyping of all experimental individuals was performed using competitive allele-specific FRET-based PCR (KASPar) assays (LGC Genomics, Hoddesdon, UK). Primer information for KASPar genotyping is shown in .
Table 1. Primer pairs designed for sheep IGFALS.
Table 2. Primer pairs designed for KASPar assay.
Statistical analysis
Finally, 1353 individuals had genotypic and phenotypic data. Based on genotyping results, the genetic index is calculated directly for IGFALS gene.Citation36 The statistical software SPSS (version 25.0) was used to analyze the associations between genotypes and scrotal circumference in sheep. An adjusted multivariate linear model was established as follows:
where Yijlnk is the observation of the scrotal circumference traits, µ is a vector of mean, Gi is the fixed effect of ith genotype, Fj is the fixed effect due to jth farm (j = 1, 2, 3, 4, 5, 6), Fl and Mn represent the family effect, Sk represents the effect of season (k = winter, summer), and ɛijlnk was random residuals. Significance of phenotypic means was tested by Duncan’s test and Tukey’s test. There was statistical significance at p < 0.05, and the results were expressed as mean ± standard error.
In this study, genetic parameters for scrotal circumference in bearded sheep were estimated using Hiblup software (https://www.hiblup.com/tutorials). To provide reference for Hu sheep breeding, a model that explains the additive effects and residuals in the model was fitted, as follows.
Y is the record vector, β and e are the vectors of fixed effects and residual effects, respectively, X is the correlation matrix, and I denotes the unit matrix, σ2a and σ2e are the additive variance and residual variance, respectively.
Results
Scrotal circumference
The scrotal circumference of 1353 male Hu sheep were measured on days 100, 120, 140, 160 and 180, respectively. The statistical results showed that the scrotal circumference at these five stages was 18.41 (± 3.30), 22.21 (± 3.75), 25.76 (± 3.32), 27.05 (± 3.37), and 27.02 (± 3.56), respectively, and the coefficient of variation of scrotal circumference in different stages was more than 10% (). The variance components and heritability estimate for scrotal circumference at different stages are shown in . The heritability of scrotal circumference at 100, 120, 140,160 and 180 days was 0.719 (± 0.116), 0.578 (± 0.126), 0.318 (± 0.123), 0.444 (± 0.126) and 0.4781 (± 0.186) for Hu sheep, respectively, which were of medium to high heritability.
Figure 1. Descriptive statistics of scrotal circumference size at different stages. (A) The violin plot of scrotal circumference. (B) The coefficient of variation of scrotal circumference.
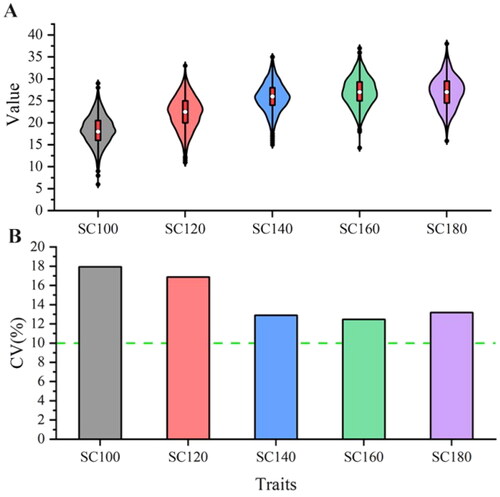
Table 3. Estimation of scrotal circumference variance components and heritability at different stages.
SNP of IGFALS gene
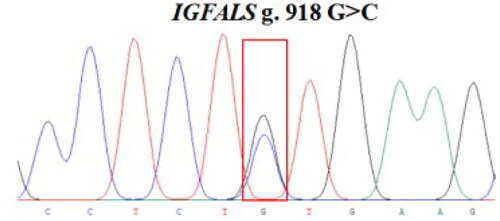
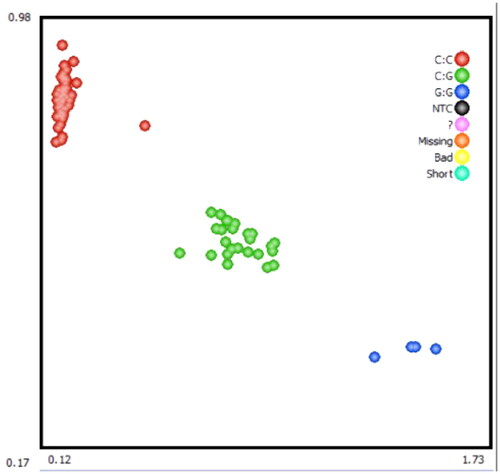
The 848 bp fragment of IGFALS gene was successfully amplified by specific primers from 10 extracted DNA samples (). By sequencing the PCR product, a SNP (G/C) was identified at position 918 in exon 1 of the IGFALS gene (). To determine the polymorphism of the IGFALS gene, KASPar assays were used to genotype the SNP at g.918 G > C of IGFALS. Three genotypes (CC, CG and GG) were detected after genotyping ().
Genetic indexes of IGFALS gene
The results of the genetic index analysis of the IGFALS gene are shown in . The results showed that the IGFALS gene was in a low level of heterozygosity in sheep population (0 < PIC < 0.25). In the experimental population, the CC genotype had the highest frequency of 0.73 among the three genotypes, so it was the main dominant genotype. C allele frequency is greater than G allele frequency and is the dominant allele. The IGFALS gene was in Hardy-Weinberg equilibrium in the experimental population.
Table 4. Genotype frequency, allele frequency, and genetic diversity of IGFALS g.918G > C sites.
Statistical associations between genotypes and the studied traits
In a statistical analysis of genotypes and scrotal circumference at different stages of development, there was a significant association between individuals with the CC genotype and the CG, GG genotypes (). The statistical results showed that the scrotal circumference of individuals with CC genotype was significantly lower than that of individuals with CG and GG genotype at 100, 120 and 180 days of age (p < 0.05). At 140 and 160 days of age, the scrotal circumference of individuals with CC genotype was significantly lower than that of individuals with CG genotype (p < 0.05).
Table 5. Association of different genotypes due to SNP in IGFALS with the scrotal circumference in sheep.
Discussion
Reasonable use of breeding rams can reduce production costs, shortens the generation interval and may increase economic benefits. Scrotal circumference is an important indicator of the reproductive capacity of rams. It has been shown that the larger the circumference of scrotum, the higher the sperm yield and the better the fertility effect.Citation36 There are many factors that affect the size of the scrotal circumference. Studies have found that there are significant differences in the biological and endocrine characteristics of the scrotum and testes among bulls of different ages in the same season. Similarly, there are significant differences in the scrotal circumference and endocrine characteristics among bulls of the same age in different seasons.Citation37 In addition to age and season, there are also factors such as body weightCitation38 and nutrition.Citation39,Citation40 In this study, 1353 Hu sheep were measured for scrotal circumference at different developmental stages under the same level of feeding management. Through statistical analysis, the results showed that the scrotal circumference increases with age from 100 to 160 days old, but remained unchanged at 160 and 180 days old. The coefficient of variation of scrotal circumference at each stage was greater than 10%, indicating that there was a certain selection space for this trait. In addition, we estimated the heritability of scrotal circumference at each stage, and the results showed that the heritability of scrotal circumference at each stage ranged from medium to high, ranging from 0.318 to 0.719. In the study of Rambouillet rams by Matos et al.,Citation41 it was found that the circumference of the scrotum increased with age from 90 to 180 days old, and the heritability at each stage was 0.22–0.60, indicating a moderate to high heritability, which is consistent with the results of this study. In this study, there was no change in the scrotal circumference at the ages of 160 and 180 days, as the growth of Hu sheep began to slow down from 3 to 6 months old, and the scrotal circumference was also related to body weight. Therefore, there was no change in the scrotal circumference at the ages of 160 and 180 days.
The size of scrotal circumference, as an important basis for judging the quality of breeding rams, will directly or indirectly affect the efficiency of sheep breeding industry. Screening the genetic variation of key candidate genes related to scrotal circumference will help to better formulate breeding planning. IGFALS (Insulin-like growth factor-binding protein acid-labile subunit) genes play an important role in regulating growth and development and other physiological processes.Citation28 In this study, the IGFALS gene was used as a candidate gene to investigate the relationship between its genetic variation and scrotal circumference. By DNA sequencing, a synonymous mutation (g.918 G > C) was detected in exon 1 of the IGFALS gene. Although synonymous mutation will not change the amino acid sequence of the protein encoded by the gene, it can affect its function by affecting cellular processes such as mRNA stability,Citation42 mRNA translation,Citation43 mRNA foldingCitation44 or splicing,Citation45 protein foldingCitation46 and so on. In addition, some studies have found that synonymous mutations in non-coding regions are highly correlated with some important economic traits of sheep.Citation47,Citation48 The results of association analysis showed that the synonymous mutation g.918G > C significantly affected the scrotal circumference in Hu sheep. The mutation at 918 locus of sheep IGFALS gene in our study did not change the amino acid sequence of the protein. As previously reported, synonymous mutation may cause the change of protein function through the processes of mRNA stabilityCitation42 and splicing events,Citation49 because GG genotype had a higher phenotype value in scrotal circumference. Secondly, the development of the scrotum is a complex process, which is affected by multiple regulatory mechanisms. The cellular processing, proliferation, differentiation and prevention of apoptosis in sheep are affected by growth factors, in which insulin growth factors I and II (IGFs) play an important role.Citation50 The protein ALS encoded by Insulin-like growth factor-binding protein acid-labile subunit (IGFALS) is an important compound, it connects IGF-I and IGFBP-3 to form a ternary complex in the cycle to regulate growth, development and other physiological processes.Citation28 A previous study identified two new recessive mutations in the IGFALS gene.Citation51 The N276S mutation led to changes in conserved amino acids, and the Q320X mutation caused severe truncation of the ALS protein. They concluded that the initial deficiency of ALS caused by IGFALS mutation reduced the formation of triple complex, thereby reducing growth after birth in these patients. In this study, we estimated the genetic parameters of scrotal circumference in five growth stages of Hu sheep and screened a molecular marker (IGFALS g.918 G > C) related to scrotal circumference in sheep, which is the advantage of this study. Unfortunately, this study did not Conjoint analysis the relationship between scrotal circumference, body weight and semen quality, and did not verify the level of cell function. Therefore, future research will involve Conjoint analysis of scrotal circumference, body weight and semen quality, Functional verification at the cell level, and verification between different varieties.
Conclusions
In this study, the measurement of scrotal circumference at different stages, descriptive statistics and heritability estimation found that the scrotal circumference of Hu sheep aged 100 to 160 days increased with age, while there was no change from 160 to 180 days old. And the coefficient of variation of scrotal circumference at each stage exceeds 10%, with heritability ranging from 0.318 to 0.719, indicating a moderate to high heritability. This indicates that scrotal circumference as an important indicator for evaluating sheep’s reproductive ability has great potential for selection. In addition, a polymorphism g.918 G > C was detected in the IGFALS gene, which is associated with sheep scrotal circumference. Association analysis showed that individuals with GG and CG genotypes have larger phenotypic values of scrotal circumference. It indicates that IGFALS gene may be a candidate gene for sheep scrotal development and has potential research value. But further research is still needed.
Author contributions
X. X. Zhang and Z. W. Ma conceived and designed the study; X. B. Yang, Y. L. Huang, P. P. Cui, J. Liu, X. W. Zeng, R. Zhai, W. M. Wang, X. J. Wang, S. R. Li and X. X. Weng collected samples and DNA extraction; Y. Zhao, Y. K. Zhang, X. L. Li, C. C. Lin, J. H. Wang, J. B. Cheng, D. Xu conducted experimental data analysis, Z. W. Ma wrote the paper, and W. M Wang, D. Y. Zhang, L. M Zhao revised the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Feng X, Li F, Wang F, et al. Genome-wide differential expression profiling of mRNAs and lncRNAs associated with prolificacy in Hu sheep. Biosci Rep. 2018;38(2):BSR20171350.
- Diao Z, Yanghua QU, Liu C, Gao Y, Luo H. Effects of forage canola mixed silage on slaughter performance and meat quality in Hu sheep. China Animal Husband Vet Med. 2018;
- Kumi-Diaka J, Adesiyun AA, Sekoni V, Ezeokoli CD. Scrotal dimensions and ejaculate characteristics of three breeds of sheep in tropical Nigeria. Theriogenology. 1985;23(4):671–677.
- Amann RP, Almquist JO. Reproductive capacity of dairy bulls. VIII. Direct and indirect measurement of testicular sperm production 1,2. J Dairy Sci. 1962;45(6):774–781.
- Hahn J, Foote RH, Seidel GE. Testicular growth and related sperm output in dairy bulls. J Anim Sci. 1969;29(1):41–47.
- Boyd LJ, Van Demark NL. Spermatogenic capacity of the male bovine. I. A measurement technique 1. J Dairy Sci. 1957;40(6):689–697.
- Willett EL, Ohms JI. Measurement of testicular size and its relation to production of spermatozoa by bulls. J Dairy Sci. 1957;40(12):1559–1569.
- Amann RP, Almquist JO. Reproductive capacity of dairy bulls. V. Detection of testicular deficiencies and requirements for experimentally evaluating testis function from semen characteristics. J Dairy Sci. 1961;44(12):2283–2291.
- Amann RP, Almquist JO. Reproductive capacity of dairy bulls. VI. Effect of unilateral vasectomy and ejaculation frequency on sperm reserves; aspects of epididymal physiology. J Reprod Fertil. 1962;3(2):260–268.
- Bongso TA, Jainudeen MR, Zahrah AS. Relationship of scrotal circumference to age, body weight and onset of spermatogenesis in goats. Theriogenology. 1982;18(5):513–524.
- Ahmad N, Noakes DE. Sexual maturity in British breeds of goat kids. Br Vet J. 1996;152(1):93–103.
- Menegassi SRO, Barcellos JOJ, Peripolli V, Pereira PRRX, Borges JBS, Lampert VN. Measurement of scrotal circumference in beef bulls in Rio Grande do Sul. Arq Bras Med Vet Zootec. 2011;63(1):87–93.
- Wikström AM, Bay K, Hero M, Andersson AM, Dunkel L. Serum insulin-like factor 3 levels during puberty in healthy boys and boys with Klinefelter syndrome. J Clin Endocrinol Metab. 2006;91(11):4705–4708.
- Johansen ML, Anand-Ivell R, Mouritsen A, et al. Serum levels of insulin-like factor 3, anti-Mullerian hormone, inhibin B, and testosterone during pubertal transition in healthy boys: a longitudinal pilot study. Reproduction. 2014;147(4):529–535.
- Kawate N, Ohnari A, Pathirana IN, et al. Changes in plasma concentrations of insulin-like peptide 3 and testosterone from birth to pubertal age in beef bulls. Theriogenology. 2011;76(9):1632–1638.
- Pathirana IN, Yamasaki H, Kawate N, et al. Plasma insulin-like peptide 3 and testosterone concentrations in male dogs: changes with age and effects of cryptorchidism. Theriogenology. 2012;77(3):550–557.
- Hannan MA, Kawate N, Fukami Y, et al. Changes of plasma concentrations of insulin-like peptide 3 and testosterone, and their association with scrotal circumference during pubertal development in male goats. Theriogenology. 2017;92:51–56.
- Ott R. Breeding soundness examination in bulls. 1986.
- Lunstra DD, Ford JJ, Echternkamp SE. Puberty in beef bulls: hormone concentrations, growth, testicular development, sperm production and sexual aggressiveness in bulls of different breeds. J Anim Sci. 1978;46(4):1054–1062.
- Killian GJ, Amann RP. Reproductive capacity of dairy bulls. IX. Changes in reproductive organ weights and semen characteristics of Holstein bulls during the first thirty weeks after puberty. J Dairy Sci. 1972;55(11):1631–1635.
- Devkota B, Koseki T, Matsui M, et al. Relationships among age, body weight, scrotal circumference, semen quality and peripheral testosterone and estradiol concentrations in pubertal and postpubertal Holstein bulls. J Vet Med Sci. 2008;70(1):119–121.
- Colas G. Seasonal variations of the quality of sperm in the Ile-de-France ram. I. Study of the cellular morphology and massal motility. Reprod Nutr Dev. 1980;20(6):1789–1799.
- Boland MP, Al-Kamali AA, Crosby TF, et al. The influence of breed, season and photoperiod on semen characteristics, testicular size, libido and plasma hormone concentrations in rams. Animal Reprod Sci. 1985;9(3):241–252.
- Kafi M, Safdarian M, Hashemi M. Seasonal variation in semen characteristics, scrotal circumference and libido of Persian Karakul rams. Small Rumin Res. 2004;53(1–2):133–139.
- Cárdenas-Gallegos MA, Aké-López JR, Centurión-Castro F, Magaña-Monforte JG. The breed and season effects on scrotal circumference and semen characteristics of hair sheep rams under tropical conditions. Reprod Domest Anim. 2012;47(6):e92-94–e94.
- Setchell BP. The Parkes Lecture. Heat and the testis. J Reprod Fertil. 1998;114(2):179–194.
- Boisclair YR, Seto D, Hsieh S, Hurst KR, Ooi GT. Organization and chromosomal localization of the gene encoding the mouse acid labile subunit of the insulin-like growth factor binding complex. Proc Natl Acad Sci USA. 1996;93(19):10028–10033.
- Alizadeh F, Moradian F, Farhadi A. Association of allelic polymorphisms of IGFALS gene with growth traits in Makouei and Ghezel sheep breeds. Trop Anim Health Prod. 2020;52(6):3027–3034.
- Domené HM, Scaglia PA, Martínez AS, et al. Heterozygous IGFALS gene variants in idiopathic short stature and normal children: impact on height and the IGF system. Horm Res Paediatr. 2013;80(6):413–423.
- Le RD. Deficiency of the circulating insulin-like growth factor system associated with inactivation of the acid-labile subunit gene. New Eng J Med. 2004;350:570–577.
- Suwanichkul A, Boisclair YR, Olney RC, Durham SK, Powell DR. Conservation of a growth hormone-responsive promoter element in the human and mouse acid-labile subunit genes. Endocrinology. 2000;141(2):833–838.
- Rhoads RP, Greenwood PL, Bell AW, Boisclair YR. Organization and regulation of the gene encoding the sheep acid-labile subunit of the 150-kilodalton insulin-like growth factor-binding protein complex. Endocrinology. 2000;141(4):1425–1433.
- Delhanty PJ, Baxter RC. Cloning and characterization of the rat gene for the acid-labile subunit of the insulin-like growth factor binding protein complex. J Mol Endocrinol. 1997;19(3):267–277.
- Li S, Ren J, Huang L. Characterization of the porcine insulin-like growth factor-binding protein, acid-labile subunit gene: full-length cDNA and DNA sequence, polymorphisms and expression profile. J Anim Breed Genet. 2007;124(3):133–138.
- Liu Y, Duan X, Liu X, et al. Genetic variations in insulin-like growth factor binding protein acid labile subunit gene associated with growth traits in beef cattle (Bos taurus) in China. Gene. 2014;540(2):246–250.
- Zhao H, Wu X, Cai H, et al. Genetic variants and effects on milk traits of the caprine paired-like homeodomain transcription factor 2 (PITX2) gene in dairy goats. Gene. 2013;532(2):203–210.
- Coulter GH, Foote RH. Bovine testicular measurements as indicators of reproductive performance and their relationship to productive traits in cattle: a review. Theriogenology. 1979;11(4):297–311.
- Perumal P, Savino N, Sangma CTR, et al. Effect of season and age on scrotal circumference, testicular parameters and endocrinological profiles in Mithun bulls. Theriogenology. 2017;98:23–29.
- Coulter GH, Larson LL, Foote RH. Effect of age on testicular growth and consistency of Holstein and Angus bulls. J Anim Sci. 1975;41(5):1383–1389.
- Barth AD, Brito LF, Kastelic JP. The effect of nutrition on sexual development of bulls. Theriogenology. 2008;70(3):485–494.
- Dance A, Thundathil J, Wilde R, Blondin P, Kastelic J. Enhanced early-life nutrition promotes hormone production and reproductive development in Holstein bulls. J Dairy Sci. 2015;98(2):987–998.
- Matos CA, Thomas DL, Nash TG, Waldron DF, Stookey JM. Genetic analyses of scrotal circumference size and growth in Rambouillet lambs. J Anim Sci. 1992;70(1):43–50.
- Duan J, Wainwright MS, Comeron JM, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12(3):205–216.
- Drummond D, Wilke CO. Mistranslation-induced protein misfolding as a dominant constraint on coding-sequence evolution. Cell. 2008;134(2):341–352.
- Goodman DB, Church GM, Kosuri S. Causes and effects of N-terminal codon bias in bacterial genes. Science. 2013;342(6157):475–479.
- Parmley JL, Chamary JV, Hurst LD. Evidence for purifying selection against synonymous mutations in mammalian exonic splicing enhancers. Mol Biol Evol. 2006;23(2):301–309.
- Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–528.
- Zhao L, Li F, Yuan L, et al. Expression of ovine CTNNA3 and CAP2 genes and their association with growth traits. Gene. 2022;807:145949.
- Wang J, Zhang X, Wang X, et al. Polymorphism and expression of the HMGA1 gene and association with tail fat deposition in Hu sheep. Anim Biotechnol. 2021;34(4):1626–1634.
- Pagani F, Raponi M, Baralle FE. Synonymous mutations in CFTR exon 12 affect splicing and are not neutral in evolution. Proc Natl Acad Sci USA. 2005;102(18):6368–6372.
- Conover CA. Regulation and physiological role of insulin-like growth factor binding proteins. Endocr J. 1996;43:S43–S48.
- Heath KE, Argente J, Barrios V, et al. Primary acid-labile subunit deficiency due to recessive IGFALS mutations results in postnatal growth deficit associated with low circulating insulin growth factor (IGF)-I, IGF binding protein-3 levels, and hyperinsulinemia. J Clin Endocrinol Metab. 2008;93(5):1616–1624.