Abstract
Matrix metalloproteinase 9 (MMP9) plays a pivotal role in mammary ductal morphogenesis, angiogenesis and glandular tissue architecture remodeling. However, the molecular mechanism of MMP9 expression in mammary epithelial cells of dairy cows remains unclear. This study aimed to explore the underlying mechanism of MMP9 expression. In this study, to determine whether the PI3K/AKT/mTORC1/NF-κB signalling pathway participates in the regulation of MMP9 expression, we treated mammary epithelial cells with specific pharmacological inhibitors of PI3K (LY294002), mTORC1 (Rapamycin) or NF-κB (Celastrol), respectively. Western blotting results indicated that LY294002, Rapamycin and Celastrol markedly decreased MMP9 expression and P65 nuclear translocation. Furthermore, we found that NF-κB (P65) overexpression resulted in elevated expression of MMP9 protein and activation of MMP9 promoter. In addition, we observed that Celastrol markedly decreases P65-overexpression-induced MMP9 promoter activity. Moreover, the results of the promoter assay indicated that the core regulation sequence for MMP9 promoter activation may be located at −420 ∼ −80 bp downstream from the transcription start site. These observations indicated that the PI3K/AKT/mTORC1 signalling pathway is involved in MMP9 expression by regulating MMP9 promoter activity via NF-κB in the mammary epithelial cells of dairy cows.
Introduction
Matrix metalloproteinases (MMPs) are a multigene family of zinc-dependent endopeptidases enzymes that degrade the extracellular matrix (ECM).Citation1 Until now, 26 different MMPs have been confirmed in humans. Based on substrate specificity and domain homologies, MMPs were divided into six groups: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs and other non-classified MMPs.Citation2 Typical MMP consists of several conserved domains including pre-domain, propeptide, catalytic domain and hemopexin domain. Among these domains, the catalytic domain consists of 170 amino acids (AAs) and contains a conserved three histidine sequence which is required for zinc chelation and plays an important role in MMP activity.Citation2
Given their role in the degradation of the ECM, MMPs and the plasminogen activator (PA) system may play also a critical role in the extensive remodeling that occurs in the bovine mammary gland during development, lactation and involution.Citation3 The mammary gland undergoes many changes in structure and function, including cyclic expansions corresponding to the hormonal changes induced by the estrous/menstrual cycle, as well as the dramatic changes that occur during pregnancy, lactation, and involution during the lifetime of the female.Citation4 MMPs play a pivotal role in mammary ductal morphogenesis, angiogenesis and glandular tissue architecture remodeling.Citation5 For example, remodeling of the mammary tissue is also linked with the destruction of the lobular-alveolar structure of the gland and the ECM during mammary involution.Citation6 At the same time, the MMP system is activated and induces the proteolysis of ECM components.Citation7
Among MMPs, MMP9 has been shown to regulate many cellular processes, such as tissue repair, angiogenesis, apoptosis, cell migration and wound healing.Citation8 In the mammary gland, MMP9 preferentially degrades ECM during angiogenesis and tissue remodeling. The previous study indicated that MMP9 activity is regulated at three levels: gene transcription and mRNA stability, enzymatic form activation and inhibition by endogenous inhibitors, such as tissue inhibitors of metalloproteinases (TIMPs).Citation9 The MMP9 gene can be induced by a variety of oncogene products, cytokines, mitogens and phorbol ester.Citation10 These stimulators can upregulate the expression of MMP9 by modulating the activation of transcription factors, such as NF-κB and AP-1 through the PI3K/AKT and MAPK signalling pathways. It is well known that human MMP9 promoter contains cis-acting regulatory elements for transcription factors including an NF-κB site (located at −600 bp), an SP-1 site (located at −558 bp) and two AP-1 sites (located at −79 bp and −533 bp).Citation11
Phosphatidylinositol-3-kinase is an intracellular protein with catalytic activity, which can regulate the phosphorylation of downstream Akt and constitutes the PI3K/Akt signalling transduction pathway.Citation12 The PI3K/AKT signalling pathway plays an important role in the regulation of cell survival, growth, proliferation, angiogenesis and metabolism. In MDA-MB-231 cells, CoQ0 suppressed MMP-9 protein expression by inhibiting the PI3K/AKT/NF-κB pathway.Citation13 PA effectively suppressed human breast cancer MDA-MB-231 cell migration, invasion and cell motility by abating the expression of NF-κB and AP-1 to reduce the expression of MMP-9 through ERK/PI3K/Akt/mTOR signalling pathway.Citation14 MMP9 secretion is mediated by 12-LOX in PC-3 cells via the activation of the PI3K/AKT/NF-κB signalling pathway.Citation15
However, the molecular mechanism governing MMP9 expression in dairy cow mammary epithelial cells has been poorly understood. In this study, we aimed to investigate the mechanism by which PI3K/AKT/mTORC1 pathway is involved in NF-κB activation and MMP9 expression. We hypothesized that the PI3K/AKT/mTORC1 signalling pathway regulates MMP9 expression through NF-κB (P65) in mammary epithelial cells of dairy cows. To test our hypothesis, we evaluated the effects of LY294002, Rapamycin, and Celastrol on NF-κB (P65) nuclear translocation and MMP9 expression in mammary epithelial cells. We then investigated the crucial role of NF-κB (P65) in the regulation of the MMP9 promoter activity.
Materials and methods
Cell culture
Mammary epithelial cells were isolated from mammary parenchymal tissues of lactating Holstein dairy cows as described previously.Citation16 All animal experimental procedures were approved by the Northeast Agricultural University Animal Care and Use Committee (No. NEAUEC20200109) (Harbin, China). Mammary epithelial cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified 5% CO2 incubator. Pure mammary epithelial cells were obtained after three passages and identified by immunofluorescence for Cytokeratin-18 and β-casein.Citation17 All experiments were conducted using cells within seven passages.
Western blotting
Mammary epithelial cells were seeded in 6-well plates at a density of 1 × 106 cells/well. Then cells were pretreated with LY294002 (PI3K inhibitor) (NA; Gibco, Grand Island, NY) (25 μM), Rapamycin (mTORC1 inhibitor) (Solarbio, Beijing, China) (0.1 μM), or Celastrol (NF-κB inhibitor) (Solarbio, Beijing, China) (0.3 μM) for 24 h, respectively. DMSO was used as a negative control. Whole-cell lysates were prepared from mammary epithelial cells using RIPA buffer. The total protein concentration was determined using a BCA protein assay kit (Beyotime, Shanghai, China). Protein was separated by 10% SDS-PAGE and then transferred to nitrocellulose (NC) membranes. Immunoblot analysis was performed using antibodies against MMP9 (1:4000 dilution; Proteintech, Rosemont, IL), p-mTOR (1:1000 dilution; Cell Signaling Technology, Shanghai, China), mTOR (1:1000 dilution; Abcam, Shanghai, China), p-AKT (1:1000 dilution; Cell Signaling Technology, Shanghai, China), AKT (1:1000 dilution; Cell Signaling Technology, Shanghai, China), p-P65 (1:1000 dilution; Bioss, Beijing, China), P65 (1:1000 dilution; Cell Signaling Technology, Shanghai, China), Histine3 (1:1000 dilution; Bioss, Beijing, China), β-actin (1:4000 dilution; Proteintech) overnight at 4 °C. Horseradish peroxidase-conjugated IgG was used as a secondary antibody (1:10000 dilution; ZSGB-BIO, Beijing, China). The protein expression levels were detected using ECL chemiluminescence (Biosharp) and analysed using Image J.
Preparation of nuclear extract
Mammary epithelial cells were seeded in 6-well plates at a density of 1 × 106 cells/well. Cells were grown to 90% confluence and treated with LY294002, Rapamycin, or Celastrol, respectively. Cells were collected after 4 h of treatment. Cytoplasmic and nuclear protein was prepared using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, China). The nuclear extracts and cytoplasmic extracts were stored at −80 °C.
Construction of dairy cow MMP9 promoters
The potential binding sites of NF-κB to the MMP9 promoter were predicted using the JASPAR database and hTFtarget database before construction of dairy cow MMP9 promoters. There were three same potential binding sites predicted by these two databases: −395/−384 (GGGGGTCTTCCG); −432/−421 (GGGAACTTTCCA); and −612/−602 (TGGAATTCCCC). Then, a series of fragments from the 5’-flanking region of the dairy cow MMP9 gene were purchased from Sangon Biotech (Shanghai, China). All MMP9 gene promoter fragments were designed to contain an appropriate restriction enzyme site: 5’-end (Kpn Ι) and 3’-end (Bgl II). These MMP9 promoter fragments had the same 3’-end. Kpn Ι and Bgl II sites were included in DNA fragments so that these DNA fragments could be digested with Kpn Ι and Bgl II, and then subcloned into the PGL3-Basic Kpn Ι/Bgl II site. The MMP9 promoter sequence was confirmed by DNA sequencing, and the resultant reporter plasmids were named MMP9-640-Luc (−640, 0), MMP9-420-Luc (−420, 0), MMP9-380-Luc (−380, 0) and MMP9-80-Luc (−80, 0), respectively.
Transient transfection and dual-luciferase reporter assay
Mammary epithelial cells were seeded in 6-well plates at a density of 1 × 106 cells/well. Cells were grown to 80% confluence and transfected with pcDNA3.1-NF-κB (P65) plasmid (500 ng) (purchased from Sangon Biotech), the pGL3 vector containing different MMP9 promoter fragments (MMP9 promoter-luciferase reporter constructs) (500 ng) and the internal reference plasmid pRL-TK (10 ng) by Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). To construct pcDNA3.1-NF-κB (P65) plasmid, the pcDNA3.1(+) plasmid was used to construct the expression vector by subcloning the PCR-amplified DNA of the NFkB (P65) CDS (NM_001080242.2) into the KpnI/NotI site. A Dual-Luciferase Reporter Assay System (Promega, Madison, WI) was used to measure the luciferase activities in cell lysates after 48 h of treatment. Renilla luciferase was used for the normalized of Luciferase activities. In addition, when mammary epithelial cells were transfected with pcDNA3.1-NF-κB (P65) plasmid, MMP9 promoter-luciferase reporter constructs and pRL-TK for 6 h, cells were treated with Celastrol for 48 h, and 0.1% DMSO was used as a negative control. All transient transfections were repeated in three independent experiments.
Statistical analysis
Data analysis was carried out using GraphPad Prism version 8.0 software (GraphPad Software, San Diego, CA). All experiments were performed in triplicate and the results are expressed as means ± SEM. t-Test was used to compare means between two groups. One-way ANOVA followed by Tukey post hoc test was used to compare means between three groups. The minimum significance level was set at a p value of less than 0.05 for all analyses.
Results
LY294002 attenuates MMP9 expression and P65 nuclear translocation in mammary epithelial cells
To determine whether PI3K/AKT signalling pathway is involved in MMP9 protein expression, mammary epithelial cells were treated with LY294002 (a PI3K inhibitor) for 24 h. Compared with control, LY294002 markedly decreased the phosphorylation level of mTOR and AKT, and the expression level of MMP9 protein (). However, there was no noticeable change in the level of AKT and mTOR protein expression under the same treatment conditions. Subsequently, we investigated whether LY294002 inhibited the translocation of NF-κB(p65) into the nucleus. Mammary epithelial cells were treated with LY294002 for 4 h and compared with the control group, LY294002 significantly reduced the level of P65 protein in the nucleus by Western blot analysis (), and markedly increased the expression of P65 protein in the cytosolic extracts ().
Figure 1. LY294002 attenuates MMP9 expression and P65 nuclear translocation in mammary epithelial cells. (A) Mammary epithelial cells were pretreated with LY294002. DMSO was used as a negative control. The expression of p-AKT, AKT, p-mTOR, mTOR and MMP9 were measured by Western blot analysis. β-actin expression was evaluated as an internal control. (B) Quantification of p-AKT, AKT, p-mTOR, mTOR and MMP9 expression from the Western blots in panel A. (C) Mammary epithelial cells were treated with LY294002 for. Nuclear and cytoplasmic extracts were prepared, and Western blot analysis was performed. Histone H3 and β-actin were used as an internal control for nuclear and cytoplasmic extracts respectively. (D) Quantification of nuclear and cytoplasmic P65 expression from the Western blots in panel C. Data are expressed as mean ± SEM of three independent experiments. *p < 0.05 and **p < 0.01 compared with the control.
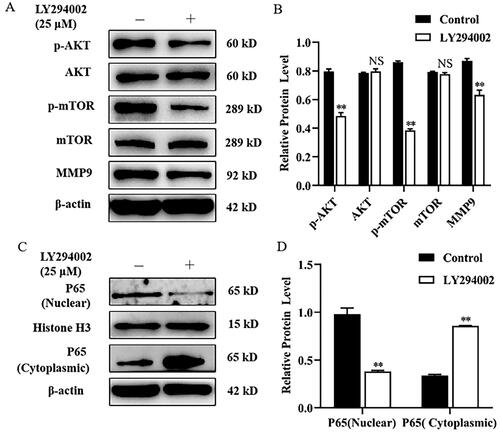
Rapamycin decreased MMP9 expression and P65 nuclear translocation in mammary epithelial cells
Mammary epithelial cells were treated with Rapamycin, an mTORC1 inhibitor, for 24 h. Compared with the control, treatment of mammary epithelial cells with Rapamycin tremendously decreased the expression of MMP9 and the p-mTOR protein level (). However, the expression level of mTOR protein was not significantly changed. To investigate whether Rapamycin can affect NF-κB (p65) nuclear translocation, mammary epithelial cells were treated with Rapamycin for 4 h. Western blot analysis showed that treatment with Rapamycin significantly increased the protein levels of P65 in the cytoplasm, and diminished nuclear P65 protein compared with the control ().
Figure 2. Rapamycin decreased MMP9 expression and P65 nuclear translocation in mammary epithelial cells. (A) Mammary epithelial cells were pretreated with Rapamycin. The expression of p-mTOR, mTOR and MMP9 were measured by Western blot analysis. β-actin expression was evaluated as an internal control. (B) Quantification of p-mTOR, mTOR and MMP9 expression from the Western blots in panel A. (C) Mammary epithelial cells were treated with Rapamycin. Nuclear and cytoplasmic extracts were prepared, and Western blot analysis was performed. Histone H3 and β-actin were used as an internal control for nuclear and cytoplasmic extracts, respectively. (D) Quantification of nuclear and cytoplasmic P65 expression from the Western blots in panel C. Data are expressed as mean ± SEM of three independent experiments. *p < 0.05 and **p < 0.01 compared with the control.
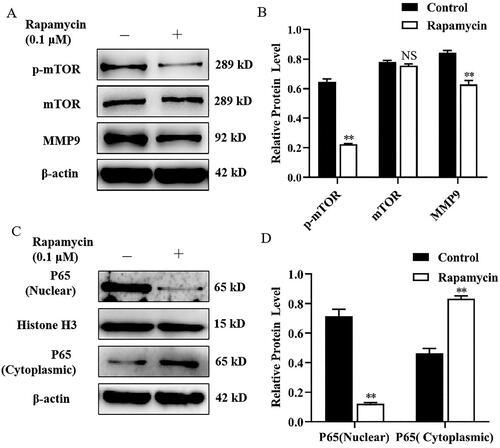
Celastrol suppressed MMP9 expression and P65 nuclear translocation in mammary epithelial cells
To test whether MMP9 protein expression can be regulated by the transcriptional factor NF-κB in the mammary epithelial cells of dairy cows, mammary epithelial cells were treated with Celastrol (an NF-κB inhibitor) for 24 h. We observed that Celastrol significantly decreased the expression of MMP9 compared with control (). Next, we found that Celastrol treatment caused a marked increase in the expression of P65 in the cytosol, whereas the expression of P65 in the nucleus was significantly decreased compared with the control group ().
Figure 3. Celastrol suppressed MMP9 expression and P65 nuclear translocation in mammary epithelial cells. (A) Mammary epithelial cells were pretreated with Celastrol. The expression of MMP9 was measured by Western blot analysis. β-actin expression was evaluated as an internal control. (B) Quantification of MMP9 expression from the Western blots in panel A. (C) Mammary epithelial cells were treated with Celastrol for. Nuclear and cytoplasmic extracts were prepared and Western blot analysis was performed. Histone H3 and β-actin were used as an internal control for nuclear and cytoplasmic extracts, respectively. (D) Quantification of nuclear and cytoplasmic P65 expression from the Western blots in Panel C. Data are expressed as mean ± SEM of three independent experiments. *p < 0.05 and **p < 0.01 compared with the control.
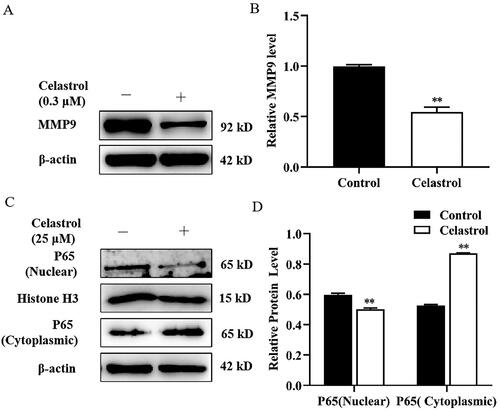
Transcription factor NF-κB (P65) overexpression enhanced MMP9 expression and MMP9 gene promoter activity in mammary epithelial cells
To determine whether MMP9 expression was directly regulated by NF-κB, mammary epithelial cells were transfected with pcDNA3.1-NF-κB (P65) plasmid. After transfection for 48 h, we observed a significant increase in P65, MMP9 and p-P65 expression in the P65 overexpression group compared with the empty vector group (). The potential binding sites of NF-κB to the MMP9 promoter were predicted using the JASPAR database and hTFtarget database (). Then, the effect of transcription factor NF-κB (P65) on the transcriptional regulation of MMP9 was examined using an MMP9 promoter-luciferase construct, which contains 640 bp, 420 bp, 380 bp and 80 bp of proximal MMP9 promoter sequence upstream of a luciferase reporter. Mammary epithelial cells were transiently co-transfected with a reporter plasmid containing MMP9 promoter sequence and pcDNA3.1-NF-κB (P65) plasmid for 48 h. Our results demonstrated that the luciferase reporter gene activity of plasmid linked to the 640 bp, 420 bp and 380 bp fragment of MMP9 promoter is significantly increased in the P65 overexpression treated group compared with the untreated group ()). Under the same conditions, there was no noticeable change in the M80-Luc group. Moreover, the luciferase activity of the M420-Luc group was highest in cells treated with P65 overexpression ().
Figure 4. Effect of transcription factor NF-κB (P65) on MMP9 promoter activity in mammary epithelial cells. (A) Mammary epithelial cells were transfected with pcDNA3.1-NF-κB (P65) plasmid. The levels of P65, p-P65 and MMP9 were determined by Western blotting. Protein expression levels of β-actin in cell lysates were used as a control. (B) Quantification of P65, p-P65 and MMP9 expression from the Western blots in panel A. (C) MMP9 promoter image. NFkB binding sites were pointed out as S1 (−612/−602), S2 (−432/−421) and S3 (−395/−384). (D) Mammary epithelial cells were co-transfected with a series of MMP9-PGL3 plasmid including MMP9-640-Luc (M640-Luc), MMP9-420-Luc (M420-Luc), MMP9-380-Luc (M380-Luc) and MMP9-80-Luc (M80-Luc), which contains a deletion of the MMP9 gene promoter 5’-flanking region, and pcDNA3.1-NF-κB (P65) plasmid for 48h. Luciferase activity was normalized to the Renilla activity in each cell lysate. (E) Mammary epithelial cells were co-transfected with M420-Luc plasmid and pcDNA3.1-NF-κB (P65) plasmid for 6h. Then cells were treated with Celastrol, and DMSO was used as a negative control. Luciferase activity was normalized with the Renilla activity in each cell. Data are expressed as mean ± SEM of three independent experiments. For B, means without a common letter (a,b) differ at p < 0.05. For C and D, *p < 0.05; **p < 0.01.
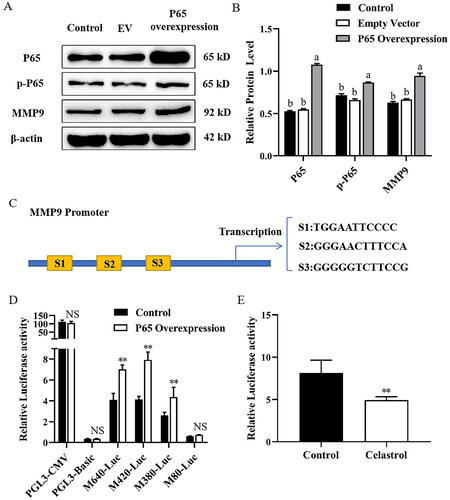
Next, to further determine the inhibitory effect of Celastrol on P65 overexpression-induced MMP9 gene transcription factor activity, we performed a promoter assay using transiently co-transfected mammary epithelial cells with a luciferase reporter gene linked to a 420 bp fragment from the MMP9 promoter region and a pcDNA3.1-NF-κB (P65) plasmid. After transfection for 6 h, cells were treated with Celastrol, and DMSO was used as a negative control. Compared with the control group, treatment with Celastrol resulted in a significant decrease in the promoter activity of the MMP9 gene ().
Discussion
The mammary gland is a unique glandular organ, which distinguishes mammals from all other animals and functions to produce and secrete milk to nourish offspring. The mammary gland undergoes many changes in structure and function during pregnancy, lactation and involution. Because of the significant amount of ECM remodeling that must take place during these different stages, MMPs would be expected to play a pivotal role in mammary morphogenesis. During normal mammary gland development, MMP9 was involved in proliferation and branching morphogenesis, and it plays a role in the controlled invasion of the fat pad.Citation18
Previous studies have shown that PI3K/AKT signalling pathway is involved in regulating MMP9 expression.Citation19 In colorectal cells, RasGRF2 promotes migration and invasion by modulating the expression of MMP9 through PI3K/AKT and NF-κB signalling pathway.Citation20 In addition, CXCL5/CXCR2 promotes bladder cancer cells metastasis and invasion progression by activating PI3K/AKT pathway-induced upregulation of MMP9.Citation21 In MCF-7, PI3K/AKT pathway was involved in E2-BSA-induced MMP9 expression, and inhibiting activity of PI3K could influence E2-BSA-induced MMP9 expression using the PI3K inhibitor LY294002.Citation22 In this study, we demonstrated that LY294002, a PI3K/AKT pharmacological inhibitor reported to be the first synthetic reversible PI3K inhibitor and can inhibit the catalytic activity of PI3K,Citation23 reduced the phosphorylation of AKT and the expression of MMP9 protein. In addition, LY294002 significantly suppressed the nuclear translocation of NF-κB (P65). Similarly, LY294002 significantly decreased MMP9 protein level and AKT phosphorylation in human HCC cells, and NF-κB activation in MDA-MB-231 human breast cancer cells.Citation13 Previous studies demonstrated that the PI3K/AKT pathway has a close connection with the NF-κB signalling pathway, in which the phosphorylation of Akt can activate NF-κB.Citation24 Inhibition of the PI3K/AKT pathway can block phosphorylation of AKT and subsequently suppress the activation of NF-κB.Citation25 Based our findings, we confirmed that the expression of MMP9 was regulated by PI3K/AKT signalling pathway.
The mTOR pathway is involved in many intracellular processes, such as translation and protein synthesis through its substrates, cell growth and apoptosis. Moreover, PI3K and/or AKT can regulated mTOR activation.Citation26 Some studies have established that AAs can activate PI3K/AKT/mTOR signalling, leading to milk fat synthesis in bovine mammary epithelial cells in an mTOR-dependent manner.Citation27,Citation28 The calpain 2 significantly promote migration and invasion progression by activating AKT/mTOR signalling, enhancing epithelial mesenchymal transition (EMT) and MMP9 levels in renal cell carcinoma.Citation29 In human hepatocellular carcinoma cells, 14-3-3β promotes migration and invasion by modulating the expression of MMP2 and MMP9 through PI3K/AKT/mTOR pathway.Citation30 In this study, we demonstrated that Rapamycin, a classic mTORC1 inhibitor that blocks mTORC1 activity through a mechanism involving an interaction with FKBP12 and a subsequent interaction with TORC1 to inhibit mTOR kinase activity,Citation31 and suppressed expression of MMP9 in mammary epithelial cells. This agrees with the results of Park, who showed that MDA-MB-231 cells pretreated with mTOR siRNA or Rapamycin strongly inhibited MMP9 expression.Citation14 A more recent study demonstrated that Rapamycin decreased the expression of MMP9 and inhibited the colony formation of tumor cells in the human colorectal cancer cell line HCT116.Citation32 These findings support the importance of the mTORC1 pathway in regulating MMP9 expression. We also showed that Rapamycin has a bigger influence on P65 nuclear translocation, and similar findings were reported by Han et al., who demonstrated that EPA could inhibit NF-κB (P65) translocation from the cytoplasm into the nucleus by blocking the phosphorylation of mTOR in SKOV-3cells.Citation33 Previous studies also showed that AKT-dependent regulation of NF-κB is controlled by mTOR through interaction with IKK in PTEN-null/inactive prostate cancer cells.Citation34 It is well established that AKT controls NF-κB activation via mTOR. These results provide good evidence that NF-κB is an important downstream effector of mTOR, which is involved in MMP9 expression in mammary epithelial cells.
NF-κB is a dimeric transcription factor that regulates a target gene’s expression through binding to its promoter. NF-κB is initially located in the cytoplasm in an inactive form complexed with IκB, an inhibitory factor, which binds NF-κB and masks its nuclear localization signal, thereby preventing NF-κB translocation into the nucleus.Citation35 Activation of NF-κB is initiated through phosphorylation of IκB by a macromolecular cytoplasmic IκB kinase (IKK). Exposure of cells to a variety of extracellular stimuli leads to the rapid phosphorylation, ubiquitination and ultimately proteolytic degradation of IκB, which frees NF-κB to translocate to the nucleus where it regulates gene transcription including MMP9.Citation36
Transcriptional regulation of MMP9 is believed to be the most important component for MMP9 expression. Previous studies have demonstrated that the MMP9 promoter has several transcription factor binding motifs, including NF-κB In human MCF-7 cells, NF-κB directly regulates the transcription of MMP-9.Citation37 Puerarin treatment effectively negated the expression of MMP9 by inhibition of the NF-κB pathway in LPS-activated MCF-7 and MDA-MB-231 cells.Citation38 Another study indicated that KA inhibits the PMA-induced activation of MMP-9 by suppressing NF-κB activation in HT-1080 cells.Citation39 In this study, we showed that P65 overexpression significantly enhanced the phosphorylation level of P65 and increased MMP9 protein expression and gene promoter activity. Celastrol, an NF-κB inhibitor, remarkably reduced MMP9 protein expression and NF-κB (P65) nuclear translocation in mammary epithelial cells. Moreover, Celastrol also suppressed P65 overexpression-induced MMP9 gene promoter activity. The results of the promoter assay indicated that dairy cows’ MMP9 gene promoter may contain cis-acting regulatory elements for transcription factors, such as NF-κB and may be located at −420∼−80 bp. It is well established that NF-κB plays a significant role in the transcriptional regulation of MMP9.
Here, we examined PI3K/AKT/mTORC1 signalling to the MMP9 promoter in a defined system. However, the MMP9 promoter could be regulated by a network of signalling cascades because the promoter contains cis-regulatory regions that are targets of multiple signalling pathways. It remains to be seen whether PI3K/AKT/mTORC1 signalling can synergize or co-operate with other signalling components to further enhance MMP9 promoter activity. Therefore, further experiments will be necessary to investigate the molecular mechanism of PI3K/AKT/mTORC1 signalling on the transcriptional regulation of MMP9.
Conclusion
In conclusion, we show that MMP9 protein expression was mediated by transcription factor NF-κB through PI3K/AKT/mTORC1 signalling pathway in mammary epithelial cells of dairy cows. In addition, our study demonstrated that the core regulation sequence of transcription factor NF-κB for MMP9 promoter activation may be located at −420 ∼ −80 bp.
Disclosure statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Funding
References
- Yu HY, Kim KS, Moon HI, Kim KM, Lee YC, Lee JH. JNP3, a new compound, suppresses PMA-induced tumor cell invasion via NF-κB down regulation in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2012;421(2):1–11.
- Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31(1):177–183.
- Rabot A, Sinowatz F, Berisha B, Meyer HH, Schams D. Expression and localization of extracellular matrix-degrading proteinases and their inhibitors in the bovine mammary gland during development, function, and involution. J Dairy Sci. 2007;90(2):740–748.
- Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development. 2015;142(6):1028–1042.
- Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14(17):2123–2133.
- Boutinaud M, Isaka N, Gandemer E, et al. Inhibiting prolactin by cabergoline accelerates mammary gland remodeling during the early dry period in dairy cows. J Dairy Sci. 2017;100(12):9787–9798.
- Tremblay G, Bernier-Dodier P, Delbecchi L, Wagner GF, Talbot BG, Lacasse P. Local control of mammary involution: is stanniocalcin-1 involved? J Dairy Sci. 2009;92(5):1998–2006.
- Nemeth JA, Yousif R, Herzog M, et al. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst. 2002;94(1):17–25.
- Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211(1):19–26.
- Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189(3):300–308.
- Cho HJ, Jeong YJ, Park KK, et al. Bee venom suppresses PMA-mediated MMP-9 gene activation via JNK/p38 and NF-kappaB-dependent mechanisms. J Ethnopharmacol. 2010;127(3):662–668.
- Sun L, Tian W, Guo X, et al. Lactobacillus gasseri JM1 with potential probiotic characteristics alleviates inflammatory response by activating the PI3K/Akt signaling pathway in vitro. J Dairy Sci. 2020;103(9):7851–7864.
- Yang HL, Thiyagarajan V, Shen PC, et al. Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J Exp Clin Cancer Res. 2019;38(1):186.
- Park JH, Cho YY, Yoon SW, Park B. Suppression of MMP-9 and FAK expression by pomolic acid via blocking of NF-κB/ERK/mTOR signaling pathways in growth factor-stimulated human breast cancer cells. Int J Oncol. 2016;49(3):1230–1240.
- Dilly AK, Ekambaram P, Guo Y, et al. Platelet-type 12-lipoxygenase induces MMP9 expression and cellular invasion via activation of PI3K/Akt/NF-κB. Int J Cancer. 2013;133(8):1784–1791.
- Cui Y, Liu Z, Sun X, et al. Thyroid hormone responsive protein spot 14 enhances lipogenesis in bovine mammary epithelial cells. In Vitro Cell Dev Biol Anim. 2015;51(6):586–594.
- Cui Y, Sun X, Jin L, et al. MiR-139 suppresses β-casein synthesis and proliferation in bovine mammary epithelial cells by targeting the GHR and IGF1R signaling pathways. BMC Vet Res. 2017;13(1):350.
- Lee PP, Hwang JJ, Murphy G, Ip MM. Functional significance of MMP-9 in tumor necrosis factor-induced proliferation and branching morphogenesis of mammary epithelial cells. Endocrinology. 2000;141(10):3764–3773.
- Jung JS, Jung K, Kim DH, Kim HS. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol Res. 2012;66(1):95–103.
- Lu P, Chen J, Yan L, et al. RasGRF2 promotes migration and invasion of colorectal cancer cells by modulating expression of MMP9 through Src/Akt/NF-κB pathway. Cancer Biol Ther. 2019;20(4):435–443.
- Gao Y, Guan Z, Chen J, et al. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol. 2015;47(2):690–700.
- Pan Y, Wang X, Zhang Y, et al. Estradiol-induced MMP-9 expression via PELP1-mediated membrane-initiated signaling in ERα-positive breast cancer cells. Horm Cancer. 2020;11(2):87–96.
- Sheridan C, Downward J. Inhibiting the RAS-PI3K pathway in cancer therapy. Enzymes. 2013;34 Pt. B:107–136.
- Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA, Chen JH. Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol. 2010;632(1-3):23–32.
- Jiang RH, Xu JJ, Zhu DC, et al. Glycyrrhizin inhibits osteoarthritis development through suppressing the PI3K/AKT/NF-κB signaling pathway in vivo and in vitro. Food Funct. 2020;11(3):2126–2136.
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293.
- Li P, Zhou C, Li X, Yu M, Li M, Gao X. CRTC2 is a key mediator of amino acid-induced milk fat synthesis in mammary epithelial cells. J Agric Food Chem. 2019;67(37):10513–10520.
- Li X, Li P, Wang L, Zhang M, Gao X. Lysine enhances the stimulation of fatty acids on milk fat synthesis via the GPRC6A-PI3K-FABP5 signaling in bovine mammary epithelial cells. J Agric Food Chem. 2019;67(25):7005–7015.
- Miao C, Liang C, Tian Y, et al. Overexpression of CAPN2 promotes cell metastasis and proliferation via AKT/mTOR signaling in renal cell carcinoma. Oncotarget. 2017;8(58):97811–97821.
- Tang Y, Lv P, Sun Z, Han L, Zhou W. 14-3-3β Promotes migration and invasion of human hepatocellular carcinoma cells by modulating expression of MMP2 and MMP9 through PI3K/Akt/NF-κB pathway. PLoS One. 2016;11(1):e0146070.
- Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4(5):343–348.
- Zhang ZH, Li MY, Wang Z, et al. Convallatoxin promotes apoptosis and inhibits proliferation and angiogenesis through crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in colorectal cancer. Phytomedicine. 2020;68:153172.
- Han L, Zhang Y, Meng M, Cheng D, Wang C. Eicosapentaenoic acid induced SKOV-3 cell apoptosis through ERK1/2-mTOR-NF-κB pathways. Anticancer Drugs. 2016;27(7):635–642.
- Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22(11):1490–1500.
- Jiang Q, Pan Y, Cheng Y, Li H, Liu D, Li H. Lunasin suppresses the migration and invasion of breast cancer cells by inhibiting matrix metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-κB signaling pathways. Oncol Rep. 2016;36(1):253–262.
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18(1):621–663.
- Ricca A, Biroccio A, Del Bufalo D, Mackay AR, Santoni A, Cippitelli M. bcl-2 over-expression enhances NF-kappaB activity and induces mmp-9 transcription in human MCF7(ADR) breast-cancer cells. Int J Cancer. 2000;86(2):188–196.
- Liu X, Zhao W, Wang W, Lin S, Yang L. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-κB and Erk pathway. Biomed Pharmacother. 2017;92:429–436.
- Choi JH, Hwang YP, Jin SW, et al. Suppression of PMA-induced human fibrosarcoma HT-1080 invasion and metastasis by kahweol via inhibiting Akt/JNK1/2/p38 MAPK signal pathway and NF-κB dependent transcriptional activities. Food Chem Toxicol. 2019;125:1–9.
