Abstract
As a protein structurally similar to insulin, relaxin3 (RLN3) plays a role in promoting arousal, suppressing depressive or anxious behaviors. Two studies revealed the increase of RLN3 expression during chicken follicle selection. In this study, by real-time quantitative PCR and luciferase assay, mRNA expression and single nucleotide polymorphisms (SNPs) of chicken RLN3 were investigated. The mRNA expression of chicken RLN3 was higher in the granulosa cell of hierarchal follicles (Post-GCs) than that of pre-hierarchal follicles (Pre-GCs). In Pre-GCs, the mRNA expression of chicken RLN3 was stimulated by FSH and progesterone; in Post-GCs, it was stimulated by higher concentration of estrogen and FSH, however, was inhibited by progesterone. Four SNPs including g.-655G > C, g-592G > A, g.-372T > A and g.-282G > C were identified in the critical promoter region from −1291 bp to −207 bp of chicken RLN3, among which g.-655G > C, and g-592G > A were associated with age at first laying and clutch size, respectively, in Zaozhuang Sunzhi chickens. At g.-655G > C and g-592G > A, allele C and allele A had higher transcriptional activity, respectively. These data suggest that RLN3 plays an important role in chicken follicle development and SNPs in its promoter region are potential DNA markers for improving egg production traits.
Introduction
Relaxin (RLN) is a peptide hormone structurally similar to insulin that plays a pivotal role in diverse physiological activities including the facilitation of pregnancy responses, metabolic regulation, stress modulation, and energy equilibrium.Citation1–3 The human relaxin family comprises seven members,Citation3 among which RLN3 was initially identified in humans and mice in 2002Citation4 It is predominantly expressed in cerebral tissuesCitation5 and is involved in several physiological processes of stress responsiveness,Citation6 motivational behavioral regulationCitation7 and appetite control.Citation8–9 RLN3 plays a role in promoting arousal and suppressing depressive or anxious behaviors.Citation10 In addition to the function of relaxin-like protein in cerebral tissues, it is also found to be involved in reproductive regulation just prior to the divergence of amphibians.Citation11 As for chicken RLN3, changes in its expression across different developmental stages, genders, and strains,Citation12 in the gonadotropic cells of the anterior pituitary,Citation13 with nesting behavior,Citation14 with egg laying,Citation15 and factors modulating its expressionCitation16,Citation17 were reported.
Follicle selection in chickens refers to the process of choosing one small yellow follicle from the prehierarchal follicles to enter hierarchy,Citation18 which is a critical stage influencing the egg-laying performance and regulated by gonadotropins and growth factors.Citation19,Citation20 Two studies both reported the increase of RLN3 expression after chicken follicle selection: One is the proteomic analysis of granulosa cells before and after chicken follicle selection showing a higher level of RLN3 protein in the granulosa cells of the most recently recruited prehierarchal follicles,Citation21 another is our comparative transcriptomic analysis between the granulosa cells from prehierarchal and hierarchal follicle revealing a significant increase of RLN3 mRNA expression in the granulosa cells of chicken hierarchal follicles.Citation22
Based on these studies, we hypothesize that, during follicle selection in chicken, RLN3 expression is possibly affected by reproductive hormones and is tightly regulated by transcription factors by binding to cis-acting elements, and single nucleotide polymorphisms (SNPs) existing in its critical promoter region is likely associated with egg laying traits in hens. To test this hypothesis, in this study, we investigated the role of RLN3 in follicle selection by analyzing the mRNA expression characteristics of RLN3, the effect of reproductive hormones on RLN3 mRNA expression in granulosa cells before and after follicle selection, and analyzed polymorphisms in the promoter region of RLN3 and their associations with egg-laying performance in hens.
Materials and methods
Birds and tissue collection
Thirty healthy Hy-Line brown commercial laying hens at the age of 40 weeks that were sampled from a commercial egg farm affiliated to Shandong Agricultural University were used for the mRNA expression analysis of chicken RLN3 in ovarian granulosa cells. These hens were housed in separate cages under a daily light of 14 h, with free access to water and feed, and slaughtered by cervical dislocation according to the guideline of the Animal Care and Use Committee of Shandong Agricultural University (No. SDAUA-2021-097). Small white follicles with a diameter of 1-4 mm, large white follicles with a diameter of 4-6 mm and small yellow follicles with a diameter of 6-8 mm were classified as prehierarchal follicles. Hierarchal follicles include F5 to F1 follicles, ranging from 9 mm to 33 mm in diameter. They were separately collected for the preparation of granulosa cells after slaughter.
For polymorphism analysis, 44 Jinghong laying hens, 37 Jining Bairi hens and 414 Zaozhuang Sunzhi hens were sampled. Approximately 2 mL blood sample from each hen was obtained from wing vein and stored at −20 °C. Genomic DNA from blood was extracted using a DNA extraction kit (DP304, Tiangen, Beijing, China) and stored at −20 °C. A subset of 208 Zaozhuang Sunzhi chickens with egg-laying records was chosen for the association analysis between RLN3 genotypes and egg-laying traits. Egg-laying age and daily egg production were recorded for each Zaozhuang Sunzhi chicken following the onset of laying.
Primary granulosa cell culture and treatment with hormones
Chicken granulosa cells from hierarchal and prehierarchal follicles were isolated according to reference.Citation23 Briefly, the theca cell layer of hierarchal follicles and the yolk was carefully removed using ophthalmic forceps and treated at 38 °C for 15 min in 0.25% trypsin-EDTA (Gibco-BRL, NY, USA) to obtain primary granulosa cells (Post-GCs). For prehierarchal follicles, 1% Type II collagenase (Coolaber, Beijing, China) was employed for a 6 min treatment at 37 °C to prepare primary granulosa cells (Pre-GCs), and the digestion was terminated by adding an appropriate amount of M199 culture medium (Camarillo, CA, United States). Post-digestion, the liquid was filtered using a 200-mesh sieve into a sterile small beaker under a laminar flow hood. The filtrate was then transferred to a new 15 mL centrifuge tube, centrifuged at 1,800 rpm for 5 min, and the supernatant was discarded. Cell pellets were washed with PBS buffer, and after centrifugation at 1,800 rpm for 5 min, an appropriate amount of M199 culture medium was added to prepare a cell suspension. The cell suspension, with a density of 2 × 105 cells, was seeded in a 24-well plate containing 7% fetal bovine serum (VivaCell, ShangHai, China) and 1% penicillin/streptomycin in M199 medium. The cells were cultured in a constant-temperature incubator at 38 °C with 5% CO2. Upon reaching a cell density of 70-80%, the medium was replaced with M199, and granulosa cells were treated with different concentrations of estradiol (Sigma-Aldrich, Missouri, USA, 0, 5, 50, 100 nmol/L), progesterone (Sigma-Aldrich, Missouri, USA, 0, 5, 50, 100 nmol/L) or FSH (NOVUS, Colorado, USA, 0, 5, 50, 100 ng/mL) for 24 h. Cells were then harvested for quantitative analysis of RLN3 mRNA when the cell density exceeded 90%. For each treatment, three replicates were performed, the range of concentration for sex hormones was used according to reference.Citation24
Extraction of total RNA and real-time quantitative PCR (RT-qPCR)
The total RNA from pre-GCs and Post-GCs was extracted using the RNA Easy Fast Animal Tissue/Cell Total RNA Extraction Kit (DP451, Tiangen, Beijing, China), and its purity and concentration were determined using UV spectrophotometry Biophotometer plus (Eppendorf, Germany). Subsequently, the total RNA was reverse-transcribed into cDNA using the Evo M-MLV RT Mix Kit with gDNA Clean for qPCR Ver.2 (ACCURATE BIOLOGY, Changsha, China). The resultant cDNA served as a template for RT-qPCR reactions using specific primers (RLN3-F and RLN3-R, as listed in ) to detect the relative expression levels of RLN3 mRNA in Pre-GCs and Post-GCs and GAPDH was used as the housekeeping gene (primers listed in ) according to reference.Citation24 The real-time quantitative PCR reaction system consisted of 10 μL of 2× SYBR Green Pro Taq HS Premix (Vazyme, Nanjing, China), 0.4 μL each of RLN3-F and RLN3-R primers ( μL of cDNA, and 7.2 μL of ddH2O. The reaction program comprised an initial denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s and annealing at 59 °C for 30 s.
Table 1. Primers for PCR and RT-qPCR.
Table 2. Genotype and allelic frequency of four SNPs of RLN3 gene in three chicken breeds.
Luciferase assay
The 5′ promoter region of chicken RLN3 spanning from −2,888 bp to +221 bp (with +1 denoting the transcription start site) was amplified using the forward primer F1 and reverse primer R (). The PCR reaction program included an initial denaturation at 95 °C for 3 min, followed by 34 cycles of denaturation at 95 °C for 15 s, annealing at 61 °C for 15 s, extension at 72 °C for 3 min and 20 s, and a final extension at 72 °C for 6 min. The PCR products were purified and recovered after gel electrophoresis, followed by a double restriction enzyme digestion of the purified fragments. The digested products were again purified after gel electrophoresis. Finally, the chicken RLN3 promoter fragment was ligated to the previously enzyme-digested and purified pGL3-Basic luciferase gene reporter vector using Ligation Solution A (ACCURATE BIOLOGY, Changsha, China). The construct was then transformed into E. coli DH5α competent cells, and single colonies were sequenced for validation. The recombinant plasmid devoid of endotoxins was extracted. Using the full-length plasmid containing the chicken RLN3 promoter region as a template, different sizes of promoter fragments were amplified with pairs of primers F2, F3, F4, F5, F6, and R () for constructing deletion vectors of promoter region. Mutant vectors pGL3-(–655C) and pGL3-(-592G) were obtained by PCR amplification using primers Mut-F-592 and Mut-R-592, and Mut-F-655 and Mut-R-655 (), respectively, with F1 as template. The annealing temperatures for PCR are specified in . These recombinant plasmids were then transfected into chicken Post-GCs. After 24-48 h of culture, luciferase activity was measured to analyze transcriptional activity using the Dual-Luciferase Reporter Assay System according to the manufacturer’s protocol (Promega, Madison, WI, USA) for at least triplicate. The enzymatic activity of luciferase was measured with a luminometer (ModulusTM, Turner Biosystems, USA) with pGL3-basic as the control, and was calculated by dividing the Firefly luciferase activity by the Renilla luciferase activity.
Genotyping
A fragment of the chicken RLN3 promoter was amplified using primers F-1291 and F + 221 (). The PCR amplification program comprised an initial denaturation at 95 °C for 3 min, followed by 34 cycles of denaturation at 95 °C for 15 s, annealing at 61 °C for 1 min and 30 s, extension at 72 °C for 2 min, and a final extension at 72 °C for 5 min. The amplified products were subjected to 1% agarose gel electrophoresis, and specifically amplified fragments were sequenced bi-directionally with ABI3730XL as DNA analyzer (Applied Biosystems, Carlsbad, CA, USA), and aligned with DNAMAN program to determine the genotypes at four sites, namely −655, −592, −372, and −282.
Statistics
For RT-qPCR, each sample was assayed in triplicate, and the results were expressed as means ± standard error. Differences in RLN3 mRNA expression and the fluorescence enzyme activity among different promoter fragments were compared using one-way analysis of variance (ANOVA), with significance set at p < 0.05. Allele frequencies and genotype frequencies for the four SNP sites in chicken population were calculated using Microsoft Excel and Hardy-Weinberg equilibrium analysis was performed. The relationship between genotypes and egg-laying traits in Zaozhuang Sunzhi hens was analyzed using the general linear model in the SAS 9.2 statistical software package as follows: Yij =μ+ Gi+ eij, where Yij is the phenotypic value of egg laying traits, μ is the population mean of egg laying traits, Gi is the fixed effect of RLN3 genotype, and eij is the random error effect.
Results
The mRNA expression of RLN3 in chicken follicle granulosa cells
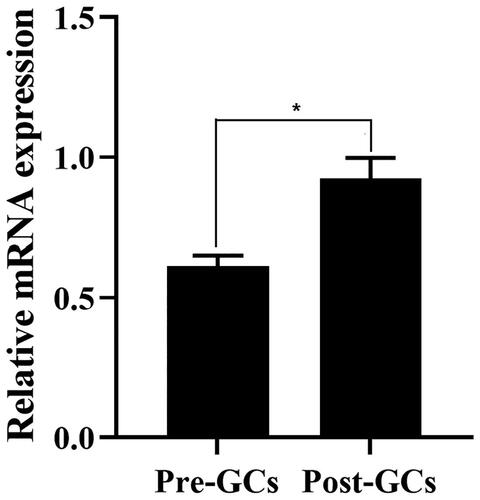
The mRNA expression of RLN3 was analyzed by RT-qPCR in chicken Pre-GCs and Post-GCs, respectively, it was significantly higher in Post-GCs than in Pre-GCs (p < 0.05) ().
Effect of reproductive hormones on the mRNA expression of RLN3 in chicken follicle granulosa cells
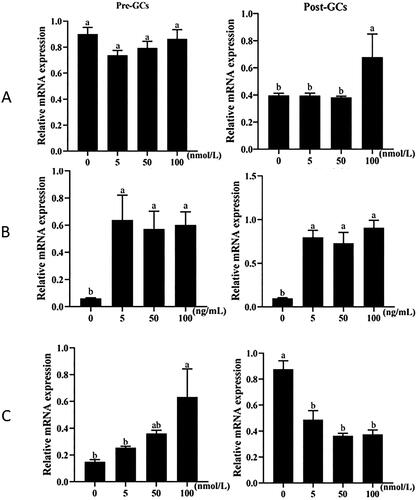
We further analyzed the effects of estradiol, follicle-stimulating hormone (FSH), and progesterone on the expression of RLN3 mRNA in chicken granulosa cells. The results indicated that, in Pre-GCs, FSH and progesterone had a significant stimulatory effect on RLN3 expression, and the effect of progesterone showed a dose-dependent response. In Post-GCs, high concentrations of estradiol could stimulate RLN3 expression, low concentrations of FSH significantly stimulated RLN3 expression, and progesterone had an inhibitory effect on RLN3 expression ().
Figure 2. Effect of estrogen (A), FSH (B) and progesterone (C) on the mRNA expression of chicken RLN3 in Pre-GCs and Post-GCs. The relative mRNA expression level of RLN3 was measured by RT-qPCR, with each sample being assayed in triplicate using GAPDH as reference and represented as means ± standard error. Bars with different superscript letters indicate significantly different (p < 0.05).
Critical promoter region of chicken RLN3 gene
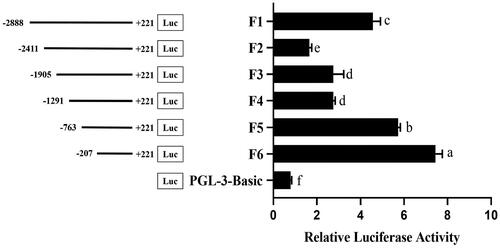
To identify the key transcriptional regulatory elements in the 5′ regulatory region of the chicken RLN3 gene, we initially cloned a fragment spanning from −2888 to +221, totaling 3019 bp. Subsequently, using different upstream primers (F2-F6, ) and the same downstream primer (R, ), we amplified fragments of varying sizes. Luciferase reporter gene vectors were constructed, and the changes in transcriptional activity were analyzed. The relative luciferase activity increased significantly from F2 to F3, F4 to F5 and F5 to F6 (p < 0.05), which suggested that the crucial regulatory regions existed in these fragments ().
Figure 3. Promoter activity analysis by Dual-Luciferase Reporter Assay System in chicken Post-GCs. Left, schematic structure of various progressive deletions in the 5′ flanking region of the chicken RLN3 gene; right, the activity of the corresponding truncated RLN3 promoters. Bars with different superscript letters indicate significantly different (p < 0.05).
Polymorphism in the critical promoter region of chicken RLN3 gene
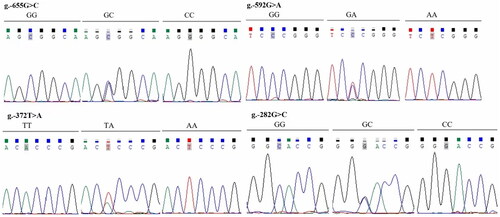
Thirty samples of genomic DNA were randomly selected from Jinghong laying hens, Jining Bairi chickens and Zaozhuang Sunzhi chickens, respectively, to prepare three DNA pools. The chicken RLN3 fragment from −1291 to +221 was amplified using the primer pair F-1291 and R + 221 and subjected to sequencing. Comparative sequence analysis revealed the presence of four SNP sites: g.-655G > C, g-592G > A, g.-372T > A, and g.-282G > A (). At these four SNP sites, all four populations deviated from Hardy-Weinberg equilibrium. Additionally, at the g-592G > A and g.-372T > A sites, the dominant alleles in Jining Bairi chickens and Zaozhuang Sunzhi chickens were significantly different from those in Jinghong laying hens ().
Association of RLN3 promoter polymorphism with chicken laying traits
Association analysis between the four polymorphic sites in the key promoter region of RLN3 and chicken egg-laying performance indicated that the two SNP sites at −655 and −592 were associated with the age at first egg and clutch size in Zaozhuang Sunzhi chickens. At the −655 site, individuals with the genotype CC started laying earlier. At the −592 site, individuals with the genotype AA had the longest clutch size (p < 0.05, ).
Table 3. Associations of the four SNPs of RLN3 with laying traits in the Zaozhuang Sunzhi chicken population.
Effect of -655 and -592 SNPs on the transcription of chicken RLN3 gene
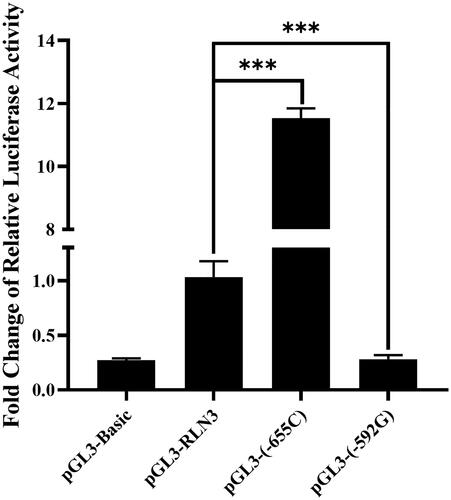
For the SNP sites at −655 and −592 in the chicken RLN3 gene, mutant luciferase reporter gene vectors were separately constructed with alleles C and G, named as pGL3-(-655C) and pGL3-(-592G), respectively. After transfection with Post-GCs, at the −655 site, the transcriptional activity of RLN3 significantly increased after the G was mutated to C (p < 0.001); while at the −592 site, the transcriptional activity of RLN3 significantly decreased after the A was mutated to G (p < 0.001) ().
Discussion
In this study, the expression of chicken RLN3, the impact of reproductive hormones on its expression and SNPs in its critical promoter region were reported for the first time. Previous study revealed that the mRNA expression of RLN3 was highest in granulosa cells of 9-12 mm follicles than in that of other chicken ovarian follicles.Citation21 We showed that after follicle selection, the expression of RLN3 in Post-GCs further increased. This suggests that RLN3 is involved in follicle selection and the development and maturation of hierarchal follicles, playing a crucial role in the process of chicken follicle development. Similarly, in Japanese quails, RLN3 mRNA expression is also detected in granulosa cells of follicles and increases with follicle development.Citation25 In mammals, RLN3 expression is detected in the mural granulosa cells of antral follicles, preovulatory follicles, and corpora lutea in pigs,Citation26 monkeys,Citation27 and humans.Citation28
As for the relationship between reproductive hormones and RLN3 expression, estrogen inhibits the expression of RLN3 in the nucleus of the type I medulla oblongata in female rats, possibly related to fluctuations in food intake during their estrous cycle.Citation29 RLN3 plays a central signaling role in connecting nutritional status and reproductive function by stimulating the hypothalamus-pituitary-gonadal axis to increase plasma LH levels.Citation30 In ducks, the expression of RLN3 is related to nesting behavior.Citation31 In male tilapia fish, the expression of RLN3a (one of RLN3 members) is a prerequisite for sperm production and fertility.Citation32 However, these studies did not analyze RLN3 expression in ovarian follicles. In chicken, we found that high concentrations of estrogen and FSH stimulated RLN3 expression in both Pre-GCs and Post-GCs, while progesterone stimulated RLN3 expression in pre-GCs and inhibited its expression in Post-GCs. Similarly, in Japanese quails, RLN3 expression is upregulated by estrogen and inhibited by progesterone and luteinizing hormone.Citation25 Follicle selection is tightly controlled by estrogen, FSH and progesterone, with FSH playing an essential role. The fact that FSH regulated RLN3 mRNA expression in both Pre-GCs and Post-GCs, and that RLN3 mRNA expression in granulosa cells increased along with follicle selection implies that RLN3 plays an important role in chicken follicle development, especially during follicle selection.
We further analyzed the transcriptional regulation of the chicken RLN3 gene and identified three critical regulatory regions and four SNP sites (g.-655G > C, g-592G > A, g.-372T > A, and g.-282G > A) within the important regulatory region from −763 to −207; and at these four SNP sites, all of the three chicken populations deviated from Hardy-Weinberg equilibrium, which suggests a role of RLN3 in affecting egg laying traits. Notably, at the g-592G > A and g.-372T > A sites, the dominant alleles in high egg-producing Jinghong laying hens were significantly different from those in indigenous chicken breeds such as Jining Bairi chickens and Zaozhuang Sunzhi chickens, which again suggested an association of these two SNPs with egg laying traits. Further analysis indicated that g.-655G > C and g-592G > A were associated with the onset of laying age and the clutch size in 208 Zaozhuang Sunzhi hens, respectively. However, due to that the effect is affected by sample size and genetic heterogeneity, further test with more individuals of Zaozhuang Sunzhi chickens and in other chicken breeds with variations at these two SNPs will testify this effect.
To explain the mechanism of these associations, luciferase assay was performed and the results indicated that at the −655 site, the transcriptional activity of allele C was significantly higher than that of allele G, while at the −592 site, the transcriptional activity of allele A was significantly higher than that of allele G. At these two SNPs, haplotype C-A is expected to increase RLN3 mRNA expression by affecting the binding of transcription factors, such as GR-β and Pax-5, which requires further investigations. The effect of RLN3 on these two egg laying traits is consistent with the study on changes in RLN3 protein during follicle selection,Citation18 which may act by promoting the differentiation or proliferation of granulosa cells.
The selection of individuals with these two alleles could contribute to improving the onset of laying age and clutch size in Zaozhuang Sunzhi chickens. The onset of laying age reflects the timing of sexual maturity in chickens, and breeding early-maturing lines as germplasm may help improve early maturity in both meat and egg production chickens. Compared to high egg-producing breeds, indigenous chicken breeds generally have lower egg production levels due to their relatively shorter peak laying period. By selectively breeding to improve the continuous laying ability of local chicken breeds, i.e., clutch size, it is possible to enhance their egg production. The onset of laying age and the clutch size in chicken are also controlled by other genes and environmental factors, different genetic background may cause variations in genetic effect of these two SNPs at −655 and −592 in other chicken breed, and therefore, it is suggested to test the effect of these two SNPs of RLN3 first before using them in breeding.
Conclusion
This study revealed that the mRNA expression of chicken RLN3 increased in the granulosa cells of ovarian follicles after selection and was differentially affected by reproductive hormones. The two SNP sites at −655 and −592 in the critical regulatory region of chicken RLN3 gene were associated with the age at first laying and clutch size of Zaozhuang Sunzhi chickens and affected its transcriptional activity, which are potential DNA markers for improving egg laying performance in hens. These data collectively indicated that RLN3 is an important factor affecting chicken follicle development and egg performance.
Author’s contributions
Yunliang Jiang conceived and designed the study. Chunfeng Zhang performed all the experiments. Li Kang and Yi Sun analyzed the data. Yunliang Jiang wrote and revised the manuscript.
Institutional review board statement
The animal procedures used in this study were reviewed and approved by the Shandong Agricultural University’s Academic Committee on the animal welfare requirements (No. SDAUA-2021-097).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- van der Westhuizen ET, Halls ML, Samuel CS, et al. Relaxin family peptide receptors–from orphans to therapeutic targets. Drug Discov Today. 2008;13(15-16):1–9.
- Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93(1):405–480.
- Patil NA, Rosengren KJ, Separovic F, Wade JD, Bathgate RAD, Hossain MA. Relaxin family peptides: structure-activity relationship studies. Br J Pharmacol. 2017;174(10):950–961.
- Bathgate RA, Samuel CS, Burazin TC, et al. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem. 2002;277(2):1148–1157.
- Smith CM, Ryan PJ, Hosken IT, Ma S, Gundlach AL. Relaxin-3 systems in the brain–the first 10 years. J Chem Neuroanat. 2011;42(4):262–275.
- McGowan BM, Minnion JS, Murphy KG, et al. Relaxin-3 stimulates the neuro-endocrine stress axis via corticotrophin-releasing hormone. J Endocrinol. 2014;221(2):337–346.
- Ryan PJ, Kastman HE, Krstew EV, et al. Relaxin-3/RXFP3 system regulates alcohol-seeking. Proc Natl Acad Sci U S A. 2013;110(51):20789–20794.
- Smith CM, Chua BE, Zhang C, et al. Central injection of relaxin-3 receptor (RXFP3) antagonist peptides reduces motivated food seeking and consumption in C57BL/6J mice. Behav Brain Res. 2014;268:117–126.
- Calvez J, de Ávila C, Matte LO, Guèvremont G, Gundlach AL, Timofeeva E. Role of relaxin-3/RXFP3 system in stress-induced binge-like eating in female rats. Neuropharmacology. 2016;102:207–215.
- Wong WLE, Dawe GS, Young AH. The putative role of the relaxin-3/RXFP3 system in clinical depression and anxiety: A systematic literature review. Neurosci Biobehav Rev. 2021;131:429–450.
- Wilkinson TN, Speed TP, Tregear GW, Bathgate RA. Evolution of the relaxin-like peptide family: from neuropeptide to reproduction. Ann N Y Acad Sci. 2005;1041:530–533.
- Delfino KR, Southey BR, Sweedler JV, Rodriguez-Zas SL. Genome-wide census and expression profiling of chicken neuropeptide and prohormone convertase genes. Neuropeptides. 2010;44(1):31–44.
- Zhang J, Lv C, Mo C, et al. Single-cell RNA sequencing analysis of chicken anterior pituitary: a bird’s-eye view on vertebrate pituitary. Front Physiol. 2021;12:562817.
- Shen X, Bai X, Xu J, et al. Transcriptome sequencing reveals genetic mechanisms underlying the transition between the laying and brooding phases and gene expression changes associated with divergent reproductive phenotypes in chickens. Mol Biol Rep. 2016;43(9):977–989.
- Sah N, Kuehu DL, Khadka VS, et al. RNA sequencing-based analysis of the magnum tissues revealed the novel genes and biological pathways involved in the egg-white formation in the laying hen. BMC Genomics. 2021;22(1):318.
- Higgins SE, Ellestad LE, Trakooljul N, et al. Transcriptional and pathway analysis in the hypothalamus of newly hatched chicks during fasting and delayed feeding. BMC Genomics. 2010;11:162.
- Lv C, Zheng H, Jiang B, et al. Characterization of relaxin 3 and its receptors in chicken: evidence for relaxin 3 acting as a novel pituitary hormone. Front Physiol. 2022;13:1010851.
- Johnson PA. Follicle selection in the avian ovary. Reprod Domest Anim. 2012;47(Suppl 4):283–287.
- Onagbesan O, Bruggeman V, Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim Reprod Sci. 2009;111(2-4):121–140.
- Johnson AL. Regulation of follicle differentiation by gonadotropins and growth factors. Poult Sci. 1993;72(5):867–873.
- Ghanem K, Johnson AL. Proteome profiling of chicken ovarian follicles immediately before and after cyclic recruitment. Mol Reprod Dev. 2021;88(8):571–583.
- Li D, Zhong C, Sun Y, Kang L, Jiang Y. Identification of genes involved in chicken follicle selection by ONT sequencing on granulosa cells. Front Genet. 2023;13:1090603.
- Chen Q, Wang Y, Liu Z, et al. Transcriptomic and proteomic analyses of ovarian follicles reveal the role of VLDLR in chicken follicle selection. BMC Genomics. 2020;21(1):486.
- Gong Y, Li D, Sun Y, Kang L, Jiang Y. Expression and regulation of Noggin4 gene in chicken ovarian follicles and its role in the proliferation and differentiation of granulosa cells. Theriogenology. 2023;212:83–90.
- Hoang KX, Matsuzaki M, Kohsaka T, Sasanami T. Expression of relaxin 3 in the ovarian follicle of Japanese quail. J Poult Sci. 2023;60:2023025.
- Ferrara N, Chen H, Davis-Smyth T, et al. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med. 1998;4(3):336–340.
- Gunnersen JM, Crawford RJ, Tregear GW. Expression of the relaxin gene in rat tissues. Mol Cell Endocrinol. 1995;110(1-2):55–64.
- Chassin D, Laurent A, Janneau JL, Berger R, Bellet D. Cloning of a new member of the insulin gene superfamily (INSL4) expressed in human placenta. Genomics. 1995;29(2):465–470.
- de Ávila C, Chometton S, Calvez J, et al. Estrous cycle modulation of feeding and relaxin-3/Rxfp3 mRNA expression: implications for estradiol action. Neuroendocrinology. 2021;111(12):1201–1218.
- McGowan BM, Stanley SA, Donovan J, et al. Relaxin-3 stimulates the hypothalamic-pituitary-gonadal axis. Am J Physiol Endocrinol Metab. 2008;295(2):E278–286.
- Ye P, Ge K, Li M, et al. Egg-laying and brooding stage-specific hormonal response and transcriptional regulation in pituitary of Muscovy duck (Cairina moschata). Poult Sci. 2019;98(11):5287–5296.
- Yang L, Li Y, Wu Y, et al. Rln3a is a prerequisite for spermatogenesis and fertility in male fish. J Steroid Biochem Mol Biol. 2020;197:105517.