Abstract
This article examines rates of nonmedical use and diversion of extended-release amphetamine and extended-release oral methylphenidate in the United States. Prescription dispensing data were sourced from retail pharmacies. Nonmedical use data were collected from the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS) System Drug Diversion Program and Poison Center Program. Drug diversion trends nearly overlapped for extended-release amphetamine and extended-release oral methylphenidate. Calls to poison centers were generally similar; however, calls regarding extended-release amphetamine trended slightly lower than those for extended-release oral methylphenidate. Data suggest similar diversion and poison center call rates for extended-release amphetamine and extended-release oral methylphenidate.
BACKGROUND
Prescription stimulants have been recommended as first-line medications in the pharmacologic treatment of attention-deficit hyperactivity disorder (ADHD).Citation 1 These medications include methylphenidate and amphetamine and they are available in traditional immediate-release formulations and in modified- or extended-release formulations (oral tablets and capsules, a methylphenidate transdermal patch, and an amphetamine prodrug), which allow for less frequent dosing. With all of these medications, the potential exists for nonmedical use (the use of prescription medications in a manner inconsistent with the prescribed indication and dosing), and ADHD stimulants are classified as Schedule II controlled substances in the United States based on their potential for abuse, dependence, and individual and public health harm, which is consistent with the Convention on Psychotropic Substances 1971.Citation 2
In this article, we focus on post-marketing surveillance in the United States for rates of abuse, misuse, and diversion of ADHD medications because extensive data are available. Time trends of abuse, misuse, and diversion of extended-release amphetamine and extended-release oral methylphenidate are compared because these products are commonly prescribed in the United States and because of interest in extended-release formulations as a possible mechanism to improve adherence among patients. Data are presented from the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS) System, a national surveillance system that monitors the abuse, misuse, and diversion of prescription controlled substances (e.g., pharmaceutical medicines with psychoactive properties).Citation 3 We are not aware of any published studies using national data sources that measure rates of nonmedical use of stimulants by formulation in the post-marketing setting.
METHODS
For the purposes of this article, diversion is defined as the unlawful channeling of regulated pharmaceuticals from legal sources to the illicit marketplace.Citation 4 Use is defined as any use of the drug, with no restrictions as to purpose or legality. Nonmedical use includes both abuse and misuse. Abuse is defined as the intentional improper or incorrect use of a drug for the purposes of experiencing psychotropic effects (e.g., to get high) that may cause harm to the user or others; conversely, misuse is the intentional improper or incorrect use of a substance for reasons other than to get high (such as taking an additional dose without consulting with a prescriber because an individual believes that his or her symptoms were not adequately controlled).Citation 5 The reporting period of quarter 3 of 2007 through quarter 2 of 2011 was chosen because RADARS first began collecting information on prescription stimulants in quarter 3 of 2007 and the most recent data available at the time of this study was from quarter 2 of 2011.
Stimulant Prescribing
The IMS Health National Prescription Audit (NPA) is a standard data commercial source that measures the retail outflow, or retail outpatient dispensing, of prescriptions in the United States, which represents the rate at which drugs move out of independent, chain, and food store retail pharmacies into the hands of consumers. According to estimates by IMS Health,Citation 6 approximately 55% of all prescriptions dispensed in the United States (including Alaska and Hawaii) are accounted for by independent, chain, and food store retail pharmacies.
The NPA sample is updated on a monthly basis and consists of approximately 36,000 to 38,000 randomly selected stores. In 2011, the sample consisted of 38,326 retail pharmacies, including 22,526 chain pharmacy stores, 8,206 independent pharmacy stores, and 7,594 food store pharmacies. This sample was drawn from 56,996 pharmacies (29,528 chain pharmacies, 17,701 independent pharmacies, and 9,767 food store pharmacies). The estimated number of dispensed prescriptions was projected nationally from this sample using proprietary methods linked to a unique patient identifier. From July 1, 2007, through June 30, 2011, the total number of U.S. prescriptions dispensed for all prescription extended-release amphetamine products and all extended-release oral methylphenidate products () were calculated by quarter.
TABLE 1 Extended-Release Amphetamine and Oral Methyl- phenidate Prescription Products in IMS NPA
Measurement of Abuse, Misuse, and Diversion of Prescription Stimulants
The RADARS System is a national surveillance system that monitors the abuse, misuse, and diversion of prescription pharmaceutical products. In this article, data are used from the RADARS System Drug Diversion and Poison Center Programs. The other RADARS System programs focus their data collection on opioids and were unable to collect data on prescription stimulants.Citation 3 The RADARS System collects and reports data on a quarterly basis with geographic specificity at the 3-digit ZIP code (U.S. postal code) level throughout the United States. There are 929 3-digit ZIP codes in the United States. Data used in this analysis were obtained from quarter 3 of 2007 through quarter 2 of 2011.
The RADARS System Drug Diversion Program is composed of prescription drug diversion investigators or state regulatory agencies that submit data quarterly and report the number of new diversion cases investigated in that quarter. In 2007, there was a quarterly average of 244 reporting investigators or agencies out of 301 participating investigators or agencies (covering an average of 486 3-digit ZIP codes per quarter), 250 of 308 in 2008 (554 3-digit ZIP codes), 256 of 299 in 2009 (511 3-digit ZIP), and 242 of 283 in 2010 (487 3-digit ZIP codes). In quarter 1 and 2 of 2011, there were 229 of 264 (458 3-digit ZIP codes) and 220 of 264 (458 3-digit ZIP codes) reporting investigators or agencies, respectively. Variability in represented 3-digit ZIP codes over time is a result of reporter jurisdiction changes and consistency of survey submissions. Cases are defined as the number of new instances of pharmaceutical diversion reported to or investigated by the diversion unit or regulatory board during the previous quarter. Cases must be formally initiated during the quarter and documented by a written complaint or report. Cases are assigned to the 3-digit ZIP code where the case occurred or, when the 3-digit ZIP code where the case occurred is not specified, cases are distributed across the 3-digit ZIP codes in the informant's jurisdiction. The reporters are requested to give as much detail as possible on the formulations and brands of the products involved in the cases. More detailed descriptions of the Drug Diversion Program have been published previously.Citation 3 , Citation 4 , Citation 7
The RADARS System Poison Center Program is composed of participating U.S. poison centers, which choose to participate in the RADARS System. These poison centers send data on cases involving RADARS System drugs of interest on a weekly basis to a central database maintained by the RADARS System. For 2007, an average of 43 poison centers participated per quarter, with 40 of those poison centers reporting per quarter (covering an average of 675 3-digit ZIP codes per quarter), 45 of 46 in 2008 (736 3-digit ZIP codes), 48 of 48 in 2009 (788 3-digit zip codes), and 49 of 49 in 2010 (789 3-digit ZIP codes); in quarter 1 and 2 of 2011, 47 of 47 poison centers (788 3-digit ZIP codes) reported. Spontaneous reports from the public, health care professionals, and other public safety professionals received by poison centers (for which the sampling frame is unspecified) are answered by nurses and pharmacists who have training in clinical toxicology and have passed a national certification examination.Citation 8 Each case is recorded in nationally standardized electronic health records and subject to quality control processes to verify the identity of the drugs involved and ensure coding accuracy. For the purposes of this analysis, cases are defined as any human intentional exposure call managed by participating poison centers involving extended-release amphetamine or extended-release oral methylphenidate.
Intentional exposures are used as surrogates for abuse and misuse and are composed of the following categories, which have standardized definitions within the poison center data collection system: suicide, intentional misuse, abuse, intentional unknown, and withdrawal cases.Citation 5 Cases are assigned to the reported 3-digit ZIP code of the exposed individual's residence. A medical outcome category assessing the effects of the exposure is assigned to each case. Moderate effect medical outcomes are defined as exposures in which “the patient exhibited symptoms as a result of the exposure which are more pronounced, more prolonged or more of a systemic nature” but not life-threatening or otherwise permanently disabling, relative to minimally bothersome minor effects.Citation 5 Major effect medical outcomes are defined as life-threatening effects or those leading to “significant residual disability or disfigurement.”Citation 5 More detailed descriptions of the Poison Center Program have been published elsewhere.Citation 9 – Citation 11
RADARS System rates are calculated per 100,000 individuals and per 1,000 unique recipients of a dispensed drug (URDD) in a 3-digit ZIP code. Population and URDD values for each program were assigned based on U.S. Census data and reports from SDI, a healthcare analytics firm (Plymouth Meeting, PA). The interpretation of population rates per 100,000 individuals provides a measure of the absolute burden on public health incurred from the abuse, misuse, and diversion of the drug, standardized for differences in population. The URDD rate provides a drug-specific estimate of unintended adverse consequences of the drug based on the amount of the drug available in the community. Each filling of a drug prescription in a given quarter is counted as a single URDD, regardless of the number of prescriptions received during that time. The use of URDD to calculate rates has been described elsewhere.Citation 12 , Citation 13 Drug diversion and intentional exposure rates per population and per URDD were calculated for extended-release amphetamines and extended-release oral methylphenidate using case counts from the Drug Diversion and Poison Center Programs, respectively. Population and URDD values are assigned to the 3-digit ZIP codes in which the cases occurred. Rates are plotted over time using the calculated rate corresponding to each calendar year quarter (quarter 3 of 2007 to quarter 2 of 2011).
FIGURE 1 ADHD prescribing information for ER amphetaminea and ER oral methylphenidateb, IMS, Q3 of 2007 through Q2 of 2011.
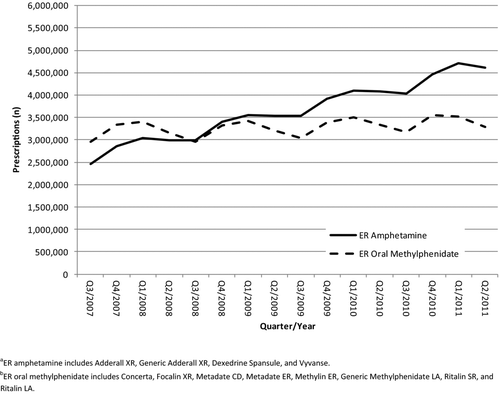
TABLE 2 RADARS System Drug Diversion Program: Total Extended-Release Amphetamine and Oral Methylphenidate Rates
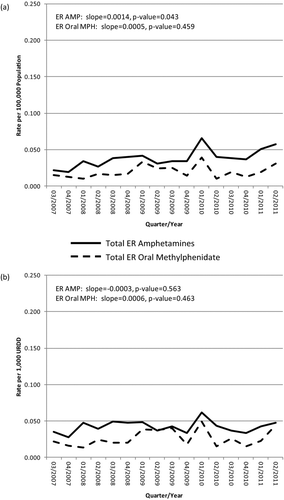
FIGURE 2 RADARS System Drug Diversion Program: Total ER amphetamine and total ER oral methylphenidate rates per (a) 100,000 population and (b) per 1,000 URDD, all sites, Q3 of 2007 through Q2 of 2011.
Graphs of rates over time were created, and 95% Poisson confidence limits were also calculated, along with mean rates (μ), for extended-release oral methylphenidate and extended-release amphetamines for both Drug Diversion and Poison Center data using SAS Enterprise Guide 4.2 (SAS Institute, Inc., Cary, NC). A simple linear regression model of the rate versus time (allowing for autocorrelation via an AR[1] error structure) is fit to the data. The slope of the regression line indicates the expected increase (if positive) or decrease (if negative) in rates per year quarter. The trend models do not include the 2 initial quarters of collected data to account for potential effects of increasing prescription rates following the introduction of a product to market or the addition of a product to RADARS System surveillance. These slopes, as well as their corresponding p-values, are reported.
The protocols for data collection and analysis in the RADARS System have been reviewed by the institutional review boards governing Nova Southeastern University (formerly University of Delaware) (RADARS System Drug Diversion Program) and the Denver Health and Hospital Authority (RADARS System Poison Center Program). Arrest data reported through the Drug Diversion Program are publicly available and are de-identified. In addition, all participating poison centers obtained institutional review board approval to participate in the RADARS System.
RESULTS
Prescribing of Extended-Release Amphetamines and Extended-Release Oral Methylphenidate
Beginning in quarter 3 of 2007, prescriptions for extended-release amphetamines have been trending steadily upward (2,460,411 in quarter 3 of 2007 to 4,618,528 in quarter 2 of 2011) (). Over the same time period, prescriptions for extended-release oral methylphenidate increased only slightly (2,957,314 in quarter 3 of 2007 to 3,291,121 in quarter 2 of 2011). Over the specified timeframe, prescriptions for extended-release amphetamine increased 87.7%, whereas those for extended-release oral methylphenidate increased 11.3 %.
Drug Diversion
Drug Diversion population-based rates for extended-release amphetamines ranged from 0.019 to 0.065 per 100,000 persons (μ = 0.038 per 100,000 persons) across the 16 quarters; URDD rates ranged from 0.028 to 0.062 per 1,000 URDD (μ = 0.042 per 1,000 URDD) (). Drug Diversion population-based rates for extended-release oral methylphenidate ranged from 0.010 to 0.039 per 100,000 persons (μ = 0.019 per 100,000 persons); URDD rates ranged from 0.014 to 0.049 per 1,000 URDD (μ = 0.026 per 1,000 URDD) (). shows Drug Diversion population rates over time, while displays Drug Diversion URDD rates over time.
Plotted over time, the estimated trend in diversion population rates for extended-release amphetamines shows a slight but significant increase (slope = 0.0014, P = .043), whereas no meaningful trends were observed in the estimated diversion URDD rates for extended-release amphetamines (slope = –0.0003, P = .563). The estimated trend in diversion population rates for extended-release oral methylphenidate shows a nonsignificant increase over time (slope = 0.0005, P = .459), as does the estimated trend in diversion URDD rates for extended-release oral methylphenidate (slope = 0.0006, P = .463). The rates for the two formulations diverge no more than 0.034 per 100,000 population or per 1,000 URDD in any given quarter.
TABLE 3 RADARS System Poison Center Program: Total Extended-Release Amphetamine and Oral Methylphenidate Rates
Poison Center Data
Poison Center intentional exposure rates per population for extended-release amphetamines ranged from 0.084 to 0.204 per 100,000 persons (μ = 0.143 per 100,000 persons); URDD rates ranged from 0.132 to 0.182 per 1,000 URDD (μ = 0.156 per 1,000 URDD) (). Intentional exposure rates per population for extended-release oral methylphenidate ranged from 0.144 to 0.194 per 100,000 persons (μ = 0.168 per 100,000 individuals); URDD rates ranged from 0.197 to 0.254 per 1,000 URDD (μ = 0.225 per 1,000 URDD) (). shows Poison Center intentional exposure population rates over time, whereas displays Poison Center intentional exposure URDD rates over time.
FIGURE 3 RADARS System Poison Center Program: Total ER amphetamine and total ER oral methylphenidate rates per (a) 100,000 population and (b) per 1,000 URDD, all sites, Q3 of 2007 through Q2 of 2011.
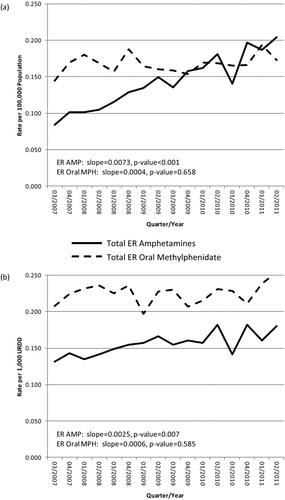
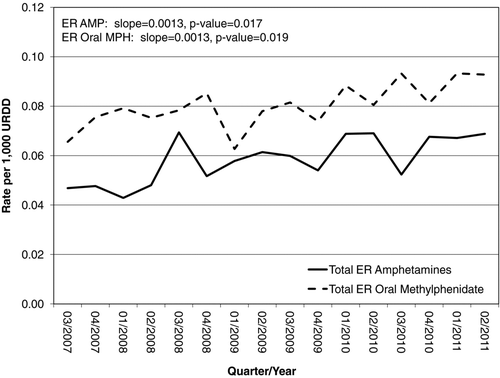
Plotted over time, the estimated trend in intentional exposure population rates for extended-release amphetamines shows a significant increase (slope = 0.0073, P < .001); similarly, the estimated trend in intentional exposure URDD rates for extended-release amphetamines shows a significant increase over time (slope = 0.0025, P = .007). The estimated trend in intentional exposure population rates for extended-release oral methylphenidate is flat and shows a nonsignificant increase over time (slope = 0.0004, P = .658), as does the estimated trend in intentional exposure URDD rates for extended-release oral methylphenidate (slope = 0.0006, P = .585). The greatest difference between extended-release oral methylphenidate and extended-release amphetamine rates per 100,000 individuals or per 1,000 URDD in any given quarter was 0.098.
As shown in , rates per 1,000 URDD for intentional exposure calls that resulted in moderate or major medical outcomes are generally slightly greater for, although comparable with, extended-release oral methylphenidate than for extended-release amphetamines. URDD rates for intentional exposures resulting in moderate or major medical outcomes for extended-release oral methylphenidate ranged from 0.063 to 0.093 per 1,000 URDD (μ = 0.080 per 1,000 URDD), whereas those for extended-release amphetamines ranged from 0.043 to 0.069 per 1,000 URDD (μ = 0.058 per 1,000 URDD). Plotted over time, the estimated trends in URDD rates for both extended-release amphetamines (slope = 0.0013, P = .017) and extended-release oral methylphenidate (slope = 0.0013, P = .019) are nearly parallel and increasing significantly.
DISCUSSION
Our findings suggest that the risk for nonmedical use and diversion is similar between extended-release amphetamine and extended-release oral methylphenidate. By using U.S. poison center data that distinguish extended-release amphetamine and extended-release oral methylphenidate and analyzing data in the context of availability of drug, our research builds on earlier studies that examined prescription stimulant exposures.Citation 14 , Citation 15 Similarly, our research is a useful context for previously published studies reporting survey-based data on the proportion of prescription stimulant abuse attributed to immediate- and extended-release formulations; these previous studies were not designed to investigate specific formulations of stimulants or the relationship with availability based on prescriptions.Citation 16 , Citation 17
Prescriptions for both extended-release formulations have increased over time, albeit at a faster rate for extended-release amphetamine. Concurrently, availability adjusted rates of nonmedical use and diversion for both extended-release amphetamine and extended-release oral methylphenidate have remained comparatively level over the past 4 years relative to population and available drug. In the Drug Diversion study, the estimated population trend for extended-release amphetamine shows a mildly significant increase over time, but when taking product availability into account (see URDD rate), no meaningful trends are apparent. In the Poison Center study, the estimated population and URDD trend rates for extended-release amphetamine show a significant increase over time. However, when taking product availability into account, URDD trend rates for extended-release amphetamines are below extended-release oral methylphenidate throughout the timeframe presented. Given the overall low rates, the narrow differences between extended-release amphetamine and extended-release oral methylphenidate are not clinically meaningful. For example, although there has been general growth in nonmedical use and diversion of prescription stimulants, rates are much lower than for opioids, which vary within the class, based on previous research with the same data sources (i.e., the RADARS Drug Diversion and Poison Center Programs).Citation 10 , Citation 12
The growth in prescriptions is not surprising given the increased diagnosis of ADHD among children and adults, resulting in an expansion of the indicated patient population for ADHD prescription stimulants. Parent-reported ADHD lifetime diagnosis of 4–17 year olds increased from 7.8% to 9.5% between 2003 and 2007.Citation 18 Based on a nationally representative survey conducted from 2001 to 2003 that determined ADHD using diagnostic criteria, prevalence among adults has been estimated at 4.4%.Citation 19
Two rates are presented in the current article that should be considered simultaneously. The interpretation of the population rate (e.g., cases per 100,000 individuals) is the absolute burden on public health incurred from the abuse, misuse, and diversion of the drug. The interpretation of the URDD rate is the drug-specific unintended adverse consequences of medical availability, accounting for the amount of drug in outpatient medical use. Both rates must be used in tandem to achieve a full picture of the public health consequences.
Similarly, law enforcement and health outcomes can be used simultaneously because together they provide perspective on the effect of nonmedical use of ADHD stimulants on public health, are useful to inform policy decisions about interventions, and support decision making by law enforcement about resource allocation. The RADARS System Drug Diversion and Poison Center Programs provide an opportunity to explore nonmedical stimulant use across law enforcement and health settings. Although there is a broader debate about the balance between a health-oriented and punitive approach to drug policy interventions, we suggest that both indicators should be examined jointly to derive a broader understanding of the unintended consequences of prescription stimulant availability.
Our results show that there is misuse, abuse, and diversion of extended-release prescription stimulants that cannot be ignored. However, the rates are low, with little difference in rates between extended-release amphetamine and extended-release methylphenidate. Continuing to monitor these rates over time will provide important information on the public health effect of these products and guide the initiation of mitigation strategies to address their misuse, abuse, and diversion.
Limitations
Study limitations extending from two types of potential confounding are inherent to the data sources available. First, individuals exposed to extended-release oral methylphenidate may be inherently different from those exposed to extended-release amphetamines, making comparisons between the two populations difficult (also known as confounding by indication or channeling).Citation 20 In the case of comparing two controlled substances for similar indications, if one drug is perceived to have lower abuse liability (historically, scientifically, or by word-of-mouth), clinicians may prescribe that drug preferentially to high-risk patients, who would be more likely to abuse it than a population where abuse risk was more heterogeneously distributed, and patients and the general population may similarly be more likely to use it nonmedically.Footnote i It would be a useful follow-up study to compare use and abuse risk patterns among patients prescribed the two medications. The second type of potential confounding arises from the lack of control of external factors that may influence the observed changes in abuse and diversion rates over time. Observational time-series analyses may be subject to influences not measured in this study.
Cases in the Drug Diversion Program may represent a broad range of law enforcement events; one case may represent a single pill or a pharmacy robbery involving hundreds or thousands of pills. Law enforcement reporting is also subject to available resources within reporting jurisdictions and the prioritization of prescription drug diversion investigations. Furthermore, national coverage for the Drug Diversion Program is influenced by both the number of informants who report during any given quarter, which varies over time, and slight variations in covered jurisdictions, which results in 3-digit ZIP code coverage fluctuations.
Poison centers are spontaneous reporting systems, which gives rise to problems of understanding how complete a representation of all exposure cases was observed. It is unclear whether the variations observed in this study can be accounted for by known causes for changes in poison center use.Citation 21 , Citation 22 However, in such scenarios, poison center use would have to have led to differential reporting between the drugs to affect changes in the conclusion. Inclusion of only RADARS System poison centers affects the generalizability of our findings. However, the 45 states (as of quarter 2 of 2011) represented in this analysis include some of the largest metropolitan areas in the country, as well as states with a considerable rural population. Collectively, cases could have arisen from approximately 85.9% of the U.S. population covered by the poison centers in this analysis. Although site selection was originally directed to include states with substantial rural areas, we believe that the final sample included a reasonable mix of metropolitan and non-metropolitan areas.
Statistical testing was conducted to identify the presence of linear trending in the observed phenomena. The appropriateness of the assumption of a linear effect over time (even accounting for autocorrelation) can be questioned. However, our previous experience with poison center and drug diversion data suggest that this is an appropriate assumption for other pharmaceutical controlled substances at this stage of market maturity.Citation 3 , Citation 7 When rates over time were inspected empirically ( and ), linear trend was a natural choice for testing.
This study focused on the rates of nonmedical use and diversion of extended-release amphetamine and extended-release oral methylphenidate in the United States because these products are commonly prescribed in the United States and because of interest in extended-release formulations as a possible mechanism to improve adherence among patients. The data show that the nonmedical use and diversion of prescription extended-release amphetamine and extended-release oral methylphenidate are low and similar; however, our analysis did not disaggregate the individual contributory effects of particular formulations on the observed chemical substance level effects. Conducting a similar analysis of specific products within each drug class might be of future interest.
Acknowledgments
The authors thank Dr. Saul Shiffman, Pinney Associates, Pittsburgh, PA, USA, for his contributions to the development of the concept.
Pinney Associates, funded by Shire Development Inc., wrote and edited this manuscript together with Ms. Buchholtz. Shire Development Inc. was involved in the concept development and fact checking, approved the manuscript, and purchased RADARS® System reports. At the time of the initial writing of this manuscript, Ms. Buchholtz was an employee of Denver Health and Hospital Authority, which operates the RADARS System. Ms. Ertischek, Mr. Sembower, and Dr. Schnoll are employees of Pinney Associates and have consulted for Shire Development, Inc. Mr. Dasgupta is a paid consultant to Pinney Associates and has consulted for Shire Development, Inc. through Pinney Associates. Mr. Dasgupta and Dr. Schnoll advise Rocky Mountain Poison & Drug Center, a department of Denver Health and Hospital Authority.
Notes
1. The prescribing information for all prescription stimulants for the treatment of ADHD have a black box warning about misuse, abuse, and dependence.
REFERENCES
- Kutcher , S , Aman , M Brooks , S J . 2004 . International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions . Eur Neuropsychopharmacol , 14 : 11 – 28 .
- Kollins , S H . 2007 . Abuse liability of medications used to treat attention-deficit/hyperactivity disorder (ADHD) . Am J Addict , 16 ( Suppl 1 ) : 35 – 42 .
- Cicero , T J , Dart , R C , Inciardi , J A , Woody , G E , Schnoll , S and Muñoz , A . 2007 . The development of a comprehensive risk-management program for prescription opioid analgesics: researched abuse, diversion and addiction-related surveillance (RADARS) . Pain Med , 8 : 157 – 70 .
- Inciardi , J A , Surratt , H L , Lugo , Y and Cicero , T J . 2007 . The diversion of prescription opioid analgesics . Law Enforc Exec Forum , 7 : 127 – 141 .
- American Association of Poison Control Centers . 2007 . “ AAPCC National Poison Data System (NPDS) ” . In Reference Manual Washington, DC: American Association of Poison Control Centers
- IMS Health . 2011 . National Prescription Audit (NPA) Basic Data Report, Q2 2011 , Plymouth Meeting, PA: IMS Health Incorporated .
- Inciardi , J A , Cicero , T J Munoz , A . 2006 . The diversion of Ultram, Ultracet, and generic tramadol HCL . J Addict Dis , 25 : 53 – 8 .
- Watson , W A , Litovitz , T L Belson , M G . 2005 . The Toxic Exposure Surveillance System (TESS): risk assessment and real-time toxicovigilance across United States poison centers . Toxicol Appl Pharmacol , 207 : 604 – 10 .
- Bailey , J E , Barton , P L , Lezotte , D , Lowenstein , S R and Dart , R C . 2006 . The effect of FDA approval of a generic competitor to OxyContin (oxycodone HCl controlled-release) tablets on the abuse of oxycodone . Drug Alcohol Depend , 84 : 182 – 7 .
- Hughes , A A , Bogdan , G M and Dart , R C . 2007 . Active surveillance of abused and misused prescription opioids using poison center data: a pilot study and descriptive comparison . Clin Toxicol (Phila) , 45 : 144 – 51 .
- Smith , M Y , Dart , R Hughes , A . 2006 . Clinician validation of Poison Control Center (PCC) intentional exposure cases involving prescription opioids . Am J Drug Alcohol Abuse , 32 : 465 – 78 .
- Dasgupta , N , Bailey , E J Cicero , T . 2010 . Post-marketing surveillance of methadone and buprenorphine in the United States . Pain Med , 11 : 1078 – 91 .
- Smith , M Y , Schneider , M F , Wentz , A , Hughes , A , Haddox , J D and Dart , R . 2007 . Quantifying morbidity associated with the abuse and misuse of opioid analgesics: a comparison of two approaches . Clin Toxicol (Phila) , 45 : 23 – 30 .
- Forrester , M B . 2006 . Methylphenidate abuse in Texas, 1998–2004 . J Toxicol Environ Health A , 69 : 1145 – 53 .
- Forrester , M B . 2007 . Adderall abuse in Texas, 1998–2004 . J Toxicol Environ Health A , 70 : 658 – 64 .
- Novak , S P , Kroutil , L A , Williams , R L and Van Brunt , D L . 2007 . The nonmedical use of prescription ADHD medications: results from a national Internet panel . Subst Abuse Treat Prev Policy , 2 : 32
- Wilens , T E , Gignac , M , Swezey , A , Monuteaux , M C and Biederman , J . 2006 . Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications . J Am Acad Child Adolesc Psychiatry , 45 : 408 – 14 .
- Centers for Disease Control and Prevention . 2010 . Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007 . Morbidity and Mortality Weekly Report , 59 : 1439 – 43 .
- Kessler , R C , Adler , L Barkley , R . 2006 . The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication . Am J Psychiatry , 163 : 716 – 23 .
- Signorello , L B , McLaughlin , J K , Lipworth , L , Friis , S , Sørensen , H T and Blot , W J . 2002 . Confounding by indication in epidemiologic studies of commonly used analgesics . Am J Ther , 9 : 199 – 205 .
- LoVecchio , F , Katz , K , Watts , D and Pitera , A . 2004 . Media influence on Poison Center call volume after 11 September 2001 . Prehosp Disaster Med , 19 : 185
- Krenzelok , E P and Mrvos , R . 2003 . Initial impact of toll-free access on poison center call volume . Vet Hum Toxicol , 45 : 325 – 7 .