ABSTRACT
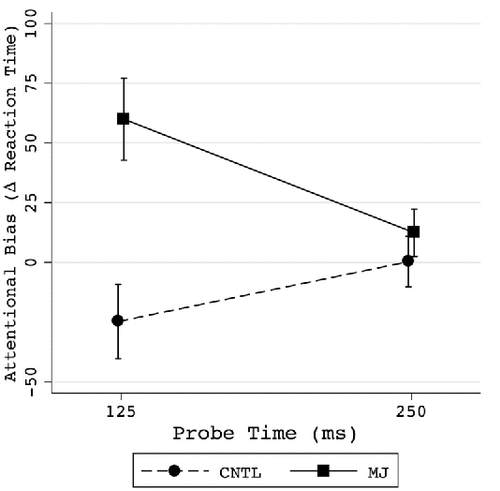
There has been modest examination of attentional bias in individuals with cannabis use disorders. Clinical implications of this work are directly relevant to better informing extant evidence-based treatment for substance use disorders (e.g., relapse prevention) and/or developing novel interventions. The overarching aim of this investigation was to examine a novel attentional bias task in adults with cannabis use disorders. Participants were comprised of 25 adults (8 women: M age = 31, SD = 6.8; range = 22–45) with cannabis use disorders (n = 12) and controls (n = 13) without any current (past month) psychopathology. Relative to controls, adults with cannabis use disorders had greater attentional bias scores. These differences were present only at the 125-ms probe time, where the cannabis use disorders group showed greater attentional bias to cannabis cues than the control group (adjusted p = .001, cannabis use disorders mean = 59.9, control mean = −24.8, Cohen's d-effect size for 125 ms = 1.03). The cannabis use disorders group also reported significantly greater perceived stress and post-task stress scores than the control group, but stress was not related to attentional bias. This study informs understanding of the influence of cannabis cues on visual detection and reaction time under different cue-target onset times, as attentional bias was most prevalent under time pressure to detect the probe.
Attentional bias (AB) in adults with cannabis use disorders (CUDs)
Cannabis has been the most commonly abused illicit substance in North America for the last four decades.Citation1 Cannabis accounts for more than four million of the estimated seven million Americans with illicit substance use disorders (SUD).Citation2 About 10% of people who use cannabis will develop a CUD, but among daily cannabis users, the estimated prevalence rises to 25–50%. Importantly, CUD is associated with cognitive and social impairments, including educational and employment difficulties, and cannabis is the second most common substance for which individuals are admitted to drug treatment programs.Citation3
As with most drugs of abuse, cue-induced relapse presents a significant barrier to cannabis abstinence. Cue reactivity is a relapse risk factor for almost every known abused substance.Citation4–6 By definition, drug-cue reactivity is a process in which, through associative conditioning, drug-related stimuli become paired with drug-reinforcement processes. Subsequently, exposure to these conditioned drug-related stimuli alters physiological and psychological processing.Citation7,8 Cue reactivity represents a constellation of events measurable in a number of domains (subjective, physiological, and neurocognitive); one of which is biased or selective attention to drug cues, or AB. Proposed sequelae of AB toward drug cues include perseverative thinking about drug use, drug seeking, and the initiation of compulsive or habitual drug use behaviors.Citation9 Further, AB predicts a number of key real-world outcomes, such as retention in treatment trials.Citation5,10,11
AB in cannabis users requires further examination. This is particularly true in users meeting criteria for CUD, in whom cue reactivity is likely to be most salient. Many previous AB studies have examined only recreational cannabis users,Citation12,13 and the paradigms employed have generally either used cannabis-related words, which are less vivid and ecologically relevant than pictorial stimuli,Citation13–15 or relatively expensive electrophysiological measures of attention to visual stimuli.Citation16,17 A simple, robust behavioral measure of AB to visual stimuli in CUD suitable for use in the clinic could contribute to greater understanding of the role of AB in CUD, and inform intervention efforts. Furthermore, as anxiety and stress are known to amplify cue reactivity and relapse risk, the extent to which any given measure is influenced by stress must also be considered.Citation8,9,18 Several studies have demonstrated that stress (acute and chronic) is related to not only increased risk for relapse and cue reactivity but also to AB for other substance classes aside from cannabis.Citation5,9,18–20
Accordingly, the present study examined a novel method for measuring AB in a group of chronic cannabis users meeting criteria for CUD and healthy controls, with the aim of establishing preliminary validation for a robust and inexpensive method of measuring AB in CUD. An AB task was used that utilized pictorial stimuli and short probe times to maximize AB effects. As documented in previous AB studies of individuals with SUD, anxiety, chronic pain, and eating disorders,Citation5,21–26 the use of vivid pictorial stimuli combined with time pressure is most likely to produce robust AB effects. It was expected that cannabis users would show greater AB than controls on this new measure, and that this effect would be evident primarily at short probe times. Further, given the role stress is known to play in relapse and AB, a preliminary exploration was also conducted of possible relationships between stress and AB as measured by this novel task.
Method
Participants
A total of 25 participants (8 women; M age = 31.3, SD = 6.8; range = 22–45) were enrolled in the current study. This study was initiated and conducted prior to the release of the Diagnostic and Statistical Manual of Mental Disorders—5th Edition (DSM-5).Citation27 Accordingly, all diagnostic criteria and phrasing are presented in terminology related to DSM—4th Edition (DSM-IV).Citation28 Twelve participants met criteria for current CUD (abuse or dependence) and 13 control participants did not meet criteria for any Axis I disorders. Please see for a summary of participant characteristics. All participants were required to be between 18–45 years of age. Approximately 50% of the sample was screened to meet diagnostic criteria for CUD (abuse or dependence), defined as (1) a score of ≥13 on the Cannabis Use Disorders Identification Test—Revised (CUDIT-R)Citation27, (2) a score of ≥4 on the Cannabis Abuse Screening Test (CAST),Citation29,30 and (3) diagnostic criteria for cannabis abuse or dependence as per the structured clinical interview for DSM-IVCitation28 Axis I Disorders (SCID-I).Citation31 Approximately 50% of the sample was screened to not meet criteria for any current (past month) Axis I disorders. Exclusionary criteria were comprised of: (1) current (past month) DSM-IV Axis I disorder other than cannabis abuse/dependence; (2) serious illness requiring ongoing medical treatment, which could affect the central nervous system, or any other medical contraindication (e.g., renal, cardiovascular, pulmonary, hematologic) as determined by medical screening; (3) a positive pregnancy test or breast feeding (women); (4) concomitant use of prescription medications that could affect the central nervous system; (5) active suicidal ideation or Beck Depression Inventory-II (BDI-II)Citation32 score greater than 19; (6) positive urine drug screen for drugs other than cannabis or positive breath alcohol screen; (7) Shipley-233 test of cognitive aptitude score outside 2 SD units of the published composite score average; and (8) smoking >10 nicotine cigarettes per day or Fagerstrom Test for Nicotine Dependence (FTND)Citation34 score >4. This study was approved by the University of Texas Health Science Center at Houston's Institutional Review Board.
Table 1. Descriptive statistics for total sample and by group. Values represent mean (SD) or number in each group (number).
Measures
The SCID-ICitation31 is a well-validated, structured clinical interview for psychiatric disorders that is commonly used in research settings for diagnostic purposes.
The CUDIT-RCitation35 is an 8-item questionnaire for screening problematic cannabis use (range 0–32, with higher numbers indicating more likelihood of problematic use). This questionnaire has strong sensitivity (91%), specificity (90%), and 96% positive predictive value for CUD as diagnosed by DSM-IV when 13 is used as the cutoff point.
The CASTCitation29,30 is a 6-item questionnaire for screening problematic cannabis use (range 0–24, with higher numbers indicating more likelihood of problematic use). It has good sensitivity (76%), specificity (85%), and 74% predictive value for CUD when four is used as the cutoff point.
The FTNDCitation34 is a well-established 6-item scale designed to assess gradations in tobacco dependence (range 1–10 with higher numbers indicating more dependence). This measure exhibits good internal consistency, positive relations with key smoking variables (e.g., salivary cotinine);Citation34,36 and high test–retest reliabilityCitation36.
The BDI-IICitation32 is a well-established 21-item self-report measure on which respondents indicate, using a 4-point Likert-style scale, the intensity with which they experience depressive symptoms (range 0–84, with higher numbers indicating more depressive symptoms).
The Shipley-233 is a well-established, brief measure of crystallized knowledge and fluid reasoning ability. Crystallized ability is assessed via administration of the 40-item vocabulary scale, while fluid reasoning is assessed using the 12-item block patterns scale. The composite vocabulary-block patterns score (range 25–145 with higher numbers indicating better cognitive functioning) was used in this study to rule out cognitive impairment that might interfere with participation (scores had to be >70).
The Perceived Stress Scale (PSS)Citation37,38 is a 10-item measure, rated on a 5-point Likert-style response format, developed to measure the degree to which individuals appraise their life as stressful (range 0–40, with higher numbers indicating more stress) during the past month. The PSS has been widely used in health studies; the items are general in nature and relatively content free with regard to specific population groups. The total score is calculated by summing the responses. This measure assesses chronic life stress, which could potentially moderate responses to drug cues.
The Visual Analogue Stress Scale–Current (VASS-C) was used to index stress prior to and after the experimental session. Each participant's current stress level was ranked on a 0–10 visual analog scale with 0 defined as “no stress” and 10 as “extreme stress,” as cued by the question: “Please rate your current stress level.” This measure was included to provide a brief measure of acute stress levels during the study, and ensure that differences in stress responses to the task did not account for any observed differences in AB.
Drug use screening
Drug and alcohol usage was monitored by obtaining urine and expired air samples on each day of participation in the research. The integrated E-Z split key cup II (Innovacon Company, San Diego, CA) was used to test for urine cocaine (benzoylecgonine), THC, opiates, amphetamine, and benzodiazepines. An Alcosensor III (Intoximeters, St. Louis, MO) was used to screen for alcohol consumption. Participants who reported smoking cigarettes were allowed to smoke 60 minutes prior to testing (but not during), in order to prevent contamination of results due to either acute nicotine or nicotine withdrawal effects. In addition, a substance use survey, developed for purposes of the present study, was administered to assess use of each class of substances (e.g., alcohol, cocaine). Participants were asked to report on lifetime and current use of each substance class. Specifically, participants reported on the following information for each substance class: number of estimated lifetime uses, estimated number of uses within the past month and week, rates of use for heaviest 30-day period of use during the lifetime, route(s) of administration, and age of first use.
Participants were asked to report on lifetime and current use of each substance class in addition to age of onset of use, years of regular use, and past month use frequency.
Cannabis AB task
Testing lasted approximately 12 minutes. The task utilized pictorial stimuli (i.e., color photographs) and short cue-probe onset timing (i.e., time pressure) in order to maximize AB effects, as suggested by previous research.Citation21–24,26 The structure of the task was as follows: The participant first read printed instructions and completed 12 practice trials with unique stimuli (nature scenes). The instructions were re-summarized, and the experiment proper began. Each trial had the following structure: (1) orienting stimulus (cross hair, 500 ms); (2) cue = cannabis picture or neutral picture (500 ms); (3) probe stimulus (125 or 250 ms) = yellow smiley ![]() or neutral
or neutral ![]() face just outside one of the four corners of the cue picture; (4) probe stimulus removed, picture (cue) remained on screen (1500 ms); inter-trial interval (1500 ms). Beginning with the introduction of the probe (face), the participant had 1500 ms to record a response on the left or right button of a Universal Serial Bus (USB) response device, based on the cue (smiley or neutral).
face just outside one of the four corners of the cue picture; (4) probe stimulus removed, picture (cue) remained on screen (1500 ms); inter-trial interval (1500 ms). Beginning with the introduction of the probe (face), the participant had 1500 ms to record a response on the left or right button of a Universal Serial Bus (USB) response device, based on the cue (smiley or neutral).
There were six cannabis pictures featuring cannabis-related stimuli, and six neutral pictures matched in size, color, and context. Three of the cannabis cues featured adults smoking cannabis (i.e., all pictures were digital color photographs of an individual smoking cannabis; two showed men smoking joints, and one showed a woman smoking from a water pipe); and three of the cannabis pictures offered close-range photographs of cannabis/cannabis “cigarettes” (i.e., joints, blunts). The six neutral images were comprised of photographs of neutral adult faces (matched in terms of gender and perspective of photograph) and objects (e.g., pens; matched in size, color, context to the cannabis photographs). Pictures are available in electronic form via request to the corresponding author. Pictures were presented on a 17-inch computer monitor and were approximately 6 inches high × 8 inches wide at 300 dpi. Participants were exposed to the pictures for 2000 ms, providing ample time to discriminate the content. The task utilized a 96-trial block design with 48 trials each of cannabis stimuli and neutral stimuli. Within a block, each stimulus appeared in random order, with no repeats on consecutive trials. Block order and probe location were counterbalanced within and across participants. One experimental advantage of the task is that it provided multiple indices, including AB, operationalized as reaction time (RT) difference (cannabis–neutral cues) and allowed, on an exploratory basis, the examination of accuracy of identification of the probe and response bias (i.e., tendency to identify the face as a “smiley” face), which were calculated using the signal detection metrics d′ and criterion c.Citation39
Procedure
Participants were recruited via local advertisements for non-treatment-seeking cannabis users. Print, radio, TV ads, and patient-to-patient sources were utilized. Interested participants were first screened via telephone to ensure that basic inclusionary/exclusionary criteria were met. Potentially eligible participants were scheduled for an in-person screening appointment. After providing verbal and written consent, participants received medical and psychiatric screening, which included provision of urine and alcohol breath samples, administration of the SCID-I, and completion of the BDI-II, Shipley-2, CAST, CUDIT-R, and substance use survey, as evaluation for enrollment into the study. Eligible participants were then scheduled for a laboratory test session to complete the experimental task. At the experimental laboratory session, participants again provided urine and alcohol breath samples, and completed the PSS. Participants completed the Cannabis AB Task, as previously described. Participants completed the VASS-C ratings prior to and immediately after the task. Participants were compensated $20 for their time, plus approximately $4.00–6.00 based on test performance (accuracy in detecting probes) at the conclusion of the testing day. Participants received a base payment of $2.00 at the start of the task, and based on performance, they could earn an additional amount up to $4.00 extra (for a total of $6.00 for the task). Therefore, taken together, participants earned between $22.00 and $26.00 for participating in the study.
Data analytic plan
The primary data analytic strategy used mixed linear modeling, as implemented in version 1.1.6 of the lme4 packageCitation40 in version 3.1.0 of the R statistical computing environment.Citation41 Three mixed linear models were conducted. The primary analysis examined AB, operationalized as the average of RTs to a probe following a cannabis cue minus the average of RTs to a probe following a neutral cue. This produced a single AB score for each type of trial measured in ms, where higher numbers indicate greater bias, which were entered into a mixed linear model with group, probe time (125 versus 250 ms) and cue type (smiley versus neutral) as fixed factors, and subject as a random factor.
On an exploratory basis d′ was also entered (sensitivity or ability to discriminate the “smiley” versus the neutral face averaged across each type of trial, where higher numbers indicate more accurate discrimination), and criterion c (bias toward identifying the probe as a “smiley” or neutral face, averaged across each type of trial, where higher numbers indicate a greater bias to identify the face as neutral) into two separate mixed linear models with group, cue type (marijuana versus neutral) and probe time (125 versus 250 ms) as fixed factors, and subject as a random factor.
Then the relationship of stress to cannabis use and AB were examined. First, for the PSS, an indicator of overall life stress measured at a single time point, a between-groups t-test was used with PSS scores as the dependent variable. For the VASS-C, an indicator of stressful feelings collected immediately before and after the task, the VASS-C scores were entered into a mixed linear model with group and time (before task versus after task) as fixed factors, and subject as a random factor. Three additional mixed linear models were then conducted on each dependent variable of interest (AB, d′, and criterion c) using in turn mean-centered PSS, average VASS-C across the study and change in VASS-C from before to after the experiment as an additional fixed factor, and modeling all interactions between the given stress variable and the other independent variables. This procedure allowed examination of whether mean levels of stress or changes in stress due to the task related to any of the outcomes, or altered the effect of cannabis use on any outcome.
p-Values for mixed linear models were derived using Satterthwaite approximations of degrees of freedom, as implemented in version 2.0–6 of the lmertest package in R.Citation41 Any significant interactions were followed by a false discovery rate (FDR) corrected post hoc tests of least-squares means, which examined all possible pairs of means involved in the interaction, also implemented in the lmerTest package. Effect sizes for linear mixed models are expressed in terms of unstandardized Bs and standard errors (SE). All variables and residuals were examined for normality. Skewness and kurtosis were within acceptable limits for all variables.
Results
shows characteristics of the sample. There were no between-group differences in age, years of education, Shipley-2 scores, or the distribution of gender or ethnicities. The CUD group reported marginally higher perceived life stress on the PSS (p = .05). There was also a significant main effect of time on the VASS-C 2, such that all participants reported less stress after the task than before (B = –.78, SE = .31, t(23) = 2.62, p = .02). There were no interactions between group and time, indicating that the CUD group did not experience a significantly different change in stress over the task compared to controls.
With regard to substance use history, more participants in the CUD group smoked cigarettes compared to the control group (66 and 15%, respectively; p < .05) and reported more years of regular cigarette use (p < .05). Among the current cigarette smokers only, the CUD and control groups did not differ significantly in the average number of cigarettes smoked per day or the age of onset of cigarette smoking. Groups did not differ with regard to alcohol use history. While more participants in the CUD group reported lifetime history of use of other substances (ns = 2–4 in the CUD group versus 0 in the control group across substance classes; please see ), these participants reported low levels of current (monthly use). Most participants in the CUD group did not report a history of other substance use.
Examining AB to cannabis cues, there was a significant main effect of group (B = 42.83, SE = 14.29, t(92) = 3.00, p < .01), such that the CUD group showed greater AB for cannabis pictures than the control group. However, this occurred in the context of the predicted significant group × probe time interaction (B = −68.39, SE = 28.58, t(92) = 2.39, p = .02). Using FDR-adjusted post hoc tests to examine this interaction, it was found that the CUD group had significantly greater AB to cannabis cues than the control group when the probe appeared for 125 ms (adjusted p = .001), but not when the probe appeared for 250 ms (adjusted p = .67), as shown in (top panel). There was also an unexpected significant interaction between group and probe type (smiley versus neutral, B = −84, 14, SE = 28.58, t(92) = −2.94, p < .01). Using FDR adjusted post hoc tests to examine this interaction, it was found that the CUD group had significantly greater AB to cannabis cues than the control group when the probe was a neutral face (adjusted p = .001), but not when the probe was a smiley face (adjusted p = .97). The predicted group × probe time (125 versus 250 ms) interaction remained significant when the PSS, VAS average, and VAS change scores were included in the model, but the group × probe type (smiley versus neutral) interaction dropped to non-significance when PSS scores were included, and a significant probe type × PSS score interaction appeared, suggesting this unexpected group × probe type interaction may have been better accounted for by differences in stress levels between the groups.
Figure 1. Attentional bias was significantly greater in the CUD group at 125 ms probe times, but not 250 ms. Attentional bias score is calculated as RT on cannabis picture trials minus neutral picture trials, thus a score above 0 indicates longer RT to respond to the probe in the presence of the cannabis pictures, and is interpreted as attentional bias to the cannabis cues relative to the neutral cues. Error bars represent the SEM.
Examining the ability to discriminate between the smiley and neutral probes, for example, d′, the only significant effects observed were a small advantage in discrimination for the CUD group overall (B = .59, SE = .27, t(23) = 2.14, p = .04), and a large advantage in discrimination for the entire sample at longer probe times (B = 1.67, SE = .13, t(69) = 12.40, p < .001). Neither of these findings were altered by inclusion of any of the stress variables. Examining bias to identify the probe as a “smiley face,” for example, criterion c, there were no significant effects of any of the independent variables.
Discussion
Reactivity to conditioned drug cues plays a key role in relapse. Laboratory models of skewed attention toward drug cues have helped to delineate important factors in the AB process, including context, drug-stimulus characteristics, and stress. Clinical implications of this work are directly relevant to better informing extant evidence-based treatment for SUD (e.g., relapse prevention) and/or developing novel interventions. One example might include the development of treatment programs that expose individuals with CUD to cannabis stimuli during the course of treatment so as to help them habituate to the cues and/or better manage associated cravings and urges to use with adaptive emotion regulatory skills. Thus, this line of work has significant potential for impacting clinical interventions, pending further advancement of the understanding of the associations between drug cue reactivity and substance use behaviors, including treatment-seeking and relapse to use.
In the present report, it is demonstrated that individuals with CUD had significantly greater AB in a simple behavioral measure of attention to visual cannabis cues when task demands (125-ms probe) put time pressure on accurate discrimination of probes. It was also unexpectedly found that AB was greater in individuals with CUD when the probe used was a “neutral” face instead of a “smiley” face, although this unexpected difference became non-significant when group-level differences in chronic stress were taken into account. The CUD group had marginally higher levels of both perceived chronic stress and acute stress during study tasks (although only PSS chronic stress was statistically significantly higher in CUD). Both groups showed an overall decline in stress across the task, and the task did not affect stress differently in CUD individuals versus controls. Further, CUD individuals were marginally more accurate in the discrimination task overall, suggesting that the observed differences in AB were not due to either greater perceived difficulty/stress during the tasks, or differences in ability to complete the task.
Task difficulty engendered by the short probe-stimulus onset asynchrony (SOA) had a marked effect on RTs and AB. SOA is the time interval between the onset of a cue (e.g., the marijuana or neutral picture) and the onset and/or duration of the probe (e.g., the face icons). The short 125-ms probe placed time pressure on the discrimination of the probe, which most likely interacted with the distracting effect of the cannabis pictures to engender the AB to cannabis cues observed in the CUD group; the effect was not present in either group when the probe was on-screen for 250 ms. Interestingly, the two groups also showed different AB depending on the type of probe: The CUD group showed greater AB when the probe was neutral, but not when the probe was smiley. It may be intriguing to interpret this as an unintended consequence of the affective component of the pervasive generic yellow “smiley” face. Although the neutral and smiley face were simply chosen as visual stimuli that were neither too difficult nor too easy to discriminate, several studies have documented that affective-based cues can modify signal detection and attentional processing.Citation42–44 Further, this difference between groups became non-significant when the small group differences in stress were taken into account, further suggesting an affective explanation. However, the affective valence of the smiley and neutral faces was not experimentally established. Thus, this possibility remains a conjecture until verified by direct experimental manipulation. Future studies should consider using a different discrimination with no affective connotation.
Perceived life stress, as measured by the PSS, was marginally higher (p = .05) in individuals with CUD. However, the task itself did not appear to constitute a stressor for either group, with stress levels actually declining from before to after the task to an equivalent extent in both groups, which—combined with the moderational analyses examining associations between stress and performance—suggest the observed differences in AB are not due to the task being more stressful for individuals with CUD. While significant associations were not observed between AB and chronic stress (PSS) or acute stress (VASS-C) in this modest sample of 12 participants with CUD, the results are consistent with other lines of inquiry demonstrating that short SOA and time pressure exacerbate AB,Citation22,24,26 and should not be interpreted as contradictory to the established finding that stress generally exacerbates cue-reactivity, including reactivity to drug cues.Citation9,45 Here, an initial exploratory analysis was conducted to examine whether pre-existing, uncontrolled levels of stress corresponded to task performance, and thus, the range of stress may have been restricted. Future studies of AB in individuals with CUD may benefit from experimental designs that incorporate pharmacological or psychological manipulations of acute stress.
Several limitations of the present study are evident. First, in this small laboratory-based sample, generalizability to the broader population of cannabis users is clearly limited due to the inclusion/exclusion criteria employed for this pilot study and the racial/ethnic composition of the sample. Stringent exclusionary criteria were employed to increase the internal validity of the pilot study and allow for more accurate, direct examination of AB to cannabis cues in a novel experimental paradigm. However, restriction of the sample to individuals with CUD only, and exclusion of all other Axis I disorders, limited generalizability to SUD populations. Further replication and extension of this study to cannabis-using adults presenting with multiple psychiatric comorbidities would be an important next step in this line of inquiry. Second, a higher proportion of individuals smoking nicotine cigarettes were observed in the CUD group as compared to the control group. However, there were no differences in level of nicotine dependence or number of cigarettes smoked, and the latency was standardized from last smoking period at 60 minutes prior to testing for all smokers, in order to prevent contamination of results due to either acute nicotine or nicotine withdrawal effects. Nonetheless, cigarette smoking could not be fully ruled out as a potential confound. Third, while higher stress levels were observed in the CUD group, no experimental manipulation of stress was employedCitation19,46,47 and no significant relationships between stress and AB were observed. However, based on the present report, it would be inappropriate to conclude that stress and AB do not interact to modulate cannabis use in CUD. Future experiments will be enhanced by directly manipulating stress and employing both repeated measures designs and larger sample sizes. Fourth, small sample sizes have inherent hazards, including low probability of identifying true effects; low positive predictive value that an obtained significant effect is a “true positive;” and an exaggerated estimation of the magnitude of significant effects that are obtained.Citation48 Finally, a novel AB task was employed, which was based on important features of previous AB studies, including picture stimuli, short probe presentations, and RT and signal detection based metrics. However, the task was not experimentally validated by including a second well-studied comparator AB task such as a traditional dot-probe or drug-stroop,Citation5,49 or alternative measures of cue-reactivity and craving.Citation4,45 Inclusion of such measures will be needed for further task validation.
Under the circumstances, a full appraisal of this novel task requires replication with larger samples and more rigorous experimental designs. However, it potentially offers a comparatively simple and robust method of assessing AB, using digital picture stimuli, which could be further individualized to each participant to enhance the sensitivity of the procedure. The current data are also congruous with previous AB studies in several SUD populations.Citation5,50 Despite the consistencies, the results require extension and replication to support the general utility of this measure in CUD, including examination in treatment-seeking populations.
Acknowledgments
The authors wish to thank Tara Watts, Sarly Vasquez, Zahra Kamdar, Ellen Desmarais, and Nuvan Rathnayaka for expert technical assistance conducting the research protocol. Portions of the data in this report were presented at the 2014 College of Problems on Drug Dependence annual convention in San Juan, Puerto Rico.
Funding
This project was supported by funding from U.S. DHHS/NIH grants R21 DA 034825 and P50 DA 09262 from the National Institute on Drug Abuse (SD Lane, JM Schmitz). Dr. Vujanovic acknowledges the support of NIH/University of Texas Clinical and Translational Sciences Award (KL2TR000370-07).
References
- SAMHSA. Results from the 2008 National Survey on Drug Use and Health: National Findings; Office of Applied Studies (NSDUH Series H-36, HHS Publication No. SMA 09–4434). Rockville, MD: Department of Health and Human Services, 2009.
- SAMHSA. Results from the 2010 National Survey on Drug Use and Health: National Findings; Office of Applied Studies (NSDUH Series H-41. HHS Publication No. SMA 11–4658). Rockville, MD: Department of Health and Human Services, 2011.
- SAMHSA. Treatment Episode Data Set (TEDS): 1994–2004. National admissions to substance abuse treatment services. Rockville, MD: Office of Applied Studies, 2006.
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction 1999; 94(3):327–40.
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend 2008; 97(1):1–20.
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev 2014; 38:1–16.
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 1993; 18(3):247–91.
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacol (Berl) 2001; 158(4):343–59.
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci 2008; 1141(1):105–30.
- Carpenter KM, Martinez D, Vadhan NP, Barnes-Holmes D, Nunes EV. Measures of attentional bias and relational responding are associated with behavioral treatment outcome for cocaine dependence. Am J Drug Alcohol Abuse 2012; 38(2):146–54.
- Vadhan NP, Carpenter KM, Copersino ML, Hart CL, Foltin RW, Nunes EV. Attentional bias towards cocaine-related stimuli: relationship to treatment-seeking for cocaine dependence. Am J Drug Alcohol Abuse 2007; 33(5):727–36.
- Cane JE, Sharma D, Albery IP. The addiction Stroop task: examining the fast and slow effects of smoking and marijuana-related cues. J Psychopharmacol (Oxf) 2009; 23(5):510–9.
- Field M, Mogg K, Bradley BP. Cognitive bias and drug craving in recreational cannabis users. Drug Alcohol Depend 2004;74(1): 105–11.
- Field M. Cannabis “dependence”and attentional bias for cannabis-related words. Behav Pharmacol 2005; 16(5–6):473–6.
- Cousijn J, Watson P, Koenders L, Vingerhoets W, Goudriaan A, Wiers R. Cannabis dependence, cognitive control, and attentional bias for cannabis words. Addict Behav 2013; 38(12):2825–32.
- Asmaro D, Carolan PL, Liotti M. Electrophysiological evidence of early attentional bias to drug-related pictures in chronic cannabis users. Addict Behav 2014; 39(1):114–21.
- Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend 2006; 85(1):75–82.
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep 2007; 9(5):388–95.
- Chaplin TM, Hong K, Fox HC, Siedlarz KM, Bergquist K, Sinha R. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Human Psychopharmacol: Clin Experiment 2010; 25(5):368–76.
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcoholism: Clinical and Experimental Research 2007; 31(3):395–403.
- Becker DV, Mortensen CR, Ackerman JM, et al. Signal detection on the battlefield: priming self-protection vs. revenge-mindedness differentially modulates the detection of enemies and allies. PLoS ONE 2011; 6(9):e23929.
- Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav Pharmacol 2004; 15(1):29–36.
- Bruce G, Jones BT. A pictorial Stroop paradigm reveals an alcohol attentional bias in heavier compared to lighter social drinkers. J Psychopharmacol (Oxf) 2004; 18(4):527–33.
- Fadardi JS, Bazzaz MM. A Combi-Stroop test for measuring food-related attentional bias. Exp Clin Psychopharmacol 2011; 19(5):371.
- Schoth DE, Nunes VD, Liossi C. Attentional bias towards pain-related information in chronic pain: a meta-analysis of visual-probe investigations. Clin Psychol Rev 2012; 32(1):13–25.
- Sharma D, McKenna FP. The role of time pressure on the emotional Stroop task. Br J Psychol 2001; 92(3):471–81.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Association, 2013.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR). Washington, DC: American Psychiatric Association, 2000.
- Legleye S, Karila L, Beck F, Reynaud M. Validation of the CAST, a general population Cannabis Abuse Screening Test. J Subst Use 2007; 12(4):233–42.
- Legleye S, Piontek D, Kraus L. Psychometric properties of the Cannabis Abuse Screening Test (CAST) in a French sample of adolescents. Drug Alcohol Depend 2011; 113(2):229–35.
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition. New York, NY: SCID-I/P, 2002.
- Beck A, Steer R, Brown G. Manual for the BDI-II. San Antonio, TX: Psychological Corporation, 1996.
- Shipley W, Gruber C, Martin T, Klein M. Shipley-2 professional manual. Torrance, CA: Western Psychological Services, 2009.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991; 86(9):1119–27.
- Adamson SJ, Kay-Lambkin FJ, Baker AL, et al. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend 2010; 110(1):137–43.
- Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM. Assessing nicotine dependence: a comparison of the Fagerström Tolerance Questionnaire (FTQ) with the Fagerström Test for Nicotine Dependence (FTND) in a clinical sample. Addict Behav 1994; 19(3):307–17.
- Cohen LH, Towbes LC, Flocco R. Effects of induced mood on self-reported life events and perceived and received social support. J Pers Soc Psychol 1988; 55(4):669.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983:385–96.
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods, Instruments, Computers 1999; 31(1):137–49.
- Bates D, Maechler M, Bolker B, Walker S. Linear mixed-effects models using Eigen and S4. Journal of Statistical Software 2014; 67(1), 1–48.
- Kuznetsova A, Brockhoff PB, Christensen RHB. Package “lmerTest.” ImerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R Package Version.
- Garland EL, Froeliger B. Mindfulness training targets addiction at the attention-emotion interface: a neurocognitive framework. In Le A., Ngnoumen CT, Langer EJ, eds. The Wiley Blackwell Handbook of Mindfulness. New York, NY: John Wiley & Sons 2014: 794–817.
- Heckman BW, Kovacs MA, Marquinez NS, et al. Influence of affective manipulations on cigarette craving: a meta-analysis. Addiction 2013; 108(12):2068–78.
- Viviani R. Emotion regulation, attention to emotion, and the ventral attentional network. Frontiers Human Neurosci 2013; 7, 746.
- Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep 2011; 13(5):398–405.
- McRae-Clark AL, Carter RE, Price KL, et al. Stress-and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacol (Berl) 2011; 218(1):49–58.
- Porcelli AJ, Lewis AH, Delgado MR. Acute stress influences neural circuits of reward processing. Front Neurosci 2012;6, 157.
- Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature Rev Neurosci 2013; 14(5):365–76.
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. The American Journal of Drug and Alcohol Abuse 2011; 37(2):117–22.
- Coskunpinar A, Cyders MA. Impulsivity and substance-related attentional bias: a meta-analytic review. Drug Alcohol Depend 2013; 133(1):1–14.
