Abstract
Background
Central sensitization is an important mechanism underlying many chronic pain conditions. Chronic pain and alcohol use disorder (AUD) are highly comorbid. Despite great scientific interest in brain mechanisms linking chronic pain and AUD, progress has been impeded by difficulty assessing central sensitization in AUD.
Objective
The present study is the first to employ a validated surrogate measure to describe central sensitization in a clinical sample with AUD.
Methods
Participants with AUD (n = 99) were recruited from an academic addiction treatment center. A well-established surrogate measure of central sensitization, The American College of Rheumatology Fibromyalgia Survey Criteria (ACRFMS) was administered. Participants also responded to questions about quality of life (RAND-36), and AUD. Descriptive analyses and Spearman’s rho correlations were performed.
Results
Chronic pain and evidence of central sensitization were prevalent. Greater central sensitization was associated with worse health-related quality of life. Participants higher in central sensitization expressed greater endorsement of pain as a reason for AUD onset, maintenance, escalation, treatment delay, and relapse.
Conclusion
The present study bolsters prior assertions that AUD and chronic pain might compound one another via progressive sensitization of shared brain circuitry. These results may inform future mechanistic research and precision AUD treatment.
1. Introduction
Chronic pain and alcohol use disorder (AUD) are detrimental to personal and public health, frequently comorbid, and characterized by significant clinical and neurobiological overlap.Citation1 Chronic pain—defined as pain lasting three months or longer—is a leading cause of disability affecting more than 30% of the global population.Citation2,Citation3 The lifetime prevalence of alcohol use disorder (AUD) is nearly 30% in the United States, and alcohol use is a leading risk factor for premature death and disability worldwide.Citation4,Citation5 Consumption of low amounts of alcohol has been associated with decreased pain, with an inverse U-shaped curve and worsening pain noted only as alcohol use become greater than two drinks per day.Citation6–9 Nevertheless, a growing number of observations demonstrate clinically impactful intersections between chronic pain and AUD.Citation10
Clinical overlap between chronic pain and AUD appears extensive. Chronic pain may antedate AUD onset, and familial studies suggest chronic pain and AUD may have converging heritability.Citation11–13 Individuals with AUD may be more likely to develop pain disorders and consume analgesics with greater frequency, duration, and potency.Citation14 Chronic pain is prevalent among treatment seeking individuals with AUD.Citation15,Citation16 Additionally, pain-coping is a common motive for continuing and escalating alcohol use in AUD and pain may predict AUD relapse.Citation17–19 Such observations have kindled growing interest in the intersecting neurobiology of chronic pain and AUD.Citation10
Central nervous system (CNS) mechanisms appear to connect chronic pain and AUD and similar neuroplastic changes accompany clinical progression of these conditions.Citation1,Citation20 Egli et al. proposed that heavy alcohol use and chronic physical pain both dysregulate the activity of the amygdala, nucleus accumbens, anterior cingulate cortex and insula—key brain structures in the neuropathology of AUD.Citation1 Dysregulation of brain systems involved in stress and negative emotionality – known as hyperkatifeia – occurs in AUD due to progressive CNS sensitization during repeated episodes of withdrawal.Citation21–23 Similar dysregulation of the nociceptive system – known as central sensitization – involves ‘“increased responsiveness of nociceptive neurons in the CNS to normal or subthreshold input.”Citation24’ Central sensitization is a mechanism underlying or exacerbating many chronic pain conditions.Citation25 Because the nociceptive system includes brain regions involved in stress, negative emotion, reward, and executive function, central sensitization may be a promising precision medicine target for patients with comorbid chronic pain and AUD.Citation23,Citation26 However, translation of basic research in this area has been impeded by difficulty assessing central sensitization in clinical populations with AUD and chronic pain. Therefore, a surrogate measure capable of assessing central sensitization among patients with AUD would be of great importance.
Recently, our group studied central sensitization among a clinical sample of patients with opioid use disorder (OUD) using an established surrogate measure, the American College of Rheumatology Fibromyalgia Survey Criteria (ACRFMS).Citation27,Citation28 We found associations between central sensitization, health-related quality of life, and self-report of pain as a reason for OUD onset, maintenance, escalation, and relapse. An implication of this study was that central sensitization might underlie previously observed relationships between chronic pain and clinically salient features of OUD. If these findings could be extended to the AUD population, this would support the AUD-pain model proposed by Egli et al.Citation1 However, it remains unclear whether these findings are replicable in an independent sample with AUD.
Therefore, the present work aims to replicate and extend our prior study by employing ACRFMS as a central sensitization surrogate among patients with AUD. We describe central sensitization among a clinical sample of patients with AUD and assess relationships between central sensitization, health-related quality of life, and agreement with original questions probing pain-related AUD onset, maintenance, escalation, and fear of pain-precipitated relapse.
2. Methods
2.1. Participants
Participants were treatment–engaged adults meeting diagnostic criteria for AUD at the time of the survey. AUD diagnosis was made per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) by trained personnel.Citation29 Potential participants were enrolled regardless of whether pain was present or absent. The only exclusion criteria were inability to provide informed consent, read, or understand survey items.
One hundred and three individuals with AUD were consecutively recruited between July 27th, 2022, and November 22nd, 2022, from patients receiving care at the Ohio State University Wexner Medical Center (OSUWMC), Talbot Hall. Talbot Hall provides AUD treatment including partial hospitalization, intensive outpatient, individual and group-based counseling, medically managed withdrawal, and medications for AUD (naltrexone, acamprosate and disulfiram). Recruitment was conducted during routine care by addiction treatment professionals (board certified Addiction Medicine Specialist Physicians and Psychiatric Nurse Practitioners) and a research coordinator trained by the principal investigator (Board Certified Addiction Medicine Physician). The research coordinator interacted with potential participants only after they had been assessed for AUD by study team clinicians. Participants accessed survey items on a tablet computer in a private examination room and were not allowed to interact during the survey. Survey data were collected using REDCap, a web platform for securely collecting personal health information and managing online databases.Citation30,Citation31 Participants provided verbal consent and were monetarily compensated for their time.
Eight individuals were offered participation but declined. Four cases were excluded for violating inclusion criteria (one in remission of AUD and another three that did not meet AUD criteria). Three cases were missing health-related quality of life or demographics (3, 3.0%). These were retained in the final sample but excluded from specific correlational analyses where necessary, as noted. The final sample was (n = 99). The study protocol (2022H0175) was approved by the OSUWMC Institutional Review Board (Located: Columbus, Ohio, inital approval granted on 06/05/2022).
2.2. Measures
2.2.1. Survey
The survey included DSM-5 AUD criteria (as assessed by trained personnel on the date of participation), demographic information (age, gender, race, ethnicity) and the following validated instruments and original items.
2.2.1.1. American college of rheumatology fibromyalgia Survey criteria
Degree of central sensitization was assessed by ACRFMS.Citation28,Citation32 ACRFMS records the location and number of sites of bodily pain (0–19) and the severity of central sensitization-related symptoms including problems thinking, fatigue and difficulty sleeping (0–12). As a continuous scale (range 0–31), ACRFMS has been extensively utilized as a surrogate for central sensitization.Citation33–37 Alternatively, it may be used with a specific cutoff point to indicate the presence of fibromyalgia (ACRFMS ≥ 13, sensitivity 96.6% and specificity 91.8%).Citation28 ACRFMS has previously been shown to be robustly predictive of pain, disability, and treatment outcomes in diverse clinical populations.Citation33,Citation38–44 This scale has exhibited good internal consistency reliability (Cronbach’s α ≥ .8) in diverse populations.Citation45–48
2.2.1.2. Health-related quality of life
Health-related quality of life was assessed by the Research and Development (RAND) Corporation RAND 36-Item Health Survey 1.0 (RAND-36).Citation49 RAND-36 is a widely adopted survey designed to assess health-related quality of life along eight domains: general health, physical functioning, mental health, social functioning, vitality, bodily pain, role limitations due to physical health and role limitations due to emotional problems. Scoring RAND-36 requires linear transformation of each of its 36 items to a range of 0–100 and averaging items by domain.Citation49,Citation50 Lower domain scores represent worse health-related quality of life. The validity and reliability of RAND-36 has been studied extensively.Citation50–53 The internal consistency (Cronbach’s α) of this scale has ranged from has ranged from .71 to .92 in prior research.Citation53,Citation54
2.2.1.3. Original items
Original items were created to reflect perceptions of pain’s potential impact on clinically salient features of AUD. These items were adapted from questions used in our previous studies of central sensitization and OUD by substituting the words ‘“alcohol’” for ‘“opioids’” and ‘“drinking’” for ‘“using’”.Citation27,Citation55 Original items two through five were directly adapted from the Pain-related Opioid Use Disorder Exacerbation Scale (PrOUD-ES). The PrOUD-ES was previously shown to have a single-factor solution, strong internal consistency (Cronbach’s α = .86), and construct validity.Citation55 All original items were scaled as they have been in our previous studies: strongly disagree (1), disagree (2), neutral (3), agree (4) or strongly agree (5). includes these original items.
Table 1. Original items.
2.3. Analyses
Descriptive analyses were conducted to characterize demographic features as well as AUD severity, central sensitization, health-related quality of life, and responses to original items. Then a series of Spearman’s rank order partial correlations were carried out to explore hypothesized associations between central sensitization (ACRFMS) and other variables of interest (DSM-5 AUD criteria, RAND-36, original items) while controlling for age and gender.Citation56,Citation57 For Spearman’s rank order partial correlations, the size of rs was interpreted 0.1 = small, 0.3 = medium, and 0.5 = large.Citation58 All tests were two-tailed and were deemed significant at α < 0.05. Statistical analyses were performed using SPSS software (Version 28.0, SPSS. Inc.).
3. Results
3.1. Sample characteristics
Demographic characteristics were provided by 96 (97.0%) participants. Mean age was 45 ± 11.0 years. Thirty-five (36.5%) were female and 61 (63.5%) were male. Thirty-two reported their race as Black (32.3%), 61 White (61.6%), and 4 any other race (4.0%). Most (94, 97.9%) claimed non-Hispanic ethnicity. Little variance was observed in AUD severity (mean number of AUD criteria present = 10.4 ± 1.4). provides sample characteristics.
Table 2. Sample characteristics.
3.2. Central sensitization (ACRFMS)
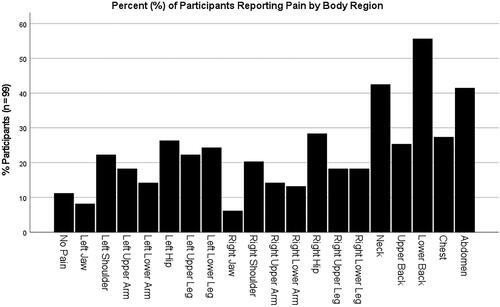
Total ACRFMS scores were non-normally distributed per Shapiro-Wilk test (p = 0.02). The scale had a high level of internal consistency, as determined by a Cronbach’s α of .85. The sample median ACRFMS score was 12.0 [interquartile range (IQR) = 8.0, 15.0]. Eighty-six participants (86.9%) reported at least one painful body region. Multi-site pain was common, with 47 (47.5%) of participants reporting four or more painful body regions. Pain was most often reported in the low back (55, 55.6%), neck (42, 42.4%), and abdomen (41, 41.4%). shows the percentage of participants who reported pain in each body region.
The number of participants reporting moderate or severe cognitive symptoms, fatigue, or waking unrefreshed was 60 (60.6%), 69 (69.7%), and 77 (77.8%) respectively. The scores of 44 participants (44.4%) met criteria for fibromyalgia (ACRFMS ≥ 13).
3.3. Health-related quality of life (RAND-36)
RAND-36 was observed to have high internal consistency reliability (Cronbach’s α = .92). Of the eight life-domains measured by RAND-36, general health, mental health, and vitality were normally distributed. Physical functioning, role limitations due to physical health, role limitations due to mental health, social functioning and bodily pain domains were non-normally distributed. Measures of central tendency indicated overall poor health-related quality of life among the sample. A score of 100 in each domain indicates optimal life quality. Mean scores were 46.0 ± 19.3, 43.5 ± 22.2, and 37.7 ± 19.9 for general health, mental health, and vitality, respectively.
Median health-related quality of life was 50 or lower in: social functioning, 37.5 (IQR = 12.5, 37.5); bodily pain, 45 (IQR = 23.1, 45.0) role limitations due to physical health, 25 (IQR = 0.0, 50.0); and role limitations due to mental health, 0.0 (IQR = 0.0, 33.3). Physical functioning was the exception, with a sample median of 62.5 (IQR = 45.0, 85.0).
3.4. Original items
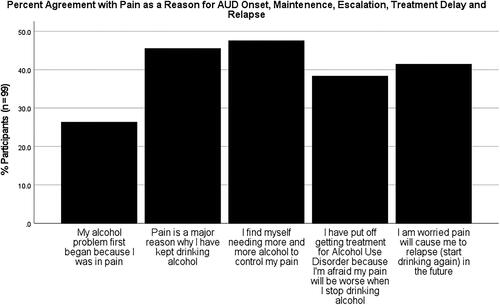
Original items exhibited a high level of internal consistency with a Cronbach α of .90. Twenty-six out of 99 participants (26.3%) agreed or strongly agreed their alcohol problem first began “because I was in pain.” Forty-five (45.5%) participants indicated that pain played an important role in maintaining their AUD by agreeing or strongly agreeing pain was “a major reason why I have kept drinking alcohol.” Forty-seven (47.5%) affirmed that pain-coping motivated their escalating alcohol use “I find myself needing more and more alcohol to control my pain.” Thirty-eight (38.3%) indicated they had delayed accessing AUD treatment because they worried their pain would be worse when they stopped drinking alcohol. Finally, pain-triggered AUD relapse was anticipated by 41 (41.4%) participants who agreed or strongly agreed “I am worried pain will cause me to relapse (start drinking again) in the future.” shows the percentage of participants who agreed or strongly agreed with each statement.
3.5. Associations between central sensitization, AUD severity, health related quality of life, and original items
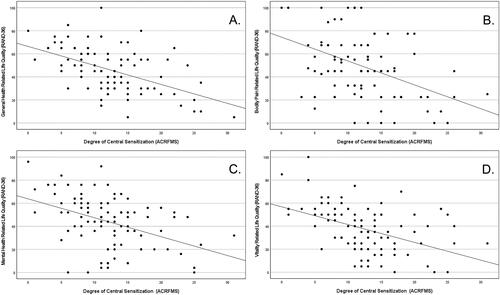
A series of Spearman’s rank order partial correlations were performed. Each of these correlational analyses controlled for age and gender. The assumption of monotonicity was confirmed by visual inspection of individual scatter plots. Central sensitization was positively correlated with DSM-5 AUD criteria (rs (92) = 0.302, p = .003). Significant negative correlations were observed between central sensitization and all eight domains of health-related quality of life. These were: general health (rs (92) = −0. 481, p < .001), mental health (rs (92) = −0.443, p < .001), social functioning (rs (92) = −0.344, p < .001), physical functioning (rs (92) = −0.394, p < .001), vitality (rs (92) = −0.491, p < .001), bodily pain (rs (92) = −0.377, p < .001), role limitations due to mental health (rs (92) = −0.374, p < .001), and role limitations due to physical health (rs (92) = −0.386, p < .001). plots four domains of health-related quality of life by ACRFMS total score.
Figure 3. Relationships between four domains of health-related quality of life (RAND-36) and Central Sensitization (ACRFMS total score): A. General Health; B. Bodily Pain; C. Mental Health; D. Vitality.
Small but significant associations were found between central sensitization and pain-related onset of AUD (rs (92) = 0.216, p = .036) and pain-related AUD treatment delay (rs (92) = 0.217, p = .036) and reporting pain was “a major reason” for continued alcohol use (rs (92) = 0.292, p = .004). There were also medium associations between pain-coping motivated escalation of alcohol use (rs (92) = 0.325, p = .001), and worry about pain precipitating AUD relapse in the future (rs (92) = 0.319, p = .002).
4. Discussion
This is the first study to assess central sensitization among individuals with AUD using ACRFMS as a surrogate measure. Substantial pain-burden was evident among the sample. Nearly ninety percent of participants reported pain in at least one body region. Almost half endorsed widespread pain (≥ 4 sites). Chronic pain (>3 months) was present in over eighty percent of the sample and endorsed by more than eighty-five percent of participants who reported having any pain. Almost half of participants met criteria for fibromyalgia (ACRFMS ≥ 13). The sample prevalence of fibromyalgia (44.4% with ACRFMS ≥ 13) greatly exceeded that of the general population (2–4%;).Citation42 Remarkably, the percentage of participants meeting fibromyalgia criteria was even greater than we recently observed among patients with OUD (27.7% of the total sample (n = 141) with ACRFMS ≥ 13).Citation27 In aggregate, these findings suggest central sensitization may be prevalent among individuals with AUD and chronic pain.
Correlational analyses revealed relationships of potential importance. Greater central sensitization was associated with more severe AUD. This finding appears concordant with the pain-AUD model proposed by Egli et al. which posited that pain and heavy alcohol use produce similar neuroplastic changes to overlapping brain circuits—simultaneously compounding AUD and central sensitization.Citation1 Central sensitization was also related to worse health-related quality of life. This relationship was consistent across all eight life domains of RAND-36, suggesting central sensitization may figure significantly into the overall disease burden of AUD. Additional research is needed to determine if treatments targeting central sensitization might improve quality of life or symptom burden among individuals with AUD.
Participants higher in central sensitization generally expressed greater agreement with statements “My alcohol problem first began because I was in pain,” “Pain is a major reason why I have kept drinking alcohol,” and “I find myself needing more and more alcohol to control my pain.” Prior research has documented that chronic pain may antedate the onset of AUD for some individuals.Citation12 Given that nearly all participants in the present study endorsed chronic pain, and that degree of central sensitization was correlated with greater agreement with pain as the precipitant of onset of AUD suggests central sensitization may uniquely contribute to AUD risk.Citation1,Citation59–61 Similarly, the present findings bolster previous assertions that central sensitization might play a role in AUD disease progression.Citation1,Citation10,Citation62 As a persistent and profoundly aversive condition, central sensitization might accelerate negative reinforcement processes integral to the development of AUD.
Central sensitization was correlated with pain-related treatment delay: “I have put off getting treatment for Alcohol Use Disorder because I’m afraid my pain will be worse when I stop drinking alcohol.” Acutely, alcohol may serve as an anesthetic.Citation62,Citation63 Low to moderate alcohol consumption is associated with improved pain and quality of life among patients with fibromyalgia and chronic pain.Citation7,Citation9 While hyperalgesia may accompany acute withdrawal, little is known about the effect of alcohol abstinence on central sensitization among individuals with AUD.Citation64 One small study found that participants with remitted AUD were not different than controls on quantitative sensory testing.Citation65 Other studies have found treatment engaged patients with AUD continue to report substantially elevated pain burden well into abstinence.Citation15,Citation16 Longitudinal data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) indicated alcohol cessation was associated with worsened bodily pain.Citation66 Longitudinal studies are needed to determine the natural history of central sensitization and its effects on pain perception among alcohol-abstaining individuals with AUD.
Finally, central sensitization was associated with fear of pain-precipitated AUD relapse: “I am worried pain will cause me to relapse (start drinking again) in the future.” It is unknown whether AUD-related dysregulation of the nociceptive system is reversible, or whether central sensitization might persist during sustained abstinence due to lasting damage to underlying neural substrates by alcohol. Prior research has suggested pain may be an important trigger for AUD relapse.Citation19 However, to our knowledge, no prior study has attempted to determine what role central sensitization might have in the relationship between pain and AUD relapse. Central sensitization might potentially mediate the increased risk of AUD relapse attributed to chronic pain, although additional research is needed.
The present work has many implications. ACRFMS may be used in future research to increase mechanistic understanding of chronic pain and AUD. To date, research into central mechanisms of pain in AUD has utilized laboratory-bound techniques such as neuroimaging and quantitative sensory testing (QST).Citation10,Citation20 ACRFMS has been well correlated with evidence of central sensitization in prior QST and neuroimaging studies among individuals without AUD.Citation25,Citation34,Citation35,Citation67–70 By extension, ACRFMS might be useful for participant selection in future basic AUD and pain research. It might also allow for the assessment of central sensitization among large samples and in AUD treatment settings where QST or neuroimaging would be impractical. Future studies employing ACRFMS should explore the role of central pain mechanisms in AUD onset, maintenance, escalation, and relapse. Finally, given substantial overlap between the neurobiology of central sensitization and hyperkatifeia (Hyperkatifeia is conceived of as progressive worsening of emotional and motivational symptoms of withdrawal and is believed to be related to addiction-related dysregulation of brain-stress-systems), ACRFMS might be a viable tool to advance the study of hyperkatifeia – a recently identified research area of high interest to the National Institute on Alcohol Abuse and Alcoholism (NIAAA).Citation23
ACRFMS might also facilitate translation of existing basic research linking central sensitization and chronic pain in AUD. It has been theorized that central sensitization might impact AUD disease trajectory.Citation1,Citation10 ACRFMS may identify subgroups of AUD patients for whom central sensitization is a barrier to AUD remission. ACRFMS score has been repeatedly shown to have high clinical importance, predicting pain, function, and analgesic use in diverse populations.Citation35,Citation36,Citation38,Citation39,Citation41,Citation43,Citation71
Additionally, the neuropharmacology of central sensitization intersects with medications for AUD in potentially impactful ways. For example, naltrexone may up-regulate the endogenous opioid system, and early evidence suggests it might improve pain among individuals with central sensitization.Citation72 Acamprosate is believed to rebalance glutamate and gamma-aminobutyric acid—two key neurotransmitters in excitatory and inhibitory nociception respectively.Citation73,Citation74 Finally, disulfiram works by aversive conditioning.Citation75 Central sensitization is associated with enhanced reactivity to non-nociceptive aversive stimuli.Citation37,Citation69,Citation76,Citation77 It follows that central sensitization might plausibly impact disulfiram acceptance, adherence, and efficacy. Therefore, ACRFMS might inform future pharmacological trials of new and existing agents to increase the precision of AUD treatment by targeting central pain mechanisms.
Study strengths included an adequate sample size and use of validated measures with low levels of missing data. There were also notable limitations. Cross-sectional design precluded direct observation of clinical outcomes, or their relation to central sensitization. We did not control for AUD treatment factors, specific pain conditions, or psychiatric diagnoses. Most participants met criteria for severe AUD and may not represent individuals with mild or moderate AUD. Despite limitations potentially affecting generalizability, alignment of the present findings with our recent similar study (two separate independent samples recruited one year apart) is promising.Citation27
The present study investigated CNS mechanisms previously theorized to underlie the relationship between AUD and chronic pain.Citation1,Citation10,Citation20 A well validated surrogate measure of central sensitization (ACRFMS) was associated with worse health-related quality of life and greater agreement with pain as a reason for the onset, maintenance, escalation, and relapse of AUD as well as for delaying AUD treatment. These findings are concordant with the pain-AUD model introduced by Egli et al. which proposed that chronic pain and AUD compound one another by progressive sensitization of overlapping brain circuits responsible for stress (hyperkatifeia) and pain perception (central sensitization).Citation1 Results also replicate and extend our previous similar study among patients with OUD, further implicating central sensitization as a potentially important mechanism with ramifications for the future study and treatment of comorbid chronic pain and addictive disorders.
| Abbreviations | ||
| AUD | = | alcohol use disorder |
| ACRFMS | = | American College of Rheumatology 2011 Fibromyalgia Survey Criteria |
| OSUWMC | = | Ohio State University Wexner Medical Center |
| DSM-5 | = | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| RAND-36 | = | RAND 36-Item Health Survey 1.0 |
| NIAAA | = | National Institute on Alcohol Abuse and Alcoholism |
Acknowledgements
The authors would like to thank Mamie Martin, RN, MSN, APRN-CNP, DNP; Leah Brown, MS, MPH, APRN-CNP; Jessica Belser, MSW, MS, PMHNP-BC, CARN-NP, and Megan Ackley APRN-CNP for their support in conducting this study.
Disclosure statement
Dr. Hall has provided expert opinion regarding the opioid crisis to the healthcare consultancy firm Lumanity. Dr. Clauw has testified in state lawsuits against opioid manufacturers for their role in the opioid overdose crisis. Dr. Harte has received research support from Aptinyx and Arbor Medical Innovations, and consultation fees from Aptinyx, Memorial Slone Kettering Cancer Center, Indiana University, and University of North Carolina – Chapel Hill; not related to the present work. The remaining authors report no relevant conflicts of interest.
Additional information
Funding
References
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36(10):2179–92. doi:10.1016/j.neubiorev.2012.07.010.
- Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–97. doi:10.1016/S0140-6736(21)00393-7.
- Rice AS, Smith BH, Blyth FM. Pain and the global burden of disease. Pain. 2016;157(4):791–6. doi:10.1097/j.pain.0000000000000454.
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, et al. Epidemiology of DSM-5 alcohol use disorder. JAMA Psychiatry. 2015;72(8):757–66. doi:10.1001/jamapsychiatry.2015.0584.
- Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, Venkateswaran V, Tapp AD, Forouzanfar MH, Salama JS, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–35. doi:10.1016/S0140-6736(18)31310-2.
- Beasley MJ, Macfarlane TV, Macfarlane GJ. Is alcohol consumption related to likelihood of reporting chronic widespread pain in people with stable consumption? Results from UK biobank. Pain. 2016;157(11):2552–60. doi:10.1097/j.pain.0000000000000675.
- Kim CH, Vincent A, Clauw DJ, Luedtke CA, Thompson JM, Schneekloth TD, Oh TH. Association between alcohol consumption and symptom severity and quality of life in patients with fibromyalgia. Arthritis Res Ther. 2013;15(2):R42. doi:10.1186/ar4200.
- Macfarlane GJ, Beasley M, Prescott GJ, McNamee P, Hannaford PC, McBeth J, et al. Alcohol consumption in relation to risk and severity of chronic widespread pain: results from a UK population‐based study. Arthritis Care Res. 2015;67(9):1297–303. doi:10.1002/acr.22604.
- Scott JR, Hassett AL, Schrepf AD, Brummett CM, Harris RE, Clauw DJ, Harte SE. Moderate alcohol consumption is associated with reduced pain and fibromyalgia symptoms in chronic pain patients. Pain Med. 2018;19(12):2515–27. doi:10.1093/pm/pny032.
- Maleki N, Tahaney K, Thompson BL, Oscar-Berman M. At the intersection of alcohol use disorder and chronic pain. Neuropsychology. 2019;33(6):795–807. doi:10.1037/neu0000558.
- Goldberg RT, Pachas WN, Keith D. Relationship between traumatic events in childhood and chronic pain. Disabil Rehabil. 1999;21(1):23–30. doi:10.1080/096382899298061.
- Katon W, Egan K, Miller D. Chronic pain: lifetime psychiatric diagnoses. Am J Psychiatry. 1985;142(10):1156–60.
- Stewart SH, Finn PR, Pihl RO. A dose-response study of the effects of alcohol on the perceptions of pain and discomfort due to electric shock in men at high familial-genetic risk for alcoholism. Psychopharmacology. 1995;119(3):261–7. doi:10.1007/BF02246289.
- Hung H-Y, Chien W-C, Chung C-H, Kao L-T, Chow L-H, Chen Y-H, Kotlińska JH, Silberring J, Huang EY-K. Patients with alcohol use disorder increase pain and analgesics use: a nationwide population-based cohort study. Drug Alcohol Depend. 2021;229(Pt A):109102. doi:10.1016/j.drugalcdep.2021.109102.
- Boissoneault J, Lewis B, Nixon SJ. Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol. 2019;75:47–54. doi:10.1016/j.alcohol.2018.05.009.
- Sheu R, Lussier D, Rosenblum A, Fong C, Portenoy J, Joseph H, Portenoy RK. Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Med. 2008;9(7):911–7. doi:10.1111/j.1526-4637.2008.00420.x.
- Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, Saitz R. Primary care patients with drug use report chronic pain and self-medicate with alcohol and other drugs. J Gen Intern Med. 2016;31(5):486–91. doi:10.1007/s11606-016-3586-5.
- Brennan PL, Schutte KK, Moos RH. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100(6):777–86. doi:10.1111/j.1360-0443.2005.01074.x.
- Witkiewitz K, Vowles KE, McCallion E, Frohe T, Kirouac M, Maisto SA. Pain as a predictor of heavy drinking and any drinking lapses in the COMBINE study and the UK Alcohol Treatment Trial. Addiction. 2015;110(8):1262–71. doi:10.1111/add.12964.
- Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S. Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav. 2013;112:34–41. doi:10.1016/j.pbb.2013.09.008.
- Koob GF. Anhedonia, hyperkatifeia, and negative reinforcement in substance use disorders. In: Pizzagalli DA, editor. Anhedonia: preclinical, translational, and clinical integration (Current Topics in Behavioral Neurosciences; vol. 58). Cham: Springer International Publishing; 2022. p. 147–65. doi:10.1007/7854_2021_288.
- Koob GF. Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev. 2021;73(1):163–201. doi:10.1124/pharmrev.120.000083.
- Patterson JT, Koob GF, Anderson RI. Understanding hyperkatifeia to inform treatment for alcohol use disorder: an assessment of the national institute on alcohol abuse and alcoholism research portfolio. Biol Psychiatry. 2022;91(12):e53–e59. doi:10.1016/j.biopsych.2022.02.011.
- (IASP) IA for the S of P. IASP Terminology. https://www.iasp-pain.org/terminology?navItemNumber=576#Centralsensitization.
- Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. J Appl Behav Res. 2018;23(2):e12137. doi:10.1111/jabr.12137.
- Nijs J, George SZ, Clauw DJ, Fernández-de-las-Peñas C, Kosek E, Ickmans K, Fernández-Carnero J, Polli A, Kapreli E, Huysmans E, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3(5):e383–e392. doi:10.1016/S2665-9913(21)00032-1.
- Hall OJ, Rood K, Phan KL, Clauw DJ. Central sensitization in opioid use disorder: a novel application of the 2011 American College of Rheumatology Fibromyalgia Survey. PAIN Reports; 2022. doi:10.1097/PR9.0000000000001016.
- Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–22. doi:10.3899/jrheum.100594.
- American Psychiatric Association D, Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. 2013.
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi:10.1016/j.jbi.2008.08.010.
- Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–29. doi:10.1016/j.semarthrit.2016.08.012.
- Aoyagi K, He J, Nicol AL, Clauw DJ, Kluding PM, Jernigan S, Sharma NK. A subgroup of chronic low back pain patients with central sensitization. Clin J Pain. 2019;35(11):869–79. doi:10.1097/AJP.0000000000000755.
- Basu N, Kaplan CM, Ichesco E, Larkin T, Harris RE, Murray A, Waiter G, Clauw DJ. Neurobiologic features of fibromyalgia are also present among rheumatoid arthritis patients. Arthritis Rheumatol. 2018;70(7):1000–7. doi:10.1002/art.40451.
- Neville SJ, Clauw AD, Moser SE, Urquhart AG, Clauw DJ, Brummett CM, Harte SE. Association between the 2011 Fibromyalgia survey criteria and multisite pain sensitivity in Knee Osteoarthritis. Clin J Pain. 2018;34(10):909–17. doi:10.1097/AJP.0000000000000619.
- Nicol A, Arnold P, Clauw D. (321) Fibromyalgia-ness in persistent low back pain after lumbar spine surgery: a preliminary investigation. J Pain. 2017;18(4):S55. doi:10.1016/j.jpain.2017.02.214.
- Williams DA. Phenotypic features of central sensitization. J Appl Biobehav Res. 2018;23(2):e12135. doi:10.1111/jabr.12135.
- Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long‐term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67(5):1386–94. doi:10.1002/art.39051.
- Brummett CM, Janda AM, Schueller CM, Tsodikov A, Morris M, Williams DA, Clauw DJ. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology. 2013;119(6):1434–43. doi:10.1097/ALN.0b013e3182a8eb1f.
- Clauw DJ. Fibromyalgia: a clinical review. Jama. 2014;311(15):1547–55. doi:10.1001/jama.2014.3266.
- Clauw DJ, Hassett AL. The role of centralised pain in osteoarthritis. Clin Exp Rheumatol. 2017;35(Suppl 107):79–84.
- Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. 2021;397(10289):2098–110. doi:10.1016/S0140-6736(21)00392-5.
- Janda AM, As-Sanie S, Rajala B, Tsodikov A, Moser SE, Clauw DJ, Brummett CM. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122(5):1103–11. doi:10.1097/ALN.0000000000000637.
- Wolfe F. Fibromyalgianess. Arthritis Care & Research: Official Journal of the American College of Rheumatology. 2009 Jun 15;61(6):715–6.
- Fitzcharles MA, Ste-Marie PA, Panopalis P, Ménard H, Shir Y, Wolfe F. The 2010 American college of rheumatology fibromyalgia survey diagnostic criteria and symptom severity scale is a valid and reliable tool in a French speaking fibromyalgia cohort. BMC Musculoskelet Disord. 2012;13(1):179. doi:10.1186/1471-2474-13-179.
- Galvez-Sánchez CM, de la Coba P, Duschek S, Reyes del Paso GA. Reliability, factor structure and predictive validity of the widespread pain index and symptom severity scales of the 2010 American College of Rheumatology Criteria of Fibromyalgia. J Clin Med. 2020;9(8):2460. doi:10.3390/jcm9082460.
- Usui C, Hatta K, Aratani S, Yagishita N, Nishioka K, Kanazawa T, Ito K, Yamano Y, Nakamura H, Nakajima T, et al. The Japanese version of the 2010 American College of rheumatology preliminary diagnostic criteria for fibromyalgia and the fibromyalgia symptom scale: reliability and validity. Mod Rheumatol. 2012;22(1):40–4. doi:10.3109/s10165-011-0462-3.
- Aguirre Cárdenas C, Oñederra MC, Esparza Benavente C, Durán J, González Tugas M, Gómez-Pérez L. Psychometric properties of the fibromyalgia survey questionnaire in Chilean women with fibromyalgia. J Clin Rheumatol. 2021;27(6S):S284–S293. doi:10.1097/RHU.0000000000001547.
- Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350–7. doi:10.3109/07853890109002089.
- Coons SJ, Alabdulmohsin SA, Draugalis JR, Hays RD. Reliability of an Arabic version of the RAND-36 Health Survey and its equivalence to the US-English version. Med Care. 1998;36(3):428–32. doi:10.1097/00005650-199803000-00018.
- Brazier JE, Harper R, Jones NM, O’’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. doi:10.1136/bmj.305.6846.160.
- Moorer P, Foets M, Molenaar IW. Suurmeije ThP Psychometric properties of the RAND-36 among three chronic disease (multiple sclerosis, rheumatic diseases and COPD) in the Netherlands. Qual Life Res. 2001;10(7):637–45. doi:10.1023/a:1013131617125.
- Vander Zee KI, Sanderman R, Heyink JW, de Haes H. Psychometric qualities of the RAND 36-Item Health Survey 1.0: a multidimensional measure of general health status. Int J Behav Med. 1996;3(2):104–22. doi:10.1207/s15327558ijbm0302_2.
- Gijsberts CM, Agostoni P, Hoefer IE, Asselbergs FW, Pasterkamp G, Nathoe H, Appelman YE, de Kleijn DPV, den Ruijter HM. Gender differences in health-related quality of life in patients undergoing coronary angiography. Open Heart. 2015;2(1):e000231. doi:10.1136/openhrt-2014-000231.
- Hall OT, Teater J, Entrup P, Deaner M, Bryan C, Harte SE. Fibromyalgia predicts increased odds of pain-related addiction exacerbation among individuals with pain and opioid use disorder. PAIN. 2022;2022:78. doi:10.1097/j.pain.0000000000002878.
- Partial rank correlations in SPSS [Internet]. 2020. [accessed 2023 Jun 17]. https://www.ibm.com/support/pages/partial-rank-correlations-spss.
- Conover WJ. Practical nonparametric statistics. Vol. 350. New York: John Wiley & Sons; 1999.
- Cohen J. Statistical power analysis for the behavioral sciences. Cambridge: Academic Press; 2013.
- George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology. 2014;231(19):3911–7. doi:10.1007/s00213-014-3623-1.
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Research & Health. 2008;31(3):185.
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Behav Neurobio Alcohol Addict. 2011;13:3–30.
- Cucinello-Ragland JA, Edwards S. Chapter one – neurobiological aspects of pain in the context of alcohol use disorder. In: Calipari ES, Gilpin NW, editors. International review of neurobiology Neurobiology of Addiction and Co-Morbid Disorders; vol. 157). Cambridge: Academic Press; 2021, p. 1–29. https://www.sciencedirect.com/science/article/pii/S0074774220301392
- Karimi R, Mallah N, Nedjat S, Beasley MJ, Takkouche B. Association between alcohol consumption and chronic pain: a systematic review and meta-analysis. Br J Anaesth. 2022;129(3):355–65. doi:10.1016/j.bja.2022.03.010.
- Gatch MB. Ethanol Withdrawal and Hyperalgesia. Curr Drug Abuse Rev. 2009;2(1):41–50. doi:10.2174/1874473710902010041.
- Jochum T, Boettger MK, Burkhardt C, Juckel G, Bär KJ. Increased pain sensitivity in alcohol withdrawal syndrome. Eur J Pain. 2010;14(7):713–8. doi:10.1016/j.ejpain.2009.11.008.
- Imtiaz S, Loheswaran G, Le Foll B, Rehm J. Longitudinal alcohol consumption patterns and health-related quality of life: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Rev. 2018;37(1):48–55. doi:10.1111/dar.12503.
- Kutch JJ, Ichesco E, Hampson JP, Labus JS, Farmer MA, Martucci KT, Ness TJ, Deutsch G, Apkarian AV, Mackey SC, et al. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. PAIN. 2017;158(10):1979–91. doi:10.1097/j.pain.0000000000001001.
- Pickering G, Achard A, Corriger A, Sickout-Arondo S, Macian N, Leray V, Lucchini C, Cardot J-M, Pereira B. Electrochemical skin conductance and quantitative sensory testing on fibromyalgia. Pain Pract. 2020;20(4):348–56. doi:10.1111/papr.12857.
- Staud R, Godfrey MM, Robinson ME. Fibromyalgia patients are not only hypersensitive to painful stimuli but also to acoustic stimuli. J Pain. 2021;22(8):914–25. doi:10.1016/j.jpain.2021.02.009.
- Wodehouse T, Poply K, Ramaswamy S, Snidvongs S, Bourke J, Tahir H, Ullrich K, Mehta V. A pilot study investigating whether quantitative sensory testing alters after treatment in patients with fibromyalgia. Br J Pain. 2018;12(4):250–6. doi:10.1177/2049463718776336.
- Dudeney J, Law EF, Meyyappan A, Palermo TM, Rabbitts JA. Evaluating the psychometric properties of the Widespread Pain Index and the Symptom Severity Scale in youth with painful conditions. Can J Pain. 2019;3(1):137–47. doi:10.1080/24740527.2019.1620097.
- Trofimovitch D, Baumrucker SJ. Pharmacology update: low-dose naltrexone as a possible nonopioid modality for some chronic, nonmalignant pain syndromes. Am J Hosp Palliat Care. 2019;36(10):907–12. doi:10.1177/1049909119838974.
- McDowell TS. Neurotransmitters involved in pain modulation. In: Abd-Elsayed A, editor. Pain: a review guide. Cham: Springer International Publishing; 2019. p. 49–51. doi:10.1007/978-3-319-99124-5_12.
- Witkiewitz K, Saville K, Hamreus K. Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility. Ther Clin Risk Manag. 2012;8:45–53. doi:10.2147/TCRM.S23184.
- Mutschler J, Grosshans M, Soyka M, Rösner S. Current findings and mechanisms of action of disulfiram in the treatment of alcohol dependence. Pharmacopsychiatry. 2016;49(4):137–41. doi:10.1055/s-0042-103592.
- Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9(5):417–22. doi:10.1016/j.jpain.2007.12.006.
- Harte SE, Ichesco E, Hampson JP, Peltier SJ, Schmidt-Wilcke T, Clauw DJ, Harris RE. Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula. Pain. 2016;157(9):1933–45. doi:10.1097/j.pain.0000000000000593.