Abstract
Rationale
The opioid crisis in North America has recently seen a fourth wave, which is dominated by drug-related deaths due to the combined use of illicitly manufactured fentanyl [IMF] and stimulants such as cocaine and methamphetamine.
Objectives
A systematic review addressing the question why drug users combine opioids and stimulants and why the combination results in such a high overdose mortality: from specific and dangerous pharmacokinetic or pharmacodynamic interactions or from accidental poisoning?
Results
Motives for the combined use include a more intensive high or rush when used at the same time, and some users have the unfounded and dangerous belief that co-use of stimulants will counteract opioid-induced respiratory depression. Overdose deaths due to combined (intravenous) use of opioids and stimulants are not likely to be caused by specific pharmacokinetic or pharmacodynamic interactions between the two drugs and it is unlikely that the main cause of overdose deaths is due to accidental poisoning.
Conclusion
The unexpectedly high overdose rates in this population could not be attributed to accidental overdosing or pharmacokinetic/pharmacodynamic interactions. The most likely explanation for the high rate of drug-related deaths in opioid-cocaine co-users is careless overdosing with either cocaine, opioid(s) or both, probably facilitated by the high level of preexisting impulsivity in these co-users and a further acute increase in impulsivity following cocaine use. The primary corollary is that cocaine users should avoid IMF use in the same time window. In addition, IMF users should refrain from cocaine use to avoid impulsive IMF overdosing.
Introduction
Epidemiology
In the US, the triple wave of the opioid crisis (1st wave: prescription opioids, 2nd wave: heroin, 3rd wave: illicitly manufactured fentanyls [IMFs]) has recently seen a 4th wave, which is dominated by drug-related deaths due to the combination of IMFs and stimulants such as cocaine and methamphetamine.Citation1,Citation2 Between 2002–2017 in the U.S., co-use of cocaine and heroin increased linearly (past-30-day use; AOR = 1.06; 95% CI: 1.03–1.10)Citation3 which is also reflected in a rising number of cocaine-related (fatal) overdoses.Citation2,Citation4
The term “opioid” is generally meant to be the broader term, and includes natural opiates (e.g.,opium, morphine, codeine), semi-synthetic derivatives of opiates (e.g., heroin, hydromorphone, oxycodone), and synthetic compounds (i.e., fentanyl, fentanyl analogues, methadone, tramadol). In this review, opioid refers to any opioid (natural, semi-synthetic or synthetic) if not further specified.
Opioids, notably IMFs, have a much smaller safety margin (between desired and lethal dose) than cocaine, and opioids are thus far more life-threatening than cocaine. So, it is understandable that the increased number of overdose-deaths involving opioids, specifically fentanyl, accounted for most of the increase in cocaine-involved overdose deaths in recent yearsCitation2,Citation5–7 and continue to contribute to the public health burden in the U.S. The rate of drug-related overdose deaths involving both cocaine and opioids increased nearly 5.5 times from 0.7 per 100,000 in 2009 to 3.8 per 100,000 in 2019, while the rate of overdose deaths involving cocaine without opioid involvement increased just over 1.5 times from 0.7 to 1.1.Citation6 Between 2016–2017, death rates involving both cocaine and opioids, particularly synthetic opioids increased dramatically from 1.3 to 2.3 per 100,000.Citation7 In 2019, any opioid and synthetic opioids were involved in 76% and 64% of cocaine-involved overdoses, respectivelyCitation6, Citation8 suggesting that increases in cocaine-involved overdose deaths in 2019 were mainly driven by the co-use of (synthetic) opioids. With regard to cocaine-involved non-fatal overdoses with and without an opioid, the rate increased between 2006–2016 annually by 14.7% and 11.3%, respectively; in 2016, 27% of non-fatal cocaine-involved overdoses including an opioid.Citation7 According to National Poison Control data (N = 15,391; reported between 2015–2021), the proportion of fatal and non-fatal fentanyl-related poisonings in the U.S. increased for co-use of methamphetamine by 669% and for cocaine by 374%.Citation9 Similarly, in British Columbia, Canada, health insurance data from a random general population sample (N = 1,089,682) showed a two-fold higher risk for fatal overdose in people who used both opioids and stimulants compared to people who used opioids only: HR= 2.02 (95% CI: 1.47–2.78, p < 0.001).Citation10 Finally, more than half of treatment-seeking opioid users in the U.S. reported regular stimulant useCitation11,Citation12 and between 2012–2019, the number of heroin-cocaine treatment admissions increased 2.3-foldCitation13 with a RRR of 2.4 (95% CI: 2.34–2.46) for non-Hispanic Black, compared to non-Hispanic White patients.Citation13
Public health concern
The growing co-use of opioids and cocaine (and other stimulants) presents significant challenges for public health. In fact, the dramatic increase in fatal opioid overdosing together with stimulants points to stimulant use as a potential risk factor in these drug-related deaths, especially if these drugs are injected. Of those who died from co-used opioids and stimulants in January–June 2019, nearly 34.8% had injected the drugs.Citation14 Used in combination, opioids and cocaine may mask typical clinical presentations of one another, impeding a rapid diagnosis of the overdose when arriving at the critical care unit and its treatment.Citation15 Furthermore, the combined use of opioids and cocaine impedes and confounds the assessment of the cause-of-death and the specific overdose risks associated with each of the two drugs. Scientific understanding on the factors contributing to opioid overdose deaths involving cocaine is lacking.Citation16 Considering that opioids, and especially fentanyl, are far more toxic than cocaine, the increase in cocaine-involved overdose deaths between 2012–2017 in the U.S. is presumably driven by fentanyl and other opioids.Citation7,Citation8 However, the available overdose studies about the co-use of cocaine and opioids do not provide information about the cause of death. In the current systematic review, we try to establish why the combined use of cocaine with opioids results in such a high overdose mortality. The main questions that we address here are why people co-use cocaine and opioids and whether overdose death from cocaine-opioid use result from specific and dangerous pharmacokinetic or pharmacodynamic interactions or from accidental poisoning or overdosing?
Motives to co-use cocaine and opioids
There has traditionally been a subset of opioid users who prefer to simultaneously inject a stimulant, typically cocaine, together with an opioid in what has been colloquially referred to as speedballing (i.e., simultaneous use of heroin and cocaine) and a common practice for decades.Citation17 Users report that the simultaneous use of stimulants and opioids is more reinforcing than either drug alone i.e., a synergistic high giving a more intense euphoria, or rush.Citation18,Citation19 However, Leri et al. (2003) claimed that simultaneous co-use of cocaine and heroin does not induce a novel set of subjective effects and that it is not more reinforcing than either drug alone, especially when the doses of heroin and cocaine are high. Although the results are not ubiquitous (for review see ref.Citation20), pre-clinical data endorse that cocaine plus heroin was in self-administration paradigms more rewarding than cocaine or heroin aloneCitation21 and self-administration of cocaine in Rhesus monkeys was enhanced by otherwise inactive doses of heroin.Citation22 Accordingly, self-administration of both cocaine and heroin produces a synergistic increase in extracellular dopamine concentration in the nucleus accumbens, the reward pathwayCitation23,Citation24 and similar supra-additive effects have been noted in comparable studies in both rodentsCitation21, Citation25,Citation26 and non-human primates,Citation22, Citation27–29 though one study in Rhesus monkeys showed a sub-additive effectCitation30 and another study reported apparently discrepant results regarding synergy.Citation31 Similarly, the combination of methamphetamine and morphine produced behavioral stimulation that was dramatically stronger than either drug alone.Citation32,Citation33
Mixing cocaine with opioids is sometimes used to ‘mellow down’ the ‘upper’ effects of cocaine or - conversely - cocaine is used to counteract the depressant after-effects of the opioid. Opioids, and especially fentanyl, induce heavy sedation and a tranquilizing effect that make daily functioning arduous, inciting the (subsequent) use of cocaine or methamphetamine to counteract the fentanyl effectCitation34 As such, this drug combination may derive from the reduction of the unwanted side effects of one drug by the other due to compensatory mechanisms of action and/or from enhanced effects of the combination.Citation17 Furthermore, cocaine may alleviate the severity of symptoms of withdrawal from heroin.Citation17 Finally, some users have the unfounded and dangerous belief that co-use of stimulants will counteract opioid-induced respiratory depression, because - as outlined below - cocaine does not impact brain hypoxia induced by high doses of heroin.Citation35
Risks of combined use
Mixing heroin and cocaine increases the risk of overdose or permanent damage to the body. It is especially risky because taking an opioid (a depressant) and cocaine (a stimulant) together causes a dangerous “push-pull” reaction in the body which can result in an irregular heart rate, heart failure, and death. In addition, regular cocaine but opioid-naïve users may suddenly die from a heroin overdose because they are not (yet) tolerant for the opioid. Moreover, qualitative interviews documented that cocaine users who have no history of opioid use are largely unprepared to recognize and respond to an overdose.Citation36 Indeed, mortality risk and excess mortality were significantly greater among subjects with cocaine-opiate use disorder than those with only cocaine use disorder.Citation37
Methods
A systematic literature review was performed on October 10th, 2023 to retrieve studies, including studies published or ahead of print about risk factors that severely increase the mortality of combined use of opioids and cocaine. Our search for relevant data was focussed on the most prominent factors and mechanisms involved in fatalities reported following use of either opioids or cocaine, like respiratory depression and cardiac effects, respectively. In a first step, handbooks on basic pharmacology and drug abuseCitation38,Citation39 were consulted to select the pathophysiological mechanisms, where opioids and cocaine could adversely interact, resulting in and explaining the excess mortality observed following the use of this combination. The mechanisms included effects on their common metabolic routes which could lead to an acute firm increase in internal dose of either opioids or cocaine when used in combination.
Using the PRISMA-protocol and focussing on these suspected interactions, appropriate key words were selected to conduct a systematic literature search using Medline (PubMed) and EMBASE to detect studies performed in humans and published since 1995. Key words selected were: “overdose”, “cardiovascular”, “arrhythmia*” “respiratory”, “carboxylesterase”, “decision-making” and “impulsivity”. This search string was combined with the string “(fentanyl or opioid or heroin or methadone or speedball) and with cocaine”. Exclusion criteria: case reports, letters or commentaries.
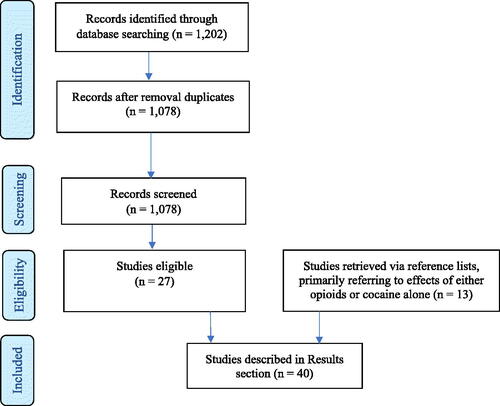
The selection of appropriate studies was performed by JvA and WvdB in two rounds. A total of 1,202 studies were identified from the initial search and 1,078 articles remained after duplicates were removed. These 1,078 studies were further processed, i.e., the title and abstract were screened to determine eligibility applying the above inclusion and exclusion criteria. In a second round, the full texts of the selected 27 studies were checked for eligibility. In addition, 12 additional relevant studies, primarily referring to effects of either opioids or cocaine alone, were retrieved via the reference lists of the previously selected 27 studies (e.g. reviews), resulting in 39 studies in total; 35 have been described in the Results section (see also ) and four in the Discussion. shows the PRISMA flow chart for the identification, screening, and inclusion of the studies. See ‘Supplementary material’ for PRISMA checklist.
Table 1. Studies retrieved with details about number of subjects and gender.
Pharmacokinetic interactions
Pharmacokinetic interactions i.e., drug-drug interactions (DDIs), occur when two substances are metabolized by the same enzymes or the same metabolic route. As a result, the two substances compete for the same metabolic enzymes so that one of the two substances can prolong and potentiate the biological effects of the other. As explained below this may be the case with cocaine and heroin when co-used.
Cocaine is a pharmacologically active compound, which is primarily further metabolized by plasma choline-esterase and two liver carboxylesterases (human carboxylesterase hCE-1 and hCE-2) to ecgonine methyl ester and benzoylecgonine which are both pharmacologically inactive.Citation40, Citation45 Heroin (diacetylmorphine) is an inactive prodrug which is rapidly converted in two steps to pharmacologically active 6-monoacetylmorphine (6-MAM) and morphineCitation43 by liver hCE-2 and hCE-1 and plasma cholinesterase.Citation40, Citation44,Citation45 Cocaine and heroin have therefore a common metabolic pathway, i.e., they compete for the same metabolizing enzymes. The metabolic steps differ, however, in kinetics: the Km-value of hCE1 and hCE2 for cocaine is 0.12 and 0.39 mM, respectively and for heroin 6.3 and 6.8 mM, respectively. This implicates that both carboxylesterases inactivate cocaine 17 to 52-times more efficient (rapid) than heroin.Citation44 Furthermore, cocaine competitively inhibits hCE-1- and hCE-2-hydrolysis of heroin to 6-MAM (Ki = 530 and 460 µM, respectively) and of 6-MAM hydrolysis to morphine (Ki = 710 and 220 µM, respectively) with very modest potency.Citation42 In conclusion: cocaine and heroin show a pharmacokinetic interaction, but considering the large differences in affinities (Km and Ki) between cocaine and heroin for their metabolizing enzymes, this interaction is very modest and does not lead to prominent elevations in heroin plasma levels when used in combination with cocaine. Another pharmacokinetic may occur between cocaine and fentanyl, as cocaine, like fentanyl, can also be metabolized via CYP3A4 into norcocaine. Drug-drug interaction studies where plasma cocaine concentrations were simulated with and without the presence of fentanyl showed, however, that the predicted AUC-profiles of cocaine were very similar and therefore unaffected by fentanyl probably, because cocaine is mainly metabolized by cholinesterase enzymes.Citation41 Therefore, the pharmacokinetic interaction between cocaine and either heroin or fentanyl is not likely to seriously contribute to the high rate of cocaine-heroin overdose deaths.
Pharmacodynamic interactions
Respiratory depression
Bronchial hyperreactivity has been reported following inhalation of heroin mixed with cocaine,Citation46 but this is unlikely to lead to fatalities.
Respiration is controlled primarily by respiratory centers in the brainstem along with input from peripheral chemoreceptors and other sources.Citation71 via direct depression of the respiratory center in the medulla of the central nervous system, opioids may induce respiratory depression that can progress to hypoventilation, apnea, brain hypoxia (low cerebral oxygen level) and ultimately death.
Small doses of cocaine cause acute respiratory stimulation while very high doses of cocaine, like opioids, may cause respiratory arrest.Citation72 At the same time, cocaine causes peripheral vasoconstriction and a dose dependent cerebral vasodilation increasing cerebral blood flow facilitating oxygen entry into the brain. For example, it has been shown that cocaine delivered to rats at doses below 0.25 mg/kg and at optimal self-administration doses (0.5 and 1.0 mg/kg) modestly increased oxygen levels in the nucleus accumbens. Heroin at a low reinforcing dose (0.05 mg/kg) also increased oxygen levels in the nucleus accumbens, but it caused a strong monophasic oxygen decrease in the nucleus accumbens during overdose (0.6 mg/kg). When combined at moderate doses, cocaine (0.25 and 0.5 mg/kg) slightly increased the oxygen increases induced by heroin alone (0.5 and 0.1 mg/kg), but when moderate doses of cocaine were combined at high doses of heroin (1 mg/kg cocaine plus 0.2–0.6 mg/kg heroin) oxygen decreases in the nucleus accumbens due to heroin were no longer compensated by oxygen increases due to cocaine, resulting in oxygen decreases similar to high doses of heroin alone.Citation35
Still, mixing heroin with a stimulant, like cocaine, is hazardous, because cocaine increases the oxygen demand while the depressant heroin reduces the breathing rate. As the opioid receptor-like receptor (ORL1) appears to be implicated in the rewarding and addictive properties of cocaine and other drugs of abuse,Citation73–76 it cannot be excluded that cocaine and opioids (also) interact at the level of respiratory control via mu and delta opioid receptors.
In summary, concurrent cocaine use cannot prevent overdose (deaths) due to respiratory depression and hypoxia in cases of opioid overdosing. However, high doses of cocaine may result in reduced cerebral oxygenation and respiratory failure giving an additive effect on respiratory arrest when cocaine and opioids are simultaneously used. This may partly explain the high mortality in opioid-cocaine co-users.
Cardiovascular complications
The use of cocaine is associated with numerous adverse cardiovascular effects, including cardiac arrythmias, myocardial infarction, and neurological effects like headache, seizure, and strokeCitation57,Citation58 while fatal cocaine overdosing is typically caused by cardiac arrhythmias, seizures or respiratory failureCitation38 with cardiovascular complications accounting for the majority of cocaine-related deaths.Citation55,Citation56, Citation58 Physiologically, acute administration of cocaine causes - in addition to tachycardia and hypertension - an increase in myocardial oxygen consumption i.e., a higher oxygen demand.Citation38
In contrast to cocaine, heroin overdose is clearly and specifically identified by reduced consciousness, miosis, and respiratory depression.Citation38,Citation39 In general, no prominent cardiovascular effects are observed following heroin use, but the following acute opioid-induced cardiovascular effects may occur: orthostasis, syncope, and bradycardia with hypotension primarily through the µ-opioid receptor-mediated vasodilatation. Experienced opiate users treated i.v. with morphine (0, 5 and 10 mg/70 kg) administered in combination with and without cocaine (0, 8, 16 and 32 mg/70 kg) showed significantly increased peak heart rate, diastolic pressure and systolic pressure, but the effects were mainly driven by cocaine.Citation47 In addition, cocaine use is a known risk factor for QTc interval prolongation, which can lead to life-threatening dysrhythmias including Torsades de Pointes.Citation54 Therefore, a possible dangerous pharmacodynamic interaction may occur in methadone maintenance patients who also use cocaine,Citation52 because through the same mechanism both methadoneCitation50,Citation51 and cocaineCitation48,Citation49, Citation54 may prolong the QTc-interval and the risk of (fatal) ventricle fibrillation.Citation52 In conclusion, with exception of patients treated with methadone and those with opioid withdrawal, which may go along with adverse cardiovascular events, no major cardiovascular complications are expected to occur when cocaine is co-used with heroin or fentanyl. Thus, hazardous cardiovascular interactions cannot explain the high incidence in opioid-cocaine fatal cases, because there was no major increase in methadone use or use of higher methadone doses during the 4th wave of the opioid epidemic.
Overall
Based on the above, it is obvious that health dangers of speedballing may result from de-compensation of vital functions due to diminished intra-brain oxygen inflow induced by high-dose heroin coupled with an enhanced (cardiac) oxygen demand induced by cocaine.Citation35, Citation53
Accidental overdosing
Impulsivity and risk-taking
Heroin and cocaine abusers, as well as heroin cocaine i.v. co-use, have higher impulsivity (discount rates for delayed rewards) than non-drug-using controls.Citation62,Citation63 A recent meta-analysis with 59 studies showed that individuals with a history of cocaine or opioid use had increased risky decision-making when compared to controls.Citation60 In particular, chronic cocaine users showed higher levels of impulsivity, sensation seeking and risk-taking propensity than heroin usersCitation59, Citation67 and cocaine-dependent subjects were more impulsive than non-drug using subjects.Citation64,Citation65 Similarly, a cross-sectional observational study showed that patients with lifetime cocaine dependence, and those with comorbid cocaine and opiate dependence were more impulsive than subjects with opiate dependence alone.Citation66 Together, these observations draw attention to the role of impulsivity in (fatal) overdosing in mixed cocaine-opioid users. In addition to these apparent personality traits of individuals with a history of cocaine use, acute cocaine use has been shown to cause an increase in extracellular dopamine with an overstimulation of the dopaminergic postsynaptic receptors, inducing excitation and the euphoric ‘rush’.Citation61 Under such circumstances, the already predisposed (impulsive) user may become reckless and careless which may facilitate overdosing with either cocaine, any opioid(s) or both. Moreover, cocaine’s relatively short half-life of 40 to 90 min may contribute to multiple dosing and thus erroneous handlings.
Adulteration of cocaine with potent opioids
Because of an emerging trend to lace cocaine with opioids,Citation68, Citation70 many cocaine users are unaware of highly potent opioids in their cocaine supply. For instance, findings from a survey among 172 participants with past-month cocaine and/or opioid use showed that only 5.4% of cocaine users without an opioid use history suspected that fentanyl was in their drugs, compared to 41.2% of their peers with an opioid use history.Citation36 The unpredictable fluctuation in quality generates uncertainty for consumers about their cocaine supply and increases the risk for unintentional exposure to highly potent opioids. Obviously, the latter population is at higher risk of opioid overdosing since they are more likely to be opioid-naïve (read: no opioid tolerance). Fortunately, there is a growing awareness among people who attend nightclubs and dance festivals that cocaine can contain fentanyl.Citation69
Summary and conclusions
Because of the much smaller safety margin (between desired and lethal dose) of opioids, notably IMFs, compared to cocaine, opioids are far more life-threatening than cocaine. Their combined use was shown to lead to a higher mortality compared to the use of either drug alone. Their combined use was shown to lead to a higher mortality compared to the use of either drug alone. For instance, their simultaneous intravenous use as injected speedballs, is a known predictor of overdose and overdose deaths.Citation77 The high mortality of combined use of cocaine and heroin/opioids does, however, not result from pharmacokinetic and probably also not from pharmacodynamic interactions between the two types of drugs.
Deception of cocaine users by offering them opioid adulterated cocaine may easily lead to fatal overdosing. However, qualitative findings, suggest that the increase in overdose deaths involving both highly potent opioids (IMFs) and stimulants is largely due to intentional co-use and not driven by systemic adulteration of illicit stimulants with IMFs.Citation78–80 Therefore, the unexpectedly high overdose rates in this population could not be attributed to accidental overdosing, pharmacokinetic or pharmacodynamic interactions, whereas trait/state impulsivity is a possible risk factor for (fatal) overdosing in this population.
It is tentatively concluded that the high overdose mortality rate in mixed cocaine-opioid users may at least partly result from careless overdosing with either cocaine, any opioid(s) or both, facilitated by a combination of already existing high (trait) impulsivity in combination with an acute increase in (state) impulsivity due to cocaine use resulting in extreme risk-taking behaviors. The issue currently addressed is not unique to cocaine and opioid use, but probably also extends to combined methamphetamine and opioid use which presumably also has an increased mortality risk compared to the use of either drug alone. However, the scientific literature of combined cocaine-opioid use is more developed and the incorporation of methamphetamine-opioid co-use would be too much for one review and affect readability. Whatever the mechanism/reason, cocaine-opioid co-users are at increased risk for mortality compared to opioid-only users. The two primary implications of this conclusion are that in the same time window (a) cocaine users should avoid IMF use, and (b) IMF users should refrain from cocaine use to avoid impulsive IMF overdosing.
Supplement.docx
Download MS Word (22.4 KB)Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Ahmed S, Sarfraz Z, Sarfraz A. A changing epidemic and the rise of opioid-stimulant co-use. Front Psychiatry. 2022; 13:918197. doi:10.3389/fpsyt.2022.918197.
- Ciccarone D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry. 2021; 34(4):344–50. doi:10.1097/YCO.0000000000000717.
- Goodwin RD, Moeller SJ, Zhu J, Yarden J, Ganzhorn S, Williams JM. The potential role of cocaine and heroin co-use in the opioid epidemic in the United States. Addict Behav. 2021; 113:106680. doi:10.1016/j.addbeh.2020.106680.
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021; 70(6):202–7. doi:10.15585/mmwr.mm7006a4.
- Nolan ML, Shamasunder S, Colon-Berezin C, Kunins HV, Paone D. Increased presence of fentanyl in cocaine-involved fatal overdoses: implications for prevention. J Urban Health. 2019; 96(1):49–54. doi:10.1007/s11524-018-00343-z.
- Hedegaard H, Miniño AM, Warner M. Co-involvement of opioids in drug overdose deaths involving cocaine and psychostimulants. NCHS Data Brief. 2021;406(406):1–8.
- Hoots B, Vivolo-Kantor A, Seth P. The rise in non-fatal and fatal overdoses involving stimulants with and without opioids in the United States. Addiction. 2020; 115(5):946–58. doi:10.1111/add.14878.
- Kariisa M, Seth P, Scholl L, Wilson N, Davis NL. Drug overdose deaths involving cocaine and psychostimulants with abuse potential among racial and ethnic groups - United States, 2004-2019. Drug Alcohol Depend. 2021; 227:109001. doi:10.1016/j.drugalcdep.2021.109001.
- Palamar JJ, Cottler LB, Goldberger BA, Severtson SG, Grundy DJ, Iwanicki JL, Ciccarone D. Trends in characteristics of fentanyl-related poisonings in the United States, 2015-2021. Am J Drug Alcohol Abuse. 2022; 48(4):471–80. doi:10.1080/00952990.2022.2081923.
- Palis H, Xavier C, Dobrer S, Desai R, Sedgemore KO, Scow M, Lock K, Gan W, Slaunwhite A. Concurrent use of opioids and stimulants and risk of fatal overdose: a cohort study. BMC Public Health. 2022; 22(1):2084. doi:10.1186/s12889-022-14506-w.
- Kidorf M, Solazzo S, Yan H, Brooner RK. Psychiatric and substance use comorbidity in treatment-seeking injection opioid users referred from syringe exchange. J Dual Diagn. 2018; 14(4):193–200. doi:10.1080/15504263.2018.1510148.
- Lee JD, Nunes EV, Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X: BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309–18. doi:10.1016/s0140-6736(17)32812-x.
- Blaes SL, Shimp KG, Betzhold SM, Setlow B, Orsini CA. Chronic cocaine causes age-dependent increases in risky choice in both males and females. Behav Neurosci. 2022; 136(3):243–63. doi:10.1037/bne0000509.
- O'Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL. Vital signs: characteristics of drug overdose deaths involving opioids and stimulants - 24 states and the district of Columbia, January-June 2019. MMWR Morb Mortal Wkly Rep. 2020; 69(35):1189–97. doi:10.15585/mmwr.mm6935a1.
- Glidden E, Suen K, Mustaquim D, Vivolo-Kantor A, Brent J, Wax P, Aldy K. Characterization of nonfatal opioid, cocaine, methamphetamine, and polydrug exposure and clinical presentations reported to the Toxicology Investigators Consortium Core Registry, January 2010-December 2021. J Med Toxicol. 2023;19(2):180–9. doi:10.1007/s13181-022-00924-0.
- Kariisa M, Scholl L, Wilson N, Seth P, Hoots B. Drug overdose deaths involving cocaine and psychostimulants with abuse potential - United States, 2003-2017. MMWR Morb Mortal Wkly Rep. 2019; 68(17):388–95. doi:10.15585/mmwr.mm6817a3.
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003; 98(1):7–22. doi:10.1046/j.1360-0443.2003.00236.x.
- Chatterjee A, Lopez D, Ramkellawan S, Brown R, Smith K, Gaeta JM, Baggett TP. That’s what we call the cocktail”: Non-opioid medication and supplement misuse among opioid users. Subst Abus. 2021; 42(2):175–82. doi:10.1080/08897077.2019.1671943.
- Lopez AM, Dhatt Z, Howe M, Al-Nassir M, Billing A, Artigiani E, Wish ED. Co-use of methamphetamine and opioids among people in treatment in Oregon: a qualitative examination of interrelated structural, community, and individual-level factors. Int J Drug Policy. 2021; 91:103098. doi:10.1016/j.drugpo.2020.103098.
- Maguire DR, Minervini V. Interactions between opioids and stimulants: Behavioral pharmacology of abuse-related effects. Adv Pharmacol. 2022; 93:1–33. doi:10.1016/bs.apha.2021.10.002.
- Ranaldi R, Munn E. Polydrug self-administration in rats: cocaine-heroin is more rewarding than cocaine-alone. Neuroreport. 1998; 9(11):2463–6. doi:10.1097/00001756-199808030-00007.
- Rowlett JK, Woolverton WL. Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacology (Berl)). 1997; 133(4):363–71. doi:10.1007/s002130050415.
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999; 288(1):274–80.
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006; 31(1):139–50. doi:10.1038/sj.npp.1300786.
- Duvauchelle CL, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacol Biochem Behav. 1998; 61(3):297–302. doi:10.1016/s0091-3057(98)00098-7.
- Martin TJ, Kahn W, Cannon DG, Smith JE. Self-administration of heroin, cocaine and their combination under a discrete trial schedule of reinforcement in rats. Drug Alcohol Depend. 2006; 82(3):282–6. doi:10.1016/j.drugalcdep.2005.11.018.
- Rowlett JK, Wilcox KM, Woolverton WL. Self-administration of cocaine-heroin combinations by rhesus monkeys: antagonism by naltrexone. J Pharmacol Exp Ther. 1998; 286(1):61–9.
- Winger G, Galuska CM, Hursh SR, Woods JH. Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. J Pharmacol Exp Ther. 2006; 318(1):223–9. doi:10.1124/jpet.105.100461.
- Woolverton WL, Wang Z, Vasterling T, Tallarida R. Self-administration of cocaine-remifentanil mixtures by monkeys: an isobolographic analysis. Psychopharmacology (Berl)). 2008; 198(3):387–94. doi:10.1007/s00213-008-1152-5.
- Negus SS. Interactions between the reinforcing effects of cocaine and heroin in a drug-vs-food choice procedure in rhesus monkeys: a dose-addition analysis. Psychopharmacology (Berl)). 2005; 180(1):115–24. doi:10.1007/s00213-004-2133-y.
- Ward SJ, Morgan D, Roberts DC. Comparison of the reinforcing effects of cocaine and cocaine/heroin combinations under progressive ratio and choice schedules in rats. Neuropsychopharmacology. 2005; 30(2):286–95. doi:10.1038/sj.npp.1300560.
- Trujillo KA, Smith ML, Guaderrama MM. Powerful behavioral interactions between methamphetamine and morphine. Pharmacol Biochem Behav. 2011; 99(3):451–8. doi:10.1016/j.pbb.2011.04.014.
- Ranaldi R, Wise RA. Intravenous self-administration of methamphetamine-heroin (speedball) combinations under a progressive-ratio schedule of reinforcement in rats. Neuroreport. 2000; 11(12):2621–3. doi:10.1097/00001756-200008210-00003.
- Duhart Clarke SE, Kral AH, Zibbell JE. Consuming illicit opioids during a drug overdose epidemic: illicit fentanyls, drug discernment, and the radical transformation of the illicit opioid market. Int J Drug Policy. 2022; 99:103467. doi:10.1016/j.drugpo.2021.103467.
- Thomas SA, Perekopskiy D, Kiyatkin EA. Cocaine added to heroin fails to affect heroin-induced brain hypoxia. Brain Res. 2020; 1746:147008. doi:10.1016/j.brainres.2020.147008.
- Hughto JM, Gordon LK, Stopka TJ, Case P, Palacios WR, Tapper A, Green TC. Understanding opioid overdose risk and response preparedness among people who use cocaine and other drugs: mixed-methods findings from a large, multi-city study. Subst Abus. 2022; 43(1):465–78. doi:10.1080/08897077.2021.1946893.
- Colell E, Domingo-Salvany A, Espelt A, Parés-Badell O, Brugal MT. Differences in mortality in a cohort of cocaine use disorder patients with concurrent alcohol or opiates disorder. Addiction. 2018; 113(6):1045–55. doi:10.1111/add.14165.
- Karch SB, Goldberger BA. Karch’s Drug Abuse. Handbook: CRC Press, Boca Baton, Florida, USA, 2022.
- Knollmann B, Brunton L. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 14th Edition. Edited by JG Hardman, LE Limbird, and AG Gilman. McGraw Hill, New York. ACS Publications 2022.
- Bencharit S, Morton CL, Xue Y, Potter PM, Redinbo MR. Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nat Struct Biol. 2003; 10(5):349–56. doi:10.1038/nsb919.
- Cheng J, Wang S, Lin W, Wu N, Wang Y, Chen M, Xie XQ, Feng Z. Computational Systems Pharmacology-Target Mapping for Fentanyl-Laced Cocaine Overdose. ACS Chem Neurosci. 2019; 10(8):3486–99. doi:10.1021/acschemneuro.9b00109.
- Kamendulis LM, Brzezinski MR, Pindel EV, Bosron WF, Dean RA. Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases. J Pharmacol Exp Ther. 1996; 279(2):713–7.
- Perekopskiy D, Kiyatkin EA. 6-monoacetylmorphine (6-MAM), not morphine, is responsible for the rapid neural effects induced by intravenous heroin. ACS Chem Neurosci. 2019; 10(8):3409–14. doi:10.1021/acschemneuro.9b00305.
- Pindel EV, Kedishvili NY, Abraham TL, Brzezinski MR, Zhang J, Dean RA, Bosron WF. Purification and cloning of a broad substrate specificity human liver carboxylesterase that catalyzes the hydrolysis of cocaine and heroin. J Biol Chem. 1997; 272(23):14769–75. doi:10.1074/jbc.272.23.14769.
- Redinbo M, Bencharit S, Potter P. Human carboxylesterase 1: from drug metabolism to drug discovery. Biochem Soc Trans. 2003; 31(Pt 3):620–4. doi:10.1042/bst0310620.
- Boto de los Bueis A, Pereira Vega A, Sánchez Ramos JL, Maldonado Pérez JA, Ayerbe García R, García Jiménez D, Pujol de la Llave E. Bronchial hyperreactivity in patients who inhale heroin mixed with cocaine vaporized on aluminum foil. Chest. 2002; 121(4):1223–30. doi:10.1378/chest.121.4.1223.
- Foltin RW, Fischman MW. The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther. 1992; 261(2):623–32.
- Haigney MC, Alam S, Tebo S, Marhefka G, Elkashef A, Kahn R, Chiang CN, Vocci F, Cantilena L. Intravenous cocaine and QT variability. J Cardiovasc Electrophysiol. 2006; 17(6):610–6. doi:10.1111/j.1540-8167.2006.00421.x.
- Havakuk O, Rezkalla SH, Kloner RA. The cardiovascular effects of cocaine. J Am Coll Cardiol. 2017; 70(1):101–13. doi:10.1016/j.jacc.2017.05.014.
- Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002; 137(6):501–4. doi:10.7326/0003-4819-137-6-200209170-00010.
- Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose‐related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy. 2003; 23(6):802–5. doi:10.1592/phco.23.6.802.32186.
- Krantz MJ, Rowan SB, Mehler PS. Cocaine-related torsade de pointes in a methadone maintenance patient. J Addict Dis. 2005; 24(1):53–60. doi:10.1300/J069v24n01_05.
- Platt JJ. Cocaine Addiction: Theory, Research, and Treatment. Harvard University Press 1997.
- Ryan K, Benz P, Zosel A, Farkas A, Theobald J. QTc prolongation in Poison center exposures to CredibleMeds list of substances with “known risk of Torsades de Pointes. Cardiovasc Toxicol. 2022; 22(9):866–77. doi:10.1007/s12012-022-09764-4.
- Roque Bravo R, Faria AC, Brito-da-Costa AM, Carmo H, Mladěnka P, Dias da Silva D, Remião F, On Behalf Of The Oemonom R. Cocaine: an updated overview on chemistry, detection, biokinetics, and pharmacotoxicological aspects including abuse pattern. Toxins (Basel). 2022; 14(4):278. doi:10.3390/toxins14040278.
- Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010; 122(24):2558–69. doi:10.1161/CIRCULATIONAHA.110.940569.
- Shanti CM, Lucas CE. Cocaine and the critical care challenge. Crit Care Med. 2003; 31(6):1851–9. doi:10.1097/01.Ccm.0000063258.68159.71.
- Vasica G, Tennant CC. Cocaine use and cardiovascular complications. Med J Aust. 2002; 177(5):260–2. doi:10.5694/j.1326-5377.2002.tb04761.x.
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez C. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005; 13(4):311–8. doi:10.1037/1064-1297.13.4.311.
- Chen S, Yang P, Chen T, Su H, Jiang H, Zhao M. Risky decision-making in individuals with substance use disorder: a meta-analysis and meta-regression review. Psychopharmacology (Berl)). 2020;237(7):1893–908. doi:10.1007/s00213-020-05506-y.
- Cunha-Oliveira T, Rego AC, Carvalho F, Oliveira CR. Medical toxicology of drugs of abuse. In: principles of Addiction, Chapter 17. Academic Press. Editors: Peter M. Miller (Ed.) 2013.
- Joe GW, Knezek L, Watson D, Simpson DD. Depression and decision-making among intravenous drug users. Psychol Rep. 1991; 68(1):339–47. doi:10.2466/pr0.1991.68.1.339.
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non‐drug‐using controls. Addiction. 2004; 99(4):461–71. doi:10.1111/j.1360-0443.2003.00669.x.
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001; 21(4):193–8. doi:10.1016/s0740-5472(01)00202-1.
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 2002; 68(1):105–11. doi:10.1016/s0376-8716(02)00106-0.
- Rodríguez-Cintas L, Daigre C, Grau-López L, Barral C, Pérez-Pazos J, Voltes N, Braquehais MD, Casas M, Roncero C. Impulsivity and addiction severity in cocaine and opioid dependent patients. Addict Behav. 2016; 58:104–9. doi:10.1016/j.addbeh.2016.02.029.
- Verdejo-García AJ, Perales JC, Pérez-García M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007; 32(5):950–66. doi:10.1016/j.addbeh.2006.06.032.
- DiSalvo P, Cooper G, Tsao J, Romeo M, Laskowski LK, Chesney G, Su MK. Fentanyl-contaminated cocaine outbreak with laboratory confirmation in New York City in 2019. Am J Emerg Med. 2021; 40:103–5. doi:10.1016/j.ajem.2020.12.002.
- Palamar JJ. Awareness that cocaine can contain fentanyl among nightclub and festival attendees in New York City, 2018–2022. Public Health Nurs. 2023;40(4):566–71. doi:10.1111/phn.13193.
- Wagner KD, Fiuty P, Page K, Tracy EC, Nocera M, Miller CW, Tarhuni LJ, Dasgupta N. Prevalence of fentanyl in methamphetamine and cocaine samples collected by community-based drug checking services. Drug Alcohol Depend. 2023; 252:110985. doi:10.1016/j.drugalcdep.2023.110985.
- Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008; 100(6):747–58. doi:10.1093/bja/aen094.
- Benowitz NL. Clinical pharmacology and toxicology of cocaine. Pharmacol Toxicol. 1993; 72(1):3–12. doi:10.1111/j.1600-0773.1993.tb01331.x.
- Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl)). 2001;154(1):1–7. doi:10.1007/s002130000609.
- Marquez P, Nguyen AT, Hamid A, Lutfy K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology. 2008; 54(3):564–8. doi:10.1016/j.neuropharm.2007.11.003.
- Unterwald EM. Regulation of opioid receptors by cocaine. Ann N Y Acad Sci. 2001;937(1):74–92. doi:10.1111/j.1749-6632.2001.tb03559.x.
- Kiyatkin EA. Dopamine mechanisms of cocaine addiction. Int J Neurosci. 1994; 78(1-2):75–101. doi:10.3109/00207459408986048.
- O'Driscoll PT, McGough J, Hagan H, Thiede H, Critchlow C, Alexander ER. Predictors of accidental fatal drug overdose among a cohort of injection drug users. Am J Public Health. 2001; 91(6):984–7. doi:10.2105/ajph.91.6.984.
- LaRue L, Twillman RK, Dawson E, Whitley P, Frasco MA, Huskey A, Guevara MG. Rate of fentanyl positivity among urine drug test results positive for cocaine or methamphetamine. JAMA Netw Open. 2019; 2(4):e192851–e51. doi:10.1001/jamanetworkopen.2019.2851.
- Twillman RK, Dawson E, LaRue L, Guevara MG, Whitley P, Huskey A. Evaluation of trends of near-real-time urine drug test results for methamphetamine, cocaine, heroin, and fentanyl. JAMA Netw Open. 2020; 3(1):e1918514–e14. doi:10.1001/jamanetworkopen.2019.0s18514.
- Zibbell JE, Aldridge AP, Cauchon D, DeFiore-Hyrmer J, Conway KP. Association of law enforcement seizures of heroin, fentanyl, and carfentanil with opioid overdose deaths in Ohio, 2014-2017. JAMA Netw Open. 2019; 2(11):e1914666–e66. doi:10.1001/jamanetworkopen.2019.14666.