ABSTRACT
Pyrethroids are a class of insecticides used widely for vector control programs. Acute pyrethroid poisoning is rare, but well documented, whereas effects of cumulative exposure are insufficiently described, including possible negative effect on glucose regulation. The objective of this study was to investigate an association between exposure to pyrethroids and abnormal glucose regulation (prediabetes or diabetes). A cross-sectional study was performed among 116 pesticide sprayers from public vector control programs in Bolivia and 92 nonexposed controls. Pesticide exposure (duration, intensity, cumulative exposure) was assessed from questionnaire data. Participants were asked about symptoms of diabetes. Blood samples were analyzed for glycosylated hemoglobin (HbA1c), a measure of glucose regulation. No association was found between pyrethroid exposure and diabetes symptoms. The prevalence of abnormal glucose regulation (defined as HbA1c ≥ 5.6%) was 61.1% among sprayers and 7.9% among nonexposed controls, corresponding to an adjusted odds ratio (OR [95% confidence interval]) for all sprayers of 11.8 [4.2–33.2] and 18.5 [5.5–62.5] for pyrethroid-exposed only. Among sprayers who had only used pyrethroids, a significant positive trend was observed between cumulative pesticide exposure (total number of hours sprayed) and adjusted OR of abnormal glucose regulation, with OR 14.7 [0.9–235] in the third exposure quintile. The study found a severely increased prevalence of prediabetes among Bolivian pesticide sprayers compared with a control group, but the relevance of the control group is critical. Within the spraying group, an association between cumulative exposure to pyrethroids and abnormal glucose regulation was seen. Further studies are needed to confirm this association.
INTRODUCTION
Pyrethroids are broad-spectrum insecticides that have become widely used, primarily due to low acute toxicity and low dermal uptake. Large amounts of pyrethroids are used in agriculture, the textile industry, as the main active substance in insecticide-treated bed nets in the tropics, and in aircraft “disinfection.” During the last 2 decades, the substances have been increasingly used worldwide in the public health vector programs.Citation1,Citation2 Residential pesticide spraying for vector control is an essential part of the prevention of malaria and other vector-borne diseases,Citation1,Citation3 and the pesticide sprayers are some of the most heavily pesticide-exposed subjects.Citation4 In Bolivia, approximately 500 persons are employed by the Ministry of Health and Sports as full-time pesticide sprayers in vector programs. These programs are carried out as campaigns in the rainy season from November to May, and during this period the sprayers are exposed 6 days a week, 8 to 10 hours per day. Since the mid-1990s, pyrethroids have almost completely replaced the environmentally persistent organochlorines (i.e., DDT [dichlorodiphenyltrichloroethane]) and the acutely toxic organophosphates in the vector control programs. Presently, the most used pyrethroids in the programs are α-cypermethrin and λ-cyhalothrin, but smaller amounts of β-cypermethrin and deltamethrin are used as well (personal communication, Rafael Cervantes Morant, Fundación Plagbol, Bolivia).
The possibility of negative chronic health effects of pyrethroids after occupational exposure, by accident or from suicidal attempts, has been widely discussed.Citation2,Citation5–Citation7 In Germany, the discussion of this long-term, low-dose exposure in households and work environments has reached a point where control operators refuse to use pyrethroids.Citation8 The debate has mainly focused on neurotoxicity and relied mostly on case stories.Citation6 However, a diabetic effect has also been suggested: Wang et al. found significantly higher prevalence of abnormal glucose regulation among pyrethroid-exposed subjects at two Chinese pesticide factories, compared with employees not exposed to pyrethroids.Citation9
As a part of a larger cross-sectional study concerning the health of the workers of the public vector controls programs in Bolivia, the authors investigated the putative association between pyrethroid exposure and abnormal glucose regulation.
METHODS
Participant Recruitment and Information
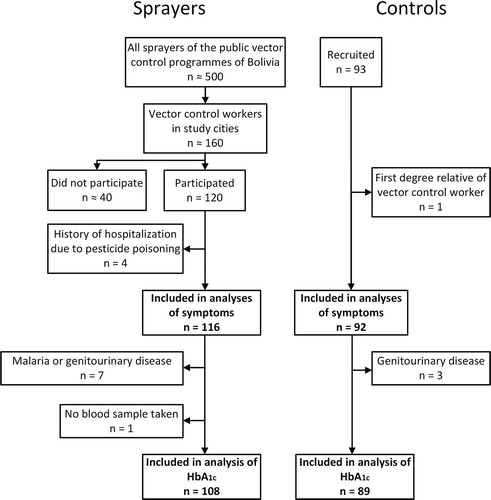
Examinations took place in each of the three largest Bolivian cities—La Paz, Santa Cruz, and Cochabamba—during June and July 2012, that is, a few months beyond the intensive spraying season, thus avoiding measuring any acute negative health effect on the sprayers. Male sprayers were recruited from the public vector control centers, which employed approximately 160 pesticide sprayers or former sprayers now working in administration. A total of 120 accepted the invitation to participate. Among the 40 nonparticipants, two sprayers specified that they had health problems related to spraying and feared being dismissed if they participated. The motives of the remaining nonparticipants were unknown. A total of 93 nonexposed controls were recruited among the nonspraying employees at the centers (≈26 persons), among university students taught by some of the examiners (41 persons), and some others (≈26 persons). Controls were male at age 20 to 60 years. As the objective of this study was to investigate effects of exposure to pyrethroids below the level where acute intoxication occurs, four sprayers were excluded due to former hospitalization due to acute pesticide poisoning. To maintain independency between observations, one nonexposed control was excluded, as he was a son of one of the sprayers. The final study groups included for analyses of symptoms (108 sprayers and 89 control subjects) are shown in .
Participation in the study was voluntary, and an informed consent form was completed by all participants before inclusion. Participants were financially compensated for their participation in the study. The investigation had been approved by the Medical Ethical Committee in Bolivia. All test materials and test administration protocols were translated to Spanish by a non-native Spanish speaker. Afterwards, the quality of translation was controlled and a backward translation to English was performed by a native Spanish speaker.
Interview and Physical Examination
An interview questionnaire was administered by an experienced specialist in occupational medicine. The questionnaire included questions about demographic variables, health status, and pesticide exposure.
Participants were asked if they had experienced any of 62 specific symptoms during the past year. Three of these symptoms were included in the study as possible signs of diabetes: urinating more often than usual (pollakisuria), urinating a greater quantity than usual (polyuria), and unintentional weight loss. Blurred vision, lethargy, and tiredness were deemed to unspecific for inclusion.
Weight and height of the participants were measured and 1.5 kg was subtracted to take clothes-wearing into consideration. Also, 2 cm was subtracted from the height for participants measured with shoes on.
Collection and Biochemical Examination of Blood Samples
Blood samples were collected from all participants using 4-mL EDTA tubes. In La Paz, samples were brought to the Genetic Institute, centrifuged, separated into cells and supernatant, and frozen at −17°C. In Santa Cruz and Cochabamba, samples were centrifuged and separated into cells and supernatant before being stored at −4°C. After 1 to 3 days, the samples were packed with dry ice and sent to the Genetic Institute of La Paz (this took 16 to 20 hours), where they were frozen at −17°C. Upon completion of the operative phase of the project, blood samples were brought to a local private biochemical laboratory where they were thawed and analyzed for hemoglobin A1c (HbA1c) by high-performance liquid chromatography (HPLC).
Subjects were initially categorized as normal (HbA1c < 5.6%), prediabetic (5.6% ≤ HbA1c ≤ 6.4%), or diabetic (HbA1c > 6.4%). Because only three subjects were diabetic, it was decided to collapse the categories of “prediabetic” and “diabetic.” Thus, the final categorization was “normal” (HbA1c < 5.6%) versus “abnormal glucose regulation” (HbA1c ≥ 5.6%).
In the analyses of HbA1c data, three controls and eight sprayers were excluded, because they had reported suffering from malaria or genitourinary diseases, and the diseases in question can influence HbA1c.Citation10 See for a summary of participant recruitment and exclusion for HbA1c analysis.
Exposure Assessment
Based on the questionnaire data, the following four measures of exposure to pesticides were derived:
Sprayer (yes/no)
Pesticide spraying duration (the total number of years working with pesticides—from the day of first use to the last day of use or to the day of the interview)
Pesticide spraying intensity (number of hours of spraying per week in the weeks with actual spraying)
Cumulative pesticide exposure (total number of hours sprayed)
Only sprayers were assigned a value for spraying duration, spraying intensity, and cumulative exposure, and the three measures were used to evaluate dose-response relationships within the group of sprayers. A total of 17 controls had used pesticides, mainly to kill insects in their own homes, but their exposure levels were judged negligible. The three continuous exposure variables were converted into quintiles, with 21 to 23 exposed subjects in each exposure group.
Statistical Analysis
Questionnaire data were double-entered by two different people using EpiData 3.1 (epidata.dk; Epidata Association, Odense, Denmark). HbA1c analysis results were delivered in Microsoft Excel format by the analyzing laboratory and had not been double entered.
All outcome variables were analyzed using both simple and multivariate logistic regressions. In the multivariate analyses, the following potential confounders were included: body mass index (BMI; continuous variable), age (continuous variable: number of years), educational level (categorical variable: less than primary school, primary school, secondary school or technical education, university), use of antidiabetics (dichotomous variable: yes/no), family history of diabetes (dichotomous variable: yes/no), location (categorical variable: La Paz, Santa Cruz, or Cochabamba; used as a proxy for ethnicity), and smoking status (categorical variable: never smoker, ex-smoker, or current smoker). Decisions to include variables in the models were made a priori, as they were judged to be possible confounders in the association between exposure and blood glucose.
TABLE 1. Demographic Characteristics of Sprayers and Controls Subjects
Exposure was initially treated as a dichotomous variable (sprayer yes/no). If a significant difference was found between sprayers and controls, dose-response relationships for sprayers were modeled by using quintiles of spraying duration, spraying intensity, and cumulative exposure as exposure measures. Each quintile of exposure was treated as a separate variable in the model. Further, a test for trend was performed where exposure quintiles were treated as a single continuous variable. Analyses were performed on all sprayers and repeated for the sprayers who had only used pyrethroids—and no other pesticides. Analyses were repeated where only age, BMI, and use of antidiabetics were included as confounders. All models were checked using the Hosmer-Lemeshow goodness-of-fit test.
The level of significance was 5%. Data cleanup and analysis were performed using Stata 12.1 (StataCorp, College Station, TX, USA).
RESULTS
Demographic variables for the sprayers and nonexposed controls can be seen in . The exposed subjects had higher BMI, were older, were more poorly educated, smoked more, and used more antidiabetics than nonexposed controls. Furthermore, the distribution of subjects with regard to city (and thus possibly ethnicity) differed between the groups.
The point prevalence of abnormal glucose regulation (defined as HbA1c ≥ 5.6%) and 1-year cumulative prevalence of subjective diabetes symptoms can be seen in . The prevalence of abnormal glucose regulation in the sprayer group was very high, 61%, compared with 8% among the nonexposed controls. No one reported having experienced “urinating a greater quantity than usual.”
TABLE 2. Point Prevalence and OR (Odds Ratio) of Abnormal Glucose Regulation and 1-Year Prevalence and OR of Diabetes Symptoms in All Sprayers and Sprayers Who Had Only Used Pyrethroids, Compared With Controls
Significantly increased odds ratios (ORs) of abnormal glucose regulation were found for the sprayers in both raw and adjusted analyses (). The ORs increased after exclusion of sprayers who had at some time used pesticides other than pyrethroids. No significant differences were found between groups with regard to the symptoms “urinating more often than usual” and “unintentional weight loss.”
The adjusted analyses for dose-response relationships between exposure levels and abnormal glucose regulation among sprayers are seen in –. In , both raw and adjusted analyses are shown. No clear dose-response relationships were found for quintiles of neither spraying duration nor spraying intensity. There was also no clear trend for cumulative exposure when looking at all sprayers. But when limiting the analysis to sprayers who had only used pyrethroids (52 out of 108 with valid HbA1c results), a statistically significant trend was revealed between cumulative exposure and odds ratio of abnormal glucose regulation (P = .01).
FIGURE 2. (a–c) Dose-response relationships between exposure and odds of abnormal glucose regulation within the exposed group. All analyses adjusted for BMI, age, education, use of antidiabetics, location, and smoking status. All = all sprayers, no matter the pesticides used; Pyr = only pyrethroids used; p = P value for trend; R2 = pseudo-R2 for trend. Numerical data corresponding to the plots can be found in .
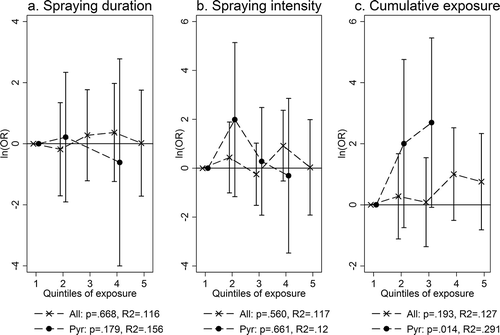
Sensitivity analyses that only included age, BMI, and use of antidiabetics as confounders gave similar estimates as the fully adjusted models, although the association between cumulative exposure and OR of abnormal glucose regulation among sprayers who had only used pyrethroids was only borderline significant at P = .08 (data not shown).
TABLE 3. Numerical Data for Dose-Response Relationships Between Pesticide Exposure and Odds Ratio of Abnormal Glucose Regulation
DISCUSSION
No differences were seen between sprayers and nonexposed controls with regards to signs of diabetes such as polyuria or unintentional weight loss. Furthermore, the consumption of antidiabetic drugs was similar in the two groups studied. All but three of the participants with abnormal glucose regulation had prediabetes and not diabetes as such. This all corresponds well with the fact that prediabetes is a preclinical condition associated with increased risk of developing diabetes (although some diabetic complications may be seen).Citation11 Our study revealed a strong association between being a vector control sprayer exposed to a mixture of insecticides and having abnormal glucose regulation, even after confounder control. The association was even stronger when limiting the analysis to sprayers who had only been exposed to pyrethroids.
However, difficulties in recruiting controls had led to inclusion of a large number of young students, and large differences in, for example, BMI, educational level, and age were found between sprayers and controls, making it likely that the two groups also differed with regards to other variables of importance for development of diabetes such as lifestyle (including diet), which is not taken into account. This calls for caution when we interpret the results. Twenty-five percent of the current or former sprayers employed at the vector control centers did not participate. This could have led to bias away from the null hypothesis (possibly explaining the results found) if affected sprayers were more likely to participate than nonaffected sprayers. This is unlikely to have happened, as no effects were found with regards to subjective symptoms. On the contrary, some sprayers with work-related health issued chose not to participate due to fear of being fired. This could lead to bias towards the null hypothesis and cannot explain the results. The only previous study on the possible association between chronic pyrethroid exposure and abnormal glucose regulation was performed by Wang et al., who compared exposed and nonexposed participants among employees in two pyrethroid factories in China. They revealed a slightly increased OR for abnormal glucose regulation among exposed persons, OR = 1.5 (95% confidence interval: 1.2–1.8).Citation9
When we restricted the analysis to persons who had only used pyrethroids, we found a significant positive trend between cumulative spraying (total number of hours sprayed) and odds of abnormal glucose regulation. An effect was demonstrated even though few of the exposed subjects had sprayed recently, supporting that long-term cumulative exposure is the exposure of interest.
A comprehensive literature search revealed no further studies on associations between chronic pyrethroid exposure and abnormal glucose regulation except that of Wang et al.Citation9 Two experimental animal studies demonstrated changed glucose metabolism during acute pyrethroid intoxication.Citation12,Citation13 The hypothesis of pyrethroids causing abnormal glucose regulation is supported by the fact that DDT, an organochlorine insecticide, has been shown to be associated with diabetes.Citation14 The main toxicological effect of DDT is in some way similar to that of pyrethroids, that is, slowing the inactivation of the voltage-gated sodium channels of excitable cells.Citation14,Citation15 One could therefore hypothesize that low-level pyrethroid exposure affected glucose regulation by this mechanism; however, this is purely speculative, as pyrethroids have been shown to affect cells in a multitude of manners. Targets for pyrethroids include protein phosphorylation, voltage-gated chloride channels, noradrenaline release, γ-aminobutyric acid (GABA)-gated chloride channels, nicotinic receptors, mitochondrial complex 1, apoptosis induction, voltage-gated calcium channels, lymphocyte proliferation, volume-sensitive anion channels, calcium adenosine triphosphatase (ATPase), intercellular gap junctions, and chromosomal damage.Citation16 Except for the first three, these targets are only affected at concentrations 2 or more orders of magnitude larger than the ones affecting voltage-gated sodium channels.
The main strength of this study is the exposure assessment. Even though no objective measures of pesticide exposure for the participants are available at the present time, detailed self-reported information was collected, allowing modeling of dose-response relationships between exposure levels (in terms of spraying duration, intensity, and cumulative exposure) and OR of abnormal glucose regulation. Exposed subjects had sprayed for an average of 10.0 years, meaning that the population was suitable for a study on long-term effects.
Glycosylated hemoglobin A (HbA1c) is a well-documented marker of glycemic status, reflecting average blood glucose level in the last 8 to 12 weeks.Citation10 It has been approved by the World Health Organization for the diagnosis of diabetes mellitus.Citation10 For this study, HbA1c analysis was chosen over the fasting glucose test and the oral glucose tolerance test because HbA1c does not vary diurnally and subjects do not have to fast. HbA1c results can be biased by a number of factors, including genitourinary disease, malaria, and variant hemoglobinCitation10; therefore, subjects with self-reported genitourinary diseases and malaria were excluded. We do not have data on the occurrence of variant hemoglobin of the participants. A literature search gave no results regarding the prevalence of variant hemoglobin in Bolivia, but a certain prevalence of sickle cell trait can be expected due to the selection pressure from malaria in the lowland areas of Cochabamba and Santa Cruz.
The main weakness in our study is the external control group that was substantially different from the sprayers. We therefore have to interpret data with great caution. Another limitation is the lack of objective exposure measurements such as analysis of urine samples for pyrethroid metabolites or measurements of airborne pyrethroid concentration in the working environment (although both are markers of acute exposure and not directly measures of long-term exposure). All measures of exposure in this study were self-reported and inaccurate recall without association to outcome would lead to bias towards the null hypothesis. We have tried to compensate for inaccurate recall by analyzing dose-response relationships based on quintiles of exposure. Because prediabetes is a subclinical condition, it is not likely to lead to systematic recall bias.
Regarding the external validity of the findings, it must be remembered that the exposed population of this study can be assumed to have high (peak) exposure to pyrethroids due to indoor spraying and suboptimal use of personal protective equipment. Care should be exerted before extrapolating the results to other populations exposed to pyrethroids, such as farmers, inhabitants of the sprayed buildings, and people sleeping under insecticide-treated bed nets. All participants were male, and although we have no reason to believe that the female response to pyrethroid exposure differs from that of the male, it cannot be ruled out beforehand.
Residential spraying with pyrethroids is an important part of vector management.Citation1 Even though the results may indicate that exposure to pyrethroids can lead to prediabetes (and thus possibly diabetes) for the vector control sprayers, at the current time we cannot recommend stopping the vector control programs or replacing pyrethroids with more acutely toxic pesticides. The most adequate response is use of personal protective equipment while spraying to minimize exposure until better data are available. Future studies should improve upon the design of this study, namely, better matching of exposed subjects and controls, larger study population, and a follow-up design. Detailed self-reported measures of pesticide exposure should be included, along with environmental monitoring of external and biological monitoring of internal exposure.
CONCLUSION
Cumulative pyrethroid exposure might be associated with abnormal glucose regulation in terms of higher risk of prediabetes. Some methodological problems in the study mean that care is needed when interpreting the results. Further research is warranted.
Data sharing statement. All raw data from this study are available in digital form for any researcher. For access, please contact Dansk Data Arkiv at www.sa.dk/dda.
FUNDING
This work was supported by The Danish Council for Independent Research—Medical Sciences grant 11-115982; The Aase and Ejner Danielsen’s Foundation; and The Diálogos Foundation.
ACKNOWLEDGMENTS
The authors wish to thank the Bolivian Ministry of Health and Sports, The Departmental Health Service of Cochabamba, The Departmental Health Service of Santa Cruz, The National Institute of Occupational Health in La Paz, and The Institute of Genetics in La Paz for their cooperation in the study. In particular, the authors would like to thank the following persons for their effort in planning and carrying out the data collection: Dr. Noemi Tirado Bustillos and Dr. Rolando Paz Bonilla (The Institute of Genetics), Dr. Maria Angelica Urzagaste Soliz (The National Institute of Occupational Health), and Dr. Giovanna Condarco Arispe (The Plagbol Foundation, La Paz, Bolivia).
REFERENCES
- World Health Organization. The Technical Basis for Coordinated Action Against Insecticide Resistance: Preserving the Effectiveness of Modern Malaria Vector Control. Geneva: World Health Organization; 2011.
- Barlow SM, Sullivan FM, Lines J. Risk assessment of the use of deltamethrin on bednets for the prevention of malaria. Food Chem Toxicol. 2001;39:407–422.
- World Health Organization. Indoor Residual Spraying—Use of Indoor Residual Spraying for Scaling Up Global Malaria Control and Elimination. Geneva: World Health Organization; 2006.
- Hardt J, Angerer J. Biological monitoring of workers after the application of insecticidal pyrethroids. Int Arch Occup Environ Health. 2003;76:492–498.
- Bradberry SM, Cage SA, Proudfoot AT, et al. Poisoning due to pyrethroids. Toxicol Rev. 2005;24:93–106.
- Kolaczinski JH, Curtis CF. Chronic illness as a result of low-level exposure to synthetic pyrethroid insecticides: a review of the debate. Food Chem Toxicol. 2004;42:697–706.
- Chen SY, Zhang ZW, He FS, et al. An epidemiological study on occupational acute pyrethroid poisoning in cotton farmers. Br J Ind Med. 1991;48:77–81.
- Müller-Mohnssen H. Chronic sequelae and irreversible injuries following acute pyrethroid intoxication. Toxicol Lett. 1999;107:161–176.
- Wang J, Zhu Y, Cai X, et al. Abnormal glucose regulation in pyrethroid pesticide factory workers. Chemosphere. 2011;82:1080–1082.
- Colagiuri S. Glycated haemoglobin (HbA1c) for the diagnosis of diabetes mellitus—practical implications. Diabetes Res Clin Pract. 2011;93:312–313.
- Karam JG, McFarlane SI. Update on the prevention of type 2 diabetes. Curr Diab Rep. 2011;11:56–63.
- Cremer JE, Cunningham VJ, Seville MP. Relationships between extraction and metabolism of glucose, blood flow, and tissue blood volume in regions of rat brain. J Cereb Blood Flow Metab. 1983;3:291–302.
- Cremer JE, Seville MP. Changes in regional cerebral blood flow and glucose metabolism associated with symptoms of pyrethroid toxicity. Neurotoxicology. 1985;6:1–12.
- Eskenazi B, Chevrier J, Rosas LG, et al. The Pine River statement: human health consequences of DDT use. Environ Health Perspect. 2009;117:1359–1367.
- Soderlund DM. Toxicology and mode of action of pyrethroid insecticides. In: Krieger R, ed. Hayes’ Handbook of Pesticide Toxicology. St. Louis, MO: Elsevier Science; 2010:1655–1686.
- Ray DE, Fry JR. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacol Ther. 2006;111:174–193.