ABSTRACT
Poultry production is an integral part of agriculture and of the U.S. economy, accounting for millions of eggs and chicken products consumed annually. Most ubiquitous to the poultry industry from farm production to research are broiler and layer poultry operations, with pullet operations at the forefront. Although essential to the cycles of production, there is a dearth of evidence regarding the occupational exposure risks of pullet production. The aim of this case study was to measure total dust and ammonia levels during the growth cycle of pullets. Ammonia and total dust concentrations were measured as single day measurements at three different points of time during the 16.5-week growth cycle of pullet flocks using two fixed sampling stations configured to represent the breathing zone height of poultry workers. As birds grew from chicks to hens, concentrations of total dust and ammonia increased. Notably, from 3 weeks-of-age to 9 weeks-of-age concentrations of total dust increased from 1.1–1.2 mg/m3 to 16.0–18.0 mg/m3; and from 9 weeks-of-age to 15 weeks-of-age, dust concentrations reached 43.0–50.0 mg/m3. Concentrations of ammonia also increased from 9 weeks to 15 weeks from 1.1–2.7 ppm to 22.0–30.0 ppm. Both levels of ammonia and total dust reached levels that have the potential to induce adverse health effects among farmers raising pullets.
Introduction
With advancements in genetics, production, and technology, more chickens and eggs are being produced annually than at any previous point in time.Citation1 It is forecasted that each American per capita will consume an average of 51.5 kilograms of poultry in 2020, exceeding beef and pork consumption combined for the fifth consecutive year in over six decades.Citation2 The upward trend of consumer demand for poultry, poultry products, and eggs has also driven more farmers to diversify their agricultural operations to include poultry. Between 2012 and 2017, the number of poultry and egg farms increased by 19%, based on census data.Citation3 Unlike other livestock productions in the United States, 90% of poultry/egg farms are operated by small- and mid-size family farms through a production contract with an integrator.Citation1 Production contracts are legal agreements between poultry companies (integrators) and poultry farmers (producers) that provide risk management in uncertain or unstable markets.Citation2,Citation4 In a vertically integrated business system, companies supply the birds and other production necessities to farmers and delegate how farmers must operate their poultry barns and care for the birds based on industry-specified parameters.Citation4,Citation5
Anecdotal evidence suggests integrators are limited in their approaches to contracted farmer’s health and safety, ultimately prioritizing animal health and welfare (2019 conversation with primary operator, unreferenced). This poses a major cause for concern, as evidence has established working in poultry confinements exposes farmers to numerous occupational respiratory hazards including gases,Citation6,Citation7 mold,Citation8 dusts,Citation9–11 volatile organic compounds (VOCs),Citation12 endotoxins,Citation13 bacteria, fungi,Citation14–16 and, in rare instances, zoonotic viruses.Citation17 Research on broiler (poultry raised for meat) and layer (poultry raised for eggs) confinements has demonstrated that frequent exposure to these airborne contaminants is associated with both acute and chronic clinical responses including but not limited to asthma, organic toxic dust syndrome (OTDS), diminished ventilatory capacity, bronchitis, chronic obstructive pulmonary disease (COPD), allergic alveolitis, and rhinitis.Citation6,Citation8,Citation9,Citation18–21
Studies on broiler and layer flock farms have shown that working with large numbers of poultry in confinement for long periods of time increases workers’ exposure to respiratory hazards and may increase the potential for adverse health effects.Citation10,Citation11,Citation18 Eliminating or reducing environmental hazards within poultry confinements is often not feasible or efficient long term, and it can be costly when multiple barns are managed.Citation20,Citation22–24 Furthermore, there is considerable variability within the types of poultry barn construction, ventilation, management practices, and flock type and duration. An in-depth review of the variability within poultry production is not within the scope of this study, but common differences in exposures are attributable to caged versus floor operations, confined enclosure versus pasture or free range, pellet versus loose feed, ventilation type and rates, and facility cleaning. This variability and, hence, occupational exposure have been examined extensively among layer and broiler productions.Citation25
Pullet operations are integral to the poultry industry, yet to our knowledge, and have rarely been considered or examined within the literature or among occupational exposure studies.Citation25 Pullets are flocks of newly hatched young female birds raised for the replacement of parental layer stocks. Pullets are raised by one grower until mature (approximately 16–17 weeks) and then sent to another poultry producer for egg production. The lack of information on occupational exposures specific to pullet production was brought to our attention by the primary operator of a family-operated pullet barn in rural Nebraska. The primary operator expressed concern regarding ammonia and dust levels in his barn, as his wife and four young children often help with chores and management. Specifically, the primary operator was interested in exposure concentrations of ammonia and dust at different stages of the pullet growth cycle and how to protect his family if or when those exposures reached concentrations that would be considered high.
As such, the current study examines a case of a family-operated pullet barn in rural Nebraska. The purpose of our case study was to determine whether growth of pullets from 3 weeks-of-age to 16.5 weeks influenced concentrations of ammonia and total dust. To evaluate whether age and, thus, growth of birds influenced ammonia and total dust concentrations, air samples for ammonia and dust were taken at three different periods during the 16.5-week growth cycle of the flock. These time points were chosen based on the primary operator’s visual perceptions and account of environmental changes in relation to the progression of flock maturity. Since the contaminants of ammonia and dust have demonstrated their synergistic impact on respiratory health through prior research and measurements collected in other poultry studies, they were our primary exposures for assessment.Citation26
Methods
Background
This exploratory case study was conducted during the fall and winter of 2019 in a family operated poultry production barn in rural Nebraska. Prior to sampling, researchers met with the primary operator to discuss his concerns, questions, and current processes, as well as his remedies for reducing personal exposures when working in the confinement. Initial conversations indicated the use of personal protective equipment (PPE), but only once visibility became increasingly diminished. The primary operator would use disposable, valved N95 respirators, eventually switching to an elastomeric dual cartridge respirator fitted with ammonia cartridges. The primary operator indicated that he supplied the PPE but alluded the integrator would provide PPE if asked. A concern of the primary operator was regarding PPE and whether the type in use was appropriate relative to exposure. Furthermore, since PPE sized for children is not readily available or approved for use by the National Institute of Occupational Health and Safety (NIOSH), an important inquiry was made: what is the tipping point during flock growth, when the amount of time young children spend in the poultry confinement, should be minimized.
Site and process
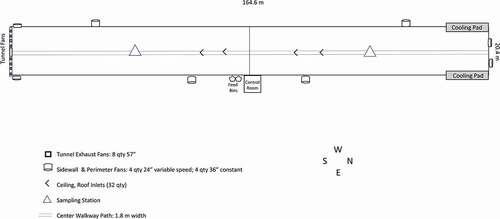
The site of study was a farm that produces 40,000 pullets in 16–17 weeks for one flock, averaging 2.5 flocks per year. The poultry barn’s confined area is approximately 3,361.2 m2, and it is partitioned in half for flock management (). The area is further partitioned into 840.3 m2 quadrants, but only until the pullets are 3 weeks of age or approximately 181.4 grams in weight. The confined area is set up this way as only half of the poultry flock (20,000 pullets) arrives during the first week of operation, with the subsequent half of the flock (20,000 pullets) arriving within 7 days. Since chicks arriving are newly hatched, they are kept in the quadrant until they essentially “grow out” of that space, upon which time they will confined, but are free to move about the floor in a 1,680.6 m2 area, or a total area stocking density of 840 cm2 per bird. Throughout the flock cycle, expected mortality is targeted at approximately 3% and typically occurs within the first 10 days. Wood shavings are used as bedding litter and cover a cement barn floor. Shavings are treated with formaldehyde by the primary operator prior to flock arrival and removed between new flocks. A 1.8-meter path runs center the length of the confinement, dividing four sets of automated drinkers and four sets of auger feed lines on either side of the path.
Internal operations such as lighting, temperature, ventilation, and heat are monitored and adjusted as necessary in a systems control room adjacent to the poultry confinement. Upon pullet arrival, ambient temperatures are approximately 35.0°C and decreased by approximately 0.5°C per day for the first 6 weeks, leveling out at a constant of 20.0°C for the remaining 10 weeks. Ventilation for the barn consists of both tunnel and minimum ventilation. Side wall fans and attic inlets are primary sources of ventilation and include four 24” variable speed and four 36” constant speed fans. Attic inlets and side-wall fans are used until internal confinement temperatures exceed targets, then switching to tunnel ventilation. Tunnel ventilation comprises eight 57” constant speed fans that aid in removing excess heat.
The primary operator, his wife, and family spend approximately 90–120 minutes per day, 7 days a week in the barn. Daily chores include checking water and feed lines, ensuring systems are working properly, and mortality collection. Due to the size of the facility and large number of poultry, biosecurity procedures are in place to prevent the introduction and spread of disease-causing organisms to the flock. All workers and visitors are required to follow shower-in and out protocols, disinfection, and donning of facility-provided clothing. PPE including gloves, safety glasses, and respirators are available for use in the office/systems area.
Environmental measurements
We evaluated ammonia and total dust concentrations as single day measurements. Two stationary sampling stations () were configured to represent the breathing zone height of the poultry workers. Sampling stations were set equidistant from either side of the central partition walkway. To avoid unsettling the flock, techniques such as walking the perimeters of the confinement or using the walkway were advised for sampling, versus stepping over feed and water lines to check on equipment. Dust samples were collected gravimetrically using preloaded tared 37 mm, 5-µm PVC filter cassettes using an AirChek 2000 (SKC Ltd., Eighty Four, PA) air sampling pump at the nominal flow rate of 2 liters per minute (SKC Ltd., Eighty Four, PA) and analyzed per NIOSH Method 0500.Citation27 For ammonia sampling, both active and passive sampling strategies were used. For active sampling, an SKC Pocket Pump 210 (SKC Ltd., Eighty Four, PA) with a flow rate set at 250 milliliter per minute was utilized with solid sorbent tube media (sulfuric acid-treated silica gel) per NIOSH Method 6016.Citation28 For passive ammonia sampling, a UMEX™ 300 passive samplerCitation29 was attached to the sampling station. For each of the three visits, field blanks were also collected for each sampling method. All sampling activities were approximately 2 hours in length. A point measurement of temperature, relative humidity, and carbon dioxide was also performed at each visit; however, sampling equipment failed to collect data at our second visit (Q-TRAK 7575, TSI, Inc., Shoreview, MN).
At our first visit, visibility was high within the confinement, and pullets were very small, without feathers. At our second and third visits, pullets had full white feathers and had matured in size and stature. A prominent haze and reduction in visibility was apparent at the second visit. At our final visit, visibility was low, and we were no longer able to see either ends of the confinement. After each visit, sampling equipment was disinfected, and collected samples were placed in a cooler for transportation to our laboratory and immediately shipped to an accredited laboratory for analysis following NIOSH methods.
Results
The concentrations of airborne pollutants increased during each visit (). At our first visit, pullets were approximately 181.4 grams and about 21 days old; baseline concentrations of total dust samples were negligible. However, concentrations of total dust increased at our intermediate visit for a range of 16.0–18.0 milligrams per cubic meter (mg/m3), as the poultry had increased in age (9 weeks) and size (839.2 g). At our final visit, when the flock was approximately 15 weeks of age, with a bird weight of 1111.3 g, concentrations of total dust had increased more than two-fold for a range of 43.0–50.0 mg/m3. We found ammonia levels at our baseline and intermediate visits were considerably low for both sampling methods. For our last visit, ammonia levels had had reached a range of 22.0–30.0 parts per million. Point measurements indicated average carbon dioxide (CO2), temperature and relative humidity (RH) levels at 764.0 ppm, 21.3° Celsius, and 67.0%, respectively, for the initial visit. Measurements for the third visit indicated an average of CO2 levels to be at 2,627.0 ppm, temperature of 21.9°C, and 52.5% RH.
Table 1. Ammonia and total dust concentrations at each sampling location from three sampling sessions.
Discussion
This case study was undertaken for investigation and analysis due to the primary operator’s unique circumstances. Over the past decade, poultry has become more abundant in the state of Nebraska, providing farmers an opportunity to diversify their agricultural enterprise. With this opportunity, challenges can arise from inexperience and inadequate support or guidance on how to protect oneself or family from the exposures incurred working in poultry confinements. While contaminants such as ammonia and dust are presumed to exist in poultry confinements, it is not fully understood how these contaminants differ, accumulate, or impact workers within lesser-known poultry production sectors like that of pullet production.
Our findings indicate as pullets increase in age and weight, contaminants of dust also increase. These findings correspond to the primary operator’s visual perception and verbal account of indoor air quality diminishment. We expected levels of dust to increase, like those in layer and broiler exposure studies, but not to the ranges observed. When comparing our findings to those of broiler and layer flock studies, a comprehensive review of dust and endotoxin exposures in livestock farming demonstrated average dust exposures between 3.6 and 10.8 mg/m3,Citation25 whereas a 2019 study by Neghab et al. reported mean concentrations of total dusts between 37.3 ± 57.8 mg/m3.Citation30 Even though our findings are comparable to those found by Neghab et al., we cannot definitively conclude these results are typical of pullet operations, just that concentrations of dust reported in poultry confinements can reach considerably high levels. Regarding ammonia, our findings are consistent with those expressed in studies on layer and broiler flock operations where mean concentrations ranged from 1.3 to 29.6 ppm.Citation6,Citation26,Citation30
Whether the findings of our study are unique to pullet operations or represent an anomaly within poultry production exposure is debatable. Exposure to contaminants remain highly variable within poultry production and cannot be summed solely on their independent effects. Although dust is a common contaminant found in poultry confinements and derived from sources such as fecal matter and dried urea, feather barbules, dander and debris, feed, and bedding materials, it is not easily removed during flock growth cycles.Citation14 Since pullets are kept in confinement, as they age these sources of dust accumulate and can easily be suspended into the air through bird and worker movement, which may provide partial explanation to our findings.Citation31 It is possible that the levels of contaminants, especially ammonia, remained relatively low, as soiled litter is removed once a flock has finished its growth cycle and not repeatedly reused. It has been indicated that climate, particularly that in winter, acts as a strong determinant for higher levels of dust and ammonia, which may partially explain the significant increase in ammonia and dust concentrations from the intermediate to final visit.Citation25 While it may be difficult to generalize our findings to other pullet poultry producers, perhaps the more profound issue is how poultry producers and their families manage and abate potential occupational risks incurred while working with poultry, especially among children and adolescent family members.
What remains evident is workers’ risks for high contaminant exposures known to pose respiratory harm and adverse health effects. Exposure standards set by the Occupational Safety and Health Administration (OSHA) for dust and ammonia are based on an 8-hour time weighted average (TWA); yet, time spent in poultry barns is variable and does not accurately reflect cumulative exposures endured performing other agricultural work outside of the poultry confinement, and thus, exposures cannot be adequately determined for risk and potential health effects using regulatory standards alone. Moreover, there is a consensus that regulatory exposure limits are not protective enough, especially for those working with livestock.Citation23 A more focused approach on determining how to support those working in poultry production is needed, as mere perceptions of risk may not be motivating enough to protect one’s health.
In this case study, we quantified exposure concentrations of dust and ammonia and provided information to the primary operator on respirator selection guidance as well as respirator fit-testing. For future studies, we plan to include a more robust sample of pullet barn operations to examine if the experiences incurred by this family farm are shared by others. Additionally, we would include personal sampling methods with continuous monitoring to quantify personal exposure risk.
Conclusion
This exploratory case study provided insight into pullet barn operations and how concentrations of dust and ammonia increase with flock age at levels potentially higher than those previously recorded on layer and broiler operations. Through our investigation, it became apparent that poultry farmers, like the family in this study, may have uncertainties on how and if they are adequately protecting their health and that of their families. Currently, research and exposure assessments on the pullet poultry producing sector are limited, and our study cannot be assumed representative of all pullet farmers. What can be extrapolated is that the exposures incurred by those working with pullets are worthy of further investigation and research. Working with poultry is multifaceted and requires more research not only into the exposures present, but greater inquiry into how poultry producers are protecting their health and to what extent.
Acknowledgments
The authors thank Dr. Zijian Qin for assistance with data collection and Ms. Rachel Maley with manuscript preparation.
Disclosure statement
The authors have nothing to disclose.
Additional information
Funding
References
- United States Department of Agriculture, Economic Research Services. America’s diverse farms: 2018 edition. United States Department of Agriculture; 2018 December 01. 1 p. Report No.: 203.
- The National Chicken Council. Per capita consumption of poultry and livestock, 1960 to forecast 2020, in pounds. Washington D.C.: 2020. [Accessed 21 Oct. 2020]. Available from: https://www.nationalchickencouncil.org/about-the-industry/statistics/per-capita-consumption-of-poultry-and-livestock-1965-to-estimated-2012-in-pounds/.
- National Agricultural Statistics Service (US). 2017 Census of agriculture highlights, poultry and egg production. Washington: Department of Agriculture (US); 2020 July. 2 p. Report No.: ACH17-15.
- Perry J, Banker D, Green R. (Farm structure and performance branch, resource economics division, economic research service, Washington DC). Broiler Farms’ Organization, Management, and Performance. Washington: Department of Agriculture (US): 1999 Mar. 35 p. Bulletin No.: 748.
- The PEW Charitable Trusts. The business of broilers: hidden costs of putting a chicken on every grill. The Pew Charitable Trusts; 2013 Available from: https://www.pewtrusts.org/en/research-and-analysis/reports/2013/12/20/the-business-of-broilers-hidden-costs-of-putting-a-chicken-on-every-grill. Accessed September 13, 2021.
- Senthilselvan A, Beach J, Feddes J, Cherry N, Wenger I. A prospective evaluation of air quality and workers’ health in broiler and layer operations. Occup Environ Med. 2011;68(2):102–107. doi:10.1136/oem.2008.045021. PMID: 20935293.
- Barrasa M, Lamosa S, Fernandez MD, Fernandez E. Occupational exposure to carbon dioxide, ammonia and hydrogen sulphide on livestock farms in north-west Spain. Ann Agric Environ Med. 2012;19:17–24.
- Rimac D, Macan J, Varnai VM, et al. Exposure to poultry dust and health effects in poultry workers: Impact of mould and mite allergens. Int Arch Occup Environ Health. 2010;83(1):9–19. doi:10.1007/s00420-009-0487-5.
- Radon K, Weber C, Iversen M, Danuser B, Pedersen S, Nowak D. Exposure assessment and lung function in pig and poultry farmers. Occup Environ Med. 2001;58(6):405–410. doi:10.1136/oem.58.6.405.
- Guillam MT, Pédrono G, Le Bouquin S, et al. Chronic respiratory symptoms of poultry farmers and model-based estimates of long-term dust exposure. Ann Agric Environ Med. 2013: 20(2): 307–311. PMID: 23772582.
- Viegas S, Faísca VM, Dias H, Clérigo A, Carolino E, Viegas C. Occupational exposure to poultry dust and effects on the respiratory system in workers. J Toxicol Environ Health A. 2013;76(4–5):230–239. doi:10.1080/15287394.2013.757199. PMID: 23514065.
- Jameel U, Batool A, Hayat TM. Assessment of occupational exposure to volatile organic compounds in poultry workers. International Journal of Research in Applied, Natural and Social Sciences. 2015;3(2):59–68.
- Huneau-Salaün A, Le Bouquin S, Bex-Capelle V, et al. Endotoxin concentration in poultry houses for laying hens kept in cages or in alternative housing systems. Br Poult Sci. 2011;52(5):523–530. doi:10.1080/00071668.2011.617728.
- Lawniczek-Walczyk A, Gorny RL, Golofit-Szymczak M, Niesler A, Wlazlo A. Occupational exposure to airborne microorganisms, endotoxins and β-glucans in poultry houses at different stages of the production cycle. Ann Agric Environ Med. 2013;20:259–268.
- Viegas S, Veiga L, Malta-Vacas J, et al. Occupational exposure to aflatoxin (AFB₁) in poultry production. J Toxicol Environ Health A. 2012;75(22–23):1330–1340. doi:10.1080/15287394.2012.721164. PMID: 23095151.
- Oppliger A, Charriere N, Droz P, Rinsoz T. Exposure to bioaerosols in poultry houses at different stages of fattening; use of real-time PCR for airborne bacterial quantification. Ann Occup Hyg. 2008;52:405–412.
- Dahms C, Hübner NO, Cuny C, Kramer A. Occurrence of methicillin-resistant staphylococcus aureus in farm workers and the livestock environment in Mecklenburg-western Pomerania, Germany. Acta Vet Scand. 2014 Aug 21;56(1):53. doi:10.1186/s13028-014-0053-3. PMID: 25142727.
- Donham KJ, Cumro D, Reynolds SJ, Merchant JA. Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: recommendations for exposure limits. J Occup Environ Med. 2000;42(3):260–269. doi:10.1097/00043764-200003000-00006.
- Viegas S, Caetano LA, Korkalainen M, et al. Cytotoxic and inflammatory potential of air samples from occupational settings with exposure to organic dust. Toxics. 2017 Mar 1;5(1):8. doi:10.3390/toxics5010008. PMID: 29051440.
- Just N, Duchaine C, Singh B. An aerobiological perspective of dust in cage-housed and floor-housed poultry operations. J Occup Med Toxicol. 2009;4(1):13. doi:10.1186/1745-6673-4-13. PMID: 19515256.
- Zuskin E, Mustajbegovic J, Schachter EN, et al. Respiratory function in poultry workers and pharmacologic characterization of poultry dust extract. Environ Res. 1995;70(1):11–19. doi:10.1006/enrs.1995.1040. PMID: 8603653.
- Williams Ischer S, Farnell MB, Tabler GT, Moreira M, O’Shaughnessy PT, Nonnenmann MW. Evaluation of a sprinkler cooling system on inhalable dust and ammonia concentrations in broiler chicken production. J Occup Environ Hyg. 2017;14(1):40–48. doi:10.1080/15459624.2016.1211285. PMID: 27869548.
- Hartung J, Schulz J. Occupational and environmental risks caused by bioaerosols in and from farm animal houses. Agricultural Engineering International. Manuscript No. 1173. 2011;13(2).
- Kearney GD, Gallagher B, Shaw R. Respiratory protection behavior and respiratory indices among poultry house workers on small, family-owned farms in north carolina: a pilot project. J Agromedicine. 2016;21(2):136–143. doi:10.1080/1059924X.2016.1143429.
- Basinas I, Sigsgaard T, Kromhout H, Heederik D, Wouters IM, Schlunssen V. A comprehensive review of levels and determinants of personal exposure to dust and endotoxin in livestock farming. J Expo Sci Environ Epidemiol. 2015;25(2):123–137. doi:10.1038/jes.2013.83.
- Donham KJ, Cumro D, Reynolds S. Synergistic effects of dust and ammonia on the occupational health effects of poultry production workers. J Agromedicine. 2002;8(2):57–76. doi:10.1300/J096v08n02_09.
- National Institute of Occupational Safety and Health. NIOSH manual of analytical methods, 4th edition. Method 0500. US:NIOSH; 1994. [4 Aug 2020]. Available from: https://www.cdc.gov/niosh/docs/2003-154/pdfs/0500.pdf.
- National Institute of Occupational Safety and Health. NIOSH manual of analytical methods, 4th edition. Method 6016. US:NIOSH; 1996. [4 Aug 2020]. Available from: https://www.cdc.gov/niosh/docs/2003-154/pdfs/6016.pdf.
- SKC Inc. UMEX™ 300 passive sampler for ammonia. Cat. No. 500-300. Eighty Four, PA. Available from: https://www.skcltd.com/images/pdfs/40131.pdf. Accessed September 13, 2021.
- Neghab M, Ebrahimi A, Soleimani E. Respiratory symptoms and lung functional impairments associated with occupational exposure to poultry house pollutants. Int J Occup Saf Ergon. 2019;11:1–7. doi:10.1080/10803548.2019.1644738.
- Jerez SB, Cheng Y, Bray J. Exposure of workers to dust and bioaerosol on a poultry farm. J Appl Poult Res. 2014;23(1):7–14. doi:10.3382/japr.2012-00710.