Abstract
The anion transport and binding properties of a series of bis-amide based compounds have been studied in POPC lipid bilayers. The compounds display evidence of aggregation in solution with single crystal X-ray crystallographic analysis showing hydrogen bonding between the amide substituents of adjacent receptors in the solid state.
Introduction
Inorganic anions play important roles throughout biological systems including regulating cellular pH, osmotic balance and cell volume (Citation1Citation2Citation3). The transport of anions, such as chloride, bicarbonate and phosphate, across lipid bilayers are key in maintaining chemical potentials across lipid bilayers, driving metabolic processes and cell signalling (Citation2). Interest in developing synthetic anion transporters with the future potential to combat ‘channelopathies’, (Citation4) diseases caused by malfunctioning anion transport channels, has gained momentum in the last few years, with many small molecule anion transporters reported (Citation5Citation6Citation7Citation8).
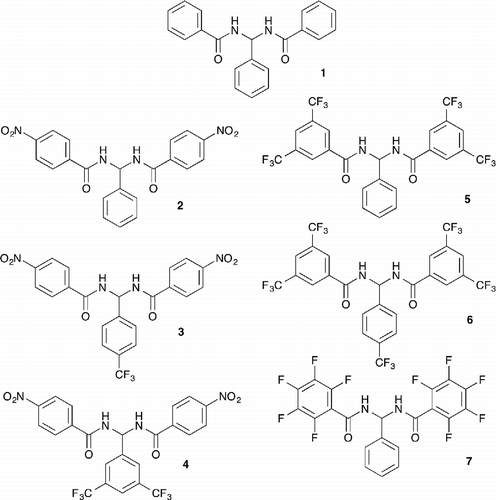
Our research interests focus on designing new small molecules containing hydrogen bond donors that function as transmembrane anion transporters. Favourable anion binding affinities and transport abilities of amides, ureas and thioureas have been reported (Citation9Citation10Citation11Citation12Citation13). QSAR studies involving a series of simple thioureas have shown that lipophilicity, molecular size and substituent effects (Hammett constant) are important molecular parameters that can be optimised to maximise transport efficiency across simple lipid bilayers. Ureas and thioureas (Citation14, Citation15) have been found to form stable complexes with a range of anions. Inspired by the effectiveness of the urea and thiourea motifs in anion receptor, transporters and self-assembling systems we report here the anion binding and transport properties of a family of N, N′-(phenylmethylene)dibenzamide (Citation16) based receptors 1–7 (Figure ). These bisamide-based receptors, like urea, possess a potentially parallel array of hydrogen bond donors. Nitro- and trifluoromethyl substituents were chosen due to their electron withdrawing properties, which is known to increase the acidity of attached hydrogen bond donors and is expected to increase the binding affinity of the receptors for anions (Citation3). Trifluoromethyl substituents have been used previously to enhance the transport properties of a variety of receptors by both enhancing the affinity of the receptor for anions and increasing its lipophilicy (Citation17).
Experimental
Synthesis
N,N′-(phenylmethylene)dibenzamide (1)
General procedure adapted from the literature (Citation16) – Benzamide (476 mg, 3.9 mmol) and benzaldehyde (203 μL, 2 mmol) were dissolved in dry DMF (8 mL). TMSCl (507 μL, 4 mmol) was added as a catalyst and was stirred at 50°C for 18 h under nitrogen. A white precipitate was formed with the addition of a few drops of water and was purified by stirring with diethyl ether for 30 min. The product was isolated using vacuum filtration. Yield 31%; m.p. 218.0–220.0°C. 1H NMR (400 MHz, DMSO-d6) δ ppm 9.05 (d, J = 7.80 Hz, 2 H), 7.89–7.96 (m, 4 H), 7.53–7.62 (m, 2 H), 7.47–7.51(m, 6 H), 7.40 (t, J = 7.40 Hz, 2 H), 7.32 (t, J = 7.40 1 H), 7.05 (t, J = 7.80 Hz, 1 H). 13C NMR (101 MHz, DMSO-d6) δ ppm 166.0 (CO), 140.8 (ArC), 134.3 (ArC), 132.0 (ArCH), 128.8 (ArCH), 128.8 (ArCH), 128.1 (ArCH), 128.0 (ArCH), 127.0 (ArCH), 59.2 (CH). LR-MS-ESI+- (m/z): 353 [M+Na+]. HR-MS-ESI+- (m/z): Calc. C21H18N2NaO2 353.1260 [M+Na+]. Meas. 353.1255 [M+Na+]. Error 1.4 ppm.
N,N′-(phenylmethylene)bis(4-nitrobenzamide) (2)
Using the general procedure with 4- nitrobenzamide (662 mg, 3.9 mmol), benzaldehyde (203 μL, 2 mmol) and TMSCl (507 μL, 4 mmol). Yield 44%; m.p. 253.1–254.4°C. 1H NMR (400 MHz, DMSO-d6) δ ppm 9.53 (d, J = 7.21 Hz, 2 H), 8.33 (d, J = 8.68 Hz, 4 H), 8.16 (d, J = 8.68 Hz, 4 H), 7.52 (d, J = 7.36 Hz, 2 H), 7.42 (t, J = 7.38 Hz, 2 H), 7.36–7.37 (m, 1 H), 7.00 (t, J = 7.27 Hz, 1 H). 13C NMR (101 MHz, DMSO-d6) δ ppm 164.8 (CO), 149.7 (ArC), 140.0 (ArC), 139.6 (ArC), 129.7 (ArCH), 128.8 (ArCH), 128.4 (ArCH), 127.3 (ArCH), 123.9 (ArCH), 59.9 (CH). LR-MS-ESI+- (m/z): 421 [M+H+]. HR-MS-ESI+-(m/z): Calc. C21H16N4NaO6 443.0962 [M+Na+]. Meas. 443.0969 [M+Na+]. Error 1.6 ppm.
N,N′-((4-(trifluoromethyl)phenyl)methylene)bis(4-nitrobenzamide) (3)
Using the general procedure with 4-nitrobenzamide (340 mg, 2 mmol), 4 (trifluoromethyl)benzaldehyde (85 μL, 0.5 mmol)) and TMSCl (200 μL, 1 mmol). Yield 42%; m.p. 280.0–281.9°C. 1H NMR (400 MHz, DMSO-d6) δ ppm 9.61 (d, J = 7.09 Hz, 2 H), 8.35 (d, J = 8.86 Hz, 4 H), 8.17 (d, J = 8.86 Hz, 4 H), 7.79 (d, J = 8.36 Hz, 2 H), 7.74 (d, J = 8.36 Hz, 2 H), 7.04 (t, J = 7.09 Hz, 1 H). 13C NMR (101 MHz, DMSO-d6) δ ppm) 165.0 (CO), 149.7 (ArC), 144.1 (ArC), 139.8 (ArC), 129.7 (ArCH), 129.1 (q, CF3), 128.2 (ArCH), 125.7 (ArC), 123.9 (ArCH), 123.3 (ArCH), 59.7 (CH). LR-MS-ESI+- (m/z): 489 [M+H+]. HR-MS-ESI+-(m/z): Calc. C22H15F3N4NaO6 511.0836 [M+Na+]. Meas. 511.0845 [M+Na+]. Error 1.8 ppm.
N, N′-((3,5-bis(trifluoromethyl)phenyl)methylene)bis(4-nitrobenzamide) (4)
Using the general procedure with 4-nitrobenzamide (1.008 g, 6 mmol), 3,5-bis (trifluoromethyl)benzaldehyde (500 μL, 3 mmol)) and TMSCl (770 μL, 6 mmol) in 10 mL DMF. Yield 81 %; m.p. 283.2–285.1°C. 1H NMR (400 MHz, DMSO-d6) δ ppm 9.68 (br d, J = 6.85 Hz, 2 H), 8.35 (br d, J = 8.56 Hz, 4 H), 8.26 (s, 2 H), 8.15 (br d, J = 8.44 Hz, 5 H) a, 7.09 (br t, J = 6.66 Hz, 1 H). 13C NMR (101 MHz, DMSO-d6) δ ppm 165.2 (CO), 149.8 (ArC), 142.8 (ArC), 139.6 (ArC), 130.8 (q, CF3), 129.7 (ArCH), 128.6 (ArC), 124.0 (ArCH), 122.6 (ArCH), 122.4 (ArCH), 59.7 (CH). LR-MS-ESI− - (m/z): 555[M-H+]. HR-MS-ESI+- (m/z): Calc. C23H14F6N4NaO6 579.0710 [M+Na+]. Meas.579.0715 [M+Na+]. Error 0.9 ppm. a two overlapping signals singlet overlapping a doublet.
N, N′-(phenylmethylene)bis(3,5-bis(trifluoromethyl)benzamide) (5)
Using the general procedure with 3,5-bis(trifluoromethyl)benzamide (600 mg, 2.3 mmol), benzaldehyde (138 μL, 1.2 mmol)) and TMSCl (296 μL, 2.3 mmol) in 10 mL DMF. Recrystallised from EtOAC to give the product as a white powder. Yield 66 %; m.p. 257.1–259°C. 1H NMR (400 MHz, DMSO-d6) δ ppm 9.73 (d, J = 7.12 Hz, 2 H), 8.60 (s, 4 H), 8.35 (s, 2 H), 7.56 (d, J = 7.34 Hz, 2 H), 7.44 (t, J = 7.34 Hz, 2H) 7.36–7.40 (m, 1 H), 7.06 (t, J = 6.97 Hz, 1 H). 13C NMR (101 MHz, DMSO-d6) δ ppm 163.6 (CO), 139.1 (ArC), 136.4 (ArC), 130.9 (q, CF3), 129.0 (br s, ArC), 128.6 (ArCH), 127.4 (ArCH), 125.6 (br s, ArCH), 124.9 (ArCH), 122.2 (ArCH), 60.3 (CH). LR-MS-ESI+- (m/z): 602 [M+H+]. HR-MS-ESI+-(m/z): Calc. C25H15F12N2O2 603.0936 [M+H+]. Meas. 603.0927 [M+H+]. Error 1.5 ppm.
N,N′-((4-(trifluoromethyl)phenyl)methylene)bis(3,5-bis(trifluoromethyl)benzamide) (6)
Using the general procedure with 3,5-bis(trifluoromethyl)benzamide (1.03 mg, 4 mmol), 4- (trifluoromethyl) benzaldehyde (272 μL, 2 mmol)) and TMSCl (507 μL, 4 mmol). Yield 8 %; m.p. 269.0–270.0°C. 1H NMR (400 MHz, DMSO-d6) δ ppm 9.82 (d, J = 6.96 Hz, 2 H), 8.60 (s, 4 H), 8.39 (s, 2 H), 7.77–7.86 (m, 4 H), 7.09 (t, J = 6.86 Hz, 1 H). 13C NMR (101 MHz, DMSO-d6) δ ppm 163.7 (CO), 143.7 (ArC), 136.2 (ArC), 130.9 (q, CF3), 129.0 (br s, ArC), 128.4 (ArCH), 127.6 (ArC), 125.8 (m, CF3), 124.9 (ArCH), 122.2 (ArCH), 119.5 (ArCH), 60.0 (CH). LR-MS-ESI− - (m/z): 669 [M-H+]. HR-MS-ESI+-(m/z): Calc. C26H14N2O2F15 671.0810 [M+H+]. Meas. 671.0806 [M+H+]. Error 0.6 ppm.
N,N′-(phenylmethylene)bis(2,3,4,5,6-pentafluorobenzamide) (7)
2,3,4,5,6-Pentafluorobenzamide (842 mg, 4 mmol) and benzaldehyde (203 μL, 2 mmol) were dissolved in dry toluene(5 mL). ZnCl2 (∼5 mg) was added as a catalyst and the reaction mixture was refluxed for 24 hours under nitrogen. A white precipitate was formed over time and the product was isolated using hot vacuum filtration washing with diethyl ether. Yield 20 %; m.p. 253.0–256.0°C. 1H NMR (400 MHz, DMSO-d6) δ ppm 9.95 (d, J = 7.96 Hz, 2 H), 7.46–7.48 (m, 4 H), 7.38–7.42 (m, 1 H), 6.90 (t, J = 7.94 Hz, 1 H). 13C NMR (101 MHz, DMSO-d6) δ ppm 156.7 (CO), 144.9 (ArC), 142.5 (ArC), 140.5 (ArC) 138.6 (ArC), 138.2 (ArC), 136.2 (ArC), 129.1 (ArCH), 128.9 (ArCH), 126.7 (ArCH), 112.5 (ArC), 58.6 (CH) LR-MS-ESI− - (m/z): 553 [M+Na+]. HR-MS-ESI+-(m/z): Calc C21H9O2N2F10 511.0499 [M+H+]. Meas 511.0511 [M+H+], Error 2.3 ppm. Calc. C21H8O2N2NaF10 533.0318 [M+Na+]. Meas. 533.0330 [M+Na+]. Error 2.25 ppm.
Results and discussion
Binding studies were carried out in two solvent systems for receptors 1–7 to determine their affinity for a variety of different anions. After determination of the binding stoichiometry, by Job plot analyses (Citation18), stability constants for chloride, bicarbonate and dihydrogen phosphate complex formation were determined from fitting 1H NMR titration data (following the downfield shift of the amide NH) to 1:1 binding isotherms using winEQNMR2 (Citation19). The association constants are shown in Table .
Table 1 Apparent stability constants (M− 1) in A) DMSO-d6/0.5 % water and B) 59.75 % acetonitrile-d3/39.75 % DMSO-d6/ 0.5 % water.
Titrations were performed using nitrate as the guest anion but these showed little or no shift of the amide NH and could not be fitted to a binding isotherm. The general trend in association constants for receptors 1–7 indicates the binding affinity for chloride is low. The compounds have a higher affinity for oxoanions particularly dihydrogen phosphate which is attributed to the favourable binding mode in which presumably two parallel hydrogen bonds are formed to the oxo-anion. Upon addition of bicarbonate, peak broadening was observed which in some cases precluded the elucidation of an association constant.
The transport ability of receptors 1–7 was investigated using phospholipid vesicle based assays (Citation20). Unilamellar 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) vesicles were prepared with an internal solution of 489 mM NaCl buffered to pH 7.2 with 5 mM sodium phosphate salts, and were suspended in an external solution of 489 mM NaNO3 buffered to pH 7.2 with 5 mM sodium phosphate salts; to obtain a final lipid concentration of 1 mM. The receptor was added as a DMSO solution, 2 mol% with respect to the lipid, and the resulting chloride efflux was measured using a chloride selective electrode. At 300 s the vesicles were lysed with 50 μL polyoxyethylene (8) lauryl ether detergent to give the 100 % chloride efflux and the results were calibrated using this final electrode reading.
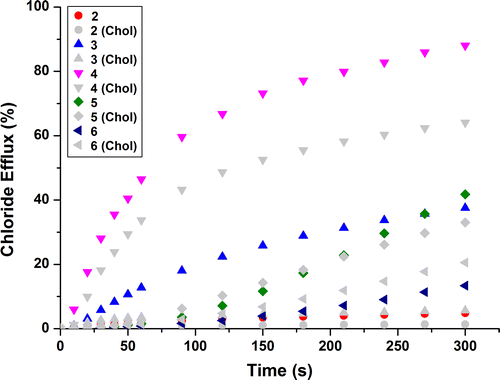
Figure demonstrates the differing abilities of receptors 1–7 to facilitate chloride efflux. Receptor 4 displays the most effective chloride transport across the course of the experiment with >80% chloride efflux over 300 s. Increasing the fluorination of the central phenyl for receptors 2–4 showed an increase in chloride efflux, which supports previous studies that attributes this to the increased lipophilicity of the transporter and therefore a greater ability to partition into the lipid bilayer (Citation17). Receptors 5 and 6, despite being highly fluorinated, do not exhibit the greatest transport abilities and this is discussed later.
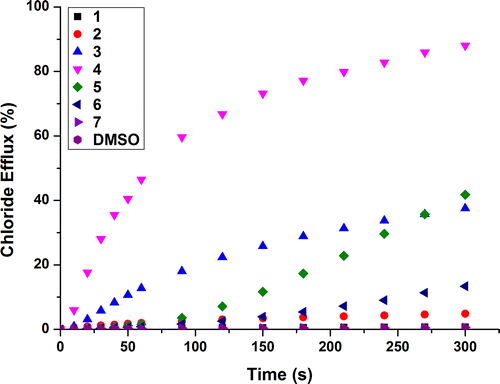
Two transport mechanisms are possible, antiport ( exchange) or symport (Na+/Cl− or H+/Cl− co-transport). To rule out the latter, the standard POPC
assay was repeated using both KCl and CsCl as the encapsulated salt and this resulted in no significant change in the chloride efflux (see ESI) – evidence that does not support a metal co-transport mechanism. To confirm the antiport mechanism and rule out the H+/Cl− symport mechanism, POPC vesicles were prepared and suspended in Na2SO4. After addition of the transporter there was little, if any, chloride efflux observed (see ESI), this was expected due to the high dehydration penalty involved in the transport of the hydrophilic sulfate anion (Citation21). This result indicated that an exchange process was likely and no H+/Cl− co-transport was occurring, this assay was repeated and at 120 s a pulse of NaHCO3 was added, switching on the chloride efflux via a chloride/bicarbonate exchange process. Receptors 2–6 were less effective as Cl− /HCO− 3 antiporters than
antiporters. For example, receptor 4 could only facilitate ca. 30% release of chloride at the end of the experiment (see Figure ).
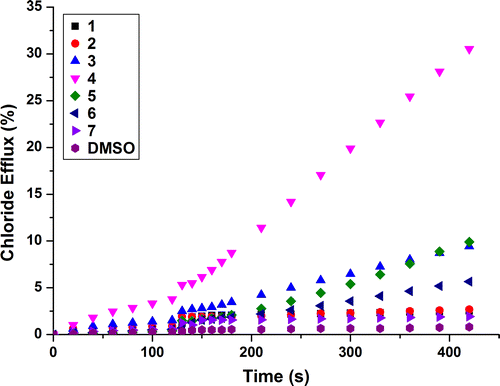
Quantification of the transport process for receptors 2–6 was attempted by Hill analysis (Citation22). Receptors 1 and 7 were excluded due to no measurable chloride transport at 2 mol% of receptor. EC50 values and effective receptor concentration required to facilitate 50% chloride efflux after 270 s, can be found in Table . Hill coefficients (n) were also calculated, giving an indication of the stoichiometry for the transported complex (Citation23).
Table 2 EC50 and Hill coefficient (n) values for receptors 2–6.
Table shows that compound 4 is quantitatively the most efficient transporter with the lowest EC50 value. Although Hill analysis was deemed unreliable and in some cases, EC50 values could not be calculated, this was due to unusual behaviour from all the receptors to varying extents. When adding the receptor in DMSO solution, the more concentrated the stock solution, the less consistent the runs and in some cases the lower the chloride efflux, despite the overall amount of receptor added being the same. This effect was most apparent for receptors 5 and 6 it was also evident that these receptors possessed a different transport profile. Figure shows their transport takes longer to begin following the addition of the receptor to the system, giving more of a sigmoidal shape to the curve, receptor 6 also displays a more sigmoidal shape in the Hill plot. This behaviour has previously been noted by Haynes et al. (Citation24) which was attributed to the receptors lying outside the optimum lipophilicity values proposed by Saggiomo et al. (Citation25) Receptors 5 and 6 possess log P values of 5.1 and 5.44 respectively as calculated by VCC labs, (Citation26) which lie above the optimum lipophilicity values proposed (log P ≈ 4) (Citation25). This would allow the slow start to transport to be rationalised, as delivery through the aqueous external solution is hindered. Another explanation of the unusual concentration dependant results is the possibility of self-aggregation of the receptors in solution which is reported to be counterproductive to transport efficacy (Citation27, Citation28).
Hill coefficients for receptors 3 and 4 were both ≈ 1, which is indicative of a mobile carrier mechanism, where one receptor transports one anion across the bilayer. Receptor 6 however has a Hill coefficient of 2.5, this indicates that more than one receptor may be in contact with the anion during the transport process, possibly the formation of a cluster or aggregate that transports the anion (Citation29). To investigate this further, vesicles were prepared with a 7:3 POPC:cholesterol composition and a assay was conducted. Cholesterol orders a lipid bilayer resulting in less fluidity (Citation30), so it would be assumed for mobile carrier mechanisms that depend on diffusion through the membrane, transport would be reduced with this increase in viscosity.
Receptors 2–4 exhibit the expected decrease in activity, Figure shows an increase in activity for receptor 6 and very little change in activity for receptor 5, this could be indicative that their transport process is not a mobile carrier mechanism; however, from U-tube tests (see ESI) a channel formation was deemed unlikely. Haynes et al. (Citation24) reported similar increases in transport with cholesterol for receptors containing highly lipophilic substituents such as CF3. It is clear that delivery and partitioning into the lipid membrane is affected by the presence of cholesterol and the lipophilic nature of the receptor (Citation31).
Investigating the unusual concentration dependant behaviour of these compounds further, a series of NMR dilution experiments were performed to try to identify if self-aggregation was a possibility in solution and hence reducing the efficacy of chloride transport. Self-aggregates typically have higher log P values and lower solubility than their monomeric components which would explain why receptors 5 and 6 exhibit more aggregate tendencies (Citation32). Typical NMR spectra of aggregators can show changes in the number of signals present, indicative of multiple aggregates species in solution. Additionally, changes in chemical shifts (δ ppm) may be detected resulting from local environmental changes in the magnetic field of the molecule if aggregates are formed. Changes in the shape of the resonance peaks, typically broadening, may indicate a change in the size and tumbling rate of the species in solution (Citation33). Proton NMR spectra were obtained for receptors 1–7 at a range of concentrations (1–100 mM). Inserts from the spectra of receptor 5 can be seen in Figure . A small change in chemical shift is evident for Ha and Hb.
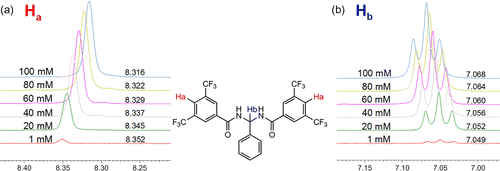
Most of the receptors showed small, but noticeable, changes in the chemical shifts for proton (Hb) and the N-H protons (see ESI). Receptor 5 was the only one to show significant changes for other proton shifts (Ha) and receptor 4 displayed the least noticeable shifts, interestingly, as this receptor showed the least concentration dependence during vesicle studies and was the most effective chloride transporter. The self-association of these receptors in solution means that the association constant determinations above will be dependent on concentration. Therefore the association constants should be regarded as apparent values only.
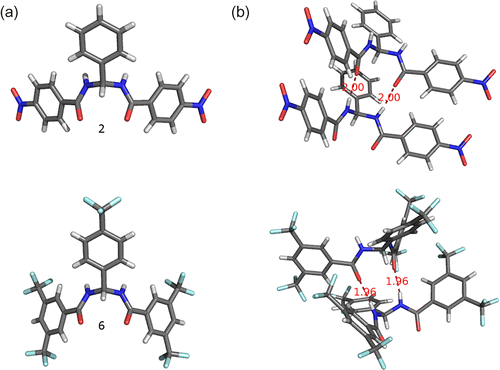
Receptors 2 and 6 were both isolated as crystalline solids by slow evaporation at room temperature of acetonitrile and DMSO solutions of the compounds respectively.Footnote1 The structures of the compounds were elucidated by single crystal X-ray diffraction. Figure shows that in the solid state the receptors adopt a conformation in which the NH hydrogen bond donors are nearly parallel. The molecules form continuous hydrogen bonded arrays in the solid state via two NH…O (1.96–2.00 Å) interactions between the amide groups on adjacent receptors.
Conclusions
A series of N, N′-(phenylmethylene)dibenzamide based receptors were found to function as and
antiporters, most likely by a mobile carrier mechanism although evidence of aggregation in solution was observed which complicated unambiguous determination of the transport mechanism. Self-aggregation was supported by NMR dilution studies and was most apparent in fluorinated receptor 5. The self-association properties of this motif may effectively increase log P and hence enhance the transport properties of the compounds. Substituent effects and lipophilicity were found to be important parameters involved in the binding and transport abilities. One can envisage this hydrogen-bonding motif being used instead of urea in a variety of self-assembled structures due to the formation of two nearly linear hydrogen-bonding interactions. We are continuing to investigate the properties of this hydrogen-bonding array.
Supplemental data
The underlying data for this article can be accessed at http://dx.doi.org/10.5258/SOTON/375695
Supplemental Material
Download MS Word (8.1 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Funding
Notes
1. X-ray data were collected on a Rigaku AFC 12 diffractometer mounted on Rigaku FR-E+ Super Bright High Flux rotating anode CCD diffractometer equipped with VariMax high flux (HF) optics and Saturn 724+ CCD detector (Citation34).Crystal data for receptor 6. CCDC 1053070, C26H13F15N2O2 (M = 670.38): orthorhombic, space group Pnma, a = 9.7085(2) Å, b = 26.9582(10) Å, c = 10.0203(2) Å, α = 90°, β = 90°, γ = 90°, V = 2622.53(12) Å3, Z = 4, T = 100(2) K, μ(MoKα) = 0.181 mm− 1, Dcalc = 1.698 g/mm3, 16362 reflections measured (2.168 ≤ Θ ≤ 31.993), 4347 unique (Rint = 0.0253) which were used in all calculations. The final R1 was 0.0581 (I>2σ(I)) and wR2 was 0.1431 (all data).Crystal data for receptor 2. CCDC 1053071, C21H16N4O6 (M = 420.38): Orthorhombic, space group Pnma, a = 16.5475(11) Å, b = 22.8442(16) Å, c = 4.9997(4) Å α = 90°, β = 90°, γ = 90°, V = 1890.0(2) Å3, Z = 4, T = 100(2) K, μ(MoKα) = 0.111 mm− 1, Dcalc = 1.477 g/mm3, 10813 reflections measured (3.040 ≤ 2Θ ≤ 27.466), 2216 unique (Rint = 0.0355) which were used in all calculations. The final R1 was 0.0365 (I>2σ(I)) and wR2 was 0.0982 (all data).
References
- Davis, J.T.; Okunola, O.; Quesada, R. Chem. Soc. Rev. 2010, 39, 3843–3862. doi:10.1039/b926164h.
- Davis, A.P.; Sheppard, D.N.; Smith, B.D. Chem. Soc. Rev. 2007, 36, 348–357. doi:10.1039/B512651G.
- Sessler, J.L.; Gale, P.A.; Cho, W.S. Anion Receptor Chemistry; Stoddart, J.F., Ed.; RSC: Cambridge, 2006.
- Ashcroft, F.M. Ion Channels and Disease Channelopathies; San Diego: Academic Press, 2000.
- Matile, S.; Vargas Jentzsch, A.; Montenegro, J.; Fin, A. Chem. Soc. Rev. 2011, 40, 2453–2474. doi:10.1039/c0cs00209g.
- Busschaert, N.; Gale, P.A. Angew. Chem. Int. Ed. Engl. 2013, 52, 1374–1382. doi:10.1002/anie.201207535.
- Gale, P.A. Acc. Chem. Res. 2011, 44, 216–226. doi:10.1021/ar100134p.
- Gale, P.A.; Pérez-Tomás, R.; Quesada, R. Acc. Chem. Res. 2013, 46, 2801–2813. doi:10.1021/ar400019p.
- Amendola, V.; Fabbrizzi, L.; Mosca, L. Chem. Soc. Rev. 2010, 39, 3889–3915. doi:10.1039/b822552b.
- Andrews, N.J.; Haynes, C.J. E.; Light, M.E.; Moore, S.J.; Tong, C.C.; Davis, J.T.; Harrell, Jr, W.A.; Gale, P.A. Chem. Sci. 2011, 2 (2), 256–260. doi:10.1039/C0SC00503G.
- Moore, S.J.; Wenzel, M.; Light, M.E.; Morley, R.; Bradberry, S.J.; Gómez-Iglesias, P.; Soto-Cerrato, V.; Pérez-Tomás, R.; Gale, P.A. Chem. Sci. 2012, 3, 2501–2509. doi:10.1039/c2sc20551c.
- Haynes, C.J.E.; Moore, S.J.; Hiscock, J.R.; Marques, I.; Costa, P.J.; Félix, V.; Gale, P.A. Chem. Sci. 2012, 3, 1436–1444. doi:10.1039/c2sc20041d.
- Gómez, D.E.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. Org. Biomol. Chem. 2005, 3, 1495–1500.
- Brooks, S.J.; Edwards, P.R.; Gale, P.A.; Light, M.E. New J. Chem. 2006, 30, 65–70. doi:10.1039/B511963D.
- Brooks, S.J.; Gale, P.A.; Light, M.E. Chem. Commun. 2005, 4696–4698. doi:10.1039/b508144k.
- Yang, P.; Myint, K.-Z; Tong, Q.; Feng, R.; Cao, H.; Almehizia, A.A.A.; Alqarni, M.H.; Wang, L.; Bartlow, P.; Gao, Y.; Gertsch, J.; Teramachi, J.; Kurihara, N.; Roodman, G.D.; Cheng, T.; Xie, X.-Q. J. Med. Chem. 2012, 55, 9973–9987. doi:10.1021/jm301212u.
- Busschaert, N.; Kirby, I.L.; Young, S.; Coles, S.J.; Horton, P.N.; Light, M.E.; Gale, P.A. Angew. Chem. Int. Ed. Engl. 2012, 51, 4426–4430. doi:10.1002/anie.201200729.
- Job, P. Ann. Chim. Appl. 1928, 9, 113–203.
- Hynes, M.J. J. Chem. Soc. Dalton Trans. 1993, 311–312. doi:10.1039/dt9930000311.
- Koulov, A.V.; Lambert, T.N.; Shukla, R.; Jain, M.; Boon, J.M.; Smith, B.D.; Li, H.; Sheppard, D.N.; Joos, J.-B.; Clare, J.P.; Davis, A.P. Angew. Chem. Int. Ed. Engl. 2003, 42, 4931–4933. doi:10.1002/anie.200351957.
- Marcus, Y. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. doi:10.1039/ft9918702995.
- Hill, A.V. Biochem. J. 1913, 7, 471–480.
- Bhosale, S.; Matile, S. Chirality 2006, 18, 849–856. doi:10.1002/chir.20326.
- Haynes, C.J. E.; Busschaert, N.; Kirby, I.L.; Herniman, J.; Light, M.E.; Wells, N.J.; Marques, I.; Félix, V.; Gale, P.A. Org. Biomol. Chem. 2014, 12, 62–72. doi:10.1039/C3OB41522H.
- Saggiomo, V.; Otto, S.; Marques, I.; Félix, V.; Torroba, T.; Quesada, R. Chem. Commun. 2012, 48, 5274–5276. doi:10.1039/c2cc31825c.
- VCClabs. http://www.vcclab.org (accessed Dec 18, 2014).
- Daschbach, M.M.; Negin, S.; You, L.; Walsh, M.; Gokel, G.W. Chem. A Eur. J. 2012, 18, 7608–7623.
- Elliott, E.K.; Daschbach, M.M.; Gokel, G.W. Chem. A Eur. J. 2008, 14, 5871–5879.
- Murillo, O.; Suzuki, I.; Abel, E.; Murray, C.L.; Meadows, E.S.; Jin, T.; Gokel, G.W. J. Am. Chem. Soc. 1997, 119, 5540–5549. doi:10.1021/ja962694a.
- Holthuis, J.C. M.; van Meer, G.; Huitema, K. Mol. Membr. Biol. 2003, 20, 231–241. doi:10.1080/09687680307082.
- Spooner, M.J.; Gale, P.A. Chem. Commun. 2015, 51, 4883–4886. doi:10.1039/C5CC00823A.
- Seidler, J.; McGovern, S.L.; Doman, T.N.; Shoichet, B.K. J. Med. Chem. 2003, 46, 4477–4486. doi:10.1021/jm030191r.
- LaPlante, S.R.; Carson, R.; Gillard, J.; Aubry, N.; Coulombe, R.; Bordeleau, S.; Bonneau, P.; Little, M.; O'Meara, J.; Beaulieu, P.L. J. Med. Chem. 2013, 56, 5142–5150. doi:10.1021/jm400535b.
- Coles, S.J.; Gale, P.A. Chem. Sci. 2012, 3, 683–689. doi:10.1039/C2SC00955B.