?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
KEYWORDS:
In a previous Eric’s Corner, I dealt with a pet peeve, that of using the proper terms for describing stereoisomers [Citation1]. This was my focus because chemists know that stereochemistry, and the associated topic of chirality, are concepts of prime importance. To further emphasise this fundamental importance, both are required topics taught in all introductory organic chemistry classes. They are as important to organic chemistry as are the required topics of transfiguration, charms, and defence against the dark arts at Hogwarts School of Witchcraft and Wizardry.
An object is chiral if it is not superimposable (congruent) with its mirror image. The definition is an absolute statement, and does not imply gradations. An object is, or is not, chiral – with no in-between. Rigorously speaking, in three dimensions a chiral geometric figure is absent improper rotation axes (Sn), most commonly mirror symmetry (σ, equivalent to an S1). We note an analogy that may seem tiresome, but chirality is akin to pregnancy. Animals either are, or are not, pregnant – with no in-between. Similarly, one is either a wizard or a muggle – there is no in-between (even a squib, albeit born into a wizard family yet lacking magical powers, is a muggle).
Fifty years ago, in a persuasive essay with a philosophical title, ‘An Epistemological Note on Chirality’ (ENC) [Citation2], Mislow and Bickart opined on the problematic judgements a chemist might render about the chirality of a system of molecules from measurements of observables. While chirality [Citation3]Footnote1 is sharply defined with respect to geometrical models, these authors used a series of carefully posed chemical scenarios to decouple chirality from any activity a chemist might undertake in the laboratory (e.g. a measurement of optical activity in solution or the enantioselectivity of a chemical reaction). The content of their ENC is approximated by the aphorism ‘the absence of evidence is not the evidence of absence’. That is, failure to detect pseudo-scalar observables can never be used to establish the chirality or achirality of a chemical system because we can imagine chiral molecules or collections of molecules with vanishingly small pseudoscalar observables, no matter the sensitivity of the method of analysis of the property consistent with molecular dissymmetry. Such systems will be cryptochiral, in the neologism of Mislow and Bickart.
That said, in the ensuing 50 years, several researchers have pondered whether chirality can be put on a scale. Further, in the supramolecular or synthetic chemistry fields, we’ll often hear about amplifying chirality (see below), as if there is a measurement of its value. In fact, the instinct towards chirality quantification can be traced to the 19th century, and efforts to correlate optical activity to chirality measures based on geometrical abstractions of molecules [Citation4]. Correlations between chirality functions and circumscribed sets of observations have been proposed. For instance, the temperature dependence of the circular birefringence along the optic axis of crystalline α-quartz was correlated with temperature dependent structural changes [Citation5] by employing ‘Continuous Chirality Measures’ [Citation6] wherein the normalised atom-for-atom distance to the nearest achiral reference structure is computed [Citation7]. But, it is clear that such correlations are destined to fail as the sets of observations included in the correlation are changed or relaxed [Citation8]. Bart Kahr at NYU recently published a News & views in Nature accentuating this same issue with an analysis of the twist in bowties [Citation8].
But chemistry marches on, and marching is especially energetic in the fields of nano- and meso- chemistry where new chiral entities are designed and discovered with great frequency. The temptations that have motivated researchers of past generations to link measures of chirality to real chemical objects beckon anew.
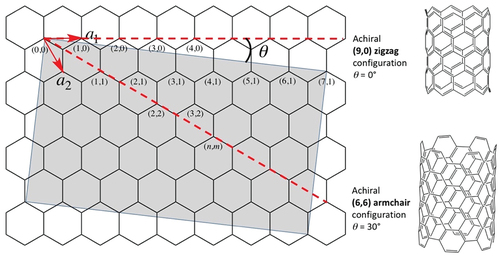
With nanotubes, it is common to speak about the ‘chiral angle’, the ‘chiral vector’, or ‘chiral indices’ [Citation9,Citation10]. Because angles and vectors have associated magnitudes, such nomenclature implies that chirality itself can be quantified by these measures. As shown in the figure below, a representation of graphene, given two basis vectors a1 and a2 in a hexagonal net separated by 60° where each vertex can be identified by indices (n,m) and the associated ‘roll-up’ vector is na1 + ma2, the chiral angle θ(n,m) can be expressed as
The chiral angle varies ± 60°. If θ = 0°, the configuration is the so-called the zigzag (n,0), and if θ = ±30° the configuration is the so-called the armchair (n,n). The zigzag has some C-C bonds that are parallel to the tube axis whereas the armchair has some C-C bonds perpendicular to the tube axis; both the zigzag and armchair configurations are achiral. In other words, the chiral angle defines an achiral object when θ = 0°, and when ± 30°. Angles between 0° and 30° generate chiral objects (the grey box). The practice is to qualify the word ‘angle’ with ‘chiral’, even when the object is not chiral! How confusing is that? It is akin to the awkwardness of meso tartaric acid having two chiral centres (more correctly, two stereocenters) but being an achiral object (see my earlier Eric’s Corner [Citation1]).
Adapted from reference 10.
Recently, Bert Meijer and Scott Denmark pointed out the dichotomy of using the term ‘amplification of chirality’ in a J. of Poly. Sci. perspective paper [Citation11]. In helical polymer self-assembly, the ‘sergeant-soldier’ and ‘majority-rules’ principles have been found in numerous works [Citation12]. Here, both concepts rely on the excess of one enantiomer to dictate the self-assembly of one helical handedness over another. The term ‘amplification of chirality’ has been used to signify the increase in helical excess, as inferred from experimental measurements of some chiroptical property, such as circular dichroism or optical activity. To quote directly from their perspective, ‘Chirality is strictly related to geometrical symmetry, or the lack thereof. As a result, chirality cannot be increased or amplified; a system can only be chiral or achiral’ - just we have opined upon above. Thus, the term ‘amplification of chirality’ was discouraged, and ‘amplification of asymmetry’ was instead recommended. Yet, once the cat is out of the bag, it is hard to put back in – efforts to quantitate chirality still abound [Citation13].
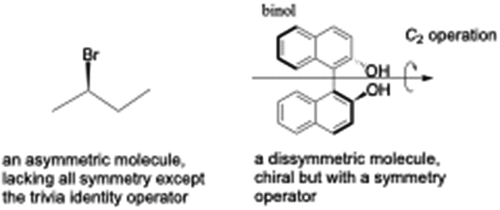
Even more strictly speaking, we believe amplification of dissymmetry is better because one could be amplifying the experimental result of the absence of improper rotation axes in otherwise highly symmetric systems (Louis Pasteur used the term ‘dissymétrie’ [Citation14], which is not the same as asymmetryFootnote2). For example, below are an asymmetric and dissymmetric molecule. Note both are chiral, but the second does possess an element of symmetry. So, to refine the terminology, I’ll go even further. I’d suggest that any phenomenological statements specifically refer to the experimental parameter being measured. For example, rather than discussing ‘amplification of chirality’, one would refer to the ‘amplification of optical activity’.
Given this discussion, why do some investigators continue to adopt the notion of measuring or quantifying chirality in their research endeavours? I believe what we think becomes reality for us. Albus Dumbledore tells Harry Potter – ‘Of course it’s happening inside your head, Harry. But why should that mean it’s not real?’ [Citation15] As one proceeds in one’s career, it is easy to forget, or even overlook, lessons of fundamental concepts taught in introductory classes. I admit to once confusing rate and rate-constants in one of my J. Am. Chem. Soc. papers as a post-doc [Citation16], and it led to considerable confusion. Simply stated, we all make mistakes. Thus, much of the confusion can be chalked up to unintentional oversights, which is how we interpret the use of ‘chirality angle’ in the nanotube field. In the case of supramolecular chirality amplification, the term nicely conjures up an image of what is occurring in the reaction vessel (and thus is insightful), albeit we discourage the use of the term. Humankind tends to want to categorise items and to seek trends, because this imparts a level of understanding that is lacking without an organisational basis. Once again, Harry Potter derived wisdom can prevail; Albus Dumbledore tells us ‘People find it far easier to forgive others for being wrong than being right.’ [Citation17] In that spirit, let’s be forgiving of these minor mishaps concerning chirality, but keep them in mind for the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes
1. Here we refer only to true chirality only exhibited by enantiomorphous systems that can be interconverted by space inversion but not time inversion.
2. If a structure lacks all symmetry elements, it is asymmetric (point group C1), and is nonsuperimposable on its mirror image and chiral. However, if a structure lacks a Sn axis but has other symmetry elements, it is dissymmetric, again nonsuperimposable on its mirror image and chiral.
References
- Ngo PH, Anslyn EV. Eric’s corner stereochemical pet peeves, terminology worthy of “all creatures great and small”. Supramol Chem. 2023;34(3–4):141–144. doi: 10.1080/10610278.2023.2271617
- Mislow K, Bickart P. An epistemological note on chirality. Isr J Chem. 1976;15(1–2):1–6. doi: 10.1002/ijch.197600002
- Barron LD. From cosmic chirality to protein structure: Lord Kelvin’s legacy. Chirality. 2012;24(11):879–893. doi: 10.1002/chir.22017
- Buda AB, Auf der Heyde T, Mislow K. Angew. Chem Int Ed. 1992;31(8):989–1007. (b) M. Petitjean, Entropy, 2003, 5, 271-312. doi: 10.1002/anie.199209891
- Yogev-Einot D, Avnir D. The temperature-dependent optical activity of quartz: from Le Châtelier to chirality measures. Tetrahedron Asymmetry. 2006;17(19):2723–2725. doi: 10.1016/j.tetasy.2006.10.004
- Zabrodsky H, Avnir D. Continuous symmetry measures. 4. Chirality. J Am Chem Soc. 1995;117(1):462–473. doi: 10.1021/ja00106a053
- Alvarez S, Alemany P, Avnir D. Continuous chirality measures in transition metal chemistry. Chem Soc Rev. 2005;34(4):313–326. doi: 10.1039/b301406c
- Langer E, Lehner H. Zur Anwendbarkeit einer Chiralitätsfunktion für verschiedene Chiralitätsbeobach-tungen. Monatsh für Chem. 1979;110(4):1003–1009. doi: 10.1007/BF00906695
- Kahr B. Bow-tie particles boast a tunable twist. Nature. 2023;615(7952):395–396. doi: 10.1038/d41586-023-00705-x
- Qin L-C. Phys Chem. 2007;9(1):31–48. doi: 10.1039/B614121H
- Palmans ARA, Meijer EW, Denmark SE. Stereochemical language in supramolecular polymer chemistry: how we can do better. J Poly Sci. 2021;59(12):1171–1174. doi: 10.1002/pol.20200814
- Yashima E, Ousaka N, Taura D, et al. Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem Rev. 2016;116(22):13752–13990. doi: 10.1021/acs.chemrev.6b00354
- Vlasov E, Heyvaert W, Ni B, et al. High-throughput morphological chirality quantification of twisted and wrinkled gold nanorods. ACS Nano. 2024;18(18):12010–12019. doi: 10.1021/acsnano.4c02757
- Pasteur L. On the Relationships between the Crystalline Form, Chemical Composition and the Direction of Optical Rotation. Annales de chimie et de physique. 1848;24(6):442–459.
- Rowling KJ. Harry Potter and the deathly hallows. New York, NY: Bloomsbury; 2007.
- Anslyn E, Breslow R. On the mechanism of catalysis by ribonuclease: cleavage and isomerization of the dinucleotide UpU catalyzed by imidazole buffers. J Am Chem Soc. 1989;111(12):4473–4482. doi: 10.1021/ja00194a050
- Rowling KJ. Harry Potter and the half blood prince. New York, NY: Bloomsbury; 2005.