Abstract
The use of antioxidants could thus prove an effective medication to prevent or facilitate recovery from oxidative stress-induced sensorineural hearing loss (SNHL). One promising strategy to prevent SNHL is developing probucol (PB)-based nanoparticles using encapsulation technology and administering them to the inner ear via the established intratympanic route. The preclinical, clinical and epidemiological studies support that PB is a proven antioxidant that could effectively prevent oxidative stress in different study models. Such findings suggest its applicability in preventing oxidative stress within the inner ear and its associated neural cells. However, several hurdles, such as overcoming the blood-labyrinth barrier, ensuring sustained release, minimising systemic side effects and optimising targeted delivery in the intricate inner ear structures, must be overcome to efficiently deliver PB to the inner ear. This review explores the background and pathogenesis of hearing loss, the potential of PB in treating oxidative stress and its cellular mechanisms, and the obstacles linked to inner ear drug delivery for effectively introducing PB to the inner ear.
Introduction
Hearing loss is becoming a major cause of disability, and it’s expected to remain a significant issue in the future [Citation1]. Hearing impairment can arise from conductive mechanisms, where the transmission of auditory information to the inner ear is compromised, or from sensorineural hearing loss (SNHL), where the sensory cells within the inner ear malfunction or sustain damage, which accounts for 90% of all hearing loss [Citation2].
The aetiology of SNHL can be broadly categorised into genetic and non-genetic factors impacting fundamental cellular processes. This complexity presents challenges in early diagnosis and symptom management. Non-genetics include middle ear infections, noise exposure, viral infections, exposure to ototoxic chemicals, ageing and autoimmune diseases. On the genetic front, conditions such as Usher syndrome and congenital defects play a significant role in predisposing individuals to SNHL. Understanding the intricate interplay of these factors is crucial for developing targeted interventions for SNHL and making it challenging to develop universal treatment strategies that address the diverse needs of affected individuals.
The World Health Organization (WHO) initially documented the global prevalence of sensorineural hearing impairment in 1985, reporting that around 1% of the global population experienced hearing impairment at that time [Citation3–5]. As of 2019, this has increased to around 6%–7% of the world’s population suffering from some level of hearing impairment, and the Global Burden of Disease forecasts a substantial increase in the upcoming years, projecting around 2.45 billion cases by 2050 if adequate interventions are not implemented [Citation6].
Patients presenting with the symptoms of hearing impairment have an increased risk of cognitive decline and social isolation, which have been related to the onset and progression of dementia [Citation7,Citation8]. Current frontline treatment is restricted to hearing aids, cochlear implants, auditory brainstem implants or occasionally pharmacological intervention, including steroids and neurotrophic, but has not been effective due to poor patient compliance, financial considerations and invasive procedures (especially for cochlea implants) and does not treat the underlying cause of hearing impairment [Citation9]. Therefore, it is crucial to understand alternative therapeutic approaches that specifically target the underlying causes of SNHL, with a particular focus on addressing root causes such as oxidative stress and diseases associated with it. This urgency arises due to the insufficient efficacy of current treatments, emphasising the necessity for more precise interventions to combat the rising prevalence and impact of SNHL. Directly addressing oxidative stress and its associated conditions is crucial for effectively managing SNHL, given its increasing role as a cause of disability.
In addition to therapeutic strategies, advancements in diagnostic techniques play a crucial role in the accurate diagnosis of SNHL. Currently, various diagnostic tools such as audiometry, otoacoustic emissions and auditory brainstem response (ABR) testing are widely employed to assess hearing function and identify SNHL. For instance, ABR measures the minimum stimulus level required to elicit identifiable electrical response waves at different frequencies. Hearing loss severity is categorised as follows: normal hearing < 20 decibel (dB), mild (21–49 dB), moderate (50–70 dB), severe–profound (>70 dB) [Citation10]. Additionally, novel imaging modalities like magnetic resonance imaging and computed tomography scans provide detailed anatomical information of the inner ear structures, aiding in the diagnosis of underlying pathologies contributing to SNHL [Citation11].
Anatomically, the ear is an intricate structure that comprises three main parts: the outer ear, the middle ear and the inner ear. Within the inner ear lies the cochlea, a spiral-shaped cavity housed in the bony labyrinth, which contains the organ of Corti. The organ of Corti is vital for auditory processing and is composed of outer hair cells (OHCs), inner hair cells (IHCs) and supporting cells. IHCs serve as sensory receptors, detecting auditory stimuli and transmitting signals to the brain via auditory neurons. In contrast, OHCs amplify auditory input to IHCs through somatic motility driven by receptor potentials [Citation7].
Many researchers are investigating how SNHL happens, and the current understanding of the causes and mechanisms behind SNHL is unclear and a focus of ongoing research. Since the early 2000s, extensive studies have been conducted, suggesting that oxidative stress on auditory cells—resulting from the excessive production of free radicals—may be a significant contributing factor in the development of SNHL [Citation12–15]. For example, a study has demonstrated a progressive increase in hearing impairment in guinea pigs, correlating with elevated levels of the free radical isoprostane. Isoprostane, which is a prostaglandin-like compound generated by free radical-catalysed peroxidation of arachidonic acid and reliably produced during oxidant stress, provides valuable insights into the connection between oxidative stress and hearing loss. This increase in isoprostane has been linked to free radical formation and damage in hair cells, as well as decreased cochlear blood flow [Citation16]. Moreover, various underlying factors such as diabetes mellitus (DM), obesity, hyperlipidaemia and dementia can activate free radical targets, contributing to the development of hearing impairment [Citation17–20].
Since understanding that oxidative stress is a key contributor to hearing loss, research has been orientated to explore the effectiveness of antioxidants in preventing hearing impairment by enhancing the endogenous antioxidant system or reducing free radical generation. Antioxidants such as sodium thiosulphate, atorvastatin, d-methionine and vitamin E have been identified as protective agents against hearing loss [Citation18,Citation21–23]. Lee et al. showed that atorvastatin prevents hearing impairment via redox balance, improves blood flow and prevents hair cell death due to reduced levels of phosphorylated protein kinase and superoxide dismutase (SOD) [Citation18]. Likewise, MitoQ, as a mitochondrial-targeted drug, has shown promise in protecting hair cells from oxidative stress [Citation24]. Likewise, metformin has been extensively studied for its potential in treating hearing impairment, in vitro research indicates protective effects on hair cells [Citation25]. Therefore, a powerful antioxidant may be a single solution to overcome the action of free radicals. However, the effects of these treatments on inner ear mitochondrial function remain uncertain, with observed mitochondrial swelling and dysfunction in kidney tissue potentially serving as precursors to various kidney diseases. Hence, ongoing research continues to investigate potent antioxidants as a means to prevent oxidative stress and mitochondrial dysfunction in the cochlea. For instance, a recent study by Wagle et al. demonstrated that probucol (PB), in the form of antioxidant-based nanoparticles, has the potential to protect auditory cell lines from oxidative stress [Citation26]. This positions it as a potential new therapeutic agent for SNHL.
Consequently, this will be the first review to summarise the possible role of oxidate stress in SNHL. Moreover, we will explore methods for countering the effect of free radicals, including using the novel free radical scavenger antioxidant drug, PB, in the context of cellular mechanism and pharmacological prevention and applications of bio-nanotechnology to break through the present obstacles faced by inner ear drug delivery.
Mitochondrial dysfunction: a primary pathophysiology of SNHL
Free radical
Free radicals are highly unstable compounds with one or more unpaired electrons in their outer shell (). These molecules are highly reactive, always seeking electrons from other atoms to become more stable. Antioxidants primarily respond to these molecules and act through numerous mechanisms. If the process of generating free radicals continues, antioxidant detoxification will overwhelm and fail to maintain radicals within an effective range. This leads to a subsequent condition called oxidative stress, which can severely damage the body and result in cellular dysfunction. This process mainly affects lipids, proteins, nucleic acid and cellular organelles such as mitochondria [Citation27]. Many studies have shown that mitochondrial damage caused by free radicals is a primary factor of SNHL [Citation23,Citation28].
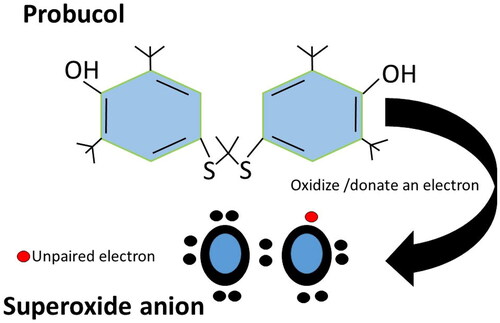
Figure 1. Molecular structure of PB and its interaction with superoxide anion. This representation enhances understanding of PB’s molecular structure and its mechanism of action in scavenging ROS.
Free radicals in hearing cells are obtained from external sources such as excitotoxicity due to overstimulation from noise, smoking cigarettes, X-rays, industrial chemicals or from normal internal essential metabolic processes in the human body, such as mitochondrial respiration, inflammatory responses, enzymatic reactions and drug metabolism in the liver [Citation29,Citation30]. For example, during cellular metabolism, the redox process utilizes oxygen and produces free radicals, primarily reactive oxygen species (ROS) and reactive nitrogen species [Citation27].
ROS are necessary to regulate normal physiological function and maintain the redox balance at low to modest levels. However, if they are generated to excess and not detoxified, they may cause cellular dysfunction. The work by Esterberg et al. suggests that aminoglycoside antibiotics may induce cytoplasmic ROS in HCs by disrupting calcium homeostasis between the endoplasmic reticulum (ER) and mitochondria [Citation29]. Their findings suggest that these drugs augment calcium flow into mitochondria, resulting in heightened mitochondrial membrane permeability, calcium release, increased ROS production and subsequent release into the cytoplasm. This cascade of events ultimately leads to damage and death of hair cells.
ROS encompass three chemical entities along the Fenton/Haber–Weiss pathway: the superoxide radical (O2•−), hydrogen peroxide (H2O2) and the hydroxyl radical (HO•). These compounds are products of the partial reduction of oxygen. The four-electron reduction of molecular oxygen results in the formation of water without generating ROS, while one-electron reduction produces the superoxide radical, hydrogen peroxide and hydroxyl radical [Citation30]. For example, O2•− can also activate cytochrome P450 reductases by ascending complexes I and III of the respiratory chain. Nitric oxide (NO•) exhibits relatively low chemical reactivity, resulting in low toxicity. However, its interaction with O2•− leads to the formation of the highly toxic species peroxynitrite (ONOO−). This compound has the potential to cause damage to lipids, proteins and DNA [Citation31]. Such powerful oxidants cause severe damage to the molecular and cellular structure of cells, which can quickly develop into cell death via apoptosis or necrosis and may result in SNHL [Citation32, Citation33]. Moreover, ROS could cause impaired blood flow damage to IHCs and OHCs, and destruction of spiral ganglion neurons [Citation18, Citation34, Citation35]. This series of events is more prevalent and frequent in the cochlea due to its excessive metabolic demand and subsequent high levels of ROS compared to other organs in the body [Citation36]. More precisely, compared to other cell types, hair cells, cells of the stria vascularis and neurons within the auditory system exhibit elevated concentrations of mitochondria, rendering them more susceptible to the impact of ROS [Citation37]. The heightened metabolic activity associated with maintaining high temporal precision in the peripheral auditory system further accentuates this vulnerability. For instance, auditory neurons display the capacity to respond at exceptionally high stimulation rates, generating numerous spikes per second. This heightened responsiveness imposes a substantial metabolic demand on sensory cells, consequently increasing their susceptibility to oxidative stress-induced damage. The intricate temporal coordination required for proper auditory function underscores the critical interplay between temporal precision and susceptibility to ROS-induced impairments in the peripheral auditory system [Citation37].
The endogenous antioxidants, such as SOD, glutathione reductase (GR), glutathione peroxidase (GPX) and catalase, neutralise the ROS in a normal physiological state. As depicted in , it donates an electron to a rampant free radical, neutralising it and thereby diminishing the free radical’s capacity to cause damage and effectively delay or hinder cellular damage primarily through its property of scavenging free radicals [Citation38]. However, the functional capacity of these enzymes can be affected during the pathological state, leading to ROS build-up and cellular damage, which can lead to SNHL via different cellular mechanisms [Citation39]. For example, SOD normally converts the O2•− into H2O2. Enzymatic dysfunction causes the accumulation of superoxide radicals, leading to increased ROS levels. Dysfunction in the mitochondria’s electron transport chain (ETC) can cause electron leakage, forming superoxide radicals and contributing to ROS. Malfunctioning antioxidant enzymes like catalase and GPX further hinder the effective neutralisation of hydrogen peroxide and other ROS, thus contributing to their accumulation within cellular environments [Citation40, Citation41].
SNHL via apoptosis
Massive ROS production impairs the mitochondria’s function, elevates ROS and causes cellular apoptosis by one of several pathways, such as dysfunction of oxidative phosphorylation, which is often associated with mitochondrial impairment [Citation42]. Studies have indicated that mitochondria are more susceptible to oxidative destruction, disturbing cellular functions, and components such as ROS produced in the mitochondria promote Ca2+ uptake and increase membrane permeability, which release cytochrome c and pro-apoptotic factors in sensory hair cells [Citation43–47]. Once released into the cytoplasm, cytochrome c activates caspase enzymes, initiating a cascade of events that ultimately results in apoptosis or programmed cell death [Citation47].
Broadly, apoptosis cascade is divided into two pathways: caspase-dependent and caspase-independent apoptosis processes [Citation37,Citation44]. Caspase-dependent apoptosis consists of the intrinsic pathway and extrinsic pathway. The intrinsic pathway, also called the mitochondrial-mediated pathway, involves the cellular stress-triggered release of cytochrome c from mitochondria, activating caspase-9 and initiating a cascade that leads to cell death. An extrinsic pathway, also called death receptor-mediated, involves death ligands binding (Fas ligand) to death receptors (Fas receptor), activating caspase-8 and initiating apoptosis in the cytosol, causing the activation of other caspases proteins, such as 3, 6 and 7. This results in changes to the morphology of cells from pyknosis to cell death [Citation45]. Once the caspase cascade is triggered, it cannot be stopped, and cell death is inevitable, particularly occurring at later stages after the critical cell death-executing activities have taken place.
In the intrinsic pathway of apoptosis, during normal physiological conditions, pro-apoptotic proteins primarily reside in the cytosol, while anti-apoptotic proteins are localised in the outer mitochondrial membrane. However, under conditions of cellular stress, the balance between pro- and anti-apoptotic proteins shifts, leading to the translocation of pro-apoptotic proteins to the outer mitochondrial membrane. Here, they form active high molecular weight homo-oligomers, inhibiting the function of anti-apoptotic proteins. Concurrently, anti-apoptotic proteins attempt to counteract this process by forming heterodimers with pro-apoptotic proteins. This disruption in protein balance results in increased mitochondrial permeability, disrupting oxidative phosphorylation and causing osmotic swelling. Consequently, pro-apoptotic proteins such as cytochrome c and endonuclease G (Endo G) are released into the cytosol through pores formed in the outer mitochondrial membrane, triggering the apoptotic cascade. For instance, the B-cell-lymphoma protein 2 (BCL-2) family is encoded in humans by the BCL2 gene. The BCL-2 family contains more than 30 proteins containing up to four conserved homology domains BH1–4 (Bcl-2 homology) [Citation48]. It is divided into anti-apoptotic protein, which shares high structural and functional homology with BCL2, and pro-apoptotic proteins, which share less homology with BCL2 and heavily control the mitochondrial pathway of apoptosis [Citation49]. Bok, Bak and Bax are the three main pro-apoptotic proteins, which are also called membrane-permeabilising effectors. Although both Bak and Bax shuttle between the cytosol and the mitochondrial outer membrane (MOM), Bax primarily localises to the cytoplasm in healthy cells, while Bak is mainly located at the MOM. On activation, effector proteins are converted from inert monomers into membrane-permeabilising oligomers and mediate cytochrome c release, interacting with anti-apoptotic proteins, leading to their inhibition and the initiation of apoptotic cascades [Citation50]. This location facilitates interactions with anti-apoptotic counterparts, influencing the cell’s fate in response to different signals or stresses.
Likewise, Bcl-2, Bcl-W and Bcl-B are some of the significant anti-apoptotic proteins. The anti-apoptotic exists as integral membrane proteins primarily localised in the MOM. Additionally, these anti-apoptotic molecules have been identified in the membranes of the ER and nucleus. Their primary function revolves around stabilising the mitochondrial membrane, thereby impeding the release of cytochrome c and its subsequent binding to apoptosis activating factor-1. Studies have demonstrated the presence of Bcl-2 in IHCs, and its overexpression has been observed to enhance the survival of hair cells by deactivating pro-apoptotic proteins [Citation51,Citation52].
Many studies have highlighted the caspase-independent pathway of hair cell death, also called the Endo G/apoptosis-inducing factor (AIF) pathway [Citation46,Citation53]. Endo G and AIF are located in the intermembrane space of mitochondria. AIF translocates to the nucleus, causing chromatin condensation and DNA fragmentation independently of caspases. Likewise, EndoG is released from mitochondria into the nucleus, inducing DNA fragmentation. Change in the mitochondria permeability by BNIP-3 (Bcl-2/adenovirus E1B 19-kDa interacting protein 3) activates the release of AIF, and Endo G results in nuclear translocation and causes mitochondrial dysfunction. BH3, one of the domains of BNIP-3, forms dimers by interacting with anti-apoptotic, which promotes and is involved in the mitochondria apoptosis pathway [Citation54]. While the caspase-independent and caspase-dependent pathways of apoptosis are distinct, evidence suggests significant cross-talk between these two pathways [Citation55].
Other mechanisms of apoptosis include the JNK (c-Jun N-terminal kinases) signalling pathway. It is also one of the mediators of apoptosis in outer hair triggered by ROS [Citation56]. The JNK signalling pathway contributes to apoptosis by phosphorylating and modulating Bcl-2 family proteins, disrupting the balance between anti-apoptotic and pro-apoptotic members. This imbalance promotes mitochondrial outer membrane permeabilisation (MOMP) and the release of cytochrome c, initiating the caspase cascade. Additionally, JNK activates transcription factors like c-Jun, influencing the expression of pro-apoptotic genes [Citation57]. Blocking the JNK pathway has been shown to prevent permanent hearing loss and hair cell death. Research has shown that mutations in apoptosis genes such as TJP2, MSRB3 and DFNA5 cause hearing impairment, which means apoptosis is related to the acquired hearing loss and also associated with genetic hearing loss [Citation58].
The transcription factor p53, which is also called tumour suppressor protein, directly influences the expression of pro-apoptotic genes, including Bax and Bak, promoting their synthesis and suppressing the expression of anti-apoptotic genes, such as Bcl-2. This contributes to the delicate balance between pro-apoptotic and anti-apoptotic signals within hair cells, influencing the apoptotic pathway and ultimately determining cell fate [Citation59]. The level of p53 expression is increased in hearing cells when the level of ROS increases [Citation60]. In the cell death stimulating condition, p53 induces mitochondria outer membrane permeabilisation and triggers the activation of pro-apoptosis factors to cause hair cell death [Citation59]. A study by Coffin et al. utilised a combination of pharmacologic and genetic manipulations to assess the contributions of p53, Bax and Bcl-2 in hair cell death following exposure to neomycin or gentamicin in the zebrafish lateral line. The study revealed that inhibiting Bax and p53 and overexpressing Bcl-2 significantly protected hair cells from drug toxicity. Further experiments using PFTα (Pifithrin-α, a p53 inhibitor) demonstrated the recovery of hair cells from gentamicin or neomycin toxicity, which emphasises the role of mitochondrial p53 activity in promoting hair cell death [Citation61].
SNHL via different cellular pathways
Exploring SNHL’s diverse cellular pathways is imperative for delving deeper into its mechanisms. Understanding these diverse pathways is essential for developing targeted interventions to prevent or treat SNHL effectively.
The cellular mechanism known as the antioxidant response element (ARE) plays a crucial role in responding to oxidative stress to restore redox balance. ARE primarily binds to the nuclear factor erythroid 2-related factor 2 (Nrf2). Under normal conditions, Nrf2 is situated in the cytoplasm, where it forms a complex with the Kelch-like ECH-associated protein 1 (Keap1), leading to its rapid degradation. However, during oxidative stress, Nrf2 translocates to the nucleus, where it binds to ARE and activates the expression of ARE-dependent protective genes, such as catalase and haem oxygenase-1 (HO-1) [Citation62,Citation63]. HO-1 is expressed widely throughout different organs, including the inner ear, and a study shows Nrf2 showed biological action and DNA repair via the expression of HO-1 [Citation64]. Current research validated that pharmacological activation of Nrf2/HO-1 protects hearing cells from the oxidative stressor allowing the cell’s DNA repair machinery to operate more effectively. [Citation65]. Moreover, Nrf2 has been identified as imperative in regulating mitochondrial homeostasis and biogenesis. A study demonstrated that pharmacological activation of Nrf2 through sulforaphane leads to the upregulation of the mitochondrial ETC component NDUFA4 (subunit of the enzyme NADH dehydrogenase (ubiquinone) (Complex I)). Conversely, genetic upregulation of Nrf2 via Keap1 knockdown results in the down-regulation of cytochrome c oxidase subunits Cyclo-oxygenase 2 and COX4I1 (Cytochrome C Oxidase Subunit 4I1) [Citation66]. Another study identified Nrf2’s role in regulating the expression of ATP synthase subunit α [Citation67]. Additionally, the mitochondrial protein DJ-1, crucial for maintaining complex I activity, is influenced by Nrf2 [Citation68]. Notably, Nrf2 deficiency has been associated with decreased oxidative phosphorylation efficiency and an elevated contribution of glycolysis to ATP synthesis [Citation69]. It promotes the expression of factors like PGC-1α (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha), which, in turn, stimulates the generation of new mitochondria [Citation70]. The reduced level of Nrf2 affects several biochemical processes contributing to decreasing ROS and subsequent SNHL [Citation71]. Single nucleotide polymorphisms in the Nrf2 promoter are potentially related to SNHL [Citation72]. Nrf2 null mice have been shown to develop more severe hearing impairment compared to the wild type, suggesting the Nrf2 gene’s activity is beneficial in reducing SNHL [Citation18,Citation73].
The NADPH oxidase (Nox) is a transmembrane protein that catalyses the production of free radicals like superoxide. NOX3 is a member of the NOX family and is expressed highly in hair cells (almost 50-fold higher than in any other tissue) [Citation74]. It has emerged as an essential source of ROS in signal transduction. It is essential for vestibular development and function. However, an increased level of NOX3 has been strongly linked with excess ROS production in the cochlea and subsequent cytotoxic damage of the hair cells by activating necrosis or apoptosis pathways [Citation74, Citation75] Mukherjea et al. suggested that transient receptor potential vanilloid 1 (TRPV1) damages the cochlea by triggering the signal transducer and activator of transcription 1 (STAT1) and NOX3 pathways [Citation76]. This cascade of events, culminating in oxidative stress and subsequent hearing loss, highlights potential targets for therapeutic intervention aimed at mitigating cochlear damage induced by oxidative stress and preventing SNHL.
SIRT1 is one member of the sirtuins family that regulates apoptosis in response to oxidative stress. It is located in the nucleus, where its function is to de-acetylate histones, enzymes and specific transcription factors to affect their activities [Citation77]. SIRT1 deficiency was associated with reduced oxidated stress-induced damage of hearing cells and spiral ganglion neurons [Citation78]. The study revealed that in cultured mouse inner ear cell lines, the reduction of SIRT1 expression enhanced cell viability during oxidative stress. This reduction prompted the nuclear translocation of Forkhead box O (Foxo3a), elevating the acetylation status of Foxo3a, a participant in the antioxidant pathway. Consequently, there was an increase in catalase activity, an antioxidant enzyme. These results suggest that SIRT1 may contribute to the early onset of hearing impairment by inhibiting FOXO3a-mediated oxidative stress resistance in the cochlea of C57BL/6 mice. Conversely, Pang et al. provided data supporting the protective role of SIRT1 in cochlear hair cells through the modulation of autophagosome induction via the de-acetylation of the core autophagy protein ATG9A [Citation79]. This reduction prompted the nuclear translocation of Forkhead box O (Foxo3a) and elevated the acetylation levels of Foxo3a, a component intricately involved in the antioxidant pathway. These findings highlight the intricate relationship between Sirt1, Foxo3a and oxidative stress response, underscoring the potential role of SIRT1 in modulating cellular resilience to oxidative challenges in the inner ear.
Recently, microRNAs have emerged as significant regulators of apoptosis, particularly miR-34a [Citation80]. Notably, Yamakuchi M et al. confirmed that overexpression of miR-34a reduces SIRT1 function, leading to increased p53 acetylation and apoptosis [Citation81]. Examination of miR-34a, p53 and SIRT1 expression levels in C57BL/6 mice revealed a notable reduction in SIRT1 expression in the cochlea with ageing. However, mRNA and miR-34a expression levels exhibited an increase with heightened oxidative stress, resulting in hearing loss. Overexpression of miR-34a in an auditory cell line inhibited SIRT1 and increased p53 acetylation and apoptosis [Citation82].
AMP-activated protein kinase (AMPK) regulates intercellular ATP levels through both anabolic and catabolic pathways. Zhao et al. revealed that accumulation of ROS activates AMPK, resulting in the loss of OHCs and IHCs in Tg-mtTFB1 transgenic mice. This ROS increase subsequently induces apoptosis [Citation83]. Additionally, they found that decreased AMPK expression reversed stria vascularis function and reduced ROS-induced apoptotic signalling in the inner ear. Mice lacking p66shc which is a member of the ShcA protein family, exhibited resistance to oxidative stress, resulting in enhanced protection against oxidative damage and reduced susceptibility to hearing loss [Citation84].
ROS causes increased release of calcium ions from the intracellular store, elevating free calcium ions in cochlear hair cells by stimulating mitogen-activated protein kinase [Citation85]. This process affects cellular components, including proteins (oxidation of residual amino acids, proteolysis, protein aggregation) and lipids (lipid peroxidation), which can alter different vital metabolic pathways in the cochlea [Citation86,Citation87]. Moreover, pro-inflammatory cytokines (tumour necrosis factor-α and interleukin-1 beta) and cell adhesion molecules (intracellular adhesion molecule-1) cause rapid infiltration and recruitment of inflammatory cells from the circulation into tissues, potentially leading to damage to cochlear cells [Citation88].
Understanding these mechanisms provides insights into potential therapeutic targets for mitigating oxidative stress-induced hearing loss. Further research is warranted to explore novel interventions that target these pathways and ultimately preserve auditory function.
SNHL via underlying conditions
There are many underlying metabolic conditions whose pathophysiology involves increased ROS and contributes to SNHL [Citation89]. Among these, DM stands out as particularly significant clinically, although its exact pathophysiological relationship with hearing loss is still being investigated. Hearing loss associated with DM is believed to stem from chronic oxidative stress, which induces neural and vascular changes in the cochlea, including thickening of capillary walls and loss of outer OHCs and inner IHCs [Citation90]. The prolonged elevation in blood glucose levels triggers the release of oxygen and nitrogen free radicals, culminating in lipid peroxidation and glucose oxidation. These processes contribute significantly to the complications of DM, including endothelial dysfunction, dyslipidaemia and microangiopathies, which can lead to SNHL [Citation17]. Moreover, hyperglycaemia induces the overproduction of superoxide, which can activate diverse cellular and biochemical pathways, potentially serving as a primary contributor to the complications associated with diabetes [Citation91].
High blood pressure and hyperlipidaemia have been implicated in the impairment of hearing cell morphology and function. Elevated blood pressure can result in inner ear haemorrhage, leading to reduced capillary blood flow and oxygen supply, thus contributing to the progression of SNHL [Citation84,Citation88,Citation89]. Moreover, hyperlipidaemia causes lipid deposition in hair cells, which could damage the cochlear neural cells [Citation92]. Owing to their plasma membrane lipid composition, OHCs are particularly sensitive to dyslipidaemic states [Citation93].
Recent clinical evaluations have uncovered a link between dementia and hearing loss [Citation20]. Several studies have identified hearing loss as a significant risk factor for both the onset and progression of dementia [Citation94–96]. The tau and amyloid cascades, central pathogenic mechanisms in Alzheimer’s disease, have been found to play a role in mitochondrial dysfunction and the damage of spiral ganglion neurons and hair cells due to ROS accumulation [Citation97,Citation98] This cascade also leads to decreased levels of vascular endothelial growth factor (VEGF). Reduced VEGF levels may contribute to abnormal deposition of tau and amyloid in OHCs and IHCs, potentially hindering the repair of spiral ganglion and hair cells [Citation20,Citation98]. Additionally, hyperphosphorylated tau proteins could also play a role in damaging the neurons that innervate the functional hair cells of the cochlea [Citation99]. Moreover, SNHL and Alzheimer’s disease share common underlying pathogenic mechanisms related to neurovascular disorders [Citation100]. Researchers have recently focused on determining whether mutual cellular processes in mitochondrial oxidative metabolism contribute to hearing loss and dementia. If confirmed, this could open up new avenues for preventing and treating both conditions.
Ageing stands as the most prevalent cause of SNHL. With advancing age, the body experiences a decline in its endogenous antioxidant capacity, accumulating free radicals [Citation101]. As discussed earlier, this increased presence of free radicals contributes to reduced blood flow to the inner ear, deterioration of cochlear hair cells and impairment of cellular mitochondrial function [Citation102].
The intricate web of underlying causes contributing to SNHL, with ROS as a central player, underscores the complexity of this condition. Recognising the interplay among these causes provides a foundation for developing comprehensive strategies to prevent and manage SNHL effectively.
Probucol is a possible antioxidant to prevent mitochondrial dysfunction and treat SNHL
ROS-related inner ear damage can potentially be mitigated through the use of antioxidants, which enhance either the internal or external antioxidant system and promote the scavenging of ROS or the upregulation of endogenous antioxidant production [Citation103]. However, current antioxidant therapies have demonstrated limited efficacy in preventing or reversing SNHL, underscoring the necessity for more targeted and efficient interventions. The identification of precise molecular targets for intervention in oxidative stress-induced SNHL remains challenging due to the complex and multifactorial nature of the condition. PB is a potential antioxidant candidate for addressing these challenges.
PB is a bisphenol/diphenolic compound with a unique pharmacodynamic profile with hydrogens attached to both sulphur atoms and is replaced by 3, 5-di-tert-butyl-4-hydroxyphenyl groups (). The compound was initially synthesised as an antioxidant by Consolidation Coal Company and subsequently developed as a therapeutic agent at The Dow Chemical Company, United States. This bisphenol acts as a free radical scavenging agent by donating its phenolic hydrogen to give phenoxyl radicals (). The drug was initially prescribed for the treatment of familial hypercholesterolaemia [Citation104]. PB has several proven benefits, including:
Proven safety profiles with a long history of clinical application.
Lipid-lowering properties.
QT (section in the electrocardiogram that represents the duration of ventricular electrical systole) interval prolongation.
However, there is limited global adoption, perhaps due to poor bioavailability and solubility. Clinical data have encouraged new interest in PB as a therapeutic drug because of its anti-atherogenic effect, immunomodulation, neuroprotection and renal protection with its antioxidant and anti-inflammatory properties [Citation105–107]. PB boosts antioxidant enzyme activity, reducing ROS accumulation in a mouse model of neurodegeneration and safeguarding neuronal cells from oxidative stress [Citation108]. This effect involves the Nrf2 pathway and protects against inflammation and neuronal apoptosis in spinal cord injury [Citation109]. Over the past decade, PB has been extremely reviewed for its uses in neurological degenerative diseases, which have been associated with oxidative stress [Citation110, Citation111]. Neurodegenerative disorders share similarities with SNHL, including misfolded protein aggregation, oxidative stress, neuroinflammation and compromised blood–brain barrier integrity. Given PB’s potential to traverse these barriers and its effectiveness in related neurological conditions, it’s plausible that PB could be beneficial in treating SNHL. Therefore, recognising these characteristics provides insight into the considerations and obstacles that need to be addressed to fully utilise PB’s effectiveness in treating SNHL. For instance, a study by Wagle et al. overcame these challenges to fully explore PB’s antioxidant properties in treating SNHL induced by oxidative stress [Citation112].
PB with pleiotropic properties is suggested to prevent a wide range of ROS-induced pathological conditions [Citation109,Citation113–116]. The mechanisms through which PB functions as a potent free radical scavenger at the cellular level, effectively halting oxidative stress-induced tissue damage, have been extensively documented across various diseases. This review aims to outline these mechanisms, with a specific focus on their relevance in preventing SNHL. A summary of these mechanisms is presented in .
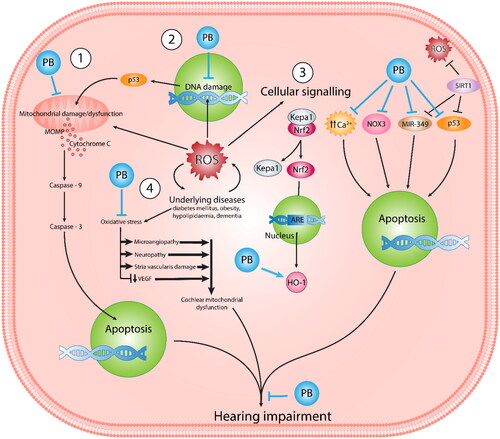
Figure 2. Schematic illustration of ROS-mediated hearing impairment highlights the therapeutic impact of PB in response to ROS and underlying diseases. This comprehensive visualisation provides insight into the multifaceted nature of oxidative stress-related hearing loss and the potential of PB as a therapeutic intervention to prevent mitochondrial dysfunction mediated by ROS.
Probucol targets Keap1/Nrf2/HO-1
As previously discussed, Keap1/Nrf2/HO-1 have been considered some of the most crucial antioxidant pathways involved in preventing cells from oxidative stress [Citation117]. Therefore, the antioxidant capacity of PB through this pathway could become remarkably important. When considering SNHL as a neurodegenerative disorder, it’s crucial to delve into its pathophysiology and cellular mechanisms, particularly in the context of oxidative stress and cellular damage within the auditory system. A recent study revealed that PB could ameliorate neuronal loss and cognitive impairment by reducing oxidative stress via Keap1/Nrf2/HO-1 pathway [Citation118].
The massive release of free radicals in spinal cord injury compromises axon regeneration and nerve recovery. Zhou et al. published a treatment for injured rats with PB that significantly increased Nrf2 and HO-1, reduced neural cell apoptosis and improved functional recovery following spinal cord injury. The study shows the neuroprotective benefits of PB following spinal cord injury, proposing that these effects are mediated through the activation of the Nrf2/ARE pathways [Citation109]. Likewise, oxidative stress is one of the foremost underlying causes of people with diabetes-induced erectile dysfunction [Citation119]. A study proved that PB improves erectile function by activating the Nrf2/HO-1 pathways [Citation120]. Therefore, activating the Nrf2/HO-1 pathways via PB could inhibit oxidative stress in the inner ear and lessen free radical activation.
Probucol targets NOXes
Careful regulation of NOX could be significant in preventing oxidative stress and maintaining a healthy level of ROS in the body, particularly the inner ear. The inhibition of NOX could be a promising approach for preventing oxidative stress in hearing cells. NOX-associated ROS production plays a vital role in the progress of glomerulonephritis. However, PB administration has been shown to fully control the progression of mesangial proliferative glomerulonephritis in rats by down-regulating the expression of Nox2 [Citation121]. Furthermore, PB significantly decreases the production of ROS by minimising Nox2 expression to protect against type-2 diabetic nephropathy caused by oxidative stress [Citation116].
Probucol targets SIRT-1
Tao et al. investigated the role of PB in osteoporosis development caused by oxidative stress [Citation122]. Their results showed that PB prevented osteoporosis development and promoted osteoblast differentiation with a substantial increase in the level of SIRT-1. Likewise, PB can interrupt the development of diabetic nephropathy [Citation123]. Yang S et al. showed that PB decreases renal injury in diabetic nephropathy through the AMPK/Sirt1/p66Shc signalling pathway [Citation115]. The study noted that PB suppresses the p66Shc expression via activation of the SIRT1-AMPK pathway. Moreover, PB has shown its potential as a pharmacological intervention by increasing the expression of SIRT1 in contrast-induced nephropathy animal models [Citation84]. One of the primary causes of contrast-induced nephropathy is the generation of excess ROS. Thus, SIRT1 can be an effective target for the clinical treatment of several different diseases, including SNHL [Citation124]. Using PB to accelerate the expression of SIRT1 might prevent the possible complications of diseases.
Drug delivery to the inner ear and different biological barriers
Oral and systemic drug delivery is one of the easiest ways to deliver the drug to the inner ear. However, the drug has to pass through several different barriers, including the blood-labyrinth barrier (BLB), before reaching its target site, which can limit the effectiveness of treatment strategies. BLB has a complex anatomical and physiological structure that has two epithelial layers alienated by a narrow interstitial space. The narrow interstitial space separates systemic circulation from the inner ear fluid [Citation125]. The tight junctions in endothelial and epithelial linings provide the barrier layer between cochlear fluids, blood and interstitial space, making it challenging to penetrate large compounds across BLB. Therefore, these challenges have limited the effectiveness of intravenous and oral dosing routes in treating inner ear conditions.
Local delivery methods aiming to overcome barriers like the tympanic membrane have been extensively explored for delivering drugs directly to the cochlea. However, challenges persist due to anatomical barriers such as the oval and round window membranes (RWM) between the middle and inner ear. Trans-tympanic and intra-tympanic methods are commonly used to achieve higher drug concentrations within the middle ear. In the trans-tympanic approach, the drug is delivered via the tympanic membrane without piercing it, although drug concentration may decrease significantly due to the intact tympanic membrane, which can be overcome through the intra-tympanic method [Citation126]. In the intra-tympanic method, the active compound, delivered via the tympanic membrane to the round window to access the inner ear, as presented in [Citation127]. The RWM consists of two layers of epithelial cells, one facing the middle ear and the other facing the inner ear, with a layer of connective tissue in between presenting a significant barrier [Citation128]. However, the semi-permeable properties of RWM give hope to drug delivery researchers. It is hypothesised that drugs with nano-sized particles may permeate this membrane through pathways like passive diffusion, while larger drugs rely on pinocytosis [Citation129]. Due to their affinity for lipid-rich environments, lipophilic drugs like PB exhibit greater ease of passive diffusion through cellular membranes than hydrophilic drugs [Citation130]. This characteristic underscores their potential advantage in drug delivery, offering improved permeation across barriers like the RWM and enhancing their efficacy in treating SNHL.
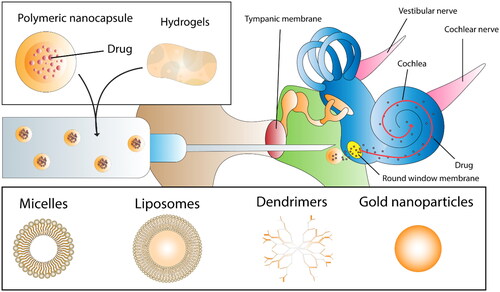
Figure 3. Intra-tympanic drug delivery nanocarriers. The figure illustrates a comprehensive overview of the diverse nanocarriers utilised for intra-tympanic drug delivery and release to the inner ear, displaying the range of delivery systems employed in auditory research.
Intra-labyrinthine or intra-cochlear is another delivery method that can bypass the RWM to deliver the drug directly into the inner ear. Thus, there are no physiological barriers for the drug to go to the inner ear. However, these methods are significantly more intrusive surgical procedures, with a risk of surgical-related trauma leading to loss of hearing, for example, disrupting the separation between perilymph and endolymph, which may impair the ion composition-dependent mechanotransduction [Citation131]. Additionally, the intracochlear fluids are linked to the fluids of the central nervous system via the cochlear aqueduct, meaning that there is potential for drugs that are delivered to the cochlea to be distributed more widely into the central nervous system [Citation132]. Other methods, such as cochleostomy, have been used to directly administer the organ of Corti and Intra-labyrinthine delivery for immediate access to the endolymph. All these methods, however, are based on invasive surgical procedures [Citation133].
When comparing delivery methods, intra-tympanic injection is a favourable route that effectively could deliver drugs to the RWM for subsequent diffusion into the inner ear. However, several fundamental factors limit drug efficiency through this route, including membrane permeability, poor drug delivery, size and the duration over which the drug is available on the RWM. One strategy to overcome the significant barrier of the RWM is to use compounds that act to modify the RWM and increase permeability, which can be achieved by adding excipients [Citation134,Citation135]. Therefore, overcoming these limitations would be more advantageous, possibly by encapsulating drugs with excipients/polymers in a nano size that can transport therapeutics across the RWM for extended and targeted drug release () [Citation136].
Encapsulation of drugs as a novel method of drug delivery
Improving the oral bioavailability and solubility of drugs, particularly those classified as Biopharmaceutics Classification System (BCS) class II like PB, poses significant challenges in drug delivery research. While various approaches have been explored to address this issue, encapsulation technology has emerged as the most promising solution and has gained widespread acceptance in recent years.
Encapsulation involves entrapping a drug within a carrier, and techniques for encapsulation are broadly categorised into mechanical/physical and chemical methods. Major physical encapsulation technologies include spray-drying and freeze-drying, while prominent chemical-based methods include ionic gelation, coacervation and interfacial techniques () [Citation137].
Table 1. Characterisation, advantages and disadvantages of different encapsulation techniques.
Every technique has pros and cons, but the encapsulation method should be chosen to maximise the availability of biologically active drugs and yield high-efficiency capsules, as outlined in . Encapsulation primarily results in the formation of microparticles and nanoparticles, with microparticles having a size range between 1 and 1000 µm and nanoparticles falling within the 1–1000 nm range. Generally, nanoparticles offer more advantages than microparticles due to their larger surface-to-volume ratio, enabling them to carry more drug payload in a nano-sized form. This property facilitates their ability to bypass various physical and chemical barriers present in the body, including those encountered in inner ear drug delivery.
In recent years, there has been growing interest in nanoparticles’ unique size-dependent properties within the drug delivery field. The efficacy of nanoparticles as drug carriers has already been demonstrated in various fields, including oncology, showcasing their potential benefits for the treatment of hearing impairment [Citation138,Citation139]. Incorporating nanoparticles into drug delivery systems holds promise for addressing the challenges associated with treating hearing impairment, offering opportunities for targeted and efficient therapies in this area of medicine. Encapsulating drugs into the nanocarrier system, with biodegradable and biocompatible properties, could represent a perfect therapeutic delivery vehicle for hearing impairment therapy.
Nanocarriers such as micelles, liposomes, dendrimers, polymeric nanoparticles and lipid-based nanoparticles have been widely used in preclinical studies to treat hearing impairment () [Citation140]. Nanocarriers have emerged as promising delivery vehicles for infiltrating the RWM and releasing the drug into the intracochlear environment where the cellular targets reside. [Citation141]. This strategy allows the encapsulation of an extensive range of lipophilic or hydrophilic drugs in a polymeric matrix. Drug-loaded nanocarriers can diffuse in the inner ear, which upsurges the concentration of drug particles in the perilymph. In contrast to free drugs, encapsulating drugs with a polymer such as Poly (lactic-co-glycolic acid), forming a nanoparticle could allow permeation into the perilymph, thus increasing local drug bioavailability [Citation142,Citation143]. For instance, mesoporous silica supraparticles prepared by Wang et al. are effective carriers for sustained drug release and are effective for chronic intracochlear implantation, which can rescue primary auditory neurons in an in vivo SNHL model [Citation144]. Dexamethasone-loaded nanoparticles were injected intratympanically in mice to prevent hearing loss induced by ototoxic drugs. These nanoparticles outperformed standard dexamethasone injections in restoring hearing across different frequencies. Additionally, they exhibited greater effectiveness in reducing proinflammatory cytokines compared to conventional treatment [Citation145]. In one study, two types of nanoparticles, one loaded with dexamethasone and the other with α-tocopheryl succinate, were synthesised. They demonstrated reduced caspase 3/7 activity, lower IL-1β release and decreased cisplatin-induced toxicity in HEI-OC1 cells. In Wistar rats, middle ear injection of these nanoparticles protected against cisplatin-induced hearing loss compared to controls [Citation146].
Polymeric nanoparticles represent a method of significant interest in delivering drugs to the inner ear. The size of the nanoparticles significantly influences the efficiency of the particles crossing the RWM. For instance, small nanoparticles of ∼95 nm have been shown to accumulate more quickly than larger ones of ∼240 nm in the inner ear [Citation147]. The size of the polymeric nanoparticles can differ significantly depending upon the polymers used, the concentration of polymers and the charge present in the polymer. For instance, neutral and anionic-charged nanocarriers have been shown to accumulate less in the inner ear compared to red catatonically charged nanoparticles. As expected, increasing the concentration of poly(lactic-co-glycolic acid) (PLGA) nanoparticles from 10 to 90 mg/ml in the administered suspension raises their concentration across the RWM [Citation142]. Similarly, PB encapsulated with the polymer sodium alginate has been shown to overcome solubility and permeability challenges, improve targeted release properties and have a possible application in oral delivery in DM [Citation114,Citation148,Citation149].
Zhang et al. prepared polymeric PLGA nanoparticles encapsulating coumarin-6 that permeated the RWM via a transcellular pathway [Citation142]. Cai et al. developed PLGA nanoparticles augmented with cell-penetrating peptides to enhance penetration, proposing a strategy for improved drug delivery to inner ear tissues. This PLGA nano-based approach, integrating hydrophilic molecule modifications, paves the way for advanced inner ear therapy [Citation150]. Wang et al. created PLGA nanoparticles with triphenylphosphonium cations to preserve mitochondrial integrity and prevent cell death. These targeted nanoparticles improved hair cell survival rates under gentamicin exposure, surpassing free drugs and untargeted nanoparticles. This underscores the promise of mitochondrial-targeted delivery for treating aminoglycoside-induced hearing loss [Citation151].
Likewise, liposome nanoparticles have successfully crossed the RWM in mice [Citation152]. Another critical barrier in targeted delivery is the tight junction between the epithelial cell sheets. Nanoparticles based on chitosan have been shown to open tight junctions reversibly and transiently, which reduces dosage requirements and toxic effects [Citation153]. Steinbach et al. showed that coating PLGA nanoparticles with chitosan improved nanoparticle cell binding and cell internalisation and dispersed them throughout the cell. This suggests favourable delivery options for applications in which internalisation presents challenges to efficacious delivery [Citation154].
Magnetic nanoparticles loaded with a drug have gained significant interest in recent times. Superparamagnetic iron oxide nanoparticles with sizes ranging from 5 to 14 nm have been encapsulated with PLGA polymer. They formed a composite nanoparticle, useful for both targeted delivery and imaging. Further, results showed that the nanoparticles were found throughout the inner ear without magnetic intervention [Citation155]. Likewise, Ramaswamy et al. developed magnetic-based nanoparticles loaded with methylprednisolone. The produced particles were deposited intra-typically into the middle ear and onto the RWM, and a Tesla external magnetic field was used to facilitate the intracochlear delivery of the particles. The drug starts to release from nanoparticles with the objective of protecting the OHC from the effect of cisplatin [Citation156]. However, such magnetic nanoparticles have also been associated with a reversible localised inflammation in the middle ear [Citation157].
There are several different strategies and methods to deliver nanoparticles for maximum effectiveness. Nanocarrier-loaded hydrogels have been intensively used in the last few years for inner ear drug delivery. Hydrogels filled in the middle ear cavity may provide additional viscosity, decrease the elimination of nanocarriers from the Eustachian tube, and increase the drug retention time in the middle ear [Citation158]. EI Kechai et al. administrated hyaluronic acid and PEGylated liposomal gel loaded with dexamethasone phosphate. They found that hyaluronic acid significantly improved the total drug concentration in the perilymph and prolonged drug retention time without adversely affecting hearing thresholds [Citation158]. Likewise, administration of poloxamer 407 loaded dexamethasone nanoparticles in guinea pig RWM supported sustained drug release and increased drug residence by ∼24-fold with more uniform drug distribution throughout all parts of the cochlea up to the apex [Citation159]. Likewise, phototherapy-based approaches such as photothermal therapy (PTT) would be an encouraging alternative for treating diseases. Currently, employing multifunctional nanoparticles improves the therapeutic properties of PTT in cancer treatment [Citation160,Citation161]. A study demonstrated that PTT efficiency with nanoparticles as mitochondrial targeting agents is enhanced by elevating the coefficient of light-to-heat conversion, reducing drug resistance and overcoming hypoxia. [Citation162]. The rapid development of fluorescent nanomaterials over the past three decades has spurred heightened interest in their potential applications in biology and medicine. These materials, including colloidal semiconductor quantum dots (QDs), typically range in size from 5 to 10 nm [Citation163]. One notable example is the utilisation of carbon or carbon-based nanomaterials in various biotechnological applications. Recent discoveries have shed light on the potential utilisation of QDs as a viable strategy for drug delivery, offering a promising avenue to enhance the efficacy of current pharmaceuticals and pave the way for the creation of innovative therapeutic approaches. For instance, a study demonstrated the enhancement of a chitosan/carboxymethyl cellulose hydrogel by zinc oxide and graphene QDs nanoparticles, creating a pH-sensitive nanocomposite for delivering quercetin in brain cancer treatment [Citation164]. These approaches hold promise and can translate into preventing ROS-induced SNHL.
Bile acid as a permeation enhancer/drug excipient/adjuvant in novel drug delivery method
Improving the bioavailability of readily available and safe medications, such as PB, may be done through the use of encapsulation. In some circumstances, further improvements in permeation are required. Thus, excipients are employed to improve permeation through biological barriers such as the RWM and targeting further. Such permeation enhancers and targeting moieties are often considered essential in the permeation of the RWM. Such excipients, ideally, are biodegradable and biocompatible. In recent years, bile acids’ (BA) unique ability to support and facilitate drug permeation via biological membranes has shown significant interest as an absorption enhancer for maximum therapeutic efficiency in drug delivery research over the last decades [Citation165–168].
BA has significant endocrine function via the intracellular nuclear receptor farnesoid X receptor and the transmembrane G protein-coupled receptor 5 (TGR5), activating signalling cascades or transcriptional networks. Vitamin D, pregnane x receptor and constitutive androstane receptor are other BA receptors [Citation169].
The primary function of BA in the human body is to solubilise dietary lipids in the gut. BA is naturally synthesised inside the human body via the liver from cholesterol catabolism. The formation of BA is a multi-organelle, complex and multi-step process. Primary BAs are those produced in the liver: cholic acid (CA) and chenodeoxycholic acid (CDCA). Primary BAs are conjugated with taurine or glycine to form conjugated BAs before excretion from the liver. Primary BAs undergo oxidation, deconjugation and dihydroxylation in the intestinal lumen to form secondary BAs, deoxycholic acid (DCA) and lithocholic acid (LCA).
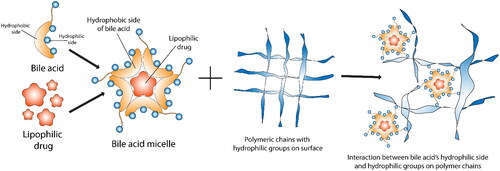
BA is an amphiphilic compound with a steroidal backbone. BAs can self-assemble into larger secondary structures over a wide concentration range, making them unique from traditional surfactants. BA has hydrophilic structures on the concave alpha side and hydrophobic groups on the convex beta side. In aqueous solutions, BA forms micelles, which can facilitate transcellular transport and increase the absorption of both hydrophobic and hydrophilic drugs (). Additionally, BAs function as permeation enhancers by fluidising the cell membranes, interacting with phospholipids and causing tight junction rearrangement by binding with calcium ions [Citation170]. BA enhances the transport of hydrophilic drugs via the paracellular route. BAs are incorporated into the cell membrane, creating reverse micelles (). Alternatively, BAs improve hydrophobic compound uptake through the transcellular and paracellular routes [Citation171]. Nanocarriers can accumulate within the cells or connective tissue of the RWM without penetrating the tympanic membrane. Liposomes and polymers may aggregate extensively within the connective tissue and the outer layer of the RWM, respectively, facilitating drug release from the accumulated nanocarriers. In such scenarios, BA micelles could enhance mucosal membrane permeability (BLB) by overcoming resistance at the aqueous diffusion layer.
Figure 4. Bile acid interaction with drugs and polymers in nanoparticle formulations. The figure illustrates the structure of BAs and proposes their interaction with hydrophilic drugs and polymers, providing insight into the potential mechanisms underlying drug–polymer interactions in nanoparticle formulations.
Further, micelle formation increases solubility and dissolution rate, enhancing a lipophilic drug’s absorption. Darkoh et al. demonstrated that BA solubilised rifaximin, making it more bioavailable [Citation172]. Lalić-Popović et al. published a paper showing that DCA enhances the drug’s permeation through the rat blood-brain barrier [Citation173]. Wagle et al. discovered that incorporating BA into PB formulations enhances the viability of hearing cells and shields them from cytotoxicity and mitochondrial dysfunction by reducing ROS levels. Additionally, BA presence was found to augment the cellular uptake of the drug [Citation26,Citation112].
Moreover, studies have revealed that BA has an anti-apoptotic character in different cell types, such as intestine and microglial cells [Citation174,Citation175]. BA, such as tauroursodeoxycholic acid, has been explored as a possible pharmaceutical intervention for apoptosis and oxidative stress to prevent residual hearing post-cochlear implantation [Citation176].
BA is cross-linked with anionic linear polysaccharide polymers in the encapsulation process to make stronger particles and prevent unwanted degradation before reaching the target site (). Therefore, in recent years, the utilisation of BA in drug encapsulation with the incorporation of polymers and delivery has gained more attention in targeted drug delivery strategies due to their dual properties, permeation enhancement and antioxidant. Wagle et al. developed alginate-based PB-LCA microcapsules that have shown improved PB release, absorption and distribution [Citation114]. Likewise, Mooranian et al. showed that incorporating Ursodeoxycholic Acid (UDCA) within PB-loaded microcapsules resulted in uniform morphology and stability and protected beta cells from oxidation, which might be suitable for optimised oral delivery of PB in DM [Citation113]. Moreover, the authors further demonstrated that adding DCA optimised the formulation by reducing particle size and increasing PB’s antidiabetic effect [Citation149,Citation177].
Similarly, integrating CDCA in alginate-based PB microcapsules resulted in more robust microcapsules supporting controlled drug release [Citation178]. Several studies outlined the importance of BA-based microcapsules in the application of oral delivery to prevent DM in the animal model [Citation179,Citation180]. However, the significance of BA in inner ear drug delivery remains incompletely understood. Therefore, there is an opportune moment to investigate BA-based formulations, producing nano-sized particles that could potentially traverse the RWM to deliver therapeutics for SNHL treatment.
Nanoparticles safety and limitations in the field of treatment strategies
The use of nanoparticles in inner ear diseases faces challenges due to nanoparticle toxicity and immune system interactions [Citation181]. Producing nanoparticles involves several necessary steps, but this process encounters numerous limitations, such as potential toxicity arising from nanoparticle components and challenges related to the site of nanoparticle injection. Therefore, safety studies are essential across a broad concentration range of nanocarriers to mitigate potential toxicities, ensuring efficient drug doses reach the intended sites. For example, a study suggests that nanoparticles, like silver nanoparticles (AgNPs), can disrupt barriers in the ear, potentially causing reversible hearing loss by affecting immune responses and inflammation pathways. This effect involves macrophage recruitment, modulation of Toll-like receptor signalling and regulation of inflammatory cytokines by proteins like A20 and Ring Finger Protein 11 (RNF11) [Citation182]. Likewise, Zou et al. investigated the toxicity of AgNPs via transtympanic injection in rats and their impact on the BALB/c 3T3 cell line. They found cochlear apoptosis at varying AgNP concentrations, with more significant effects in the vestibule than the cochlea, particularly with the 200 μg/ml solution. Lower concentrations primarily affected specific cochlear cells, while higher concentrations led to widespread cell death in both the vestibule and cochlea [Citation183]. Similarly, a study involving chitosan prednisolone nanocarriers revealed minor cochlea-related inflammatory changes among animals administered nanoparticles, yet no lasting hearing impairment was observed [Citation184].
Currently, no single method can completely cure SNHL due to multiple causes. Long-term studies assessing the safety, tolerability and efficacy of novel treatment strategies for SNHL are limited, which underscores the necessity for comprehensive clinical trials. Such trials are essential to establish the viability of these treatments as therapeutic options for individuals affected by SNHL.
Few clinical trial studies have showcased the induction of new hair cells by pharmacologically inhibiting various pathways. For instance, Schilder et al. conducted a phase I/IIa safety and efficacy trial to investigate the inhibition of Notch signalling with the use of intratympanic gamma-secretase inhibitor LY3056480 as a regenerative drug treatment for SNHL. The study demonstrated that intratympanic delivery of LY3056480 in adults with mild–moderate SNHL is safe and well tolerated. Additionally, the local administration of LY3056480 restored OHC function, improving speech perception of noise, a primary challenge for individuals with hearing loss [Citation185]. However, despite promising nanoparticle treatment approaches demonstrated in vitro and in animal models of SNHL, no studies have been registered or passed on human clinical trials using nanoparticle-based drugs, as per the authors’ search. It could be successfully translating these laboratory-based findings into human clinical trials, but this remains a significant challenge, underscoring the critical need for further preclinical research and validation to ensure the efficacy and safety of nanoparticles before proceeding to clinical studies.
Conclusion and future direction
The incidence of SNHL is increasing annually, yet effective treatments are hindered by the lack of suitable drug delivery methods. Oxidative stress is a key factor in SNHL pathogenesis, prompting research into antioxidant drugs. This review proposes utilising the potent antioxidant PB as a promising treatment for SNHL. PB’s potential mechanism of action in combating oxidative stress across various cell lines and animal models is summarised. PB’s clinical efficacy may be improved through structural modifications and novel formulations, particularly in combination with BA and polymer encapsulation technologies. Nanoparticle-based gene delivery to the inner ear is rapidly advancing, although trans-tympanic delivery of polymer-based PB nanoparticles through the RWM is deemed more relevant for achieving controlled and targeted drug release. Future studies should focus on optimising nanoparticle properties, such as shape, size and charge, to enable the loading of multiple therapeutic drugs for sustained release.
Additionally, combining drugs via adjuvant therapy, as demonstrated by Asadollahi et al. and Zeinali et al. using nanostructure lipid carriers, shows promise in preventing ROS-induced apoptosis [Citation186,Citation187]. Furthermore, the potential of ultrasound imaging and carbon dots as novel disease diagnosis and treatment approaches warrants further investigation [Citation188,Citation189]. While challenges remain in translating these findings into clinical practice, the study underscores the importance of continued research and collaboration in advancing inner ear therapeutics. As outlined in this review, many studies have successfully delivered nanoparticle-based drugs to the inner ear in an animal model, but we lack outcomes relating to nanoparticles applied in the human ear. One key direction involves further refining and optimising PB-based formulations, leveraging advanced drug delivery techniques to enhance targeted delivery and efficacy. Additionally, the development of personalised treatment approaches tailored to individual patient profiles may hold great potential in maximising therapeutic outcomes. The comprehensive analysis presented in this review provides a foundation for future studies aimed at addressing the complex mechanisms underlying SNHL and developing effective treatment strategies. This formulation is much more optimised and, thus, highly promising. Considering current data and particularly major failures of transtympanic drug injections in hearing treatment, our approach to using BA in the PB formulations is fundamentally different and highly novel. Overall, future clinical directions for SNHL treatment are likely to be multidisciplinary, incorporating innovative approaches from fields to address the complex and multifactorial nature of hearing loss.
Disclosure statement
Al-Salami H is currently receiving funding from Austin Biotec Pty, Ltd and Glanis PTY Ltd. All other authors have no completing interest to declare.
Data availability
No data were used for the research described in the article.
Additional information
Funding
References
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2.
- Hinton AS, Yang-Hood A, Schrader AD, et al. Approaches to treat sensorineural hearing loss by hair-cell regeneration: the current state of therapeutic developments and their potential impact on audiological clinical practice. J Am Acad Audiol. 2021;32(10):661–669. doi: 10.1055/s-0042-1750281.
- WHA39.14. Resolution on prevention of deafness and hearing impairment. In Forty-eighth World Health Assembly, Geneva, 27 March 1986. Resolutions and decisions, annexes. Geneva: World Health Organization; 1986. Report. 27 March 1986.
- Olusanya BO, Davis AC, Hoffman HJ. Hearing loss: rising prevalence and impact. Bull World Health Organ. 2019;97(10):646–646A. doi: 10.2471/BLT.19.224683.
- WHA70.13. World Health Assembly resolution on prevention of deafness and hearing loss. In: Seventieth World Health Assembly, Geneva, 31 May 2017. Resolutions and decisions, annexes. Geneva: World Health Organization; 2017. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_R13-en.pdf. 2017
- Haile LM, Kamenov K, Briant PS, et al. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. The Lancet. 2021;397(10278):996–1009. doi: 10.1016/S0140-6736(21)00516-X.
- Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. The Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6.
- Ciorba A, Bianchini C, Pelucchi S, et al. The impact of hearing loss on the quality of life of elderly adults. Clin Interv Aging. 2012;7:159–163. doi: 10.2147/CIA.S26059.
- Ren H, Hu B, Jiang G. Advancements in prevention and intervention of sensorineural hearing loss. Ther Adv Chronic Dis. 2022;13:20406223221104987. doi: 10.1177/20406223221104987.
- McCreery RW, Kaminski J, Beauchaine K, et al. The impact of degree of hearing loss on auditory brainstem response predictions of behavioral thresholds. Ear Hear. 2015;36(3):309–319. doi: 10.1097/AUD.0000000000000120.
- Plontke SK. Diagnostics and therapy of sudden hearing loss. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2017;16:Doc05. doi: 10.3205/cto000144.
- Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010;1201(1):183–188. doi: 10.1111/j.1749-6632.2010.05634.x.
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13(6):343–348. doi: 10.1097/01.moo.0000186799.45377.63.
- Seidman MD, Ahmad N, Bai U. Molecular mechanisms of age-related hearing loss. Ageing Res Rev. 2002;1(3):331–343. doi: 10.1016/s1568-1637(02)00004-1.
- Ohinata Y, Yamasoba T, Schacht J, et al. Glutathione limits noise-induced hearing loss. Hear Res. 2000;146(1–2):28–34. doi: 10.1016/s0378-5955(00)00096-4.
- Ohinata Y, Miller JM, Altschuler RA, et al. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878(1–2):163–173. doi: 10.1016/s0006-8993(00)02733-5.
- Ashkezari SJ, Namiranian N, Rahmanian M, et al. Is hearing impairment in diabetic patients correlated to other complications? J Diabetes Metab Disord. 2018;17(2):173–179. doi: 10.1007/s40200-018-0357-3.
- Lee YY, Choo O-S, Kim YJ, et al. Atorvastatin prevents hearing impairment in the presence of hyperlipidemia. Biochim Biophys Acta Mol Cell Res. 2020;1867(12):118850. doi: 10.1016/j.bbamcr.2020.118850.
- Lalwani AK, Katz K, Liu YH, et al. Obesity is associated with sensorineural hearing loss in adolescents. Laryngoscope. 2013;123(12):3178–3184. doi: 10.1002/lary.24244.
- Shen Y, Ye B, Chen P, et al. Cognitive decline, dementia, Alzheimer’s disease and presbycusis: examination of the possible molecular mechanism. Front Neurosci. 2018;12:394. doi: 10.3389/fnins.2018.00394.
- Brock PR, Maibach R, Childs M, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018;378(25):2376–2385. doi: 10.1056/NEJMoa1801109.
- Laurell G, Teixeira M, Sterkers O, et al. Local administration of antioxidants to the inner ear: kinetics and distribution(1). Hear Res. 2002;173(1–2):198–209. doi: 10.1016/S0378-5955(02)00613-5.
- Menardo J, Tang Y, Ladrech S, et al. Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse cochlea. Antioxid Redox Signal. 2012;16(3):263–274. doi: 10.1089/ars.2011.4037.
- Kim YR, Baek JI, Kim SH, et al. Therapeutic potential of the mitochondria-targeted antioxidant MitoQ in mitochondrial-ROS induced sensorineural hearing loss caused by Idh2 deficiency. Redox Biol. 2019;20:544–555.
- Oishi N, Kendall A, Schacht J. Metformin protects against gentamicin-induced hair cell death in vitro but not ototoxicity in vivo. Neurosci Lett. 2014;583:65–69. doi: 10.1016/j.neulet.2014.09.028.
- Wagle SR, Ionescu CM, Kovacevic B, et al. Pharmaceutical characterization of probucol bile acid-lithocholic acid nanoparticles to prevent chronic hearing related and similar cellular oxidative stress pathologies. Nanomedicine (Lond). 2023;18(12):923–940. doi: 10.2217/nnm-2023-0092.
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89–96. doi: 10.59566/IJBS.2008.4089.
- Evans P, Halliwell B. Free radicals and hearing. Cause, consequence, and criteria. Ann N Y Acad Sci. 1999;884(1):19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x.
- Esterberg R, Hailey DW, Rubel EW, et al. ER–mitochondrial calcium flow underlies vulnerability of mechanosensory hair cells to damage. J Neurosci. 2014;34(29):9703–9719. doi: 10.1523/JNEUROSCI.0281-14.2014.
- Salisbury D, Bronas U. Reactive oxygen and nitrogen species: impact on endothelial dysfunction. Nurs Res. 2015;64(1):53–66. doi: 10.1097/NNR.0000000000000068.
- Martemucci G, Costagliola C, Mariano M, et al. Free radical properties, source and targets, antioxidant consumption and health. Oxygen. 2022;2(2):48–78. doi: 10.3390/oxygen2020006.
- Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19(9):1807–1819. doi: 10.1016/j.cellsig.2007.04.009.
- Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012.
- Fukushima H, Cureoglu S, Schachern PA, et al. Cochlear changes in patients with type 1 diabetes mellitus. Otolaryngol Head Neck Surg. 2005;133(1):100–106. doi: 10.1016/j.otohns.2005.02.004.
- Fukushima H, Cureoglu S, Schachern PA, et al. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006;132(9):934–938. doi: 10.1001/archotol.132.9.934.
- Böttger EC, Schacht J. The mitochondrion: a perpetrator of acquired hearing loss. Hear Res. 2013;303:12–19. doi: 10.1016/j.heares.2013.01.006.
- Shalini S, Dorstyn L, Dawar S, et al. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22(4):526–539. doi: 10.1038/cdd.2014.216.
- Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902.
- Wong AC, Ryan AF. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front Aging Neurosci. 2015;7:58. doi: 10.3389/fnagi.2015.00058.
- Forrester SJ, Kikuchi DS, Hernandes MS, et al. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401.
- Ribas V, García-Ruiz C, Fernández-Checa JC. Glutathione and mitochondria. Front Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151.
- Pirvola U, Xing-Qun L, Virkkala J, et al. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20(1):43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000.
- Watanabe K-I, Inai S, Jinnouchi K, et al. Expression of caspase-activated deoxyribonuclease (CAD) and caspase 3 (CPP32) in the cochlea of cisplatin (CDDP)-treated Guinea pigs. Auris Nasus Larynx. 2003;30(3):219–225. doi: 10.1016/s0385-8146(03)00049-x.
- Martinou J-C, Youle Richard J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017.
- Orrenius S, Gogvadze V, Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun. 2015;460(1):72–81. doi: 10.1016/j.bbrc.2015.01.137.
- Jiang H, Sha SH, Forge A, et al. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13(1):20–30. doi: 10.1038/sj.cdd.4401706.
- Ross WN. Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci. 2012;13(3):157–168. doi: 10.1038/nrn3168.
- Giam M, Huang D, Bouillet P. BH3-only proteins and their roles in programmed cell death. Oncogene. 2008;27 Suppl 1(S1):S128–S136. doi: 10.1038/onc.2009.50.
- Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43(1):1–67. doi: 10.1080/10408360500295626.
- García-Sáez AJ. The secrets of the Bcl-2 family. Cell Death Differ. 2012;19(11):1733–1740. doi: 10.1038/cdd.2012.105.
- Cunningham LL, Matsui JI, Warchol ME, et al. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004;60(1):89–100. doi: 10.1002/neu.20006.
- Huang Q, Xiong H, Yang H, et al. Differential expression of Bcl-2 in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Audiol Neurootol. 2016;21(5):326–332. doi: 10.1159/000450937.
- Liu G, Zou H, Luo T, et al. Caspase-dependent and caspase-independent pathways are involved in cadmium-induced apoptosis in primary rat proximal tubular cell culture. PLoS One. 2016;11(11):e0166823. doi: 10.1371/journal.pone.0166823.
- Vande Velde C, Cizeau J, Dubik D, et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20(15):5454–5468. doi: 10.1128/MCB.20.15.5454-5468.2000.
- Dawson VL, Dawson TM. Deadly conversations: nuclear-mitochondrial cross-talk. J Bioenerg Biomembr. 2004;36(4):287–294. doi: 10.1023/B:JOBB.0000041755.22613.8d.
- Wang J, Ruel J, Ladrech S, et al. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol. 2007;71(3):654–666. doi: 10.1124/mol.106.028936.
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–6251. doi: 10.1038/onc.2008.301.
- de Beeck KO, Schacht J, Van Camp G. Apoptosis in acquired and genetic hearing impairment: the programmed death of the hair cell. Hear Res. 2011;281(1–2):18–27. doi: 10.1016/j.heares.2011.07.002.
- Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458(7242):1127–1130. doi: 10.1038/nature07986.
- Tadros SF, D’Souza M, Zhu X, et al. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008;13(11):1303–1321. doi: 10.1007/s10495-008-0266-x.
- Coffin AB, Rubel EW, Raible DW. Bax, Bcl2, and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line. J Assoc Res Otolaryngol. 2013;14(5):645–659. doi: 10.1007/s10162-013-0404-1.
- Pandey P, Singh AK, Singh M, et al. The see-saw of Keap1-Nrf2 pathway in cancer. Crit Rev Oncol Hematol. 2017;116:89–98. doi: 10.1016/j.critrevonc.2017.02.006.
- Niture SK, Jaiswal AK. Nrf2 protein up-regulates antiapoptotic protein bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012;287(13):9873–9886. doi: 10.1074/jbc.M111.312694.
- Yi ZW, Xia YJ, Liu XF, et al. Antrodin a from mycelium of Antrodia camphorata alleviates acute alcoholic liver injury and modulates intestinal flora dysbiosis in mice. J Ethnopharmacol. 2020;254:112681. doi: 10.1016/j.jep.2020.112681.
- Yang Y, Chen X, Tian K, et al. Heme oxygenase-1 protects hair cells from gentamicin-induced death. Front Cell Neurosci. 2022;16:783346. doi: 10.3389/fncel.2022.783346.
- Agyeman AS, Chaerkady R, Shaw PG, et al. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat. 2012;132(1):175–187. doi: 10.1007/s10549-011-1536-9.
- Abdullah A, Kitteringham NR, Jenkins RE, et al. Analysis of the role of Nrf2 in the expression of liver proteins in mice using two-dimensional gel-based proteomics. Pharmacol Rep. 2012;64(3):680–697. doi: 10.1016/s1734-1140(12)70863-0.
- Hayashi T, Ishimori C, Takahashi-Niki K, et al. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem Biophys Res Commun. 2009;390(3):667–672. doi: 10.1016/j.bbrc.2009.10.025.
- Holmström KM, Baird L, Zhang Y, et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. 2013;2(8):761–770. doi: 10.1242/bio.20134853.
- Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435.
- Oishi T, Matsumaru D, Ota N, et al. Activation of the NRF2 pathway in Keap1-knockdown mice attenuates progression of age-related hearing loss. NPJ Aging Mech Dis. 2020;6(1):14. doi: 10.1038/s41514-020-00053-4.
- Honkura Y, Matsuo H, Murakami S, et al. NRF2 is a key target for prevention of noise-induced hearing loss by reducing oxidative damage of cochlea. Sci Rep. 2016;6(1):19329. doi: 10.1038/srep19329.
- Yamamoto T, Yoh K, Kobayashi A, et al. Identification of polymorphisms in the promoter region of the human NRF2 gene. Biochem Biophys Res Commun. 2004;321(1):72–79. doi: 10.1016/j.bbrc.2004.06.112.
- Bánfi B, Malgrange B, Knisz J, et al. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279(44):46065–46072. doi: 10.1074/jbc.M403046200.
- Le Prell C, Dolan D, Schacht J, et al. Pathways for protection from noise induced hearing loss. Noise Health. 2003;5(20):1–17.
- Mukherjea D, Jajoo S, Sheehan K, et al. NOX3 NADPH oxidase couples transient receptor potential vanilloid 1 to signal transducer and activator of transcription 1-mediated inflammation and hearing loss. Antioxid Redox Signal. 2011;14(6):999–1010. doi: 10.1089/ars.2010.3497.
- Schultz MB, Rinaldi C, Lu Y, et al. Molecular and cellular characterization of SIRT1 allosteric activators. Methods Mol Biol. 2019;1983:133–149. doi: 10.1007/978-1-4939-9434-2_8.
- Han C, Linser P, Park H-J, et al. Sirt1 deficiency protects cochlear cells and delays the early onset of age-related hearing loss in C57BL/6 mice. Neurobiol Aging. 2016;43:58–71. doi: 10.1016/j.neurobiolaging.2016.03.023.
- Pang J, Xiong H, Ou Y, et al. SIRT1 protects cochlear hair cell and delays age-related hearing loss via autophagy. Neurobiol Aging. 2019;80:127–137. doi: 10.1016/j.neurobiolaging.2019.04.003.
- Subramanian S, Steer CJ. MicroRNAs as gatekeepers of apoptosis. J Cell Physiol. 2010;223(2):289–298. doi: 10.1002/jcp.22066.
- Yamakuchi M, Lowenstein CJ. MiR-34, SIRT1 AND p53: the feedback loop. Cell Cycle. 2009;8(5):712–715. doi: 10.4161/cc.8.5.7753.
- Xiong H, Pang J, Yang H, et al. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol Aging. 2015;36(4):1692–1701. doi: 10.1016/j.neurobiolaging.2014.12.034.
- Zhao J, Li G, Zhao X, et al. Down-regulation of AMPK signaling pathway rescues hearing loss in TFB1 transgenic mice and delays age-related hearing loss. Aging (Albany NY). 2020;12(7):5590–5611. doi: 10.18632/aging.102977.
- Fetoni AR, Eramo SLM, Paciello F, et al. The redox protein p66shc mediates cochlear vascular dysfunction and transient noise-induced hearing loss. Sci Rep. 2016;6(1):25450. doi: 10.1038/srep25450.
- Maeda Y, Fukushima K, Omichi R, et al. Time courses of changes in phospho- and total- MAP kinases in the cochlea after intense noise exposure. PLoS One. 2013;8(3):e58775. doi: 10.1371/journal.pone.0058775.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438.
- Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734.
- Tan WJ, Thorne PR, Vlajkovic SM. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem Cell Biol. 2016;146(2):219–230. doi: 10.1007/s00418-016-1436-5.
- Rim H-S, Kim M-G, Park D-C, et al. Association of metabolic syndrome with sensorineural hearing loss. J Clin Med. 2021;10(21):4866. doi: 10.3390/jcm10214866.
- Makishima K, Tanaka K. Pathological changes of the inner ear and central auditory pathway in diabetics. Ann Otol Rhinol Laryngol. 1971;80(2):218–228. doi: 10.1177/000348947108000208.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a.
- Mohammed AAM. Lipid profile among patients with sudden sensorineural hearing loss. Indian J Otolaryngol Head Neck Surg. 2014;66(4):425–428. doi: 10.1007/s12070-014-0744-0.
- Laury AM, Casey S, McKay S, et al. Etiology of unilateral neural hearing loss in children. Int J Pediatr Otorhinolaryngol. 2009;73(3):417–427. doi: 10.1016/j.ijporl.2008.11.012.
- Idrizbegovic E, Hederstierna C, Dahlquist M, et al. Central auditory function in early Alzheimer’s disease and in mild cognitive impairment. Age Ageing. 2011;40(2):249–254. doi: 10.1093/ageing/afq168.
- O’Leary TP, Shin S, Fertan E, et al. Reduced acoustic startle response and peripheral hearing loss in the 5xFAD mouse model of Alzheimer’s disease. Genes Brain Behav. 2017;16(5):554–563. doi: 10.1111/gbb.12370.
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35(1):21–23. doi: 10.1038/ng1226.
- Kaur A, Webster MR, Marchbank K, et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532(7598):250–254. doi: 10.1038/nature17392.
- Picciotti P, Torsello A, Wolf FI, et al. Age-dependent modifications of expression level of VEGF and its receptors in the inner ear. Exp Gerontol. 2004;39(8):1253–1258. doi: 10.1016/j.exger.2004.06.003.
- Panza F, Lozupone M, Sardone R, et al. Sensorial frailty: age-related hearing loss and the risk of cognitive impairment and dementia in later life. Ther Adv Chronic Dis. 2019;10:2040622318811000. doi: 10.1177/2040622318811000.
- Nyberg S, Abbott NJ, Shi X, et al. Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med. 2019;11(482):eaao0935. doi: 10.1126/scitranslmed.aao0935.
- Bowl MR, Dawson SJ. Age-related hearing loss. Cold Spring Harb Perspect Med. 2019;9(8):a033217. doi: 10.1101/cshperspect.a033217.
- Huang Q, Tang J. Age-related hearing loss or presbycusis. Eur Arch Otorhinolaryngol. 2010;267(8):1179–1191. doi: 10.1007/s00405-010-1270-7.
- Gao Z, Schwieger J, Matin-Mann F, et al. Dexamethasone for inner ear therapy: biocompatibility and bio-efficacy of different dexamethasone formulations in vitro. Biomolecules. 2021;11(12):1896. doi: 10.3390/biom11121896.
- Yamamoto A. A unique antilipidemic drug–probucol. J Atheroscler Thromb. 2008;15(6):304–305. doi: 10.5551/jat.e621.
- Heinecke JW. Lipoprotein oxidation in cardiovascular disease: chief culprit or innocent bystander? J Exp Med. 2006;203(4):813–816. doi: 10.1084/jem.20060218.
- Iqbal M, Sharma SD, Okada S. Probucol as a potent inhibitor of oxygen radical-induced lipid peroxidation and DNA damage: in vitro studies. Redox Rep. 2004;9(3):167–172. doi: 10.1179/135100004225005174.
- Wang H, Zhang K, Ruan Z, et al. Probucol enhances the therapeutic efficiency of mesenchymal stem cells in the treatment of erectile dysfunction in diabetic rats by prolonging their survival time via Nrf2 pathway. Stem Cell Res Ther. 2020;11(1):302. doi: 10.1186/s13287-020-01788-3.
- Santos DB, Colle D, Moreira ELG, et al. Probucol protects neuronal cells against peroxide-induced damage and directly activates glutathione peroxidase-1. Mol Neurobiol. 2020;57(8):3245–3257. doi: 10.1007/s12035-020-01963-w.
- Zhou Z, Liu C, Chen S, et al. Activation of the Nrf2/ARE signaling pathway by probucol contributes to inhibiting inflammation and neuronal apoptosis after spinal cord injury. Oncotarget. 2017;8(32):52078–52093. doi: 10.18632/oncotarget.19107.
- Sharif A, Mamo J, Lam V, et al. The therapeutic potential of probucol and probucol analogues in neurodegenerative diseases. Transl Neurodegener. 2024;13(1):6. doi: 10.1186/s40035-024-00398-w.
- Zhu J, Du J, Kou W, et al. Probucol protects against brain damage caused by intra-neural pyroptosis in rats with vascular dementia through inhibition of the Syk/Ros pathway. Aging (Albany NY). 2024;16(5):4363–4377. doi: 10.18632/aging.205593.
- Wagle SR, Kovacevic B, Ionescu CM, et al. Probucol-bile acid based nanoparticles protect auditory cells from oxidative stress: an in vitro study. Ther Deliv. 2024;15(4):237–252. doi: 10.4155/tde-2023-0099.
- Mooranian A, Raj Wagle S, Kovacevic B, et al. Bile acid bio-nanoencapsulation improved drug targeted-delivery and pharmacological effects via cellular flux: 6-months diabetes preclinical study. Sci Rep. 2020;10(1):106. doi: 10.1038/s41598-019-53999-1.
- Wagle SR, Kovacevic B, Ionescu CM, et al. Pharmacological and biological study of microencapsulated probucol-secondary bile acid in a diseased mouse model. Pharmaceutics. 2021;13(8):1223. doi: 10.3390/pharmaceutics13081223.
- Yang S, Zhao L, Han Y, et al. Probucol ameliorates renal injury in diabetic nephropathy by inhibiting the expression of the redox enzyme p66Shc. Redox Biol. 2017;13:482–497. doi: 10.1016/j.redox.2017.07.002.
- Zhou G, Wang Y, He P, et al. Probucol inhibited Nox 2 expression and attenuated podocyte injury in type 2 diabetic nephropathy of db/db mice. Biol Pharm Bull. 2013;36(12):1883–1890. doi: 10.1248/bpb.b12-00634.
- Deshmukh P, Unni S, Krishnappa G, et al. The Keap1–Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev. 2017;9(1):41–56. doi: 10.1007/s12551-016-0244-4.
- Huang J-L, Yu C, Su M, et al. Probucol, a “non-statin” cholesterol-lowering drug, ameliorates D-galactose induced cognitive deficits by alleviating oxidative stress via Keap1/Nrf2 signaling pathway in mice. Aging (Albany NY). 2019;11(19):8542–8555. doi: 10.18632/aging.102337.
- Chen Y, Li X-X, Lin H-C, et al. The effects of long-term administration of tadalafil on STZ-induced diabetic rats with erectile dysfunction via a local antioxidative mechanism. Asian J Androl. 2012;14(4):616–620. doi: 10.1038/aja.2012.22.
- Hu L-L, Zhang K-Q, Tian T, et al. Probucol improves erectile function via activation of Nrf2 and coordinates the HO-1/DDAH/PPAR-γ/eNOS pathways in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 2018;507(1–4):9–14. doi: 10.1016/j.bbrc.2018.10.036.
- Kondo S, Shimizu M, Urushihara M, et al. Addition of the antioxidant probucol to angiotensin II type I receptor antagonist arrests progressive mesangioproliferative glomerulonephritis in the rat. J Am Soc Nephrol. 2006;17(3):783–794. doi: 10.1681/ASN.2005050519.
- Tao Z-S, Li T-L, Wei S. Probucol promotes osteoblasts differentiation and prevents osteoporosis development through reducing oxidative stress. Mol Med. 2022;28(1):75. doi: 10.1186/s10020-022-00503-7.
- Eid AA, Ford BM, Block K, et al. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285(48):37503–37512. doi: 10.1074/jbc.M110.136796.
- Qi W, Hu C, Zhao D, et al. SIRT1–SIRT7 in diabetic kidney disease: biological functions and molecular mechanisms. Front Endocrinol (Lausanne). 2022;13:801303. doi: 10.3389/fendo.2022.801303.
- Nin F, Hibino H, Doi K, et al. The endocochlear potential depends on two K + diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc Natl Acad Sci U S A. 2008;105(5):1751–1756. doi: 10.1073/pnas.0711463105.
- Yang R, Sabharwal V, Shlykova N, et al. Treatment of Streptococcus pneumoniae otitis media in a chinchilla model by transtympanic delivery of antibiotics. JCI Insight. 2018;3(19):e123415. doi: 10.1172/jci.insight.123415.
- Zhang Z, Li X, Zhang W, et al. Drug delivery across barriers to the middle and inner ear. Adv Funct Mater. 2021;31(44):2008701. doi: 10.1002/adfm.202008701.
- Goycoolea MV, Lundman L. Round window membrane. Structure function and permeability: a review. Microsc Res Tech. 1997;36(3):201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R.
- Juhn SK, Hamaguchi Y, Goycoolea M. Review of round window membrane permeability. Acta Otolaryngol Suppl. 1989;457(sup457):43–48. doi: 10.3109/00016488809138883.
- Wang X, Dellamary L, Fernandez R, et al. Principles of inner ear sustained release following intratympanic administration. Laryngoscope. 2011;121(2):385–391. doi: 10.1002/lary.21370.
- Park Y-H. Stem cell therapy for sensorineural hearing loss, still alive? J Audiol Otol. 2015;19(2):63–67. doi: 10.7874/jao.2015.19.2.63.
- Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res. 2018;362:25–37. doi: 10.1016/j.heares.2017.12.010.
- Liu SS, Yang R. Inner ear drug delivery for sensorineural hearing loss: current challenges and opportunities. Front Neurosci. 2022;16:867453. doi: 10.3389/fnins.2022.867453.
- Creber NJ, Eastwood HT, Hampson AJ, et al. Adjuvant agents enhance round window membrane permeability to dexamethasone and modulate basal to apical cochlear gradients. Eur J Pharm Sci. 2019;126:69–81. doi: 10.1016/j.ejps.2018.08.013.
- Kovacevic B, Jones M, Ionescu C, et al. The emerging role of bile acids as critical components in nanotechnology and bioengineering: pharmacology, formulation optimizers and hydrogel-biomaterial applications. Biomaterials. 2022;283:121459. doi: 10.1016/j.biomaterials.2022.121459.
- Praetorius M, Brunner C, Lehnert B, et al. Transsynaptic delivery of nanoparticles to the central auditory nervous system. Acta Otolaryngol. 2007;127(5):486–490. doi: 10.1080/00016480600895102.
- Singh M, Hemant K, Ram M, et al. Microencapsulation: a promising technique for controlled drug delivery. Res Pharm Sci. 2010;5(2):65–77.
- Gonçalves M, Mignani S, Rodrigues J, et al. A glance over doxorubicin based-nanotherapeutics: from proof-of-concept studies to solutions in the market. J Control Release. 2020;317:347–374. doi: 10.1016/j.jconrel.2019.11.016.
- Foster T, Ionescu C, Walker D, et al. Chemotherapy-induced hearing loss: the applications of bio-nanotechnologies and bile acid-based delivery matrices. Ther Deliv. 2021;12(10):723–737. doi: 10.4155/tde-2021-0050.
- Jaudoin C, Agnely F, Nguyen Y, et al. Nanocarriers for drug delivery to the inner ear: physicochemical key parameters, biodistribution, safety and efficacy. Int J Pharm. 2021;592:120038. doi: 10.1016/j.ijpharm.2020.120038.
- Agrahari V, Agrahari V, Mitra AK. Inner ear targeted drug delivery: what does the future hold?: future science. Ther Deliv. 2017;8(4):179–184. doi: 10.4155/tde-2017-0001.
- Zhang L, Xu Y, Cao W, et al. Understanding the translocation mechanism of PLGA nanoparticles across round window membrane into the inner ear: a guideline for inner ear drug delivery based on nanomedicine. Int J Nanomedicine. 2018;13:479–492. doi: 10.2147/IJN.S154968.
- Cai H, Wen X, Wen L, et al. Enhanced local bioavailability of single or compound drugs delivery to the inner ear through application of PLGA nanoparticles via round window administration. Int J Nanomedicine. 2014;9:5591–5601. doi: 10.2147/IJN.S72555.
- Wang Y, Wise AK, Tan J, et al. Mesoporous silica supraparticles for sustained inner-ear drug delivery. Small. 2014;10(21):4244–4248. doi: 10.1002/smll.201401767.
- Yang K-J, Son J, Jung SY, et al. Optimized phospholipid-based nanoparticles for inner ear drug delivery and therapy. Biomaterials. 2018;171:133–143. doi: 10.1016/j.biomaterials.2018.04.038.
- Martín-Saldaña S, Palao-Suay R, Aguilar MR, et al. Polymeric nanoparticles loaded with dexamethasone or α-tocopheryl succinate to prevent cisplatin-induced ototoxicity. Acta Biomater. 2017;53:199–210. doi: 10.1016/j.actbio.2017.02.019.
- Zou J, Sood R, Ranjan S, et al. Size-dependent passage of liposome nanocarriers with preserved posttransport integrity across the middle-inner ear barriers in rats. Otol Neurotol. 2012;33(4):666–673. doi: 10.1097/MAO.0b013e318254590e.
- Mooranian A, Negrulj R, Takechi R, et al. The biological effects of the hypolipidaemic drug probucol microcapsules fed daily for 4 weeks, to an insulin-resistant mouse model: potential hypoglycaemic and anti-inflammatory effects. Drug Deliv Transl Res. 2018;8(3):543–551. doi: 10.1007/s13346-017-0473-5.
- Mooranian A, Negrulj R, Chen-Tan N, et al. An optimized probucol microencapsulated formulation integrating a secondary bile acid (deoxycholic acid) as a permeation enhancer. Drug Des Devel Ther. 2014;8:1673–1683. doi: 10.2147/DDDT.S68247.
- Cai H, Liang Z, Huang W, et al. Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int J Pharm. 2017;532(1):55–65. doi: 10.1016/j.ijpharm.2017.08.084.
- Wang Z, Kuang X, Shi J, et al. Targeted delivery of geranylgeranyl acetone to mitochondria by triphenylphosphonium modified nanoparticles: a promising strategy to prevent aminoglycoside-induced hearing loss. Biomater Sci. 2017;5(9):1800–1809. doi: 10.1039/c7bm00224f.
- Buckiová D, Ranjan S, Newman TA, et al. Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine (Lond). 2012;7(9):1339–1354. doi: 10.2217/nnm.12.5.
- Enslin GM, Hamman JH, Kotze AF. Intestinal drug absorption enhancers: synergistic effects of combinations. Drug Dev Ind Pharm. 2008;34(12):1343–1349. doi: 10.1080/03639040802098185.
- Steinbach JM, Seo Y-E, Saltzman WM. Cell penetrating peptide-modified poly (lactic-co-glycolic acid) nanoparticles with enhanced cell internalization. Acta Biomater. 2016;30:49–61. doi: 10.1016/j.actbio.2015.11.029.
- Ge X, Jackson RL, Liu J, et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg. 2007;137(4):619–623. doi: 10.1016/j.otohns.2007.04.013.
- Ramaswamy B, Roy S, Apolo AB, et al. Magnetic nanoparticle mediated steroid delivery mitigates cisplatin induced hearing loss. Front Cell Neurosci. 2017;11:268. doi: 10.3389/fncel.2017.00268.
- Shimoji M, Ramaswamy B, Shukoor MI, et al. Toxicology study for magnetic injection of prednisolone into the rat cochlea. Eur J Pharm Sci. 2019;126:33–48. doi: 10.1016/j.ejps.2018.06.011.
- El Kechai N, Mamelle E, Nguyen Y, et al. Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J Control Release. 2016;226:248–257. doi: 10.1016/j.jconrel.2016.02.013.
- Salt AN, Hartsock J, Plontke S, et al. Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol Neurootol. 2011;16(5):323–335. doi: 10.1159/000322504.
- Kadkhoda J, Tarighatnia A, Nader ND, et al. Targeting mitochondria in cancer therapy: insight into photodynamic and photothermal therapies. Life Sci. 2022;307:120898. doi: 10.1016/j.lfs.2022.120898.
- Kadkhoda J, Tarighatnia A, Tohidkia MR, et al. Photothermal therapy-mediated autophagy in breast cancer treatment: progress and trends. Life Sci. 2022;298:120499. doi: 10.1016/j.lfs.2022.120499.
- Pandey A, Singh K, Subramanian S, et al. Heterogeneous surface architectured pH responsive metal-drug nano-conjugates for mitochondria targeted therapy of glioblastomas: a multimodal intranasal approach. Chem Eng J. 2020;394:124419. doi: 10.1016/j.cej.2020.124419.
- Nabil M, Megahed F. Quantum dot nanomaterials: preparation, characterization, advanced bio-imaging and therapeutic applications. J Fluoresc. 2023;25:1–8. doi: 10.1007/s10895-023-03472-0.
- Ostovar S, Pourmadadi M, Zaker MA. Co-biopolymer of chitosan/carboxymethyl cellulose hydrogel improved by zinc oxide and graphene quantum dots nanoparticles as pH-sensitive nanocomposite for quercetin delivery to brain cancer treatment. Int J Biol Macromol. 2023;253(Pt 4):127091. doi: 10.1016/j.ijbiomac.2023.127091.
- Mooranian A, Negrulj R, Chen-Tan N, et al. Advanced bile acid-based multi-compartmental microencapsulated pancreatic β-cells integrating a polyelectrolyte-bile acid formulation, for diabetes treatment. Artif Cells Nanomed Biotechnol. 2016;44(2):588–595. doi: 10.3109/21691401.2014.971806.
- Mooranian A, Negrulj R, Arfuso F, et al. The effect of a tertiary bile acid, taurocholic acid, on the morphology and physical characteristics of microencapsulated probucol: potential applications in diabetes: a characterization study. Drug Deliv Transl Res. 2015;5(5):511–522. doi: 10.1007/s13346-015-0248-9.
- Mooranian A, Negrulj R, Chen-Tan N, et al. Novel artificial cell microencapsulation of a complex gliclazide-deoxycholic bile acid formulation: a characterization study. Drug Des Devel Ther. 2014;8:1003–1012.
- Mooranian A, Jones M, Ionescu CM, et al. Advancements in assessments of bio-tissue engineering and viable cell delivery matrices using bile acid-based pharmacological biotechnologies. Nanomaterials. 2021;11(7):1861. doi: 10.3390/nano11071861.
- Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11(1):55–67. doi: 10.1038/nrgastro.2013.151.
- Lo Y-L, Huang J-D. Effects of sodium deoxycholate and sodium caprate on the transport of epirubicin in human intestinal epithelial Caco-2 cell layers and everted gut sacs of rats. Biochem Pharmacol. 2000;59(6):665–672. doi: 10.1016/s0006-2952(99)00377-9.
- Kim K, Yoon I, Chun I, et al. Effects of bile salts on the lovastatin pharmacokinetics following oral administration to rats. Drug Deliv. 2011;18(1):79–83. doi: 10.3109/10717544.2010.512024.
- Darkoh C, Lichtenberger LM, Ajami N, et al. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob Agents Chemother. 2010;54(9):3618–3624. doi: 10.1128/AAC.00161-10.
- Lalić-Popović M, Vasović V, Milijašević B, et al. Deoxycholic acid as a modifier of the permeation of gliclazide through the blood brain barrier of a rat. J Diabetes Res. 2013;2013:598603–598608. doi: 10.1155/2013/598603.
- Li P, Fu D, Sheng Q, et al. TUDCA attenuates intestinal injury and inhibits endoplasmic reticulum stress-mediated intestinal cell apoptosis in necrotizing enterocolitis. Int Immunopharmacol. 2019;74:105665. doi: 10.1016/j.intimp.2019.05.050.
- Yanguas‐Casás N, Barreda‐Manso MA, Nieto‐Sampedro M, et al. TUDCA: an agonist of the bile acid receptor GPBAR1/TGR5 with anti‐inflammatory effects in microglial cells. J Cell Physiol. 2017;232(8):2231–2245. doi: 10.1002/jcp.25742.
- Shah V, Mittal R, Shahal D, et al. Evaluating the efficacy of taurodeoxycholic acid in providing otoprotection using an in vitro model of electrode insertion trauma. Front Mol Neurosci. 2020;13:113. doi: 10.3389/fnmol.2020.00113.
- Mooranian A, Negrulj R, Al-Salami H, et al. Designing anti-diabetic beta-cells microcapsules using polystyrenic sulfonate, polyallylamine, and a tertiary bile acid: morphology, bioenergetics, and cytokine analysis. Biotechnol Prog. 2016;32(2):501–509. doi: 10.1002/btpr.2223.
- Mooranian A, Negrulj R, Mikov M, et al. Novel chenodeoxycholic acid-sodium alginate matrix in the microencapsulation of the potential antidiabetic drug, probucol. An in vitro study. J Microencapsul. 2015;32(6):589–597. doi: 10.3109/02652048.2015.1065922.
- Mathavan S, Mikov M, Golocorbin-Kon S, et al. Diabetes development increased concentrations of the conjugated bile acid, taurocholic acid in serum, while treatment with microencapsulated-taurocholic acid exerted no hypoglycaemic effects. Eur J Pharm Sci. 2017;106:1–9. doi: 10.1016/j.ejps.2017.05.041.
- Mathavan S, Chen-Tan N, Arfuso F, et al. The role of the bile acid chenodeoxycholic acid in the targeted oral delivery of the anti-diabetic drug gliclazide, and its applications in type 1 diabetes. Artif Cells Nanomed Biotechnol. 2016;44(6):1508–1519. doi: 10.3109/21691401.2015.1058807.
- Harris JP, Tomiyama S. Experimental immune system of the inner ear. ORL J Otorhinolaryngol Relat Spec. 1987;49(5):225–233. doi: 10.1159/000275942.
- Feng H, Pyykkö I, Zou J. Involvement of ubiquitin-editing protein A20 in modulating inflammation in rat cochlea associated with silver nanoparticle-induced CD68 upregulation and TLR4 activation. Nanoscale Res Lett. 2016;11(1):240. doi: 10.1186/s11671-016-1430-9.
- Zou J, Feng H, Mannerström M, et al. Toxicity of silver nanoparticle in rat ear and BALB/c 3T3 cell line. J Nanobiotechnol. 2014;12(1):52. doi: 10.1186/s12951-014-0052-6.
- Lafond J-F, Shimoji M, Ramaswamy B, et al. Middle ear histopathology following magnetic delivery to the cochlea of prednisolone-loaded iron oxide nanoparticles in rats. Toxicol Pathol. 2018;46(1):101–106. doi: 10.1177/0192623317732028.
- Schilder AGM, Wolpert S, Saeed S, et al. A phase I/IIa safety and efficacy trial of intratympanic gamma-secretase inhibitor as a regenerative drug treatment for sensorineural hearing loss. Nat Commun. 2024;15(1):1896. doi: 10.1038/s41467-024-45784-0.
- Asadollahi L, Mahoutforoush A, Dorreyatim SS, et al. Co-delivery of erlotinib and resveratrol via nanostructured lipid carriers: a synergistically promising approach for cell proliferation prevention and ROS-mediated apoptosis activation. Int J Pharm. 2022;624:122027. doi: 10.1016/j.ijpharm.2022.122027.
- Zeinali M, Abbaspour-Ravasjani S, Ghorbani M, et al. Nanovehicles for co-delivery of anticancer agents. Drug Discov Today. 2020;25(8):1416–1430. doi: 10.1016/j.drudis.2020.06.027.
- Tarighatnia A, Fouladi MR, Nader ND, et al. Recent trends of contrast agents in ultrasound imaging: a review of the classifications and applications. Mater Adv. 2022;3(9):3726–3741. doi: 10.1039/D1MA00969A.
- Liu J, Li R, Yang B. Carbon dots: a new type of carbon-based nanomaterial with wide applications. ACS Cent Sci. 2020;6(12):2179–2195. doi: 10.1021/acscentsci.0c01306.
- Tran TH, Poudel BK, Marasini N, et al. Preparation and evaluation of raloxifene-loaded solid dispersion nanoparticle by spray-drying technique without an organic solvent. Int J Pharm. 2013;443(1–2):50–57. doi: 10.1016/j.ijpharm.2013.01.013.
- Sosnik A, Seremeta KP. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv Colloid Interface Sci. 2015;223:40–54. doi: 10.1016/j.cis.2015.05.003.
- Arpagaus C, Collenberg A, Rütti D, et al. Nano spray drying for encapsulation of pharmaceuticals. Int J Pharm. 2018;546(1–2):194–214. doi: 10.1016/j.ijpharm.2018.05.037.
- Šturm L, Osojnik Črnivec IG, Istenič K, et al. Encapsulation of non-dewaxed propolis by freeze-drying and spray-drying using gum arabic, maltodextrin and inulin as coating materials. Food Bioprod Process. 2019;116:196–211. doi: 10.1016/j.fbp.2019.05.008.
- Choi M-J, Hong G-P, Briançon S, et al. Effect of a high-pressure-induced freezing process on the stability of freeze-dried nanocapsules. Drying Technol. 2008;26(10):1199–1207. doi: 10.1080/07373930802306979.
- Minemoto Y, Adachi S, Matsuno R. Comparison of oxidation of methyl linoleate encapsulated with gum arabic by hot-air-drying and freeze-drying. J Agric Food Chem. 1997;45(12):4530–4534. doi: 10.1021/jf970465h.
- Desobry SA, Netto FM, Labuza TP. Comparison of spray‐drying, drum‐drying and freeze‐drying for β‐carotene encapsulation and preservation. J Food Sci. 1997;62(6):1158–1162. doi: 10.1111/j.1365-2621.1997.tb12235.x.
- Chen J, Li F, Li Z, et al. Encapsulation of carotenoids in emulsion-based delivery systems: enhancement of β-carotene water-dispersibility and chemical stability. Food Hydrocolloids. 2017;69:49–55. doi: 10.1016/j.foodhyd.2017.01.024.
- Mooranian A, Negrulj R, Al-Salami H. The effects of ionic gelation-vibrational jet flow technique in fabrication of microcapsules incorporating β-cell: applications in diabetes. Curr Diabetes Rev. 2017;13(1):91–96. doi: 10.2174/1573399812666151229101756.
- Mooranian A, Negrulj R, Mathavan S, et al. An advanced microencapsulated system: a platform for optimized oral delivery of antidiabetic drug-bile acid formulations. Pharm Dev Technol. 2015;20(6):702–709. doi: 10.3109/10837450.2014.915570.
- Wagle SR, Kovacevic B, Walker D, et al. Alginate-based drug oral targeting using bio-micro/nano encapsulation technologies. Expert Opin Drug Deliv. 2020;17(10):1361–1376. doi: 10.1080/17425247.2020.1789587.
- Muhoza B, Xia S, Wang X, et al. Microencapsulation of essential oils by complex coacervation method: preparation, thermal stability, release properties and applications. Crit Rev Food Sci Nutr. 2022;62(5):1363–1382. doi: 10.1080/10408398.2020.1843132.
- Mohanty B, Aswal V, Kohlbrecher J, et al. Synthesis of gelatin nanoparticles via simple coacervation. J Surf Sci Technol. 2005;21(3/4):149.
- Battaglia L, Gallarate M, Cavalli R, et al. Solid lipid nanoparticles produced through a coacervation method. J Microencapsul. 2010;27(1):78–85. doi: 10.3109/02652040903031279.
- Dong W, Bodmeier R. Encapsulation of lipophilic drugs within enteric microparticles by a novel coacervation method. Int J Pharm. 2006;326(1–2):128–138. doi: 10.1016/j.ijpharm.2006.07.013.
