Abstract
Human arboviral diseases have emerged or re-emerged in numerous countries worldwide due to a number of factors including the lack of progress in vaccine development, lack of drugs, insecticide resistance in mosquitoes, climate changes, societal behaviours, and economical constraints. Thus, Aedes aegypti is the main vector of the yellow fever and dengue fever flaviviruses and is also responsible for several recent outbreaks of the chikungunya alphavirus. As for the other mosquito species, the A. aegypti control relies heavily on the use of insecticides. However, because of increasing resistance to the different families of insecticides, reduction of Aedes populations is becoming increasingly difficult. Despite the unquestionable utility of insecticides in fighting mosquito populations, there are very few new insecticides developed and commercialized for vector control. This is because the high cost of the discovery of an insecticide is not counterbalanced by the ‘low profitability’ of the vector control market. Fortunately, the use of quantitative structure–activity relationship (QSAR) modelling allows the reduction of time and cost in the discovery of new chemical structures potentially active against mosquitoes. In this context, the goal of the present study was to review all the existing QSAR models on A. aegypti. The homology and pharmacophore models were also reviewed. Specific attention was paid to show the variety of targets investigated in Aedes in relation to the physiology and ecology of the mosquito as well as the diversity of the chemical structures which have been proposed, encompassing man-made and natural substances.
1. Introduction
Millions of people worldwide are infected by dengue, yellow fever, West Nile, and chikungunya viruses mainly transmitted by Aedes aegypti, leading to hundreds of thousands of deaths annually. Thus, for example, dengue fever is caused by one of four subtypes (DENV-1 to DENV-4) of an arbovirus belonging to the Flaviviridae family. The infection causes flu-like illness and can develop into a potentially lethal complication called severe dengue. Incidence of dengue has risen 30-fold in the last 50 years, and it is now endemic in more than 100 countries in Africa, the Americas, the Eastern Mediterranean, South-east Asia and the Western Pacific. The emergence and re-emergence of dengue fever have been associated with a number of factors that include insecticide resistance, climate changes, societal behaviours, and changes in public health policy. Each year, about 50–100 million people contract dengue fever with at least 500,000 cases of severe dengue leading to death in about 2.5% of cases, especially among children [Citation1–3].
Except for yellow fever, to date, there is no operational vaccine for the other diseases transmitted by A. aegypti. Consequently, vector control represents the only way to limit Aedes populations. However, vector control is threatened by the increasing resistance of mosquitoes to insecticides. This resistance, which is mediated by several mechanisms, has been observed with each of the major insecticide classes used in the past and present for vector control. As a result, there is a critical need to find new, environmentally safe but effective insecticides that address these problems of resistance. The search for new substances effective against mosquitoes has beneficiated from the use of in silico techniques. Quantitative structure–activity relationship (QSAR) modelling is particularly suited to propose candidate molecules for further evaluation by laboratory tests and to identify structural features and/or physicochemical properties explaining an activity [Citation4–10]. Homology modelling approximates 3D structures to gain insights into structure–function relationships and predict binding geometries and affinities [Citation11–14]. Structural and functional genomics, which commonly use in silico tools, provide keys to find new ways to prevent the propagation of the viruses, to better understand mechanisms of insecticide resistance and to identify putative targets for the design of new insecticides [Citation15–20].
The aim of this study was to review all the in silico strategies that have been used to find new chemicals active on A. aegypti. Here, the term ‘active’ has to be understood in the sense of leading to death directly or indirectly. This means that the search for new repellents has been excluded from this review notwithstanding their great interest and the relatively high number of QSAR and related approaches focused on them [Citation21–25].
A. aegypti is a holometabolous insect, meaning that it goes through a complete metamorphosis. The juvenile form undergoes a series of moults as it becomes larger. Each developmental stage is called instar. After the last instar, the larva undergoes a metamorphic moult to become a pupa, and then an imago. While the larval development is made in the aquatic environment, the adult, which has a life span ranging from 2 weeks to a month depending on the environmental conditions, is aerial, preferentially living in urban habitats, with a daytime activity. The ecology and physiology of the mosquitoes lead to their control at either the larval or adult stage. Larvicides are chemicals (or bioinsecticides) used to reduce immature mosquito populations when they are still in the aquatic media. They are applied directly to water sources that hold mosquito larvae. Adulticides are compounds aiming at rapidly reducing adult mosquito populations. These two categories generally include chemicals with different structural and physicochemical characteristics. They also show different environmental constraints. However, because some search strategies for new chemicals have been applied to both categories, conveniently a focus was made on the chemical categories rather than the targets to discuss the different in silico models that were derived for predicting new compounds active on A. aegypti. In addition, to better pinpoint the most innovative discovery strategies, a specific section has been made for such studies.
2. Juvenile hormone mimics
Metamorphosis in holometabolous insects is a complex process in which physiological, morphological, and behavioural events occur in a precisely timed manner to result in the transformation of a larva into an adult called an imago. During larval development the presence of juvenile hormone (JH) in the haemolymph at a sufficient level maintains the insect in a juvenile status, and when the JH titre falls to low levels, it signals the insect to undergo metamorphosis that is under the control of 20-hydroxyecdysone (Figure ) titre that has increased [Citation26–28]. Reduction in JH titre occurs through a combination of a decrease in JH biosynthesis and increase in JH metabolism. JH metabolism occurs through cleavage of either its epoxide or ester moiety. The relative importance of these two pathways varies with insect species, development stage, and tissue [Citation29,30]. In A. aegypti, the corpora allata synthesize and secrete JH III (Figure ) that regulates the development of the mosquitoes but also intervenes in the reproduction of the females [Citation31,32].
Figure 2. Structure of juvenile hormone III (JH3), hydroprene, kinoprene, methoprene, pyriproxyfen, and fenoxycarb.
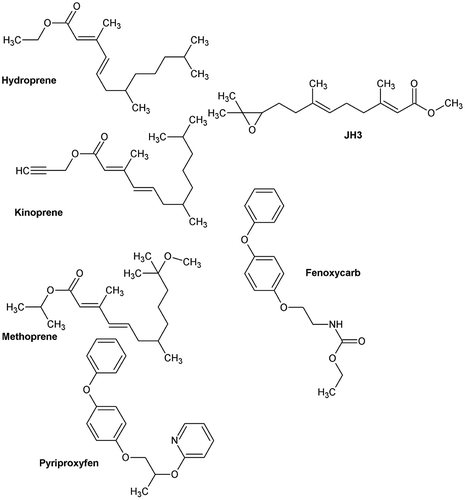
Juvenile hormone mimics, also termed juvenoids, are man-made chemicals that behave similarly to the JHs. Thus, applied at critical periods, they will disrupt metamorphosis and lead to various deleterious effects in the insects. These chemicals are much more target specific than conventional synthetic pesticides, their environmental fate profile is also broadly better, and they are safe to vertebrates. The history of the juvenoids, including the main chemicals currently in use, such as hydroprene, kinoprene, methoprene, pyriproxyfen, and fenoxycarb (Figure ) has been reviewed recently [Citation33]. Different attempts have been made to propose new JH mimics from QSAR modelling.
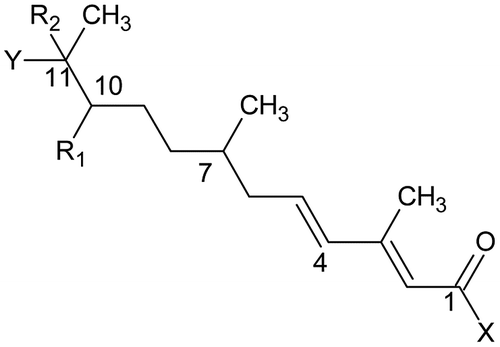
Juvenile hormone activity (JHA) values on last larval instars of A. aegypti, originally reported by Henrick et al. [Citation34] for about 100 structurally diverse (2E,4E)-3,7,11-trimethyl-2,4-dodecadienoates, were used by Nakayama et al. [Citation35] for computing different QSAR models. The JHA values originally reported by Henrick et al. [Citation34] in ppm were converted into log 1/IC50 = pI50 (mmol). Figure shows the common skeleton of the 2,4-dodecadienones in which X expresses the substituents at the carbonyl C1 atom of the dodecadienone skeleton and Y is the longest one of the C11 substituents in terms of length Ly along the bond axis. The molecules were described by different steric and hydrophobic parameters and used to compute step by step various QSAR models based on different functional groups. Equation (1) represents the final QSAR model on A. aegypti selected by Nakayama et al. [Citation35].(1)
Figure 3. 2,4-dodecadienone skeleton. X = OR, SR, NHR, NR2, Alkyl; R1 = H, OR, SEt, 10-ene, 11-ene, 10-epoxy, oxo; Y = OR, SR, OCOR, Me, Et; R2 = H, Me, Cl.
n = 85, s = 0.53, r = 0.89
In Equation (1), r is the coefficient of correlation and s is the standard error of estimate. Lx is the Sterimol length of the X end along the bond axis (C1–X). D is the maximum length of the whole molecule. Bx expresses the bulkiness toward the carbonyl group of α-substituents in the alcohol moiety of ester derivatives and/or thiol ester derivatives. IN is an indicator variable that takes the value of 1 for the amides and 0 for the other chemicals. IOR is an indicator variable that takes the value of 1 for the compounds whose Y moiety is an alkoxy or a hydroxy group and otherwise zero. Ibr is an indicator variable for the chemicals having a branch at any position in the X moiety of ketone derivatives. I(-) is an indicator variable coding enantiomeric effects. It takes the value of 1 for the (R)-(-)-isomer and zero for the (±) mixture. The hydrophobicity of the whole molecule is estimated by log P [Citation35,36].
Equation (1) is quite complex, including a lot of parameters with their square terms while leading to rather modest results. Hansch and Leo [Citation37] have tried to obtain a simpler model than Equation (1) by deriving a bilinear model [Citation38] from the descriptors calculated by Nakayama et al. [Citation35]. To do so, the Lx, Lx2, D, and D2 descriptors were omitted and 10 chemicals were removed without justification. This led to Equation (2).(2)
n = 75, s = 0.52, r = 0.86
According to Hansch and Leo [Citation37], the relatively high standard deviations and low correlation coefficients of Equations (1) and (2) clearly called for further analyses. Consequently, recently, Devillers [Citation39] has tried to compute a model presenting better performances while including a fewer number of descriptors. To do so, a three-layer perceptron (TLP) [Citation40] was used as statistical engine to optimize the search for complex relationships between the pI50 values recorded on A. aegypti and the molecular descriptors selected by Nakayama et al. [Citation35] but without accounting for the squared terms. The original data set was randomly split into a learning set (LS), test set (TS), and validation set (VS) of 69, 8, and 8 chemicals, respectively. Use of Lx, D, Bx, log P, IN, IOR, and I(−) as input neurons in the TLP resulted in a model with good performances. Because with the Statistica™ software (StatSoft, Fr) a Boolean descriptor is encoded as two input neurons, a 10/4/1 TLP was designed. Such a TLP with a bias connected to the hidden and output layers and the use of the Broyden–Fletcher–Goldfarb–Shanno (BFGS) second-order training algorithm led to satisfying prediction results. The hidden and output activation functions were a hyperbolic tangent function and a negative exponential function, respectively. The convergence was obtained after 138 cycles. With such a configuration, the correlation coefficients for the LS, TS, and VS were equal to 0.92, 0.94, and 0.95, respectively. An overall correlation coefficient of 0.92 was calculated from the prediction results obtained with the three sets. The TLP model has been more robustly derived than Equation (1) and it shows better predictive performances. Thus, for example, inspection of the prediction results (not shown) revealed that 13 chemicals (15%) have residuals ≥0.8 (in absolute values) when Equation (1) is used to estimate the pI50 values of the 85 studied 2,4-dodecadienones, while the use of the TLP model leads to more than twice fewer chemicals with a residual value ≥0.8 (7%). Other trials with random selections of 80% (LS), 10% (TS), and 10% (VS) of the data set and with the same TLP architecture led to broadly the same prediction results. In the same way, use of five or six neurons on the hidden layer increased the performances of the models. It is noteworthy that with such TLP architecture, most of the time, good performances were obtained on the TS and VS despite the high number of connections within the networks [Citation39].
Basak et al. [Citation41] also used the biological data reported by Henrick et al. [Citation34] to derive various QSAR models, focusing on the selection of the most informative descriptors as well as the most efficient statistical method. They included a higher number of chemicals in their modelling process than Nakayama et al. [Citation35], Hansch and Leo [Citation37], and Devillers [Citation39]. Thus, they considered 143 JHA values obtained on last larval instars of yellow fever mosquitoes and 35 censored data reported to be less than a given data.
The molecules were described by means of topostructural indices (e.g. Wiener number, Randic indices), topochemical indices (e.g. valence and bond connectivity indices, E-state indices), triplet indices and Balaban’s J indices, geometric or 3D indices (Kappa shape indices) [Citation42], and atom pairs. Briefly, an atom pair was defined as a substructure consisting of two non-hydrogen atoms i and j and their interatomic separation: {atom descriptori} − {separation} − {atom descriptorj}, where {atom descriptor} contains the information regarding atom type, the number of non-hydrogen neighbours, and the number of π electrons. Thus, for example, C1X2-6-O0X2 represents a carbon atom with one π electron and two non-hydrogen neighbours (atom #1) and an oxygen atom with no π electrons and two non-hydrogen neighbours (atom #2), with an interatomic separation (including both the atoms) of 6. Initially, 1173 descriptors were computed, including 915 atom pairs. Ridge regression (RR) [Citation43], principal component regression (PCR) [Citation44], and partial least squares (PLS) regression [Citation45] were used as statistical tools, and the modified Gram–Schmidt orthogonalization was employed to trim the 258 global molecular descriptors to a size of 100. The leave-one-out cross-validated q2 values obtained with the RR, PCR, and PLS methods equalled 0.361, 0.053, and 0.327 respectively. RR giving the best results, it was used to compute the models from atom pairs and from the whole set of descriptors after their trimming by means of the soft threshold method. On the basis of q2 values, atom pairs provided the best results.
Basak et al. [Citation41] suspected that the use of a nonlinear method could improve the prediction performances. Thus, recursive partitioning [Citation46] was used to capture any nonlinear relation between the JHA measured on A. aegypti and the global descriptors. The r2 values obtained for the dendrograms and predictors that lead to significant splits in recursive partitioning equalled 0.54. While the different results obtained by Basak et al. [Citation41] are interesting, the performances of their numerous models remain rather modest and the models obtained on A. aegypti do not outperform those obtained by Nakayama et al. [Citation35] (Equation (1)), by Hansch and Leo [Citation37] (Equation (2)), and by Devillers [Citation39].
It is noteworthy that while attempts were made to use 2D and 3D QSARs to propose chemical structures acting on the enzymes responsible for the degradation of JH of other insects [Citation47–52], to our knowledge, no such strategies were used on A. aegypti as well as on the other species of mosquitoes.
3. Ecdysteroids
Ecdysteroids are steroidal structures, exemplified by the 20-hydroxyecdysone (Figure ), involved in moulting and in other important events in A. aegypti adult females [Citation53–55]. In addition to the animals, the ecdysteroids are also present in fungi and plants. In the latter group, more than 300 of them have been described so far, and the number of newly detected variants is about 10–20 analogues per year [Citation55]. After the discovery and characterization of ecdysone it was expected that steroidal moulting hormone agonists might have insecticidal properties. In fact, the first commercialized ecdysteroid agonist was tebufenozide, a nonsteroidal structure (Figure ), affecting the moulting process of A. aegypti and A. taeniorhynchus, through anomalies of the head capsules, the incorrect unfolding of the body after ecdysis, and a significant mortality at the highest concentrations, identified by the presence of a partially attached exuvium. Similar abnormal larval moults were observed in the crustaceans Artemia salina and Daphnia magna exposed to tebufenozide, but they were not as obvious as in the mosquito species [Citation56]. Ecdysteroids bind to the ecdysone receptor (EcR) that functions through heterodimerization with a homologous ultraspiracle protein (USP). In A. aegypti, two EcR isoforms and two USP isoforms have been identified [Citation57]. Attempts have been made to pinpoint the structural requirements for binding of chemicals to the A. aegypti EcR. A library of 35 cis-1-benzoyl-2-methyl-4-(phenylamino)-1,2,3,4-tetrahydroquinolines was prepared by Smith et al. [Citation58]. The chemicals showed various substituents on the benzoyl ring, at the 4-position of the phenylamino ring, and at the 6-position of tetrahydroquinoline ring. The chemicals were tested for their ability to cause expression of a reporter gene downstream of an ecdysone response element in a mammalian cell line engineered to express the A. aegypti EcR [Citation58]. Generally, compounds with small lipophilic substituents at the meta and para-positions of the benzoyl ring and hydrogen or fluorine at the 4-position of the phenylamino ring and the 6-position of the tetrahydroquinoline ring were the most potent [Citation58]. In the same way, a set of 32 natural and 10 semi-synthetic ecdysteroids was assayed in murine 3T3 cells across 10 different ecdysteroid receptor (EcR) ligand-binding domains derived from A. aegypti and eight other arthropod species in an engineered gene switch format [Citation59]. Some parallel ecdysteroid SARs were observed across species but no important facts were noted in A. aegypti.
4. Organotin compounds
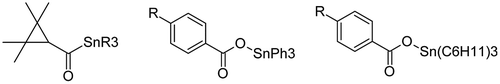
Use of organotin chemicals in insect control is not new, and their effects on mosquitoes are rather well documented [Citation60]. A series of triorganotin 2,2,3,3-tetramethylcyclopropanecarboxylates (Figure – left, R = CH3, C2H5, n-C3H7, n-C4H9, C6H5, and C6H11) were synthesized by Song et al. [Citation61] and were evaluated for their larvicidal activity against the second instar stage of the A. aegypti (IC50 = concentration at which the test compounds killed 50% of the larvae). A simple QSAR model was then derived using the principal moment of inertia along the z-axis of the chemicals as molecular descriptor [Citation61]. The data, after their conversion in molar concentrations, were used by Hansch and Verma [Citation62] to derive Equation (3).(3)
Figure 5. Structure of triorganotin 2,2,3,3-tetramethylcyclopropanecarboxylates (left), triphenyltin para-substituted benzoates (middle), and tricyclohexyltin para-substituted benzoates (right).
n = 6, r2 = 0.811, s = 0.237, q2 = 0.568, Q = 3.802, F = 17.164
where πR encodes the hydrophobicity of the R substituents. Equation (3) suggests that chemicals with highly hydrophobic R-groups will be more active. In Equation (3), r2 is the coefficient of determination, q2 is the cross-validated r2 obtained by using leave-one-out, Q is the quality ratio that is obtained by dividing the value of the correlation coefficient (r) by the standard deviation (s), and F is the Fisher statistics.
In a second step, two chemicals, C1 with R = n-C3H7 and C2 with R = C6H5) were randomly selected as external test set and the remaining chemicals were used to constitute a learning set that led to the design of Equation (4).(4)
n = 4, r2 = 0.925, s = 0.184, q2 = 0.737, Q = 5.228, F = 24.667
Using Equation (4), residual values equal to 0.24 and 0.42 were obtained for C1 and C2, respectively.
Hansch and Verma [Citation62] found a high mutual correlation (r = 0.944) between πR and the molar refractivity (MR) of the R group (Equation (5)).(5)
n = 6, r2 = 0.891, s = 0.180, q2 = 0.781, Q = 5.244, F = 32.697
Duong et al. [Citation63] synthesized a series of 11 triphenyltin para-substituted benzoates (Figure – middle) and a series of 11 tricylohexyltin para-substituted benzoates (Figure – right). In both series, R in para position was equal to H, F, Cl, Br, I, OCH3, OH, NO2, NH2, CH3, or C(CH3)3. These chemicals were tested for their larvicidal activity against the second instar stage of the A. aegypti.
The data on triphenyltin para-substituted benzoates were used by Hansch and Verma [Citation62] to derive Equation (6) after deletion of the chlorosubstituted chemical.(6)
n = 10, r2 = 0.789, s = 0.078, q2 = 0.646, Q = 11.385, F = 13.088
The triphenyltin para-substituted benzoates with R = I, NO2, and C(CH3)3 were randomly selected as external test set compounds. The seven remaining compounds were used to compute Equation (7).(7)
n = 7, r2 = 0.896, s = 0.063, q2 = 0.748, Q = 15.016, F = 17.231
Use of Equation (7) led to residual values of −0.20, 0.08, and −0.19 for the test set chemicals substituted by I, NO2, and C(CH3)3, respectively.
The data on tricylohexyltin para-substituted benzoates were used by Hansch and Verma [Citation62] to derive Equation (8) after deletion of the chemical substituted by OCH3.(8)
n = 10, r2 = 0.849, s = 0.080, q2 = 0.592, Q = 11.513, F = 19.679
In Equation (8), ES−R is the Taft’s steric parameter for R substituents. Chemicals with R = F, NO2, and C(CH3)3 were randomly selected to constitute an external test set. The seven remaining compounds were used to compute Equation (9).(9)
n = 7, r2 = 0.941, s = 0.040, q2 = 0.651, Q = 24.25, F = 31.898
Use of Equation (9) led to residual values of −0.15, −0.24, and −0.08 for the test set chemicals substituted by F, NO2, and C(CH3)3, respectively.
Song et al. [Citation64] synthesized a series of 15 tris-(para-substituted phenyl)tins (X(para)-C6H4)3SnY, were X = H, Cl, F, CH3 and SCH3 and Y = Cl, OH, and OAc) that were tested for their larvicidal activity against the second instar stage of the A. aegypti. The obtained toxicity data were used to derive a model including the kappa shape index and a modified form of this topological index, as molecular descriptors. Hansch and Verma [Citation62] re-evaluated this QSAR leading to Equation (10). Compounds X = SCH3 and Y = Cl, OH, and OAc were not used to derive the model due to missing toxicity values.(10)
n = 12, r2 = 0.851, s = 0.115, q2 = 0.664, Q = 8.017, F = 12.23
The parabolic relationship in πX in Equation (10) suggests that the lethal activity of the chemicals on larvae of A. aegypti increases with an increase in hydrophobicity of X-substituents up to an optimal value of πX = 0.36 and then decreases. It is noteworthy that a mono and biparametric QSAR model with πX and πX, led to r2 values of 0.016, and 0.553, respectively. The positive sign of σ+X in Equation (10) implies that highly electron-withdrawing substituents at position X (e.g., NO2, CN, CHO, CF3, COCF3) will enhance the lethal activity of the chemicals [Citation62].
Chemicals with X = CH3 or X = F or X = Cl and Y = OH were randomly selected to constitute an external test set. The nine remaining compounds were used to compute Equation (11).(11)
n = 9, r2 = 0.857, s = 0.124, q2 = 0.503, Q = 7.468, F = 9.988
Use of Equation (11) led to residual values of 0.12, 0.04, and 0.16 for the test set chemicals substituted by X = CH3, F, and Cl, respectively. Equations (10) and (11) were used to predict the larvicidal activity of chemicals substituted with X = C≡CH, COOC2H5, or 4-pyridinyl and Y = Cl, OH or OAc. Chemicals with X = 4-pyridinyl were predicted as the most toxic against A. aegypti.
Hansch and Verma [Citation62] combined the data of Song et al. [Citation61,64] and Duong et al. [Citation63] to derive Equation (12) after elimination of three chemicals acting as outliers.(12)
n = 37, r2 = 0.711, s = 0.184, q2 = 0.645, Q = 4.582, F = 27.062
where log P is the 1-octanol/water partition coefficient of the whole molecules. I3 is an indicator variable taking the value of 1 or 0 for the presence or absence of tricyclohexyltin para-substituted benzoates. I4 is an indicator variable that encodes the presence or absence of tris-(para-substituted phenyl)tins. It is noteworthy that the simple equation with log P showed an r2 value of 0.325.
Nine chemicals were randomly selected as external test set. The 28 remaining compounds were used to compute Equation (13).(13)
n = 28, r2 = 0.667, s = 0.189, q2 = 0.549, Q = 4.323, F = 16.024
Use of Equation (13) for predicting the larvicidal activity of the test set chemicals led to residual values ranged from 0.05 to 0.43. It is noteworthy that Hansch and Verma [Citation62] derived similar QSAR equations for predicting the toxicity of organotin compounds against Anopheles stephensi.
A QSAR modelling approach based on a PLS analysis of multivariate image analysis (MIA) descriptors was applied to the combined data of Song et al. [Citation61,64] and Duong et al. [Citation63]. The best model included five PLS components (r2 = 0.805, q2 = 0.525, r2test = 0.656) [Citation65].
5. Biopesticides
5.1 Bacteria
Bacillus thuringiensis (Bt) is a Gram-positive bacterium characterized by the production of parasporal crystalline proteic inclusions during its sporulation phase [Citation66]. These inclusions contain proteins, called δ-endotoxins, which have proven to be specifically effective against crop pests and against insect vectors of diseases. Most δ-endotoxins belong to the Cry (crystal) family of proteins, but, in the diptericidal strains of Bt, they also include the Cyt (cytolytic) family of proteins [Citation67–69]. Thus, B. thuringiensis subspecies israelensis (Bti) is applied worldwide for the control of mosquito larvae [Citation70,71]. Cry toxin proteins must be activated to be functional. Following ingestion of the inactive protoxin by the larva, the crystals are solubilized by the alkaline conditions in the insect midgut and are proteolytically converted into a toxic core fragment. The activated toxin binds to receptors located on the apical microvillus membranes of epithelial midgut cells. The activated toxin disrupts the membranes and allows ions and water to enter the cells leading to swelling, lysis, and ultimately the death of the larva [Citation72–74]. Cyt toxins, which are not related phylogenetically to Cry toxins, do not bind to protein receptors. They directly interact with membrane lipids forming pores or destroying the membrane by a detergent-like interaction. Cyt proteins synergize the mosquitocidal activity of Cry toxins [Citation75].
The structures of several Cry toxins, including Cry4Aa [Citation76] and Cry4Ba [Citation77], have been solved by X-ray crystallography. Despite differences in their primary sequences and insect specificities, all the Cry toxins share a similar three-domain structure. Domain I consists of an α-helical bundle of six amphipathic helices that surround an inner hydrophobic helix. Domain II appears as a β-prism and consists of three antiparallel β-sheets. Domain III shows a β-sandwich structure formed by two antiparallel β-sheets. Domain I intervenes in toxin oligomerization, membrane insertion and pore formation. Domains II and III are both implicated in receptor binding. A role for domain III in pore formation has been also found [Citation78,79]. Specific receptors are necessary for Cry toxin action. In A. aegypti, alkaline phosphatase and cadherin proteins have been reported as putative Cry receptors [Citation72,80]. Receptor recognition by Cry toxins is the crucial step in their mode of action and hence, the limiting factor. In silico techniques offer valuable tools for identifying the key binding regions but also for improving the activity of toxins, and for developing strategies to cope with problems of resistances. Because Cry11Aa is the most active toxin found in the Bti crystal against A. aegypti, Fernández et al. [Citation81] computed its 3-D structure from the Cry2A structure. They showed that the loop α-8 region in domain II was an important epitope involved in receptor interaction. B. thuringiensis serotype H-14 is a commercial mosquitocidal strain including Cry4Aa, Cry4Ba, Cry11Aa, and Cyt1Aa [Citation71]. Cry4Aa is poorly active against the Aedes, Anopheles, and Culex genera while Cry11Aa is very active in all of them. Cry4Ba is active against Aedes and Anopheles but shows no activity against Culex [Citation82]. The H-28a28c serotype is equipped differently in toxins and its Cry19Aa has been shown to be toxic against Anopheles stephensi and Culex pipiens but it is totally inactive against Aedes aegypti [Citation83]. Abdullah and Dean [Citation82] demonstrated that Aedes toxicity could be transferred to Cry19Aa by exchanging the loop regions of domain II to mimic Cry4Ba. First, homology modelling was used to compute the structure of Cry4Ba by using Cry3Aa and Cry4Aa as templates and the structure of Cry19Aa by using the derived Cry4Ba as template. The two model structures were aligned and the residues in domain II loops were noted. Loop residues of Cry19Aa were replaced with loop residues of Cry4Ba by site-directed mutagenesis. Two-day-old larvae of A. aegypti, C. pipiens, and C. quiquefasciatus and three-day-old larvae of Anopheles quadrimaculatus were used to test the mosquitocidal activities of the wild-type Cry19Aa and the mutant called 19AL1L2. The improvement of Aedes toxicity in Cry19Aa was 42,000 fold while the toxicity against the other species of mosquitoes was not affected (Table ) [Citation82].
Table 1. Comparative toxicity (24 h-LC50 in ng/ml) of Cry19Aa and 19AL1L2 toxins against four species of mosquitoes [Citation82].
5.2 Plant extracts and their constituent compounds
Large collections of plant extracts and their constituents have been screened against different species and stages of mosquitoes in order to estimate their potential repellent, larvicidal, pupicidal or adulticidal activity. Thus, a recent survey of the literature [Citation84] showed that among 361 essential oils extracted from 269 plant species and tested for their larvicidal activity against A. aegypti, more than 60% of them were considered active with LC50 values <100 mg/l. They were mainly extracted from species belonging to Myrtaceae, Lamiaceae, and Rutaceae. The most active essential oils showed effective concentrations comparable with the dosage recommended for using temephos in container breeding. Last, about 27% of the plants studied for their activity against larvae of A. aegypti were collected in Brazil. Dias and Moraes [Citation84] also identified some key structural characteristics for larvicidal activity. They showed that lipophilicity played a key role in evaluating larvicidal activity. Interesting reviews were also published by Kishore et al. [Citation85,86].
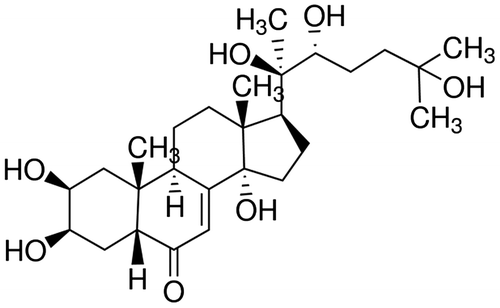
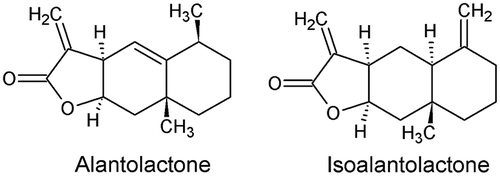
A set of compounds representing different classes of natural products, including polyacetylenes, phytosterols, flavonoids, sesquiterpenoids, and triterpenoids, was tested against larvae of A. aegypti [Citation87]. Among these compounds, alantolactone and isoalantolactone (Figure ) showed an activity against first instar larvae of A. aegypti, the latter being more toxic than the former. Structural modifications were made on both chemicals in order to understand the functional groups necessary to maintain and expected to increase activity. These modifications included epoxidations, reductions, catalytic hydrogenations, and Michael additions to α,β-unsaturated lactones. Only the analogues of alantolactone showed interesting results. A relationship was made between larval toxicity and the number of C-atoms in Michael addition. In general, activity trends observed in the larval screening were not consistent with observations made with the adulticidal screening. The propylamine Michael addition analogue of alantolactone was the most active adulticide, showing an LC50 value of 1.07 μg/mosquito [Citation87].
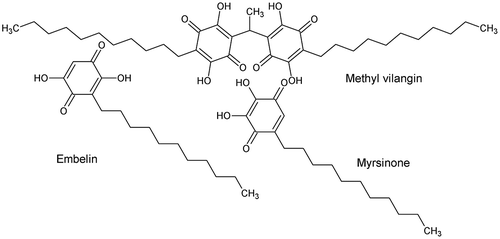
Methyl vilangin [Citation88], embelin, and myrsinone [Citation89] (Figure ) are examples of p-benzoquinones present in plants. Because these compounds have shown larvicidal activity against A. aegypti, de Sousa et al. [Citation90] investigated the SAR of six benzoquinones, namely p-benzoquinone, 2-methyl-p-benzoquinone, 2-isopropyl-p-benzoquinone, 2,6-dimethyl-p-benzoquinone, 2,5-dimethyl-p-benzoquinone, and thymoquinone. LC50 values on third-instar larvae of A. aegypti ranged from 33 to 90 ppm. They showed that the number, position, and size of the substituent were the key factors governing the activity of these compounds [Citation90].
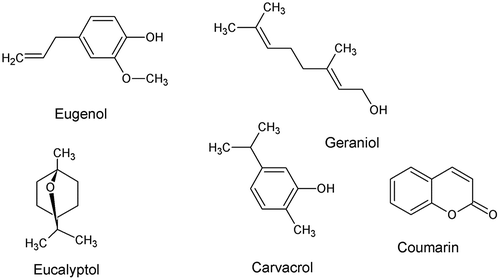
Santos et al. [Citation91] identified structural characteristics responsible for the larvicidal activity of 14 monoterpenes against A. aegypti. The presence of heteroatoms in the basic hydrocarbon structure decreases the larvicidal activity. Conjugated and exo double bonds increase the activity. Replacement of double bonds by more reactive epoxides decreases the larvicidal activity [Citation91]. QSAR equations were designed by Lucia et al. [Citation92] from a set of six monoterpenes for predicting their larvicidal activity against A. aegypti. Unfortunately, the QSAR models were not valid. Eugenol, geraniol, coumarin, eucalyptol, and carvacrol (Figure ) were docked on the octopamine receptor and acetyl cholinesterase protein models of A. aegypti and Homo sapiens for comparison purposes [Citation93]. On the basis of the total energy value, the order of binding efficiency of ligands to octopamine receptor model of H. sapiens was geraniol < octopamine < eugenol < eucalyptol < carvacrol < coumarin. Regarding the acetyl cholinesterase model in H. sapiens, the order was geraniol < acetylcholine < eugenol < coumarin < carvacrol < eucalyptol. In A. aegypti, the two sequences were geraniol < eugenol < octopamine < eucalyptol < carvacrol < coumarin and acetylcholine <geraniol < eugenol < coumarin < carvacrol < eucalyptol, respectively. Khanikor et al. [Citation93] hypothesized that these differences could be exploited for designing safer mosquitocides.
Principal component analysis (PCA), consensus-PCA, and PLS analysis were used by Scotti et al. [Citation94] to investigate the physicochemical properties governing the larvicidal activity against A. aegypti of 55 structurally diverse chemicals mostly found in plants. They showed that hydrophobicity was strongly correlated with activity.
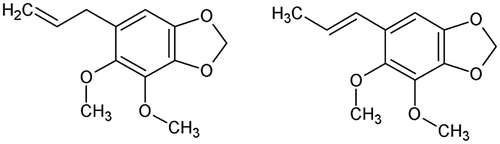
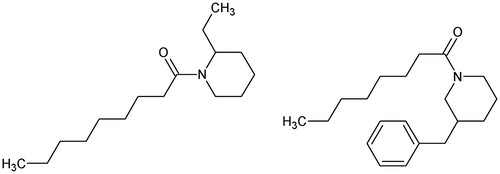
In the same way, attempts have been made for identifying structural characteristics responsible for the adulticidal activity of chemicals found in plants against A. aegypti. Thus, dillapiol (Figure – left) isolated from leaves of Piper aduncum L. and 13 semi-synthetic, C3 side-chain modified dillapiol derivatives were tested against adult females of A. aegypti [Citation95]. It was shown that the length/structure of the C3 chain governed the activity of these molecules. Isodillapiol (Figure – right) and methyl, propyl, and butyl derivatives exhibited greater mosquitocidal activity than dillapiol [Citation95]. Pridgeon et al. [Citation96] investigated SARs of 33 piperidines against adult females of A. aegypti. The toxicity of the piperidine derivatives was significantly decreased when a benzyl moiety was attached to the carbon of the piperidine ring (ethyl- > methyl- > benzyl-derivatives). When the same moiety was attached to the piperidine ring, the location of the substituted carbon conferred different toxicity and the toxicity order was second > third > fourth carbon. Thus, for example, the LC50-24h after topical application of 1-nonanoyl-2-ethyl-piperidine (Figure – left) equals 0.84 μg per adult female of A. aegypti while the LD50 value of 1-octanoyl-3-benzyl-piperidine (Figure – right) equals 29.20 μg per mosquito [Citation96].
6. Molecule repurposing
6.1 Insecticide repurposing
In the frame of a collective expertise directed by ANSES (French Agency for Food, Environmental and Occupational Health & Safety), an attempt was made to evaluate whether insecticides already marketed for specific usages could be used in vector control [Citation97]. The SIRIS (system of integration of risk with interaction of scores) method [Citation98] was used to rationalize the selection of these potential insecticides. Thus, 129 substances potentially active on mosquitoes were described by their (eco)toxicity (i.e. oral LD50 in rat, carcinogenicity, endocrine disruption potential, LD50 in honey bee, EC50 in Daphnia, LC50 in fish) and environmental fate (log P, vapour pressure, level of use, primary and ultimate biodegradation potential). Data were retrieved from literature, obtained from expert judgments or computed from QSAR and QSPR models. In the SIRIS method [Citation98], the values of the selected variables, which can be qualitative or quantitative, are transformed into modalities coded as favourable (f) or unfavourable (d) or as favourable (f), moderately favourable (m), or unfavourable (d). The variables are then ranked according to their decreasing order of importance. Indeed, unlike other multicriteria methods, it is not necessary to introduce coefficients, very often difficult to justify, in the calculation procedure to modify the final weight of the selected variables. Finally a min/max scale of scores is calculated according to specific incremental rules [Citation98]. Both categories of parameters were considered separately to construct two scales of SIRIS scores, from which a SIRIS map can be drawn. In addition two scenarios, one for the larvicides and another for the adulticides, were considered [Citation97] due to the constraints imposed by the corresponding vector control strategies.
Thus, for example, the typology of the 129 insecticides characterized by their two SIRIS scores calculated from the two hierarchies selected for the larvicide scenario is displayed in Figure . The SIRIS scores are ranged from 0 to 81 on the toxicity/ecotoxicity scale and from 10 to 52 on exposure/environmental fate scale. The insecticides located in the left bottom part of Figure are the most interesting because they present low score values on the toxicity/ecotoxicity and exposure/environmental fate scales. This is the case, for example, for the insecticides #58 (cyromazine) and #86 (dicyclanil). At the opposite, the insecticides located in the top right part of Figure are not suited for the selected scenario due to the high values of their SIRIS scores on both scales. Thus, for example, insecticide #117 (dichlofenthion) shows bad scores on both the toxicity/ecotoxicity and exposure/environmental fate scales. A substance can be correctly classified on one scale and with a high score on the other scale. Thus, formetanate (#61) is well located on the exposure/environmental fate scale but shows a rather high score on the toxicity/ecotoxicity scale. This is also the case for deltamethrin (#11) located in the right bottom part of Figure . Bs (#4) and Bti (#5) are perfectly ranked on the toxicity/ecotoxicity scale, having SIRIS scores equal to zero, but they are a little bit less well ranked on the other scale. This is even more the case for the insecticides #88 (azadirachtin) and #127 (silafluofen). The insecticides located in the middle part of Figure , such as metaflumizone (#68) or tau-fluvalinate (#85), are less interesting because they show rather high SIRIS scores on both scales. However, this does not mean that they have to be excluded from a future selection. Indeed, a map of SIRIS scores has to be considered as decision support tool. There are obvious selections for insecticides with low SIRIS score values on both scales, revealing favourable modalities for most of the criteria. At the opposite, a rejection is also obvious for insecticides with high SIRIS score values on the toxicity/ecotoxicity and exposure/environmental fate scales. Between these two extremities, all the intermediate situations exist. An insecticide with a low SIRIS score only on one scale can be selected after a reasoned decision. In this case, the SIRIS map allows us to pinpoint the criteria showing the worst modalities and, hence, for which it will be necessary to pay a special attention. In order to interpret more thoroughly the SIRIS map, specific information can be projected on it. The same strategy was used for the scenario for the adulticides (not shown here). However, it is noteworthy that the SIRIS analysis was performed on 114 insecticides because in our initial list, some insecticides can only be used as larvicides (e.g., Bti) [Citation97].
Figure 11. SIRIS map of the 129 insecticides used or potentially usable as larvicides (See text for the correspondence between some numbers and chemical names).
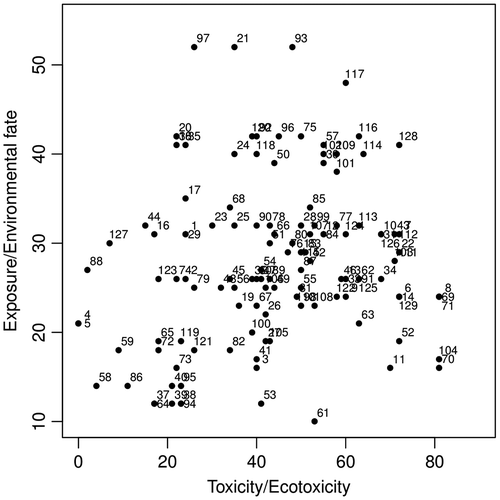
Use of this methodological strategy allowed us to propose a list of insecticides which could be used as adulticides and/or larvicides (e.g. formothion, acetamiprid, thiacloprid, cyromazine, thiamethoxam). Obviously, the different insecticides, which have emerged from the SIRIS analysis, are only potential candidates and risk assessment procedures are required before their use can be envisaged [Citation97].
6.2 Drug repurposing
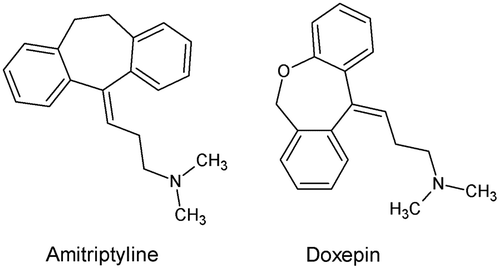
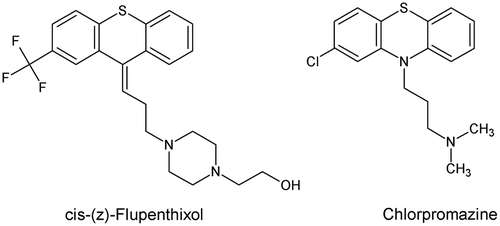
G protein-coupled receptors (GPCRs) are highly targeted for the development of human drugs. They account for the main best-selling drugs and about 40% of all prescription pharmaceuticals on the market [Citation99]. GPCRs have been identified in the genome of A. aegypti [Citation100] and other mosquitoes [Citation101]. These findings have provided a basis for the design of new insecticides. Biogenic amine-binding GPCRs are components of the nervous system of eukaryotes and include receptors that bind various neurotransmitters, among them dopamine which plays key roles in arthropods. Andersen et al. [Citation102] showed that dopamine levels increased in females of A. aegypti following a blood meal, suggesting that dopamine might be involved in ovarian and/or egg development. Dopamine receptors (DRs) are classified as either D1- and D2-like based on their functional roles. Both have been identified in the A. aegypti genome and used as starting point by Meyer et al. [Citation103] for the development of new mode-of-action insecticides. Molecular analysis was used to functionally characterize the putative DRs in A. aegypti, named AaDOP1 and AaDOP2. Their deduced amino acid (AA) sequences were analyzed to identify conserved structural features typically associated with biogenic amine-binding GPCRs and unique regions that could be potentially used for the design of new insecticides. Only a modest level of similarity was observed between the A. aegypti and human D1-like DRs, which shared between 47–54% AA identity among the transmembrane (TM) domains that represent the most conserved regions of GPCRs. In addition, comparison of the predicted TM domains from numerous invertebrate and vertebrate D1-like DRs showed that only 34% of the AAs were shared among all species included in the alignment. Heterologous expression of AaDOP1 and AaDOP2 in HEK293 cells showed dose-dependent responses to dopamine. Only AaDOP1 exhibited sensitivity to epinephrine and norepinephrine. AaDOP1 and AaDOP2 were not stimulated by histamine, octopamine, serotonin or tyramine. Meyer et al. [Citation103] selected the AaDOP2 receptor for an antagonist screen of the Library of Pharmacologically Active Compounds (LOPAC1280) due to its low constitutive activity and strong dopamine response. Fifty-one potential antagonists of the AaDOP2 receptor were identified, and among them 20 are known antagonists of mammalian dopamine receptors. Ten compounds were selected and tested for their activity in cAMP accumulation assays. Three of them revealed no significant antagonistic effects against AaDOP2. The 10 selected hit compounds were also tested against the human D1 receptor (hD1) for species comparison purposes. Analysis of the results showed that amitriptyline and doxepin (Figure ) presented more than 30-fold selectivity for AaDOP2 compared with hD1. Both compounds were tested in A. aegypti larval bioassays. A single dose point test at 400 μM led to 93% of mortality for amitriptyline and 72% of mortality for doxepin both after 24h of exposure. LC50 = 78 μM and LC90 = 185 μM were observed for amitriptyline. In another study [Citation104], these authors also identified cis-(z)-flupenthixol and chlorpromazine (Figure ) with LC50 values of 88 μM and 92 μM, respectively.
7. Challenging new sources of insecticides
7.1. Search for new neurotoxics
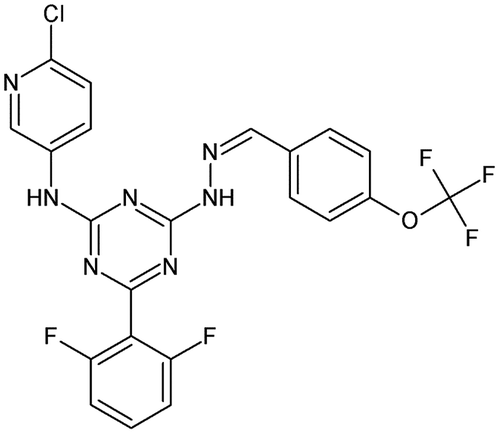
The ω-hexatoxin-1s are a family of 36–37-residue peptide neurotoxins found in spiders, and which block insect but not mammalian voltage-gated calcium channels. Because these hexatoxins show a high phylogenetic specificity, they are good candidates to develop new insecticides. In this context, Tedford et al. [Citation105] used ω-hexatoxin-Hv1a as lead due to its low toxicity against vertebrates. 3D models of ω-hexatoxin-Hv1a pharmacophore were used as search terms for a screening procedure against a proprietary database of millions of compounds. This led to 8773 hits of commercially available molecules that were ranked and sorted based on their structural match to the ω-hexatoxin-Hv1a pharmacophore, log P, Lipinski’s rules, and predicted solubility. Molecules were clustered by structural similarity leading to 2500 unique candidates. Among them, 1370 were easy to purchase and tested on larvae of A. aegypti and Spodoptera exigua. This led to characterization of a class of aryl triazines highly effective against both species. An example of compound very active against A. aegypti is given in Figure .
7.2 Targeting the blood meal
A. aegypti but also Culex quinquefasciatus and Anopheles gambiae are anautogenous mosquitoes requiring a blood meal for egg development. Trypsin and chymotrypsin are the major digestive enzymes that digest the protein-rich blood meal in the midgut of the mosquito into free AAs that are used for vitellogenin biosynthesis and egg development. The control of trypsin synthesis in mosquitoes has been the subject of intensive investigations, some being based on in silico approaches [Citation106]. Recently, Isoe et al. [Citation107] showed that blood-fed mosquitoes were very sensitive to defects in COPI vesicle transport leading to about 90% by 72 h post blood meal. COPI refers to a specific coat protein complex initiating the budding process in the cis-Golgy membrane. Because golgicide A inhibits the COPI vesicle transport [Citation108], a collection of golgicide A analogues (GCAs) were synthesized and tested on mosquitoes by Mack et al. [Citation109]. SAR studies showed that the pyridine ring was most easily manipulated without affecting the activity. Among the 18 synthesized GCAs, GCA-18 (Figure ), with a significant structural change from GCA, showed the highest LC50 value (i.e. 0.05 mM) in the second instar larvae of A. aegypti and Anopheles stephensi [Citation109].
7.3 Targeting chorion peroxidase
Although the chorion of mosquito eggs is a highly protected structure, it is susceptible to desiccation and appears non-resistant to the action of detergents. While oviposited eggs become rapidly dehydrated when moved to an extremely dry environment immediately following oviposition, in moist environments, the chorion becomes highly resistant to desiccation within 2 hours after oviposition [Citation110]. This process is called chorion hardening and results from the structural modifications of chorion proteins, leading to their insolubilization. Li et al. [Citation111,112] have shown that both chorion peroxidase and phenoloxidase-catalyzed reactions are critical for the formation of an insoluble, desiccation-resistant chorion in A. aegypti. Recently, the A. aegypti chorion peroxidase was investigated as potential target by Alcantara [Citation113] from homology modelling. The amino acid sequence of A. aegypti chorion peroxidase was downloaded from the NCBI website and uploaded to four homology modelling servers. Each of them automatically assigned a protein template for the uploaded AA sequence. The percentages of identity ranged from 30% to 33%. The highest percentage of identity was obtained with bovine lactoperoxidase (PDB ID 3q9k) as template. The protein model consisted of 36.1% α-helices and 1% β-strand. A putative ligand-binding site corresponding to the heme binding site in 3q9k was identified. It included 26 AA residues. Two decoy sites with smaller volume were also identified. The heme bound to bovine lactoperoxidase (PDB ID 3q9k) was uploaded to the ZINCPharmer server [Citation114] to generate pharmacophoric features for screening the subset of natural products in the ZINC database [Citation115]. Three chemicals were predicted to have tight binding to A. aegypti chorion peroxidase [Citation113] (Figure ).
8. Challenges of A. aegypti genomics
The A. aegypti genome [Citation100] constitutes an invaluable resource facilitating the understanding of biological mechanisms at their basic molecular level and holds potential applications in the discovery of new targets to control the larvae and adults as well as new ways to prevent the diseases transmitted by this mosquito. However, genomic information would be of limited utility without the availability of databases and in silico tools to retrieve, clean, structure, compare, rank, cluster the data for specific applications [Citation116].
To date, significant advances have been made in the biology and physiology of the A. aegypti through the analysis of genomics information [Citation117–120]. It is also the case regarding the understanding of the mechanisms of resistance to insecticides. In insects, these mechanisms are complex including changes in the penetration, sequestration, target site recognition, and metabolism of the insecticides. Changes in behavioural traits leading to insecticide avoidance can also be observed [Citation121–123]. In mosquitoes, resistance is mainly associated with target site modification and degradation alteration [Citation124]. The former event involves mutations while the latter occurs through the increase of insecticide degradation by means of an overproduction of detoxification enzymes such as cytochrome P450 monooxygenases (P450s or CYPs), glutathione-S-transferase, and carboxy/choline esterases [Citation124,125]. P450s are widely associated with resistance to insecticides, especially the pyrethroids used as adulticide [Citation126–129]. However, it is noteworthy that industrial xenobiotics are also inducers of cytochrome P450s in A. aegypti [Citation130]. Nevertheless, as recently stressed by David et al. [Citation131], the understanding of which insecticides are metabolized by what cytochrome P450s would be of invaluable value in the design process of insecticides. Use of homology models and related modelling approaches play a key role in this process. They can also be used to better understand the mechanism action of cytochrome P450s. Thus, Chandor-Proust et al. [Citation132] tried to characterize A. aegypti CYP6Z8 substrate selectivity and its ability to metabolize insecticides and industrial xenobiotics. For this purpose, a yeast expression system allowing the co-expression of any mosquito microsomal P450 along with its associated cytochrome P450-reductase (CPR) was created. Using this system, a functional microsomal membrane complex of CYP6Z8 and A. aegypti CPR was created and used to study the metabolism of xenobiotics including insecticides. The authors showed that permethrin, deltamethrin, DTT, temephos, diflubenzuron, imidacloprid, and propoxur were not significantly metabolized by CYP6Z8 while it was not the case for diethylstilbestrol or resveratrol. Importantly, they showed that CYP6Z8 metabolized 3-phenoxybenzoic alcohol and 3-phenoxybenzaldehyde, two metabolites produced by the action of carboxylesterase on the parent pyrethroid molecule leading to 3-phenoxybenzoic acid, which was shown to be the major metabolite produced in vivo and excreted without modification. To better understand why pyrethroids and their metabolites were metabolized or not by the CYP6Zs, homology modelling was used. Models were derived for A. aegypti CYP6Z6, CYP6Z8_bora, CYP6Z8_liv, and CYP6Z9 and for Anopheles gambiae CYP6Z1, CYP6Z2, CYP6Z3, and CYP6Z4. For each model, the docking modes of 3-phenoxybenzoic alcohol, 3-phenoxybenzaldehyde, 3-phenoxybenzoic acid, and deltamethrin were predicted. Experimental findings on CYP6Z8 and CYP6Z2 were confirmed as well as the same role of CYP6Zs in other mosquitoes and the production of similar metabolites. The inability of CYP6Z8 and CYP6Z2 to metabolize deltamethrin was also supported by homology modelling showing good binding scores but high clashes between active-site residues and substrate or within substrate itself [Citation132]. It is noteworthy that homology modelling was also used by Lertkiatmongkol et al. [Citation133] to study the role of P450s in the metabolism of pyrethroids in Anopheles minumus and by Chiu et al. [Citation134] to study the metabolism of DDT by Anopheles gambiae CYP6Z1.
9. Concluding remarks
The geographical distribution of A. aegypti is expanding worldwide, leading to the emergence and re-emergence of the infectious diseases transmitted by this mosquito. This worrying situation is associated with a number of factors that include insecticide resistance, climate changes, societal behaviours, genetic modifications in pathogens, and changes in public health policy [Citation97]. The spread of insecticide resistance poses a critical threat to the sustainability of the efforts made in vector control. As a result, there is a need to find molecules with new mechanisms of actions and, if possible, with a large spectrum of activity and a low toxicity against non-target organisms.
In silico approaches play a key role in this search strategy. From de novo design applied to existing structures or from natural substances extracted from plants or animals (directly or after chemical optimization), or from drug and agrochemical repurposing, various modelling strategies have been applied to find new larvicides and adulticides targeting mosquitoes. Indeed, while the present review focuses on A. aegypti, similar works, also interesting to consider, have been made on other mosquito species [Citation135–Citation140]. The goal of our review was more to account for the diversity of the strategies used to find new chemicals active on A. aegypti rather than to catalogue all the studies in this domain. This diversity encompasses the chemical structures investigated, their true or supposed mechanism of action, the molecular descriptors, the used statistical method, etc.
For facilitating the analysis of the different published studies and avoiding some repetitions, the review was not split into larvicides versus adulticides while it would had been logical to do so. Most of the mosquito species spend a significant time in larval stage in aquatic media where they are rather accessible by the larvicides. Adult mosquitoes, in contrast, fly in search of mates, blood meals, or water for egg laying. They are widely distributed and not easily accessible by the adulticides. The treatment of larvae and/or pupae is at the basis of preventive vector control, while the treatment of adults corresponds with epidemic situations. It is obvious that there is a strong difference between both categories of chemicals. The targets are very often different, leading to different chemical structures. The environmental constraints are also different. Adulticides are directly in contact with human populations, while this is not the case with larvicides. Potential toxicological effects are much more important to consider for the former than the latter type of substance. Interestingly, it is worthy to note that environmental constraints can change over the time. Thus, for example, the JH mimics were considered as belonging to green chemistry; however, recently, their potential adverse effects on the development of non-target invertebrates has led to them being considered as endocrine disruptors [Citation33,141,142].
While various in silico approaches have shown their potential for estimating the activities and properties of nanomaterials [Citation143–149], and in the meantime nanomaterials are increasingly used in vector control [Citation150–153], to our knowledge, no modelling methods have yet been used to optimize the design of nanostructures active on A. aegypti or other mosquito species.
Alternatives to insecticide-based vector control are the subject of intensive research worldwide [Citation154–157]. Nevertheless, the rational use of chemical substances remains the most operational strategy for controlling mosquito populations. Because the market for such substances is limited, it is obvious that in silico tools allow us to save time and money in the design of new insecticides.
Acknowledgements
This paper was presented at the 7th CMTPI conference in Seoul, 8–12 October 2013. The financial support from the French Agency for Food, Environmental and Occupational Health & Safety (Anses) is gratefully acknowledged (contract QSAR-LAV: #EST-2012/2/64).
References
- WHO, Dengue and severe dengue, Fact sheet n°117, World Health Organisation, 2013. Available at http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed 2 December 2013).
- D.J. Gubler, Epidemic dengue/dengue hemorrhagic fever as a public health, social and economical problem in the 21st century, Trends Microbiol. 10 (2002), pp. 100–103.10.1016/S0966-842X(01)02288-0
- N.E.A. Murray, M.B. Quam, and A. Wilder-Smith, Epidemiology of dengue: Past, present and future prospects, Clin. Epidemiol. 5 (2013), pp. 299–309.
- L. Su, L. Fu, J. He, W. Qin, L. Sheng, M.H. Abraham, and Y.H. Zhao, Comparison of Tetrahymena pyriformis toxicity based on hydrophobicity, polarity, ionization and reactivity of class-based compounds, SAR QSAR Environ. Res. 23 (2012), pp. 537–552.10.1080/1062936X.2012.666567
- Y. Sakuratani, H.Q. Zhang, S. Nishikawa, K. Yamazaki, T. Yamada, J. Yamada, and M. Hayashi, Categorization of nitrobenzenes for repeated dose toxicity based on adverse outcome pathways, SAR QSAR Environ. Res. 24 (2013), pp. 35–46.10.1080/1062936X.2012.728995
- A. Yan, H. Liang, Y. Chong, X. Nie, and C. Yu, In-silico prediction of blood–brain barrier permeability, SAR QSAR Environ. Res. 24 (2013), pp. 61–74.10.1080/1062936X.2012.729224
- Y. Brito-Sánchez, J.A. Castillo-Garit, H. Le-Thi-Thu, Y. González-Madariaga, F. Torrens, Y. Marrero-Ponce, and J.E. Rodríguez-Borges, Comparative study to predict toxic modes of action of phenols from molecular structures, SAR QSAR Environ. Res. 24 (2013), pp. 235–251.10.1080/1062936X.2013.766260
- E. Papa, S. Kovarich, and P. Gramatica, QSAR prediction of the competitive interaction of emerging halogenated pollutants with human transthyretin, SAR QSAR Environ. Res. 24 (2013), pp. 333–349.10.1080/1062936X.2013.773374
- N. Kireeva, S.L. Kuznetsov, A.A. Bykov, and A. Yu Tsivadze, Towards in silico identification of the human ether-a-go-go-related gene channel blockers: Discriminative vs. generative classification models, SAR QSAR Environ. Res. 24 (2013), pp. 103–117.10.1080/1062936X.2012.742135
- S. Gharaghani, T. Khayamian, and M. Ebrahimi, Molecular dynamics simulation study and molecular docking descriptors in structure-based QSAR on acetylcholinesterase (AChE) inhibitors, SAR QSAR Environ. Res. 24 (2013), pp. 773–794.10.1080/1062936X.2013.792877
- N. Marchand-Geneste and J. Devillers, Comparative modeling review of nuclear receptor superfamily, in Endocrine Disruption Modeling, J. Devillers, ed., CRC Press, Boca Raton, FL, pp. 97–142.
- M. Saxena, S.S. Bhunia, and A.K. Saxena, Docking studies of novel pyrazinopyridoindoles class of antihistamines with the homology modelled H1-receptor, SAR QSAR Environ. Res. 23 (2012), pp. 311–325.10.1080/1062936X.2012.664561
- A.K. Saxena and K.K. Roy, Hierarchical virtual screening: Identification of potential high-affinity and selective β3-adrenergic receptor agonists, SAR QSAR Environ. Res. 23 (2012), pp. 389–407.10.1080/1062936X.2012.664824
- B. Vyas, O. Silakari, M. Singh Bahia, and B. Singh, Glutamine: Fructose-6-phosphate amidotransferase (GFAT): Homology modelling and designing of new inhibitors using pharmacophore and docking based hierarchical virtual screening protocol, SAR QSAR Environ. Res. 24 (2013), pp. 733–752.10.1080/1062936X.2013.797493
- D.G. Heckel, L.J. Gahan, J.C. Daly, and S. Trowell, A genomic approach to understanding Heliothis and Helicoverpa resistance to chemical and biological insecticides, Phil. Trans. R. Soc. Lond. B 353 (1998), pp. 1713–1722.10.1098/rstb.1998.0323
- W. Tan, X. Wang, P. Cheng, L. Liu, H. Wang, M. Gong, X. Quan, H. Gao, and C. Zhu, Cloning and overexpression of transferrin gene from cypermethrin-resistant Culex pipiens pallens, Parasitol. Res. 110 (2012), pp. 939–959.10.1007/s00436-011-2580-4
- T. Yang and N. Liu, Genome analysis of cytochrome P450s and their expression profiles in insecticide resistant mosquitoes, Culex quinquefasciatus, PLoS One 6 (2011),12,e29418. doi: 10.1371/journal.pone.0029418.
- Y. Gong, T. Li, L. Zhang, X. Gao, and N. Liu, Permethrin induction of multiple cytochrome P450 genes in insecticide resistant mosquitoes, Culex quinquefasciatus, Int. J. Biol. Sci. 9 (2013), pp. 863–871.10.7150/ijbs.6744
- M. Paris and L. Despres, Identifying insecticide resistance genes in mosquito by combining AFLP genome scans and 454 pyrosequencing, Mol. Ecol. 21 (2012), pp. 1672–1686.10.1111/mec.2012.21.issue-7
- A. Bonin, M. Paris, G. Tetreau, J.P. David, and L. Després, Candidate genes revealed by a genome scan for mosquito resistance to a bacterial insecticide: Sequence and gene expression variations, BMC Genomics 21, 10 (2009) 551. doi: 10.1186/1471-2164-10-551.10.1186/1471-2164-10-551
- A.R. Katritzky, D.A. Dobchev, I. Tulp, M. Karelson, and D.A. Carlson, QSAR study of mosquito repellents using Codessa Pro, Bioorg. Med. Chem. Lett. 16 (2006), pp. 2306–2311.10.1016/j.bmcl.2005.11.113
- J.B. Bhonsle, A.K. Bhattacharjee, and R.K. Gupta, Novel semi-automated methodology for developing highly predictive QSAR models: Application for development of QSAR models for insect repellent amides, J. Mol. Model. 13 (2007), pp. 179–208.
- R. Natarajan, S.C. Basak, D. Mills, J.J. Kraker, and D.M. Hawkins, Quantitative structure-activity relationship modeling of mosquito repellents using calculated descriptors, Croat. Chem. Acta 81 (2008), pp. 333–340.
- U.R. Bernier and M. Tsikolia, Development of novel repellents using structure–activity modeling of compounds in the USDA Archival Database, in Recent Developments in Invertebrate Repellents, G.E. Paluch and J.R. Coats, eds., American Chemical Society, ACS Symposium Series, volume 1090, 2011, pp. 21–46.
- P.V. Oliferenko, A.A. Oliferenko, G.I. Poda, D.I. Osolodkin, G.G. Pillai, U.R. Bernier, M. Tsikolia, N.M. Agramonte, G.G. Clark, K.J. Linthicum, and A.R. Katritzky, Promising Aedes aegypti repellent chemotypes identified through integrated QSAR, virtual screening, synthesis, and bioassay, PLoS ONE 8 (2013) 9, e64547. doi: 10.1371/journal.pone.0064547.10.1371/journal.pone.0064547
- K. Hartfelder, Insect juvenile hormone: From “status quo” to high society, Braz. J. Med. Biol. Res. 33 (2000), pp. 157–177.10.1590/S0100-879X2000000200003
- L.I. Gilbert, N.A. Granger, and R.M. Roe, The juvenile hormones: Historical facts and speculations on future research directions, Insect Biochem. Mol. Biol. 30 (2000), pp. 617–644.10.1016/S0965-1748(00)00034-5
- S.R. Palli, Recent advances in the mode of action of juvenile hormones and their analogs, in Biorational Control of Arthropod Pests, I. Ishaaya and A.R. Horowitz, eds., Springer, New York, 2009, pp. 111–129.10.1007/978-90-481-2316-2
- S.G. Kamita and B.D. Hammock, Juvenile hormone esterase: Biochemistry and structure, J. Pestic. Sci. 35 (2010), pp. 265–274.10.1584/jpestics.R10-09
- S.G. Kamita, A.C. Hinton, C.E. Wheelock, M.D. Wogulis, D.K. Wilson, N.M. Wolf, J.E. Stok, B. Hock, and B.D. Hammock, Juvenile hormone (JH) esterase: Why are you so JH specific?, Insect Biochem. Mol. Biol. 33 (2003), pp. 1261–1273.10.1016/j.ibmb.2003.08.004
- A.B. Shapiro, G.D. Wheelock, H.H. Hagedorn, F.C. Baker, L.W. Tsai, and D.A. Schooley, Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti, J. Insect Physiol. 32 (1986), pp. 867–877.10.1016/0022-1910(86)90102-2
- Y. Li, S. Hernandez-Martinez, G.C. Unnithan, R. Feyereisen, and F.G. Noriega, Activity of the corpora allata of adult female Aedes aegypti: Effects of mating and feeding, Insect Biochem. Molec. Biol. 33 (2003), pp. 1307–1315.10.1016/j.ibmb.2003.07.003
- J. Devillers, Juvenile hormones and juvenoids: A historical survey, in Juvenile Hormones and Juvenoids. Modeling Biological Effects and Environmental Fate, J. Devillers, ed., CRC Press, Boca Raton, 2013, pp. 1–14.10.1201/CRCQSARENVHEA
- C.A. Henrick, W.E. Willy, and G.B. Staal, Insect juvenile hormone activity of alkyl (2E,4E)-3,7,11-trimethyl-2,4-dodecadienoates. Variations in the ester function and in the carbon chain, J. Agric. Food Chem. 24 (1976), pp. 207–218.10.1021/jf60204a018
- A. Nakayama, H. Iwamura, and T. Fujita, Quantitative structure-activity relationship of insect juvenile hormone mimetic compounds, J. Med. Chem. 27 (1984), pp. 1493–1502.10.1021/jm00377a019
- H. Iwamura, K. Nishimura, and T. Fujita, Quantitative structure-activity relationships of insecticides and plant growth regulators: Comparative studies toward understanding the molecular mechanism of action, Environ. Health Perspect. 61 (1985), pp. 307–320.10.1289/ehp.8561307
- C. Hansch and A. Leo, Exploring QSAR. Fundamentals and Applications in Chemistry and Biology, ACS Professional Reference Book Series, Washington, DC, 1995.
- S. Bintein, J. Devillers, and W. Karcher, Nonlinear dependence of fish bioconcentration on n-octanol/water partition coefficient, SAR QSAR Environ. Res. 1 (1993), pp. 29–39.10.1080/10629369308028814
- J. Devillers, SAR and QSAR modeling of juvenile hormone mimics, in Juvenile Hormones and Juvenoids. Modeling Biological Effects and Environmental Fate, J. Devillers, ed., CRC Press, Boca Raton, 2013, pp. 145–174.10.1201/CRCQSARENVHEA
- J. Devillers, Neural Networks in QSAR and Drug Design, Academic Press, London, U.K., 1996.
- S.C. Basak, R. Natarajan, D. Mills, D.M. Hawkins, and J.J. Kraker, Quantitative structure-activity relationship modeling of insect juvenile hormone activity of 2,4-dienoates using computed molecular descriptors, SAR QSAR Environ. Res. 16 (2005), pp. 581–606.10.1080/10659360500468526
- J. Devillers and A.T. Balaban, Topological Indices and Related Descriptors in QSAR and QSPR, Gordon and Breach Science Publishers, Amsterdam, the Netherlands, 1999.
- A.E. Hoerl and R.W. Kennard, Ridge regression: Applications to nonorthogonal problems, Technometrics 12 (1970), pp. 69–82.10.1080/00401706.1970.10488635
- J. Devillers, D. Zakarya, M. Chastrette, and J.C. Doré, The stochastic regression analysis as a tool in ecotoxicological QSAR studies, Biomed. Environ. Sci. 2 (1989), pp. 385–393.
- H. Abdi, Partial least squares regression and projection on latent structure regression (PLS Regression), WIREs Comput. Stat. 2010, doi: 10.1002/wics.051.
- S.S. Young and D.M. Hawkins, Using recursive partitioning to analyze a large SAR data set, SAR QSAR Environ. Res. 8 (1998), pp. 183–193.10.1080/10629369808039140
- A. Székács, B. Bordás, and B.D. Hammock, Transition state analog enzyme inhibitors: Structure–activity relationships of trifluoromethyl ketones, in Rational Approaches to Structure, Activity, and Ecotoxicology of Agrochemicals, W. Draber and T. Fujita, eds., ACS Symp. Ser, American Chemical Society, Washington, DC, 1992, pp. 219–249.
- C.E. Wheelock, Y. Nakagawa, M. Akamatsu, and B.D. Hammock, Use of classical and 3-D QSAR to examine the hydration state of juvenile hormone esterase inhibitors, Bioorg. Med. Chem. 11 (2003), pp. 5101–5116.10.1016/j.bmc.2003.08.023
- J. Devillers, J.P. Doucet, A. Doucet-Panaye, A. Decourtye, and P. Aupinel, Linear and non-linear QSAR modelling of juvenile hormone esterase inhibitors, SAR QSAR Environ. Res. 23 (2012), pp. 357–369.10.1080/1062936X.2012.664562
- J. Devillers, A. Doucet-Panaye, and J.P. Doucet, Predicting highly potent juvenile hormone esterase inhibitors from 2D QSAR modeling, in Juvenile Hormones and Juvenoids. Modeling Biological Effects and Environmental Fate, J. Devillers, ed., CRC Press, Boca Raton, 2013, pp. 241–266.10.1201/CRCQSARENVHEA
- J. Caballero, Receptor-guided structure–activity modeling of inhibitors of juvenile hormone epoxide hydrolases, in Juvenile Hormones and Juvenoids. Modeling Biological Effects and Environmental Fate, J. Devillers, ed., CRC Press, Boca Raton, 2013, pp. 267–289.
- J.P. Doucet, A. Doucet-Panaye, and J. Devillers, Structure–activity relationship study of trifluoromethylketones: Inhibitors of insect juvenile hormone esterase, SAR QSAR Environ. Res. 24 (2013), pp. 481–499.10.1080/1062936X.2013.792499
- J. Zhu, K. Miura, L. Chen, and A.S. Raikhel, AHR38, a homolog of NGFI-B, inhibits formation of the functional ecdysteroid receptor in the mosquito Aedes aegypti, EMBO J. 19 (2000), pp. 253–262.10.1093/emboj/19.2.253
- L. Swevers and K. Iatrou, Ecdysteroids and ecdysteroid signaling pathways during insect oogenesis, in Ecdysone: Structures and Functions, G. Smagghe, ed., Springer, New York, 2009, pp. 127–164.
- K.D. Spindler, C. Hönl, C. Tremmel, S. Braun, H. Ruff, and M. Spindler-Barth, Ecdysteroid hormone action, Cell. Mol. Life Sci. 66 (2009), pp. 3837–3850.10.1007/s00018-009-0112-5
- M.Y. Song, J.D. Stark, and J.J. Brown, Comparative toxicity of four insecticides, including imidacloprid and tebufenozide, to four aquatic arthropods, Environ. Toxicol. Chem. 16 (1997), pp. 2494–2500.10.1002/etc.v16:12
- J. Cruz, D.H. Sieglaff, P. Arensburger, P.W. Atkinson, and A.S. Raikhel, Nuclear receptors in the mosquito Aedes aegypti, Annotation, hormonal regulation and expression profiling, FEBS J. 276 (2009), pp. 1233–1254.
- H.C. Smith, C.K. Cavanaugh, J.L. Friz, C.S. Thompson, J.A. Saggers, E.L. Michelotti, J. Garcia, and C.M. Tice, Synthesis and SAR of cis-1-benzoyl-1,2,3,4-tetrahydroquinoline ligands for control of gene expression in ecdysone responsive systems, Bioorg. Med. Chem. Lett. 13 (2003), pp. 1943–1946.10.1016/S0960-894X(03)00317-2
- S. Lapenna, J. Friz, A. Barlow, S.R. Palli, L. Dinan, and R.E. Hormann, Ecdysteroid ligand-receptor selectivity-exploring trends to design orthogonal gene switches, FEBS J. 275 (2008), pp. 5785–5809.10.1111/j.1742-4658.2008.06687.x
- N. Awang, N.A. Kosnon, and H. Othman, The effectiveness of organotin (IV) benzylisopropyldithiocarbamate compounds as insecticide against Aedes aegypti Linn. (Diptera: Culicidae) in laboratory, Middle-East, J. Sci. Res. 13 (2013), pp. 907–912.
- X. Song, A. Zapata, J. Hoerner, A.C. de Dios, L. Casabianca, and G. Eng, Synthesis, larvicidal, QSAR and structural studies of some triorganotin 2,2,3,3-tetramethylcyclopropanecarboxylates, Appl. Organomet. Chem. 21 (2007), pp. 545–550.10.1002/(ISSN)1099-0739
- C. Hansch and R.P. Verma, Larvicidal activities of some organotin compounds on mosquito larvae: A QSAR study, Eur. J. Med. Chem. 44 (2009), pp. 260–273.10.1016/j.ejmech.2008.02.040
- Q. Duong, X. Song, E. Mitrojorgji, S. Gordon, and G. Eng, Larvicidal and structural studies of some triphenyl- and tricyclohexyltin para-substituted benzoates, J. Organomet. Chem. 691 (2006), pp. 1775–1779.10.1016/j.jorganchem.2005.12.005
- X. Song, Q. Duong, E. Mitrojorgji, A. Zapata, N. Nguyen, D. Strickman, J. Glass, and G. Eng, Synthesis, structure characterization and larvicidal activity of some tris-(para-substitutedphenyl)tins, Appl. Organomet. Chem. 18 (2004), pp. 363–368.10.1002/(ISSN)1099-0739
- M.P. Freitas, T.C. Ramalho, E.F.F. da Cunha, and R.A.Cormanich, Multivariate image analysis applied to QSAR as a tool for mosquitoes control: Dengue and yellow Fever, in Chemoinformatics: Directions Toward Combating Neglected Diseases, T.C. Ramalho, M.P. Freitas, and E.F.F. da Cunha, eds., Bentham Science Publishers Ltd. 2010, pp. 172–182.
- A. Aronson, Sporulation and δ-endotoxin synthesis by Bacillus thuringiensis, Cell. Mol. Life Sci. 59 (2002), pp. 417–425.10.1007/s00018-002-8434-6
- N. Crickmore, D.R. Zeigler, J. Feitelson, E. Schnepf, J. van Rie, D. Lereclus, J. Baum, and D.H. Dean, Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins, Microbiol. Mol. Biol. Rev. 62 (1998), pp. 807–813.
- C. Berry, S. O’Neil, E. Ben-Dov, A.F. Jones, L. Murphy, M.A. Quail, M.T.G. Holden, D. Harris, A. Zaritsky, and J. Parkhill, Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis, Appl. Environ. Microbiol. 68 (2002), pp. 5082–5095.10.1128/AEM.68.10.5082-5095.2002
- C. Stein, G.W. Jones, T. Chalmers, and C. Berry, Transcriptional analysis of the toxin-coding plasmid pBtoxis from Bacillus thuringiensis subsp. israelensis, Appl. Environ. Microbiol. 72 (2006), pp. 1771–1776.10.1128/AEM.72.3.1771-1776.2006
- V. Guidi, N. Patocchi, P. Lüthy, and M. Tonolla, Distribution of Bacillus thuringiensis subsp. israelensis in soil of a Swiss wetland reserve after 22 years of mosquito control, Appl. Environ. Microbiol. 77 (2011), pp. 3663–3668.10.1128/AEM.00132-11
- L. Després, C. Lagneau, and R. Frutos, Using the bio-insecticide Bacillus thuringiensis israelensis in mosquito control, in Pesticides in the Modern World – Pests Control and Pesticides Exposure and Toxicity Assessment, M. Stoytcheva, ed., InTech, Rijeka, Croatia 2011, pp. 93–126.
- J. Chen, K.G. Aimanova, L.E. Fernandez, A. Bravo, M. Soberon, and S.S. Gill, Aedes aegypti cadherin serves as a putative receptor of the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis, Biochem. J. 424 (2009), pp. 191–200.10.1042/BJ20090730
- V. Vachon, R. Laprade, and J.L. Schwartz, Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: A critical review, J. Invert. Pathol. 111 (2012), pp. 1–12.10.1016/j.jip.2012.05.001
- V.N. Jisha, R.B. Smitha, and S. Benjamin, An overview of the crystal toxins from Bacillus thuringiensis, Adv. Microbiol. 3 (2013), pp. 462–472.10.4236/aim.2013.35062
- L. Pardo-López, C. Muñoz-Garay, H. Porta, C. Rodriguez-Almazán, M. Soberón, and A. Bravo, Strategies to improve the insecticidal activity of Cry toxins from Bacillus thuringiensis, Peptides 30 (2009), pp. 589–595.10.1016/j.peptides.2008.07.027
- P. Boonserm, M. Mo, C. Angsuthanasombat, and J. Lescar, Structure of the functional form of the mosquito larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.8-angstom resolution, J. Bacteriol. 188 (2006), pp. 3391–3401.10.1128/JB.188.9.3391-3401.2006
- P. Boonserm, P. Davis, D.J. Ellar, and J. Li, Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications, J. Mol. Biol. 348 (2005), pp. 363–382.10.1016/j.jmb.2005.02.013
- R.A. de Maagd, A. Bravo, and N. Crickmore, How Bacillus thuringiensis has evolved specific toxins to colonize the insect world, Trends Genet. 17 (2001), pp. 193–199.10.1016/S0168-9525(01)02237-5
- C.R. Pigott, M.S. King, and D.J. Ellar, Investigating the properties of Bacillus thuringiensis Cry proteins with novel loop replacements created using combinatorial molecular biology, Appl. Environ. Microbiol. 74 (2008), pp. 3497–3511.10.1128/AEM.02844-07
- S. Likitvivatanavong, J. Chen, A.M. Evans, A. Bravo, M. Soberon, and S.S. Gill, Multiple receptors as targets of Cry toxins in mosquitoes, J Agric Food Chem. 59 (2011), pp. 2829–2838.10.1021/jf1036189
- L.E. Fernández, C. Pérez, L. Segovia, M.H. Rodriguez, S.S. Gill, A. Bravo, and M. Soberón, Cry11Aa toxin from Bacillus thuringiensis binds its receptor in Aedes aegypti mosquito larvae through loop α-8 of domain II, FEBS Letters 579 (2005), pp. 3508–3514.10.1016/j.febslet.2005.05.032
- M.A.F. Abdullah and D.H. Dean, Enhancement of Cry19Aa mosquitocidal activity against Aedes aegypti by mutations in the putative loop regions of domain II, Appl. Environ. Microbiol. 70 (2004), pp. 3769–3771.10.1128/AEM.70.6.3769-3771.2004
- M.L. Rosso and A. Delécluse, Contribution of the 65-kilodalton protein encoded by the cloned gene cry19A to the mosquitocidal activity of Bacillus thuringiensis subsp. jegathesan, Appl. Environ. Microbiol. 63 (1997), pp. 4449–4455.
- C.N. Dias and D.F.C. Moraes, Essential oils and their compounds as Aedes aegypti L. (Diptera: Cilicidae) larvicides: Review, Parasitol. Res. 113 (2014), pp. 565–592.10.1007/s00436-013-3687-6
- N. Kishore, B.B. Mishra, V.K. Tiwari, and V. Tripathi, A review on natural products with mosquitosidal potentials, in Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry, V.K. Tiwari and B.B. Mishra, eds., Research Signpost, Kerala, India, 2011, pp. 335–365.
- N. Kishore, B.B. Mishra, V.K. Tiwari, V. Tripathi, and N. Lall, Natural products as leads to potential mosquitocides, Phytochem. Rev. (2013), pp. 1–41.
- C.L. Cantrell, J.W. Pridgeon, F.R. Fronczek, and J. Becnel, Structure-activity relationship studies on derivatives of Eudesmanolides from Inula helenium as toxicants against Aedes aegypti larvae and adults, Chem. Biodiv. 7 (2010), pp. 1681–1697.10.1002/cbdv.201000031
- C.P. Kiprono, J.O. Midiwo, P.K. Kipkemboi, and S. Ladogana, Larvicidal benzoquinone from Embelia schimperi, Bull. Chem. Soc. Ethiop. 18 (2004), pp. 45–49.
- J.O. Midiwo, Y. Ghebremeskel, and R.W. Mwangi, Insect antifeedant, growth-inhibiting and larvicidal compounds from Rapanea melanophloes (Myrsinaceae), Insect Sci. Appl. 16 (1995), pp. 163–166.
- D.P. de Sousa, Y.W. Viera, M.P. Uliana, M.A. Melo, T.J. Brocksom, and S.C.H. Cavalcanti, Larvicidal activity of para-benzoquinone, Parasitol. Res. 107 (2010), pp. 741–745.10.1007/s00436-010-1942-7
- S.R.L. Santos, M.A. Melo, A.V. Cardoso, R.L.C. Santos, D.P. de Sousa, and S.C.H. Cavalcanti, Structure-activity relationships of larvicidal monoterpenes and derivatives against Aedes aegypti Linn, Chemosphere 84 (2011), pp. 150–153.10.1016/j.chemosphere.2011.02.018
- A. Lucia, E. Zerba, and H. Masuh, Knockdown and larvicidal activity of six monoterpenes against Aedes aegypti (Diptera: Culicidae) and their structure-activity relationships, Parasitol. Res. 112 (2013), pp. 4267–4272.10.1007/s00436-013-3618-6
- B. Khanikor, P. Parida, R.N.S. Yadav, and D. Bora, Comparative mode of action of some terpene compounds against octopamine receptor and acetyl cholinesterase of mosquito and human system by the help of homology modeling and docking studies, J. Appl. Pharm. Sci. 3 (2013), pp. 6–12.
- L. Scotti, M.T. Scotti, V.B. Silva, S.R.L. Santos, S.C.H. Cavalcanti, and F.J.B. Mendonça Jr, Chemometric studies on potential larvicidal compounds against Aedes aegypti, Med. Chem. 10 (2014), pp. 201–210.10.2174/15734064113099990005
- A.C.S. Pinto, K.L. Nogueira, F.C.M. Chaves, L.V. Souza da Silva, W.P. Tadei, and A.M. Pohlit, Adulticidal activity of dillapiol and semi-synthetic derivatives of dillapiol against Aedes aegypti (L.) (Culicidae), J. Mosqu. Res. 2 (2012), pp. 1–7.
- J.W. Pridgeon, K.M. Meepagala, J.J. Becnel, G.G. Clark, R.M. Pereira, and K.J. Linthicum, Structure-activity relationships of 33 piperidines as toxicants against female adults of Aedes aegypti (Diptera: Culicidae), J. Med. Entomol. 44 (2007), pp. 263–269.10.1603/0022-2585(2007)44[263:SROPAT]2.0.CO;2
- J. Devillers, L. Lagadic, O. Yamada, F. Darriet, R. Delorme, X. Deparis, J.P. Jaeg, C. Lagneau, B. Lapied, F. Quiniou, and A. Yébakima, Use of multicriteria analysis for selecting candidate insecticides for vector control, in, Juvenile Hormones and Juvenoids. Modeling Biological Effects and Environmental Fate, J. Devillers, ed., CRC Press, Boca Raton, 2013, pp. 341–381.
- M. Vaillant, J.M. Jouany, and J. Devillers, A multicriteria estimation of the environmental risk of chemicals with the SIRIS method, Toxicol. Model. 1 (1995), pp. 57–72.
- D. Filmore, It’s a GPCR world, Mod. Drug Discov. 7 (2004), pp. 24–28.
- V. Nene, J.R. Wortman, D. Lawson, B. Haas, C. Kodira, Z.J. Tu, B. Loftus, Z. Xi, K. Megy, M. Grabherr, Q. Ren, E.M. Zdobnov, N.F. Lobo, K.S. Campbell, S.E. Brown, M.F. Bonaldo, J. Zhu, S.P. Sinkins, D.G. Hogenkamp, P. Amedeo, P. Arensburger, P.W. Atkinson, S. Bidwell, J. Biedler, E. Birney, R.V. Bruggner, J. Costas, M.R. Coy, J. Crabtree, M. Crawford, B. Debruyn, D. Decaprio, K. Eiglmeier, E. Eisenstadt, H. El-Dorry, W.M. Gelbart, S.L. Gomes, M. Hammond, L.I. Hannick, J.R. Hogan, M.H. Holmes, D. Jaffe, J.S. Johnston, R.C. Kennedy, H. Koo, S. Kravitz, E.V. Kriventseva, D. Kulp, K. Labutti, E. Lee, S. Li, D.D. Lovin, C. Mao, E. Mauceli, C.F. Menck, J.R. Miller, P. Montgomery, A. Mori, A.L. Nascimento, H.F. Naveira, C. Nusbaum, S. O’leary, J. Orvis, M. Pertea, H. Quesneville, K.R. Reidenbach, Y.H. Rogers, C.W. Roth, J.R. Schneider, M. Schatz, M. Shumway, M. Stanke, E.O. Stinson, J.M. Tubio, J.P. Vanzee, S. Verjovski-Almeida, D. Werner, O. White, S. Wyder, Q. Zeng, Q. Zhao, Y. Zhao, C.A. Hill, A.S. Raikhel, M.B. Soares, D.L. Knudson, N.H. Lee, J. Galagan, S.L. Salzberg, I.T. Paulsen, G. Dimopoulos, F.H. Collins, B. Birren, C.M. Fraser-Liggett, and D.W. Severson, Genome sequence of Aedes aegypti, a major arbovirus vector, Science 316 (2007), pp. 1718–1723.10.1126/science.1138878
- C.A. Hill, A.N. Fox, R.J. Pitts, L.B. Kent, P.L. Tan, M.A. Chrystal, A. Cravchik, F.H. Collins, H.M. Robertson, and L.J. Zwiebel, G protein-coupled receptors in Anopheles gambiae, Science 298 (2002), pp. 176–178.10.1126/science.1076196
- J.P. Andersen, A. Schwartz, J.B. Gramsbergen, and V. Loeschcke, Dopamine levels in the mosquito Aedes aegypti during adult development, following blood feeding and in response to heat stress, J. Insect Physiol. 52 (2006), pp. 1163–1170.10.1016/j.jinsphys.2006.08.004
- J.M. Meyer, K.F.K. Ejendal, L.V. Avramova, E.E. Garland-Kuntz, G.I. Giraldo-Calderón, T.F. Brust, V.J. Watts, and C.A. Hill, A ‘‘genome-to-lead’’ approach for insecticide discovery: Pharmacological characterization and screening of Aedes aegypti D1-like dopamine receptors, PLoS Negl. Trop. Dis. 6 (2012), p. e1478.10.1371/journal.pntd.0001478
- C.A. Hill, J.M. Meyer, K.F.K. Ejendal, D.F. Echeverry, E.G. Lang, L.V. Avramova, J.M. Conley, and V.J. Watts, Re-invigorating the insecticide discovery pipeline for vector control: GPCRs as targets for the identification of next gen insecticides, Pest. Biochem. Physiol. 106 (2013), pp. 141–146.10.1016/j.pestbp.2013.02.008
- H.W. Tedford, B.A. Steinbaugh, L. Bao, B.D. Tait, A. Tempczyk-Russell, W. Smith, G.L. Benzon, C.A. Finkenbinder, and R.M. Kennedy, In silico screening for compounds that match the pharmacophore of omega-hexatoxin-Hv1a leads to discovery and optimization of a novel class of insecticides, Pest. Biochem. Physiol. 106 (2013), pp. 124–140.10.1016/j.pestbp.2013.01.009
- D. Borovsky, Biosynthesis and control of mosquito gut proteases, Life 55 (2003), pp. 435–441.
- J. Isoe, J. Collins, H. Badgandi, W.A. Day, and R.L. Miesfeld, Defects in coatomer protein I (COPI) transport cause blood feeding-induced mortality in yellow fever mosquitoes, Proc. Natl. Acad. Sci. USA 108 (2011), pp. E211–217.10.1073/pnas.1102637108
- J.B. Sáenz, W.J. Sun, J.W. Chang, J. Li, B. Bursulaya, N.S. Gray, and D.B. Haslam, Golgicide A reveals essential roles for GBF1 in Golgi assembly and function, Nat. Chem. Biol. 5 (2009), pp. 157–165.10.1038/nchembio.144
- D.J. Mack, J. Isoe, R.L. Miesfeld, and J.T. Njardarson, Distinct biological effects of golgicide A derivatives on larval and adult mosquitoes, Bioorg. Med. Chem. Lett. 22 (2012), pp. 5177–5181.10.1016/j.bmcl.2012.06.076
- J.S. Li and J. Li, Major chorion proteins and their crosslinking during chorion hardening in Aedes aegypti mosquitoes, Insect Biochem. Mol. Biol. 36 (2006), pp. 954–964.10.1016/j.ibmb.2006.09.006
- J. Li, B.A. Hodgeman, and B.M. Christensen, Involvement of peroxidase in chorion hardening in Aedes aegypti, Insect Biochem. Mol. Biol. 26 (1996), pp. 309–317.10.1016/0965-1748(95)00099-2
- J.S. Li, S.R. Kim, and J. Li, Molecular characterization of a novel peroxidase involved in Aedes aegypti chorion protein crosslinking, Insect Biochem. Mol. Biol. 34 (2004), pp. 1195–1203.10.1016/j.ibmb.2004.08.001
- E.P. Alcantara, In silico identification of potential inhibitors of dengue mosquito, Aedes aegypti chorion peroxidase, Comput. Biol. Bioinform. 2 (2014), pp. 38–42.
- D.R. Koes and C.J. Camacho, ZINCPharmer: Pharmacophore search of the ZINC database, Nuc. Acids Res. 40 (2012), pp. W409–W414.10.1093/nar/gks378
- J.J. Irwin and B.K. Shoichet, ZINC – A free database of commercially available compounds for virtual screening, J. Chem. Inf. Model. 45 (2005), pp. 177–182.10.1021/ci049714+
- D.W. Severson and S.K. Behura, Mosquito genomics: Progress and challenges, Annu. Rev. Entomol. 57 (2012), pp. 143–166.10.1146/annurev-ento-120710-100651
- B. Bryant, C.D. Blair, K.E. Olson, and R.J. Clem, Annotation and expression profiling of apoptosis-related genes in the yellow fever mosquito, Aedes aegypti, Insect Biochem. Molec. Biol. 38 (2008), pp. 331–345.
- Z. Xi, J.L. Ramirez, and G. Dimopoulos, The Aedes aegypti toll pathway controls dengue virus infection, PLoS Pathog. 4 (2008), e1000098. doi: 10.1371/journal.ppat.1000098.10.1371/journal.ppat.1000098
- L.L. Drake, D.Y. Boudko, O. Marinotti, V.K. Carpenter, A.L. Dawe, and I.A. Hansen, The aquaporin gene family of the yellow fever mosquito, Aedes aegypti, PLoS One 5 (2010), p. e15578. doi: 10.1371/journal.pone.0015578.10.1371/journal.pone.0015578
- A.A. Ptitsyn, G. Reyes-Solis, K. Saavedra-Rodriguez, J. Betz, E.L. Suchman, J.O. Carlson, and W.C. Black, Rhythms and synchronization patterns in gene expression in the Aedes aegypti mosquito, BMC Genomics 12, 153 (2011), pp. 1–16.
- O.J.T. Briët and N. Chitnis, Effects of changing mosquito host searching behaviour on the cost effectiveness of a mass distribution of long-lasting, insecticidal nets: A modelling study, Malaria J. 12 (2013), pp. 1–11.
- C. Sokhna, M.O. Ndiath, and C. Rogier, The changes in mosquito vector behaviour and the emerging resistance to insecticides will challenge the decline of malaria, Clin. Microbiol. Infect. 19 (2013), pp. 902–907.10.1111/1469-0691.12314
- H. Pates and C. Curtis, Mosquito behavior and vector control, Annu. Rev. Entomol. 50 (2005), pp. 53–70.10.1146/annurev.ento.50.071803.130439
- T.E. Nkya, I. Akhouayri, W. Kisinza, and J.P. David, Impact of environment on mosquito response to pyrethroid insecticides: Facts, evidences and prospects, Insect Biochem. Molec. Biol. 43 (2013), pp. 407–416.10.1016/j.ibmb.2012.10.006
- J. Hemingway and H. Ranson, Insecticide resistance in insect vectors of human disease, Annu. Rev. Entomol. 45 (2000), pp. 371–391.10.1146/annurev.ento.45.1.371
- G. Bingham, C. Strode, and L. Tran, PT Khoa, and H.P. Jamet, Can piperonyl butoxide enhance the efficacy of pyrethroids against pyrethroid-resistant Aedes aegypti? Trop. Med. Int. Health 16 (2011), pp. 492–500.10.1111/tmi.2011.16.issue-4
- C. Strode, C.S. Wondji, J.P. David, N.J. Hawkes, N. Lumjuan, D.R. Nelson, D.R. Drane, S.H.P. Karunaratne, J. Hemingway, W.C. Black 4th, and H. Ranson, Genomic analysis of detoxification genes in the mosquito Aedes aegypti, Insect Biochem. Mol. Biol. 38 (2008), pp. 113–123.10.1016/j.ibmb.2007.09.007
- S. Marcombe, R. Poupardin, F. Darriet, S. Reynaud, J. Bonnet, C. Strode, C. Brengues, A. Yébakima, H. Ranson, V. Corbel, and J.P. David, Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: A case study in Martinique Island (French West Indies), BMC Genomics 10 494 (2009), pp. 1–14.
- S. Marcombe, R.B. Mathieu, N. Pocquet, M.A. Riaz, R. Poupardin, S. Sélior, F. Darriet, S. Reynaud, A. Yébakima, V. Corbel, J.P. David, and F. Chandre, Insecticide resistance in the dengue vector Aedes aegypti from Martinique: Distribution, mechanisms and relations with environmental factors, PLoS ONE 7 (2012), p. e30989. (doi: 10.1371/journal.pone.0030989).10.1371/journal.pone.0030989
- J.P. David, E. Coissac, C. Melodelima, R. Poupardin, M.A. Riaz, A. Chandor-Proust, and S. Reynaud, Transcriptome response to pollutants and insecticides in the dengue vector Aedes aegypti using next-generation sequencing technology, BMC Genomics 11, 216 (2010), pp. 1–12.
- J.P. David, H.M. Ismail, A. Chandor-Proust, and M.J.I. Paine, Role of cytochrome P450s in insecticide resistance: Impact on the control of mosquito-borne diseases and use of insecticides on Earth, Phil. Trans. R. Soc. B 368 (2013), 20120429.10.1098/rstb.2012.0429
- A. Chandor-Proust, J. Bibby, M. Régent-Kloeckner, J. Roux, E. Guittard-Crilat, R. Poupardin, M.A. Riaz, C. Dauphin-Villemant, S. Reynaud, and J.P. David, The central role of mosquito cytochrome P450 CYP6Zs in insecticide detoxification revealed by functional expression and structural modelling, Biochem. J. 455 (2013), pp. 75–85.10.1042/BJ20130577
- P. Lertkiatmongkol, E. Jenwitheesuk, and P. Rongnoparut, Homology modeling of mosquito cytochrome P450 enzymes involved in pyrethroid metabolism: Insights into differences in substrate selectivity, BMC Res. Notes 4 (2011), p. 321.10.1186/1756-0500-4-321
- T.L. Chiu, Z. Wen, S.G. Rupasinghe, and M.A. Schuler, Comparative molecular modeling of Anopheles gambiae CYP6Z1, a mosquito P450 capable of metabolizing DDT, Proc. Natl Acad. Sci. USA 105 (2008), pp. 8855–8860.10.1073/pnas.0709249105
- R.J. Marles, R.L. Compadre, C.M. Compadre, C. Soucy-Breau, R.W. Redmond, F. Duval, B. Mehta, P. Morand, J.C. Scaiano, and J.T. Arnason, Thiophenes as mosquito larvicides: Structure–toxicity relationship analysis, Pest. Biochem. Physiol. 41 (1991), pp. 89–100.10.1016/0048-3575(91)90063-R
- D.G. Hammond and I. Kubo, Structure-activity relationship of alkanols as mosquito larvicides with novel findings regarding their mode of action, Bioorg. Med. Chem. 7 (1999), pp. 271–278.10.1016/S0968-0896(98)00248-X
- S.C. Basak, R. Natarajan, D. Mills, D.M. Hawkins, and J.J. Kraker, Quantitative structure-activity relationship modeling of juvenile hormone mimetic compounds for Culex pipiens larvae, with a discussion of descriptor-thinning methods, J. Chem. Inf. Model. 46 (2006), pp. 65–77.10.1021/ci050215y
- N.A. Begum, N. Roy, R.A. Laskar, and K. Roy, Mosquito larvicidal studies of some chalcone analogues and their derived products: Structure–activity relationship analysis, Med. Chem. Res. 20 (2011), pp. 184–191.10.1007/s00044-010-9305-6
- R. Sun, Y. Li, M. Lü, L. Xiong, and Q. Wang, Synthesis, larvicidal activity, and SAR studies of new benzoylphenylureas containing oxime ether and oxime ester group, Bioorg. Med. Chem. Lett. 20 (2010), pp. 4693–4699.10.1016/j.bmcl.2010.04.144
- H.E.D.M. Zahran and S.A.M. Abdelgaleil, Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L. (Diptera: Culicidae), J. Asia-Pacific Entomol. 14 (2011), pp. 46–51.10.1016/j.aspen.2010.11.013
- J. Devillers, N. Marchand-Geneste, A. Carpy, and J.M. Porcher, SAR and QSAR modeling of endocrine disruptors, SAR QSAR Environ. Res. 17 (2006), pp. 393–412.10.1080/10629360600884397
- J. Devillers, H. Devillers, A. Decourtye, J. Fourrier, P. Aupinel, and D. Fortini, Agent-based modeling of the long term effects of pyriproxyfen on honey bee population, in In Silico Bees, J. Devillers, ed., CRC Press, Boca Raton, 2014, pp. 179–208.10.1201/b16453
- R. Carbó-Dorca and E. Besalú, Construction of coherent nano quantitative structure–properties relationships (nano-QSPR) models and catastrophe theory, SAR QSAR Environ. Res. 22 (2011), pp. 661–665.10.1080/1062936X.2011.623319
- L. Lubinski, P. Urbaszek, A. Gajewicz, M.T.D. Cronin, S.J. Enoch, J.C. Madden, D. Leszczynska, J. Leszczynski, and T. Puzyn, Evaluation criteria for the quality of published experimental data on nanomaterials and their usefulness for QSAR modelling, SAR QSAR Environ. Res. 24 (2013), pp. 995–1008.10.1080/1062936X.2013.840679
- D.A. Winkler, F.R. Burden, B. Yan, R. Weissleder, C. Tassa, S. Shaw, and V.C. Epa, Modelling and predicting the biological effects of nanomaterials, SAR QSAR Environ. Res. 25 (2014), pp. 161–172.10.1080/1062936X.2013.874367
- J.E. Riviere and C.L. Tran, Pharmacokinetics of nanomaterials, in Nanotoxicology. Characterization, Dosing, and Health Effects, N.A. Monteiro-Riviere and C.L. Tran, eds., Informa, NY, 2007, pp. 127–140.10.3109/9781420045154
- F. Gottschalk, R.W. Scholz, and B. Nowack, Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles, Environ. Model. Soft. 25 (2010), pp. 320–332.10.1016/j.envsoft.2009.08.011
- X. Liu, K. Tang, S. Harper, B. Harper, J.A. Steevens, and R. Xu, Predictive modeling of nanomaterial exposure effects in biological systems, Int. J. Nanomed. 8 (2013), pp. 31–43.10.2147/IJN
- A. Zollanvari, M.J. Cunningham, U. Braga-Neto, and E.R. Dougherty, Analysis and modeling of time-course gene-expression profiles from nanomaterial-exposed primary human epidermal keratinocytes, BMC Bioinf. 10 (2009), sup. 11, S10.10.1186/1471-2105-10-S11-S10
- T.K. Barik, R. Kamaraju, and A. Gowswami, Silica nanoparticle: A potential new insecticide for mosquito vector control, Parasitol. Res. 111 (2012), pp. 1075–1083.10.1007/s00436-012-2934-6
- A. Sooresh, H. Kwon, R. Taylor, P. Pietrantonio, M. Pine, and C.M. Sayes, Surface functionalization of silver nanoparticles: Novel applications for insect vector control, Appl. Mater. Interf. 3 (2011), pp. 3779–3787.10.1021/am201167v
- N. Soni and S. Prakash, Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae, Parasitol. Res. 110 (2012), pp. 175–184.10.1007/s00436-011-2467-4
- M. Kah and T. Hofmann, Nanopesticide research: Current trends and future priorities, Environ. Intern. 63 (2014), pp. 224–235.10.1016/j.envint.2013.11.015
- C.J.M. Koenraadt, M. Kormaksson, and L.C. Harrington, Effects of inbreeding and genetic modification on Aedes aegypti larval competition and adult energy reserves, Parasit. Vect. 3 (2010), p. 92.10.1186/1756-3305-3-92
- D.O. Carvalho, D. Nimmo, N. Naish, A.R. McKemey, P. Gray, A.B.B. Wilke, M.T. Marrelli, J.F. Virginio, L. Alphey, and M.L. Capurro, Mass production of genetically modified Aedes aegypti for field releases in Brazil, J. Vis. Exp. 83 (2014), p. e3579.
- L.E. Yauch and S. Shresta, Dengue virus vaccine development, Adv. Virus Res. 88 (2014), pp. 315–372.10.1016/B978-0-12-800098-4.00007-6
- I. Rodriguez-Barraquer, L. Mier-y-Teran-Romero, I.B. Schwartz, D.S. Burke, and D.A.T. Cummings, Potential opportunities and perils of imperfect dengue vaccines, Vaccine 32 (2014), pp. 514–520.10.1016/j.vaccine.2013.11.020