ABSTRACT
Aim of this study was to compare cardiovascular risk in women with and without inheritable thrombophilia after hypertensive disorders of pregnancy (HDP). Blood pressure, anthropometrics and blood samples were measured 9-13 years after early-onset (<34 weeks) HDP. Amongst the 114 women included, no differences in hypertension (31.1% vs. 33.7%, OR 0.90 95% CI (0.29–2.79)), body mass index > 25 kg/m2 (43.8% vs. 53.1%, OR 0.69 95% CI (0.24–2.00)) or metabolic syndrome (18.8% vs. 13.3%, OR 1.51 95% CI (0.38–6.02)) were found. These data show similar cardiovascular risk profile in women with and without inheritable thrombophilia.
Introduction
Worldwide, cardiovascular diseases (CVD), such as myocardial infarction and ischemic stroke, are the leading cause of death for women (Citation1). In the Netherlands, CVD are the second largest cause of death for women (Citation2). It is widely established that there is a correlation between hypertensive disorders of pregnancy (HDP, including pregnancy-induced hypertension and preeclampsia (Citation3)) and developing risk factors for CVD later in life, such as hypertension, metabolic syndrome, hypercholesterolemia, diabetes mellitus (DM), and body mass index (BMI) > 25 kg/m2 (Citation4–Citation12). Within the group of women who develop HDP, some women are diagnosed with inheritable thrombophilia; a group of genetic disorders including: factor V Leiden (FVL), prothrombin gene G2021A mutation, protein C deficiency, protein S deficiency, and common polymorphisms (C677 T and A1298 C) of the gene for methylenetetrahydrofolate reductase (MTHFR) (Citation13). A possible explanation for the development of HDP in women with inheritable thrombophilia could be the occurrence of placental thrombosis, causing placental insufficiency and consequently leading to preeclampsia (Citation3). However, a causal relationship between thrombophilia and HDP has not been identified and results remain contradictory (Citation14–Citation20).
In general, thrombophilia is a known risk factor for the development of venous thrombosis or venous pulmonary embolism (Citation21). Within this context, it is reasonable to hypothesize that women with inheritable thrombophilia are more likely to develop CVD after HDP. However, it must be noted that this has not yet been proven by the available literature (Citation21–Citation24). In one observational study, the cardiovascular risk factors of twenty women were compared 5 years after preeclampsia (Citation25). Ten women had either acquired or inheritable thrombophilia and 10 women had no thrombophilia. Notably, the results of this study showed lower cardiovascular risk factors in women with thrombophilia in comparison to women without thrombophilia. In an attempt to further investigate these findings, the aim of this study is to evaluate the development of cardiovascular risk factors in women with and without thrombophilia with a history of HDP by including more participants and by making use of a longer follow-up period.
Materials and methods
Study design
We used two databases of women with a history of early-onset HDP (<34 weeks) with and without thrombophilia (Citation3,Citation4). The first database contains data from women originally included in the FRUIT-RCT: FRactionated heparin in women with Uteroplacental Insufficiency and Thrombophilia- Randomized controlled trial (Citation3). This study compared the development of HDP in women with thrombophilia, some of whom received low-molecular-weight heparin added to aspirin 80 mg during pregnancy. All participants had a history of uteroplacental insufficiency, that had resulted in either HDP or in a small for gestational age infant during their previous pregnancy (defined as a birth weight below the 10th percentile), resulting in a delivery before 34 weeks gestation. Participants of the FRUIT-RCT were required to be above the age of 18 years and were included before the 12th week of gestation in their pregnancy. Additionally, all participants were diagnosed before inclusion in the FRUIT-RCT with one or more thrombophilia disorders: protein C deficiency, protein S deficiency, activated protein C resistance, heterozygous FVL mutation and/or prothrombin gene G20210A mutation (heterozygous) (Citation3). Women with an unknown coagulation status were excluded. Testing for inheritable thrombophilia was performed twice, at least 10 weeks postpartum and at least 6 weeks apart. In the FRUIT-RCT, recurrent early-onset HDP gestation was the primary outcome. For the current study, we used data of women who participated in the follow-up study of the FRUIT-RCT (Citation26).
The second database consists of women without inheritable thrombophilia with a history of early-onset preeclampsia (<34 weeks gestation) (Citation4). These non-thrombophilia participants were specifically included in the study of Bokslag et al., by scanning medical records from the obstetrical database of the Amsterdam University Medical Center. Women with a history of early-onset preeclampsia were therefore identified and invited for this study. Women with chronic hypertension (a diastolic blood pressure of 90 mmHg in the first trimester of pregnancy, or outside of pregnancy), cardiovascular disease or thrombophilia at the time of their pregnancy were excluded from this study. In addition, women with an unknown coagulation status were excluded so as to ensure that the presence of thrombophilia would not influence the outcome of the study. Women with early-onset HDP were compared to healthy controls (defined as an uncomplicated pregnancy with delivery at term). The aim of the study of Bokslag et al. was to investigate the impact of early-onset preeclampsia on the development of cardiovascular risk in the fifth decade of life. The control group of Bokslag et al. was not used in the current study. The participants who were included in both studies were examined in the Netherlands in the period between 2014 and 2016 (Citation4,Citation26). The data of these examinations were used in the current study. We excluded all participants with chronic hypertension. All participants gave written informed consent and both follow-up studies were approved by the Institutional Review Board of the Amsterdam UMC.
Participant characteristics
A follow-up screening visit was performed 9–13 years after HDP. Initially, participants filled in a questionnaire that included lifestyle-related questions. Furthermore, details were obtained about obstetric history, current medical conditions, use of medication, history of CVD and family history of CVD. Coagulation status was defined as either the presence or absence of inheritable thrombophilia. In obstetric history, the following aspects were obtained: parity, maternal age at time of delivery, gestational age at delivery (GA), type of HDP (preeclampsia, eclampsia and/or HELLP syndrome) and recurrence of HDP. The follow-up screening consisted of a physical examination, venous blood sampling, and urine analysis. Blood pressure was manually measured twice with the participant in an upright position. The mean value of the two measurements was used for analysis. Body height and body weight were measured. BMI was subsequently calculated as kg/m2. Waist and hip circumferences were measured and waist-hip-ratio was calculated. After an overnight fast, venous blood samples were taken for analysis of lipid spectrum, glucose, and renal function. Morning urine was collected for microalbuminuria detection.
Definitions of clinical diagnoses
Inheritable thrombophilia was defined as the presence of either: protein C deficiency, protein S deficiency, activated protein C resistance, heterozygous FVL and/or heterozygous prothrombin gene G2021A mutation. Preeclampsia was defined as pregnancy-induced hypertension (diastolic blood pressure ≥ 140/90 mmHg) accompanied by proteinuria (≥300 mg/24 h). In the study of Bokslag et al. (Citation4), spot urine albumin creatinine ≥ 30 mg/mmol was also a definition of proteinuria. Eclampsia was defined as generalized convulsions in pregnancy in the absence of epilepsy and HELLP syndrome is a combination of hemolysis (LDH ≥ 600 IU/L), elevated liver enzymes (increased serum glutamic oxaloacetic transaminase/serum glutamic pyruvic transaminase ≥70 IU/L) and thrombocytopenia (<100 x 109/L) (Citation3). Increased cardiovascular risk was defined as the presence of either hypertension, hypercholesterolemia, BMI > 25 kg/m2 or DM (Citation26). Hypertension was defined as the current use of blood pressure lowering medication or de novo systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mmHg. Hypercholesterolemia is a total cholesterol ≥ 5 mmol/L or current use of statins. DM is defined as fasting glucose ≥ 7 mmol/L or treatment with diet, oral antidiabetics, or insulin treatment. Metabolic syndrome is defined as the presence of three or more of the following: blood pressure ≥ 130/≥ 85 mmHg, serum triglycerides ≥ 1.69 mmol/L, serum high-density lipoprotein cholesterol (HDL-c) < 1.29 mmol/L, waist circumference ≥ 88 cm, fasting plasma glucose ≥ 6.1 mmol/L.
Statistical analysis
Baseline characteristics of participants were reported as mean ± standard deviation for normally distributed continuous data and median and interquartile ranges for not normally distributed continuous data. For dichotomous data, numbers and percentages were depicted. Differences were analyzed using independent T-test, Mann–Whitney test, Chi-square test, or Fisher’s exact test when appropriate. Outcomes were presented as mean difference (MD) for continuous data or odds ratio (OR) for dichotomous data with added confidence intervals and p-values. Cardiovascular risk factors at follow-up screening visits were examined using an independent T-test or Mann–Whitney test for continuous data. Dichotomous data were analyzed using a Chi-square test and, if appropriate, a Fisher’s exact test. For clinical diagnoses, ORs were reported alongside their respective confidence intervals. Possible confounding variables were analyzed by using linear or logistic regression (for, respectively, continuous or dichotomous outcomes). A ≥ 10% difference in crude or adjusted MD/OR was defined to be statistical significant confounding. A p-value of <0.05 was considered to be statistically significant with the assumption that no difference was to be found in the comparison between both groups. Statistical analysis was performed using IBM SPSS version 22.0 (SPSS Inc, Chicago, USA) (Citation27).
Results
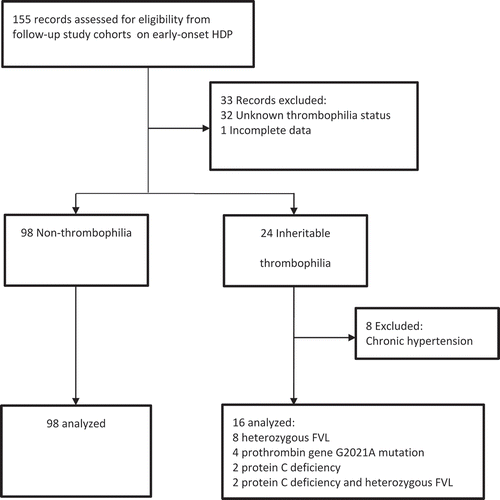
In total, the records of 155 women with a history of early-onset HDP were assessed. The inclusion process is presented in . The initial group consisted of 98 participants without thrombophilia and 24 participants with thrombophilia. In the group of women with thrombophilia, eight participants were excluded because of the presence of chronic hypertension. Ultimately, the measurements of the 16 remaining participants with inheritable thrombophilia were analyzed. Of the participants with inheritable thrombophilia, eight participants were diagnosed with heterozygous FVL, four with heterozygous prothrombin gene G2021A mutation, two with protein C deficiency and two women with both protein C deficiency and FVL.
Baseline characteristics of the study population at the follow-up screening visit including obstetric history and family history are demonstrated in . In both groups, at the time of the follow-up screening, the women’s average age was 44. Women with inheritable thrombophilia were, on average, 5 years older at the time of their index pregnancy (median 35.3 years compared to 30.5 years in women without inheritable thrombophilia, p = <0.001), and had more often experienced recurrent HDP (p < 0.001). Family history was comparable for both groups, for both venous and arterial events. One woman with thrombophilia and 14 women without thrombophilia used blood pressure lowering medication. Biometrics and biochemical results at screening visit are demonstrated in .
Table 1. Characteristics of study population.
Table 2. Cardiovascular risk factors at follow-up screening visit: physical examination, biochemical results, and clinical diagnoses.
The outcome of increased cardiovascular risk was similar between the groups: 31.1% in women with inheritable thrombophilia versus 33.7% in women without thrombophilia, as shown in . In women with inheritable thrombophilia, all five participants with hypertension at follow-up had experienced recurrent HDP. The variable recurrent HDP was not shown to be a confounding agent in the relationship between inheritable thrombophilia and the development of risk factors for CVD after HDP for any of the clinical diagnoses ().
Discussion
This study shows no difference in the presence of cardiovascular risk factors 9–13 years after HDP between women with and without inheritable thrombophilia. We could not confirm the findings of a previous observational study, which demonstrated an attributable decrease in cardiovascular risk factors in women with thrombophilia compared to those without thrombophilia and a history of HDP (Citation25). In comparison, in addition to various types of inheritable thrombophilia, women with acquired thrombophilia were not excluded from this study, where two women had positive anticardiolipin antibodies and two women had hyperhomocysteinemia (Citation25). End points in this observational study were arterial microvascular and macrovascular function, whilst compared to the clinical diagnoses evaluated in our study.
In general, development of cardiovascular risk factors in women with thrombophilia is researched thoroughly, but so far studies have shown contradictory results (Citation21–Citation24). We found that 31.3% of women with inheritable thrombophilia and 33.7% of women without inheritable thrombophilia had hypertension 9–13 years after preeclampsia. Interestingly, Drost et al. (Citation28) and Berends et al. (Citation29) reported higher rates of hypertension in women without thrombophilia with a history of early-onset HDP (43.1 and 66.7%, respectively) compared to women with uncomplicated pregnancies. This high presence of hypertension in their studies may be partially explained by the fact that women with chronic hypertension were not excluded. Chronic hypertension is known to be an independent risk factor for the development of both HDP and CVD, with a relative risk factor of more than five for the development of HDP (Citation30). In Western countries, 1-5% of pregnant women are diagnosed with chronic hypertension and the incidence increases with increasing age (Citation31,Citation32). In our study, eight of 24 participants with inheritable thrombophilia were excluded from the analysis because chronic hypertension was present. As a confounding variable, chronic hypertension could overstate the attribution of HDP to cardiovascular risk factors.
Notably, in our study, percentage of metabolic syndrome was higher in women with inheritable thrombophilia (18.8%) compared to women in the non-thrombophilia group (13.3%); however, there was no statistical difference. In women with a history of HDP, irrespective of thrombophilia, there is a wide range in data regarding the presence of metabolic syndrome years after the complicated pregnancy, described in 18-39% of women (Citation28,Citation29). A possible explanation is the use of a cutoff point of ≥80 cm for the definition of central obesity by Berends et al. (Citation29), which then reflects higher rates of metabolic syndrome (38.3%), as compared to an obesity cutoff point of ≥88 cm utilized in our study. Our results do not support the known phenomenon that women with a history of HDP are more prone to develop metabolic syndrome, as our results were are very comparable to the average Dutch female population aged 40–49 years, reflecting a risk of 17% for metabolic syndrome (Citation33).
The strength of our study is the known presence or absence of inheritable thrombophilia and therefore provides a reliable comparison between women with and without thrombophilia in cardiovascular risk factors after HDP (Citation3,Citation4). Secondly, to our knowledge, the mean follow-up period of 9–13 years when examining risk factors for CVD is lengthy and no previous studies using this follow-up period in this specific population are yet available. This extended follow-up period enables us to give insights into the long-term development of cardiovascular risk in women with a history of HDP with and without inheritable thrombophilia. In acquiring insights into the relationship between inheritable thrombophilia and the development of cardiovascular risk factors after HDP, we have tried to pay attention to the underlying confounding factors, such as the presence of chronic hypertension and recurrence of HDP. Women in the thrombophilia group were more vulnerable for recurrent HDP as they were included in the FRUIT-RCT (Citation3) only during a second or third pregnancy. These subsequent pregnancies complicated by HDP can enlarge the risk for developing cardiovascular risk factors. We have taken this into account by correcting for this possible confounding variable in the relationship between thrombophilia and the development of risk factors for cardiovascular disease and have not been able to identify recurrence of HDP as a confounding variable, even though women with thrombophilia more often experienced recurrent HDP (56.3% in women with thrombophilia versus 19.4% without thrombophilia, p < 0.001). The development of risk factors for CVD in our study may have been influenced by the anticoagulant treatment in the group of women with inheritable thrombophilia, for these women were included in the FRUIT-RCT (Citation3) and thus were treated with aspirin or aspirin in combination with heparin during pregnancy. We can imagine that the use of anticoagulants acts as a protective agent against the development of risk factors for CVD, as anticoagulants may prevent vascular obstetric complications such as preeclampsia. However, a large meta-analysis on individual patient data has shown that use of low-molecular weight heparin did not have a protective effect on the development of placenta-mediated pregnancy outcomes (Citation34). Possible selection bias may have arisen in our study, considering that women with current medical problems may have felt more willing to participate in the follow-up studies than healthy women, thus overstating risk factors for CVD in the current data. On the other hand, considering the impact of HDP on pregnant woman and their medical network, it is also plausible to think that more healthy women agreed to participate.
However, the most important limitation of our study was the small number of participants, which has had an impact on both the statistical analysis and the weight of the actual results. Thrombophilia is a rare condition and because of the exclusion of participants with unknown coagulation status and the presence of chronic hypertension, numbers were small in both groups. Therefore, our relatively small research groups varied in size.
In conclusion, the present study shows that women with and without thrombophilia have comparable cardiovascular risk factors more than 10 years after early-onset HDP. A causal role for the presence of thrombophilia could not be identified in the present study.
Given these findings, we suggest that women with thrombophilia should not be treated in a different manner than women without thrombophilia. All women should have a follow-up later in life to examine risk-factors for CVD after a pregnancy complicated by (early-onset) HDP.
Acknowledgments
We thank all women for their participation in the original studies that have enabled us with our data on cardiovascular risk factors. We also thank A. Arduç for his help during the visits of all participants with inheritable thrombophilia in physical examination and blood sample collection.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- World Health Organization. 10 leading causes of death in females [Website]. Geneva: World Health Organization; 2019. Available from: www.who.int
- StatLine.cbs.nl [internet]. Den Haag: CBS; [28-01-2019]. Available from: https://opendata.cbs.nl/statline/#/CBS/nl/
- de Vries JI, van Pampus MG, Hague WM, et al. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT. J Thromb Haemost. 2012 Jan;10(1):64–72. PubMed PMID: 22118560.
- Bokslag ATP, Teunissen PW, Franssen C, et al. Effect of early-onset preeclampsia on cardiovascular risk in the fifth decade of life. Am J Obstetrics Gynaecol. 2017;216(523):1–7.
- Hermes W, Franx A, van Pampus MG, et al. Cardiovascular risk factors in women who had hypertensive disorders late in pregnancy: a cohort study. Am J Obstet Gynecol. 2013 Jun;208(6):474 e1–8. PubMed PMID: 23399350.
- Visser VS, Hermes W, Franx A, et al. High blood pressure six weeks postpartum after hypertensive pregnancy disorders at term is associated with chronic hypertension. Pregnancy Hypertens. 2013 Oct;3(4):242–247. PubMed PMID: 26103803.
- Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet. 2016 Mar 5;387(10022):999–1011. PubMed PMID: 26342729.
- van Rijn BB, Nijdam ME, Bruinse HW, et al. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet Gynecol. 2013 May;121(5):1040–1048. PubMed PMID: 23635741.
- Benschop L, Duvekot JJ, Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019 Aug;105(16):1273–1278. PubMed PMID: 31175138; PubMed Central PMCID: PMCPMC6678044.
- Groenhof TKJ, Zoet GA, Franx A, et al. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019 Jan;73(1):171–178. PubMed PMID: 30571544.
- Markovitz AR, Stuart JJ, Horn J, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J. 2019 Apr 7;40(14):1113–1120. PubMed PMID: 30596987; PubMed Central PMCID: PMCPMC6451770.
- Timpka S, Stuart JJ, Tanz LJ, et al. Postpregnancy BMI in the progression from hypertensive disorders of pregnancy to type 2 diabetes. Diabetes Care. 2019 Jan;42(1):44–49. PubMed PMID: 30455328; PubMed Central PMCID: PMCPMC6300702.
- Kist WJ, Janssen NG, Kalk JJ, et al. Thrombophilias and adverse pregnancy outcome - A confounded problem! Thromb Haemost. 2008 Jan;99(1):77–85. PubMed PMID: 18217138.
- Mello G, Parretti E, Marozio L, et al. Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension. 2005 Dec;46(6):1270–1274. PubMed PMID: 16246971.
- Lin J, August P. Genetic thrombophilias and preeclampsia: a meta-analysis. Obstet Gynecol. 2005 Jan;105(1):182–192. PubMed PMID: 15625161.
- Said JM, Higgins JR, Moses EK, et al. Inherited thrombophilias and adverse pregnancy outcomes: a case-control study in an Australian population. Acta Obstet Gynecol Scand. 2012 Feb;91(2):250–255. PubMed PMID: 21985600.
- Howley HE, Walker M, Rodger MA. A systematic review of the association between factor V Leiden or prothrombin gene variant and intrauterine growth restriction. Am J Obstet Gynecol. 2005 Mar;192(3):694–708. PubMed PMID: 15746660.
- Kahn SR, Platt R, McNamara H, et al. Inherited thrombophilia and preeclampsia within a multicenter cohort: the Montreal Preeclampsia Study. Am J Obstet Gynecol. 2009 Feb;200(2):151 e1-9; discussion e1-5. PubMed PMID: 19070828.
- Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004 Aug;191(2):412–424. PubMed PMID: 15343215.
- De Groot CJ, Bloemenkamp KW, Duvekot EJ, et al. Preeclampsia and genetic risk factors for thrombosis: a case-control study. Am J Obstet Gynecol. 1999 Oct;181(4):975–980. PubMed PMID: 10521764.
- Siegerink B, Maino A, Algra A, et al. Hypercoagulability and the risk of myocardial infarction and ischemic stroke in young women. J Thromb Haemost. 2015 Sep;13(9):1568–1575. PubMed PMID: 26178535.
- Bentley P, Peck G, Smeeth L, et al. Causal relationship of susceptibility genes to ischemic stroke: comparison to ischemic heart disease and biochemical determinants. PLoS One. 2010 Feb 9;5(2):e9136. PubMed PMID: 20161734; PubMed Central PMCID: PMCPMC2817726.
- Boekholdt SM, Kramer MH. Arterial thrombosis and the role of thrombophilia. Semin Thromb Hemost. 2007 Sep;33(6):588–596. PubMed PMID: 17768691.
- Pasupathy S, Air T, Dreyer RP, et al. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015 Mar 10;131(10):861–870. PubMed PMID: 25587100.
- van der Horst M, van Weissenbruch MM, de Vries JI. Thrombophilia mediates lowering cardiovascular risk factors in women with a history of preeclampsia. Hypertens Pregnancy. 2011;30(4):421–432. PubMed PMID: 20860491.
- Abheiden CN, Thijs A, de Vries JI, et al. Cardiovascular risk factors in women with inheritable thrombophilia a decade after single or recurrent hypertensive disorder of pregnancy. Hypertens Pregnancy. 2016 Nov;35(4):461–469. PubMed PMID: 27322349.
- Corp. I. IBM SPSS statistics for windows. 22.0. Armonk (NY): IBM Corp.; 2013.
- Drost JT, Arpaci G, Ottervanger JP, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk Evaluation in FEmales study (PREVFEM). Eur J Prev Cardiol. 2012 Oct;19(5):1138–1144. PubMed PMID: 21859777.
- Berends AL, de Groot CJ, Sijbrands EJ, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008 Apr;51(4):1034–1041. PubMed PMID: 18259037.
- Arabin B, Baschat AA. Pregnancy: an underutilized window of opportunity to improve long-term maternal and infant health-an appeal for continuous family care and interdisciplinary communication. Front Pediatr. 2017;5:69. PubMed PMID: 28451583; PubMed Central PMCID: PMCPMC5389980. eng.
- Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014 Apr 15;348:g2301. PubMed PMID: 24735917; PubMed Central PMCID: PMCPMC3988319.
- Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995 Mar;25(3):305–313. PubMed PMID: 7875754.
- Blokstra AVP, Venmans LMAJ, Holleman P, et al. Nederland de Maat genomen 2009-2010. Monitoring van risicofactoren in de algemene bevolking. RIVM Rapport 260152001/2011. 2011.
- Rodger MA, Gris JC, de Vries JIP, et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: a meta-analysis of individual patient data from randomised controlled trials. Lancet. 2016 Nov 26;388(10060):2629–2641. PubMed PMID: 27720497.