ABSTRACT
Objective
To evaluate the causes behind the association between hypothyroidism and the risk of preeclampsia.
Methods
Checking of individual hospital and birth records from 149 levothyroxine users among 2,508 women in the preeclampsia case–control study (2002-2016).
Results
There were significant association between levothyroxine medication and preeclampsia (OR 1.48, 95th CI 1.06–2.07; p ≤ 0.022). The presence of comorbid diseases was associated with a significantly higher risk for the development of preeclampsia in women using levothyroxine.
Conclusion
Levothyroxine use during pregnancy was associated with 1.5-times higher risk for preeclampsia, but it is also linked to the other comorbid risk factors.
Introduction
Thyroid diseases including hypothyroidism, hyperthyroidism, and subclinical hypothyroidism with various etiologies are some of the most common diseases experienced by reproductive-aged women. During pregnancy, hypothyroidism, including overt (OH) and subclinical hypothyroidism (SCH), are the most frequent thyroid dysfunctions, (Citation1) being present in approximately 0.4% (OH) to 3% (SCH) (Citation2) of pregnant women, but it is believed that they are more common than generally acknowledged (Citation3). The range of prevalence of undiagnosed subclinical hypothyroidism during pregnancy has been estimated to be even higher from 3% up to 15% with the difference being attributable to the various diagnostic criteria (Citation4). Levothyroxine (LT₄) is the most widely used drug to treat hypothyroidism, and consequently, it is one of the most frequently prescribed drugs during pregnancy. In different studies, the prevalence of fertile-aged women, who had used levothyroxine, has ranged from 2% to 15%. (Citation4–6)
When women are diagnosed with hypothyroidism, they are invariably medicated with LT₄, whether they are pregnant. At the onset of pregnancy, dosage should be raised as high as 30–50% from the dose being taken prior to pregnancy. (Citation1,Citation4,Citation6–8) If hypothyroidism is diagnosed during pregnancy, LT₄ medication is initiated with the usual dosage ranging between 50 and 100 µg/day (Citation9). However, it is controversial whether subclinical hypothyroidism, diagnosed during pregnancy, should be treated with levothyroxine (Citation1). Untreated overt hypothyroidism is associated with various well-known complications both for the pregnant woman and her unborn child. (Citation2,Citation7,Citation10)
Preeclampsia is a possible pregnancy-related complication in women with hypothyroidism. (Citation2) In its most severe cases, preeclampsia may cause serious organ damage and fetal growth restriction leading to preterm birth, and increased risk of neonatal morbidity and mortality. (Citation11) The etiology of the disease is heterogeneous (Citation12). Globally the incidence of preeclampsia is approximately 5–15% of pregnancies although the risk is assumed to be higher in developing countries. (Citation13–15)
In some previous studies, women with thyroid diseases (mostly hypothyroidism) have had a higher risk of developing preeclampsia in an ongoing pregnancy. (Citation2,Citation4,Citation7) However, little is known about the background of the increased risk. The factors contributing to this risk are still unclear – is the background explained by the simultaneous presence of other diseases or confounders like diabetes, or possibly other risk factors like obesity, high maternal age, or prior infertility treatments?
In our recent study of drug use during pregnancy and the development of preeclampsia (Citation16) we observed that pregnant women who developed preeclampsia had used thyroid medication (mainly levothyroxine) significantly more often during their pregnancy than women without preeclampsia. In this investigation, we wanted to evaluate more detailed if the risk for preeclampsia could be explained by the use of levothyroxine, confounders or some of the other related diseases mentioned above or whether hypothyroidism is an individual risk for the development of preeclampsia. In addition, we wanted to survey whether there is a certain type of hypothyroidism that would be riskier than the others to trigger the development of preeclampsia.
Materials and methods
Study cohort
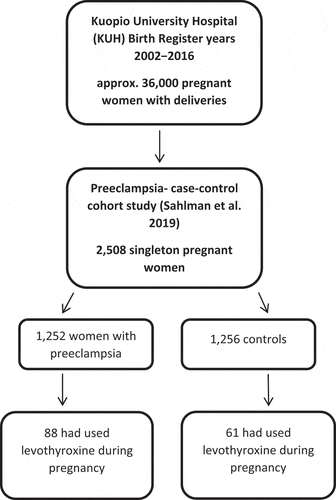
The original study population was collected from the Kuopio University Hospital Birth Register, which includes information about all of the women who gave birth during the years of 2002─2016 in Kuopio University Hospital. There were approximately 36,000 pregnant women with deliveries during these 15 years and information was collected prospectively. A more detailed data of this study have been reported by Sahlman. (Citation16) In brief, the original study evaluated whether there were differences in the use of various drugs during pregnancy between women who developed preeclampsia and controls. From this study population, we have now selected 149 pregnant women who had used levothyroxine during pregnancy and examined more closely their hospital and birth records. We evaluated the following clinical factors: (1) what was the type of hypothyroidism (OH or SCH) or the cause behind the use of levothyroxine, (2) when were the pregnant women diagnosed with hypothyroidism or when was the need of LT₄ medication discovered (before or during pregnancy) and (3) the onset and dosage of LT₄ medication (before or during pregnancy). Nearly all women with LT₄ medication (data missing 12%), had levels of TSH and free thyroxine (T4V) evaluated serially throughout the pregnancy and their values and gestational weeks at the time of sampling were collected. The diagnosis of hypothyroidism and subclinical hypothyroidism was made based on TSH- and T4V-levels. Diagnostic criteria for hypothyroidism were TSH value > 4.2 mU/l and T4V value < 11 pmol (Citation17–19). The diagnostic criteria for subclinical hypothyroidism were TSH value > 2.5 mU/l in the first trimester and TSH value > 3 mU/l in the second and third trimester and normal T4V value. (Citation4,Citation8) International Statistical Classification of Disease and Related Health Problems 10th revision codes (ICD-10) for hypothyroidism were E02, E03.2, E03.3, E03.8, E03.89, and E03.9.
The diagnostic criteria in Finland for gestational diabetes mellitus (GDM) were as follows: until 2008, the lower limits of abnormal fasting, one hour, and two hours’ capillary whole-blood glucose were 4.8, 11.2 and 9.9 mmol/l (corresponding 86.4, 201.6, and 178.2 mg/dl) (Citation20) and since 2008, the lower limits of abnormal fasting, one hour, and two hours’ capillary whole-blood glucose were 5.3, 10.0, and 8.6 mmol/l (corresponding 95.4, 180.0 and 154.8 mg/dl). The diagnosis of hypertensive and diabetic disorders was collected from the hospital records. We relied on the diagnosis made by the primary care physician. The diagnostic codes (ICD-10) for hypertensive disorders were O10.0, O10.1, O10.2, O10.3, O10.4, O10.9, O11, and O16 and for diagnosed diabetes O24.0, O24.1, and O24.9 or gestational diabetes (GDM) O24.4.
This study was approved by Central Finland Health Care District ethical committee (18 U/2011, 6.10.2016). describes the flowchart of the study population.
Statistical analysis
Numbers, percentages, means, and standard deviations of cases and controls were analyzed using descriptive statistics or crosstab analysis. P-values were calculated by Pearson Chi-square test or Independent samples t-test and logistic regression was used to compute odds ratio (OR) and 95th confidence intervals (95th CI). A p-value ≤ 0.05 was considered statistically significant. The database was analyzed using SPSS (version 25).
Results
Among 2,508 original preeclampsia case–control women, 149 (5.9%) women had used LT₄ medication during pregnancy. Preeclampsia occurred significantly more often in women with LT₄ medication than in the controls (OR 1.48, 95th CI 1.06–2.07; p ≤ 0.022). Of these women, 103 (69.1%) had OH and 46 (30.9%) had SCH. The disease was diagnosed before pregnancy in 95 (63.8%) women, during pregnancy in 49 (32.9%) women, and 5 (3.4%) women did not report the time of diagnosis. Levothyroxine treatment was started prior to pregnancy in 85 (57.0%) women, during pregnancy in 54 (36.2%) women and 10 women did not report when medication was started. In the 54 women, in whom medication was started during pregnancy, it was started on approximately the 18th (range 6–37) gestational week (GW). Among women with prior medication for hypothyroidism, the initial mean daily dose of levothyroxine in the first trimester was 100 µg and during the pregnancy, it varied from 25 µg to 300 µg. Only 38 (44.7%) women with a prior diagnosis had information on the need to increase the LT₄ dose during pregnancy. In 49 (32.9%) women with a diagnosis of OH or SCH during pregnancy, the mean individual dose per day in the first trimester was 59 µg, and later during the pregnancy, it varied between 25 µg and 180 µg. In 14 (28.6%) of these women with new onset of medication, the dosage of LT₄ was increased during pregnancy.
Altogether, TSH-levels were measured during pregnancy in 88% (131/149) of women receiving levothyroxine treatment. In general, the levels had been estimated on average four (range 1–8) times during the pregnancy. Of those 85 women who had been prescribed medication before pregnancy, 74 had had their TSH values analyzed; the mean levels of the first measurement were 2.05 ± 1.63 (median: 1.78; range 0.01–8.99) mU/l. The mean levels of TSH while pregnant but before the onset of medication among the 49 (32.9%) women who were prescribed the medication during their pregnancy were 3.45 (median: 2.90; range 1.25–11.4) mU/l. Later, after onset of medication, the mean values declined to 2.24 (median: 1.89; range 0.01–10.49) mU/l. The individual highest TSH-levels during pregnancy among all the women with LT₄ therapy were significantly higher among women who started medication during pregnancy as compared to women who had medication prior pregnancy, 4.25 ± 2.65 mU/l vs 3.19 ± 3.20 mU/l (p ≤ 0.05).
The majority, 59.1% (88/149), of women who had LT₄ treatment developed preeclampsia (p ≤ 0.05). From all these women 10.1% (17/149) had had preeclampsia in previous pregnancies, significantly more often cases (17%) than in controls (3.3%) (p ≤ 0.009). The women treated with LT₄ during pregnancy were significantly older, more often obese (BMI > 30 kg/m2) and had more comorbid diseases, like hypertensive disorder and/or diabetes/GDM as compared to other women (). Furthermore, women receiving LT₄ treatment who also developed preeclampsia were even more often obese and had more hypertension and/or diabetes/GDM than those women using LT₄ treatment but not developing preeclampsia. In the preeclamptic women undergoing LT₄ therapy, almost all, i.e. 90.9% (80/88), reported the first symptoms of preeclampsia on average at 35.1 ± 3.4 GWs. In those women who had started this medication during pregnancy, the symptoms were likely to occur one week later than in those who had already been prescribed LT4 before their pregnancy (35.9 ± 3.0 vs. 34.6 ± 3.5; p 0.081).
Table 1. Demographic and clinical characteristics of 149 women using levothyroxine, 88 women with preeclampsia and using levothyroxine, 1252 women with preeclampsia, and 1195 control women
There were no significant differences in the type of hypothyroidism between women with preeclampsia and in those in whom this condition did not develop (). Additionally, no significant change was evident in the first measured TSH-levels or on the time of the measurement or the highest levels of TSH during pregnancy between LT₄ treated women with or without preeclampsia. It did seem that there was a higher risk of developing preeclampsia when levothyroxine treatment was started during pregnancy. No significant difference was detected in the severity of the preeclampsia, the gestational age at delivery, or the birthweights in women with preeclampsia either with or without levothyroxine therapy ().
Table 2. Type of hypothyroidism, results of thyrotropin stimulating hormone measurements and medication among 149 women using levothyroxine during pregnancy in relation to the development of preeclampsia
Table 3. Comparison of 1 252 preeclamptic women with and without levothyroxine medication during pregnancy
There was scanty data available on the etiology of hypothyroidism or LT₄ treatment in the hospital records. More exact etiology was available only in nine (6.0%) women; one woman had autoimmune thyroiditis, two women had goiters (one of which was surgically treated), two women had Basedow’s disease (one of which was surgically treated), two had thyroid cancers (after surgery) and two women had developed hyperthyroidism after radioiodine treatment. The women receiving LT₄ therapy during their pregnancy had the following other chronic diseases: diabetes (N = 20), hypertension (N = 22), obesity (BMI >30 kg/m2) (N = 39), Hodgkin’s lymphoma (both in remission N = 2) and Addison’s disease (N = 1). In contrast, 73 women (49.0%) had no chronic disease other than hypothyroidism.
Discussion
We showed that women who received LT₄ treatment during pregnancy and who developed preeclampsia were significantly older, more obese, and had more comorbid diseases, such as hypertension and diabetes/GDM compared to controls, but also a difference was detected in relation to the other women who developed preeclampsia. It was probable that the risk of developing preeclampsia among women with LT₄ therapy was mainly explained by these factors and not because of LT₄ therapy or hypothyroidism per se. The risk for preeclampsia among these women was not related to the type of hypothyroidism (OH or SCH), maternal serum TSH-levels, or dosage of levothyroxine during pregnancy. However, it was more likely that women who started LT₄ treatment during pregnancy tended to have more preeclampsia as compared to women who had had LT₄ treatment prior to their pregnancy.
There have been only a few similar studies investigating the association between preeclampsia and hypothyroidism in pregnant women during the last decade. Similar data have emerged from earlier studies, but no separate evaluation of maternal characteristics or type of hypothyroidism has been reported previously. (Citation2,Citation4,Citation21) In these studies, it has been reported that women using levothyroxine have a higher occurrence of preeclampsia, varying between 4% and 7%. (Citation16,Citation21,Citation22) In addition, maternal high TSH-levels during pregnancy have been associated with the development of preeclampsia in one retrospective cross-sectional and one prospective cohort study. (Citation23,Citation24) However, we observed that neither the dose of levothyroxine medication nor maternal serum levels of TSH were associated with the development of preeclampsia in women who had used levothyroxine during pregnancy.
The information of the diagnosis between OH and SCH was collected from the hospital records. We relied on the diagnosis which was made by an attending physician. It is possible that some patients with OH have been diagnosed with SCH and vice versa. This creates one potential source of error. It is also possible that the dosage of LT₄ had been recorded incorrectly, either by a physician or a nurse. Another possible source of error is human error in collecting and analyzing the data. Nonetheless, we doubt that these different sources of error have any statistical significance.
Preeclampsia tended to occur more often in women who started medication during pregnancy as compared to women who had medication prior to pregnancy. In the light of this data, we suggest that maternal serum levels of TSH should be routinely monitored at the beginning of pregnancy, at least in women with risk factors such as diabetes, hypertension, and obesity or those who develop gestational diabetes during pregnancy. Lee and associates showed recently that high maternal TSH-levels were associated with many adverse pregnancy complications such as prematurity and preeclampsia. (Citation25) However, it is still unclear whether LT₄ therapy started at the beginning of pregnancy can reduce pregnancy-related complications. In future studies, the impact of TPO-antibodies with high TSH-levels in relation to preeclampsia should be assessed, since the role of antibodies in the development of preeclampsia remains to be clarified. (Citation7)
Conclusion
In the light of data collected and the way errors were mitigated, we suggest that from those women who are obese, have hypertension and/or diabetes/gestational diabetes mellitus, maternal markers of hypothyroidism such as TSH-levels should be controlled at early stages of pregnancy to minimize risk for not only hypothyroidism but also for the development of preeclampsia. Further assessments will be needed to define whether there is potentially an increased risk of developing preeclampsia when levothyroxine treatment is initiated during pregnancy. In addition, it is unclear whether there are other pregnancy-related complications beside preeclampsia that occur more frequently in women in whom levothyroxine treatment is started during pregnancy.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to the risk of identifying patients.
Ethics approval
This study was approved by Central Finland Health Care District ethical committee (18U/2011, 6.10.2016)
Disclosure of interest
The authors report no conflict of interest.
Acknowledgments
We thank Ewen McDonald for editing the language of the manuscript.
Additional information
Funding
References
- Shan Z, Teng W. Thyroid hormone therapy of hypothyroidism in pregnancy. Endocrine. 2019;66(1):35–42.
- Krassas G, Karras SN, Pontikides N. Thyroid diseases during pregnancy: A number of important issues. Hormones (Athens). 2015;14(1):59–69.
- Blatt AJ, Nakamoto JM, Kaufman HW. National status of testing for hypothyroidism during pregnancy and postpartum. J Clin Endocrinol Metab. 2012;97(3):777–784.
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ. 2014;349(oct06 4):g4929.
- Akram FH, Johansson B, Mollerstrom G, et al. Incidence of subclinical hypothyroidism and hypothyroidism in early pregnancy. J Womens Health (Larchmt). 2017;26(11):1231–1235.
- Yassa L, Marqusee E, Fawcett R, et al. Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J Clin Endocrinol Metab. 2010;95(7):3234–3241.
- Sullivan SA. Hypothyroidism in pregnancy. Clin Obstet Gynecol. 2019;62(2):308–319.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389.
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543–2565.
- Nazarpour S, Ramezani Tehrani F, Amiri M, et al. Levothyroxine treatment and pregnancy outcomes in women with subclinical hypothyroidism: A systematic review and meta-analysis. Arch Gynecol Obstet. 2019;300(4):805–819.
- Kajantie E, Eriksson JG, Osmond C, et al. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–1180.
- Malik A, Jee B, Gupta SK. Preeclampsia: disease biology and burden, its management strategies with reference to india. Pregnancy Hypertens. 2019;15:23–31.
- Hod T, Cerdeira AS, Karumanchi SA. Molecular mechanisms of preeclampsia. Cold Spring Harb Perspect Med. 2015;5(10):10.
- Backes CH, Markham K, Moorehead P, et al. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365.
- Rana S, Lemoine E, Granger J, et al. Preeclampsia. Circ Res. 2019;124(7):1094–1112.
- Sahlman H, Koponen M, El-Nezami H, et al. Maternal use of drugs and preeclampsia. Br J Clin Pharmacol. 2019;85(12):2848–2855.
- Salmela P, Metso S, Moilanen L, et al. Treatment of primary hypothyroidism in adult patients. Duodecim. 2016;132(1):33–42.
- Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751.
- Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev. 2014;35(3):433–512.
- Hakkarainen H, Huopio H, Cederberg H, et al. Delivery of an LGA infant and the maternal risk of diabetes: A prospective cohort study. Prim Care Diabetes. 2018;12(4):364–370.
- Mannisto T, Karumanchi SA, Pouta A, et al. Preeclampsia, gestational hypertension and subsequent hypothyroidism. Pregnancy Hypertens. 2013;3(1):21–27.
- Wikner BN, Sparre LS, Stiller CO, et al. Maternal use of thyroid hormones in pregnancy and neonatal outcome. Acta Obstet Gynecol Scand. 2008;87(6):617–627.
- Ashoor G, Rotas M, Maiz N, et al. Maternal thyroid function at 11-13 weeks of gestation in women with hypothyroidism treated by thyroxine. Fetal Diagn Ther. 2010;28(1):22–27.
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab. 2012;97(12):4464–4472.
- Lee SY, Cabral HJ, Aschengrau A, et al. Associations between maternal thyroid function in pregnancy and obstetric and perinatal outcomes. J Clin Endocrinol Metab;2019;105(5):e2015-e2023. doi: dgz275 [pii]..