ABSTRACT
This study aimed to establish in vitro hemodilution and resupplementation assays for obstetric hemorrhage in pregnancy-induced hypertension (PIH) and to monitor the coagulation function dynamically using a coagulation and platelet function analyzer. Forty-seven singleton pregnant women were divided into normal (n = 24) and PIH (n = 23) groups. Peripheral blood samples were used to construct the assays, and the activated clotting time (ACT), clotting rate (CR), and platelet function index (PF) were measured. The results showed that the baseline ACT was higher in the PIH group (p < 0.01). Hemodilution assays showed decreased ACT and increased CR and PF, with ACT changes significantly lower in the PIH group (p < 0.05). CR changed most in both groups at lower dilution ratios (35% to 50%), while ACT changed most at a higher dilution ratio (75%). In the resupplementation assay, ACT exhibited the most significant response. The analyzer effectively detected differences between pregnant women with and without PIH. Thus, we need to pay more attention to the changes of ACT in the actual clinical application to assess the coagulation status of parturients.
Introduction
Obstetric hemorrhage is a dangerous and potentially life-threatening complication that can occur during delivery. It is defined as blood loss exceeding 1000 ml within 1 h after delivery or requiring a blood transfusion to sustain life (Citation1). Obstetric hemorrhage can be classified into two types: early obstetric hemorrhage, which occurs during labor or within 24 h after delivery, and delayed obstetric hemorrhage, which occurs more than 24 h after delivery (Citation2). Both types of obstetric hemorrhage pose significant risks to maternal and fetal health and contribute significantly to maternal mortality rates. Thus, obstetric research has long focused on preventing and treating obstetric hemorrhage.
Pregnancy-induced hypertension (PIH) is a critical risk factor for obstetric hemorrhage (Citation3). The mechanisms involved in obstetric hemorrhage caused by PIH during pregnancy mainly include abnormal vascular wall function, abnormal coagulation function, and placental dysfunction. PIH can cause vascular wall damage and functional abnormalities, which can increase blood vessel fragility, making them more susceptible to damage during delivery. Abnormal coagulation functions, such as increased platelet aggregation and thrombus formation, can also increase the risk of endothelial cell damage and vascular wall injury (Citation4). Impaired placental function can affect fetal survival and development, leading to fetal distress and an increased risk of uterine wall vessel rupture during delivery. Healthcare providers should closely monitor blood pressure changes and related conditions in pregnant women with PIH and take necessary intervention measures to reduce the occurrence of obstetric hemorrhage.
When dealing with maternal bleeding in women with PIH, timely and effective therapeutic measures are needed to control the progression of the condition depending on comprehensive and accurate monitoring of their coagulation function. Nevertheless, the traditional coagulation experimental method only tests isolated endpoints from the plasma, and cannot reflect the important role of platelets in the process of hemostasis or the dynamic changes in coagulation (Citation5). Thus, it cannot achieve the purpose of accurate monitoring of coagulation. Blood coagulation and platelet function analysis is a comprehensive method for evaluating blood coagulation function that offers several advantages, such as speed, convenience, and non-invasiveness (Citation6). Additionally, it provides dynamic monitoring of blood clotting and changes in coagulation, which can assist healthcare providers in understanding disease progression and taking necessary intervention measures (Citation6). Nevertheless, so far there are very few reports on the use of this analysis in instances of obstetric bleeding or PIH.
This study aims to address the critical issue of obstetric bleeding in women with PIH by developing in vitro assays and monitoring coagulation function dynamically using the Century Clot coagulation and platelet function analyzer. The objective is to provide experimental evidence and support for subsequent research and practice in this field.
Materials and methods
Patients
This study was approved by the Institutional Review Board (IRB2022-YX-191-01) of Tianjin Medical University General Hospital. Written and informed consent was obtained from all participants included in the current study. Singleton parturients who underwent cesarean section at Tianjin Medical University General Hospital from July 2022 to February 2023 were selected. Among them, there were 24 cases of normal blood pressure group and 23 cases of parturients with PIH.
The diagnostic criteria for PIH are as follows: PIH is diagnosed after 20 weeks of gestation if systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg are found in two consecutive measurements (at least 4 h apart).
Exclusion criteria include: pregnant women with moderate to severe anemia, liver and kidney dysfunction, immune diseases, thyroid diseases, HELLP syndrome, history of coagulation disorders, or use of drugs that affect coagulation within a week.
Sampling
For each patient included in the study, blood was withdrawn via puncture of the antecubital vein with an 18 G needle at the time of intravenous line insertion upon admission to labor and delivery. The samples were collected into 3.0 mL polypropylene tubes containing citrate (BD). One sample was kept undiluted as the control and subjected to baseline measurements. Two other samples were centrifuged at 2600 g for 8 min for blood resupplementation. Blood plasma was separated from the tubes, and the buffy coat layer was removed via pipettes.
In vitro hemodilution assays
We assumed that obstetric hemorrhage causes blood loss and fluid infusion, which alter the concentration and function of coagulation and platelet factors. To conduct in vitro dilution assays, sterile Eppendorf (EP) tubes were prepared using varying volumes of blood and sodium lactate Ringer’s solution. This allowed us to establish the following graded dilution ratios, to simulate different scenarios of hemorrhage and fluid replacement, from mild to severe, as referenced in the literature (Citation7): 20% dilution (0.4 ml blood and 0.1 ml solution), 35% dilution (0.325 ml blood and 0.175 ml solution), 50% dilution (0.25 ml blood and 0.25 ml solution), 65% dilution (0.175 ml blood and 0.325 ml solution), and 75% dilution (0.125 ml blood and 0.375 ml solution).
In vitro resupplementation assays
To conduct in vitro resupplementation assays, a second 75% dilution sample was prepared in a similar manner. We determined the necessary volume of concentrated red blood cells (RBCs) to attain a hematocrit of 27% for each patient, a level deemed acceptable for surgical patients (Citation8,Citation9). Briefly, the combined volume of supplemental RBCs and the original blood cell volume (calculated as 0.5 mL × hematocrit × 0.25) constitutes 27% of the total volume. Concentrated red blood cells and plasma were then added to the dilution sample at a 1:1 ratio to simulate blood transfusion.
Coagulation and platelet function analysis
All measurements were performed with Century Clot blood coagulation and platelet function analyzer (Shijiyikang, Tianjin). Century Clot blood coagulation and platelet function analysis have three main indicators: activated clotting time (ACT), which reflects the function of coagulation factors; clot rate (CR), which reflects the function of fibrin production; and platelet function index (PF), which reflects the functions of platelet adhesion, aggregation, release, contraction, and procoagulation combined. The differences in the levels of these three indices in the peripheral blood of the control and test groups were compared at baseline, at each dilution level, and after blood resupplementation.
In order to correct for differences in the baseline levels and pre-supplementation levels of these indicators, the normalized values of each indicator in the dilution assay were calculated by dividing the original values by the corresponding baseline values, and the normalized values of each indicator in the resupplementation test were calculated by dividing the original values by the corresponding pre-supplementation values.
Statistical methods
Baseline demographic data including age, gestational age, gravidity, height, pre-pregnancy weight, and prenatal weight were collected for all patients. Routine blood data and routine coagulation data were obtained by reviewing the medical records. Mean and standard deviation (SD) were used to measure normally distributed data, and statistical significance was determined with the Student’s t-test. Median and interquartile range (IQR) were used to summarize non-normally distributed data, and statistical significance was determined with the nonparametric Mann–Whitney U-test. Original values of Century Clot were reported as mean and SD and normalized values of Century Clot were reported as median and IQR. SPSS statistics v22.0 software was used for statistical analysis. Values of p ≤ 0.05 were regarded as statistically significant.
Results
General patient information and baseline levels of ACT, CR, and PF in both groups
In total, 24 pregnant women were included in group N and 23 women were included in group H. As shown in , in terms of general characteristics, the pre-pregnancy and antenatal body mass index (BMI) of group H were significantly higher than those of group N, suggesting a positive correlation between obesity and the occurrence of PIH. There were no significant differences in blood routine indicators (red blood cells, hemoglobin, platelets) and traditional coagulation indicators (fibrinogen, D-dimer, thrombin time, prothrombin time, activated partial thromboplastin time) between the two groups ().
Table 1. Comparison between the general characteristics, routine blood test indicators, and traditional coagulation indicators of the two groups.
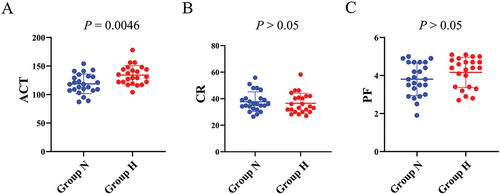
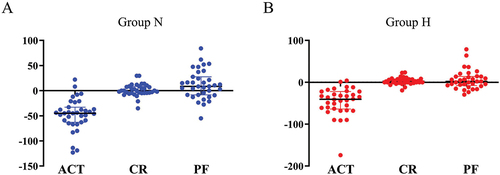
We conducted a comparison of the baseline levels of ACT, CR, and PF between two groups. We observed that the ACT levels were significantly elevated in group H as compared to group N (mean ± SD, group N: 118.7 ± 17.3, group H: 134.0 ± 16.8, p = 0.0046). However, the differences in the levels of CR (mean ± SD, group N: 37.7 ± 7.4, group H: 36.6 ± 7.6, p = 0.51) and PF (mean ± SD, group N: 3.8 ± 0.9, group H: 4.2 ± 0.8, p = 0.18) between the two groups were not statistically significant ().
Figure 1. Comparison of baseline levels of ACT, CR and PF between the two groups.
Comparison of ACT, CR, and PF between the two groups in the dilution assay
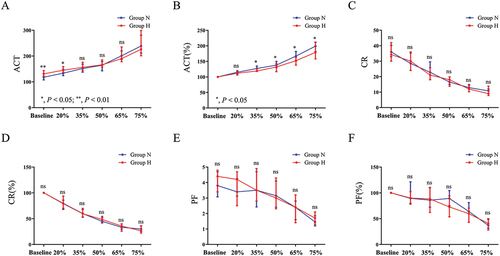
To determine differences in the effect of hemodilution on these three indicators between the two groups, we compared the original and normalized values of ACT, CR, and PF between the two groups at varying dilution ratios. Following hemodilution, both groups exhibited increased changes in their original ACT values (). The normalized values of ACT were lower in group H than in group N at the same dilution ratios, and this difference was statistically significant at dilution ratios ranging from 35% to 75% (). After blood dilution, the original values of CR and PF of the blood of the women in both groups showed a decrease, but there was no significant difference in the normalized values of CR () and PF () in both groups at all dilution ratios.
Figure 2. Comparison of ACT, CR and PF between the two groups in the hemodilution assay.
Subsequently, we calculated the percentage changes in the normalized values of three indicators under different dilution ratios. Next, we calculated the median (absolute) values of the changes. By cross-sectionally comparing the trends of the changes of three indicators in different degrees of hemodilution, we found that in dilution ratios ranging from 35% to 50%, CR exhibited the most significant change in both groups, followed by ACT, with PF showing the smallest change. However, the change in ACT increased under high percentage dilution conditions. Under 75% dilution conditions, ACT exhibited the greatest change in both groups, followed by CR, while PF remained the least variable ().
Table 2. Cross-sectional comparison of the normalized values of ACT, CR, and PF in the two groups at different dilution concentrations.
These data suggest that the trends in ACT during hemodilution were significantly different between the two groups, with group H showing relatively less sensitivity to hemodilution. Furthermore, under extreme hemodilution conditions, the most significant changes in ACT parameters were observed in both groups.
Evaluation of ACT, CR, and PF between the two groups in the resupplementation assay
In clinical practice, particularly for patients with massive bleeding, a 1:1 ratio of red blood cells to plasma is commonly used for blood supplementation. In our blood resupplementation research assay, we transfused blood components at a 1:1 ratio of red blood cells to plasma and then evaluated changes in each parameter of blood coagulation and platelet function analysis. To determine if there were any differences in the effects of blood resupplementation on ACT, CR, and PF between the two groups, we calculated the percentage change in the normalized values of the three indicators before and after resupplementation to compare the levels of change between the two groups. Our results showed that there were no significant differences in the changes in ACT, CR, and PF levels between the two groups (). However, when we compared the levels of change in the three indicators before and after blood resupplementation, we found that the average change in ACT was the most significant in both groups (). These data suggest that during blood resupplementation, the ACT exhibited the most significant response, but the overall response was not significantly different between the two groups.
Figure 3. Changes comparison of ACT, CR and PF between the two groups.
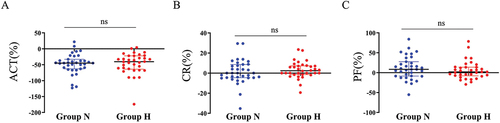
Figure 4. Cross-sectional comparison of the normalized values of ACT, CR and PF in the two groups after blood resupplementation.
Discussion
In pregnancy, PIH usually occurs after 20 weeks of gestation and is a common complication that poses a significant risk for obstetric hemorrhage. Meanwhile, abnormal coagulation is an important factor linking PIH and obstetric hemorrhage. Patients with PIH have an increased risk of platelet aggregation and thrombosis due to damage to the vascular endothelium and enhanced inflammatory response, leading to the development of coagulation abnormalities, which are mainly characterized by abnormal coagulation factors, defective platelet aggregation, and abnormal fibrinolysis (Citation10). Therefore, timely monitoring of coagulation function, detecting abnormalities, and taking appropriate therapeutic measures are crucial in the prevention and treatment of obstetric hemorrhage. In this study, we initially established a hemodilution and resupplementation assay of obstetric hemorrhage with PIH and monitored changes in coagulation function using a coagulation and platelet function analyzer.
Coagulation and platelet function analysis has not been widely used in clinical settings, including obstetrics. In this field, most research has been restricted to observations in healthy pregnant women (Citation11). Pregnant women with PIH exhibit additional coagulation abnormalities, which require heightened attention. In this study, we found that baseline levels of blood ACT were significantly higher in the PIH group than in the normal group. This suggests a more pronounced risk of bleeding in hypertensive women during pregnancy compared to normal women (Citation12–14). It is known that PIH is related to platelet dysfunction (Citation15). Platelet activation triggers the release of platelet-derived factors, including Platelet-Derived Growth Factor (PDGF) and Transforming Growth Factor-beta (TGF-β) (Citation16). These factors have been implicated in the pathophysiology of PIH and preeclampsia (Citation17–19). A more comprehensive exploration of these factors in future studies holds promise for enhancing our understanding and management of PIH.
Hemodilution-induced coagulation dysfunction refers to a decrease in the concentration of coagulation factors within the blood, resulting in impaired clotting ability (Citation20). During pregnancy, women experience a significant increase in blood volume, leading to the dilution of these essential factors. In clinical scenarios involving hemorrhage, transfusing large volumes of non-blood products without timely supplementation of RBC, plasma, platelets, and other coagulation factors can exacerbate hemodilution-induced coagulation dysfunction, potentially exacerbating blood loss (Citation21). Consequently, it becomes crucial to investigate the dynamic changes in maternal blood coagulation function across varying degrees of blood dilution ratios, particularly among women with PIH (Citation22). In our study, we simulated varying degrees of blood dilution in vivo using dilution ratios ranging from 20% to 75%. The ACT increased after blood dilution in both women with and without PIH. However, intriguingly, under the same dilution ratio, the percentage increase in ACT was significantly lower in women with PIH compared to those without PIH, particularly when blood was diluted to levels between 35% and 75%. This “inversion” phenomenon occurred after dilution to 35%, the post-dilution ACT levels were significantly lower in women with PIH than in normotensive women, despite their higher baseline ACT levels, suggesting that ACT is relatively insensitive to blood dilution in women with PIH. Additionally, CR changed most in both groups at lower dilution ratios (35% to 50%), while ACT changed most at a higher dilution ratio (75%). In the resupplementation assay, ACT exhibited the most significant response. These are some of the issues that should be noted in the clinical use of coagulation and platelet function analysis in obstetrics.
This study has several limitations. The small sample size employed may have impacted the reliability and stability of our statistical results. Consequently, we were unable to grade the severity of PIH, representing an additional constraint. Moreover, the complexity of coagulation changes cannot be overlooked. However, our study focused solely on modeling dilutional coagulation dysfunction in vitro, neglecting other systemic influences on coagulation. Finally, the blood resupplementation assay used in this study did not include platelet resupplementation, which differs from actual clinical practice and may have biased the findings. In future studies, we will use more rigorous and comprehensive research methods to address these limitations.
To summarize, we developed an assay of hemodilution and resupplementation in PIH and demonstrated that the coagulation and platelet function analyzer can distinguish between pregnant women with and without PIH, both before and after blood dilution. ACT is the main parameter that differentiates the two groups. Therefore, we should monitor this parameter closely in the clinical practice to evaluate the coagulation status of parturients.
Article highlights
Established dynamic coagulation assays for obstetric hemorrhage in PIH.
Baseline ACT was elevated in PIH, indicating coagulation dysfunction.
Hemorrhage simulation showed distinct coagulation responses between PIH and normal groups.
Coagulation and platelet function analyzer distinguished coagulation status in PIH effectively.
Acknowledgments
The authors thank all participants included in this study and the personnel who collected the data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used during the current study are available from the corresponding authors on reasonable requests.
Additional information
Funding
References
- O’Brien KL, Shainker SA, Lockhart EL. Transfusion management of obstetric hemorrhage. Transfus Med Rev. 2018;32(4):249–8. doi: 10.1016/j.tmrv.2018.05.003
- Feduniw S, Warzecha D, Szymusik I, et al. Epidemiology, prevention and management of early postpartum hemorrhage — a systematic review. Ginekol Pol. 2020;91(1):38–44. doi: 10.5603/GP.2020.0009
- Stones RW, Paterson CM, Saunders NJ. Risk factors for major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 1993;48(1):15–18. doi: 10.1016/0028-2243(93)90047-G
- Durmaz A, Komurcu N. Relationship between maternal characteristics and postpartum hemorrhage: a meta-analysis study. J Nurs Res. 2018;26(5):362–372. doi: 10.1097/jnr.0000000000000245
- Ågren A, Wikman AT, Holmström M, et al. Thromboelastography (TEG®) compared to conventional coagulation tests in surgical patients–a laboratory evaluation. Scand J Clin Lab Invest. 2013;73(3):214–220. doi: 10.3109/00365513.2013.765960
- Wan P, Yu M, Qian M, et al. Sonoclot coagulation analysis: a useful tool to predict mortality in overt disseminated intravascular coagulation. Blood Coagulat & Fibrinoly: An Int J In Haemostas Thrombos. 2016;27(1):77–83. doi: 10.1097/MBC.0000000000000345
- Katz DJ, Hira SK, Sison ML, et al. Impact of fibrinogen and prothrombin complex concentrate on clotting time in a model of obstetric hemorrhage. J Clin Anesth. 2022;78:110687. doi: 10.1016/j.jclinane.2022.110687
- Messmer KF. Acceptable hematocrit levels in surgical patients. World J Surg. 1987;11(1):41–46. doi: 10.1007/BF01658458
- Lundsgaard-Hansen P. Safe hemoglobin or hematocrit levels in surgical patients. World J Surg. 1996;20(9):1182–1188. doi: 10.1007/s002689900180
- Shamshirsaz AA, Paidas M, Krikun G. Preeclampsia, hypoxia, thrombosis, and inflammation. J Pregnancy. 2012;2012:1–6. doi: 10.1155/2012/374047
- Kjellberg U, Hellgren M. Sonoclot signature during normal pregnancy. Intensive care Med. 2000;26(2):206–211. doi: 10.1007/s001340050047
- Zhang Y, Li H, Guo W, et al. Predictive value of coagulation function and D-dimer for pregnancy outcome in pregnancy-induced hypertension. Am J Transl Res. 2023;15(2):1150–1158.
- Li &, Wang &, Wang &, et al. Correlation of component blood transfusion of hypertension patients with pregnancy and postpartum hemorrhage. Clin Lab. 2021;67(07/2021):67. doi: 10.7754/Clin.Lab.2020.200849
- Shi F, Yu A, Yuan L. Clinical significance of detection of coagulation indexes, immune factors and inflammatory factors in patients with pregnancy-induced hypertension syndrome in China. ijph. 2019;48:681–687. doi: 10.18502/ijph.v48i4.989
- Lambert G, Brichant JF, Hartstein G, et al. Preeclampsia: an update. Acta Anaesthesiol Belg. 2014;65(4):137–149.
- Liu Y, Kalén A, Risto O, et al. Time- and pH-dependent release of PDGF and TGF-ß from platelets in vitro. Platelets. 2003;14(4):233–237. doi: 10.1080/0953710031000118876
- Morita H, Mizutori M, Takeuchi K, et al. Abundant expression of platelet-derived growth factor in spiral arteries in decidua associated with pregnancy-induced hypertension and its relevance to atherosis. Eur J Endocrinol. 2001;144:271–276. doi: 10.1530/eje.0.1440271
- Muy-Rivera M. Transforming growth factor-β1 (TGF-β1) in plasma is associated with preeclampsia risk in Peruvian women with systemic inflammation. Am J Hypertens. 2004;17(4):334–338. doi: 10.1016/j.amjhyper.2003.12.010
- Yang D, Dai F, Yuan M, et al. Role of transforming growth factor-β1 in regulating fetal-maternal immune tolerance in normal and pathological pregnancy. Front Immunol. 2021;12:689181. doi: 10.3389/fimmu.2021.689181
- Nair S, Nair BR, Vidyasagar A, et al. Importance of fibrinogen in dilutional coagulopathy after neurosurgical procedures: a descriptive study. Indian J Anaesth. 2016;60(8):542–545. doi: 10.4103/0019-5049.187778
- Getrajdman C, Sison M, Lin HM, et al. The effects of hemodilution on coagulation in term parturients: an in vitro study utilizing rotational thromboelastometry. J Matern-Fetal Neonatal Med. 2022;35(10):1969–1977. doi: 10.1080/14767058.2020.1776250
- Tanaka KA, Bharadwaj S, Hasan S, et al. Elevated fibrinogen, von Willebrand factor, and factor VIII confer resistance to dilutional coagulopathy and activated protein C in normal pregnant women. Br J Anaesth. 2019;122(6):751–759. doi: 10.1016/j.bja.2019.02.012