ABSTRACT
The appropriate target blood pressure (BP) in elderly patients with hypertension remains uncertain. We investigated the relationship between morning home systolic blood pressure (MHSBP) during follow-up and cardiovascular (CV) risk in outpatients receiving olmesartan-based treatment aged <75 years (n = 16799) and ≥75 years (n = 4792) in the HONEST study. In the follow-up period (mean 2.02 years), the risk for major CV events was significantly higher in patients with MHSBP ≥155 mmHg compared with <125 mmHg in both age groups in Cox proportional hazards model adjusted for other risk factors and there was no significant difference in trend between the two groups (interaction P = 0.9917 for MHSBP). Hazard ratios for CV events for 1-mmHg increase in MHSBP were similar in patients aged <75 years and in patients aged ≥75 years. The incidence of adverse drug reactions related to excessive BP lowering was lower in patients <75 years than in patients ≥75 years (0.73 vs 1.02%, P = 0.0461).
In conclusion, the study suggests even in patients ≥75 years antihypertensive treatment targeting the same MHSBP levels in patients <75 years may be beneficial in reducing CV risk when treatment is tolerated.
Introduction
Antihypertensive treatment reduces cardiovascular (CV) morbidity and mortality in very elderly patients with hypertension, as it does in patients of other age groups (Citation1). In hypertension guidelines in Japan and western countries (Citation2–Citation5), target systolic blood pressure (SBP) levels for very elderly patients are higher than younger patients and it is common practice in a clinical setting. The Japanese guideline (Citation2) recommends further reduction of SBP when treatment is tolerated. The Systolic Blood Pressure Intervention Trial (Citation6) to compare intensive treatment (target SBP < 120 mmHg) or standard treatment (target SBP <140 mmHg) in participants aged 75 years or older showed intensive treatment resulted in significantly lower CV events than standard treatment.
Home blood pressure (HBP) is useful to evaluate out-of-clinic blood pressure (BP) control, and it is also reproducible (Citation7). It is well known that HBP measurements provide a more accurate prognosis for survival compared with clinic blood pressure (CBP) (Citation8–Citation11). However, there are few studies to evaluate the optimal on-treatment HBP in very elderly hypertensive patients.
The Home blood pressure measurement with Olmesartan Naive patients to Establish Standard Target blood pressure (HONEST) study is a large-scale prospective observational study involving >20 000 outpatients receiving olmesartan-based antihypertensive treatment for 2 years. The principal results have demonstrated reduction of morning home systolic blood pressure (MHSBP) to <145 mmHg is beneficial for prevention of major CV events (Citation12), and the importance of monitoring HBP in clinical practice.
In this pre-specified subanalysis of the HONEST study, we further investigated the relationship between MHSBP during the follow-up period and the incidence of major CV events in patients aged ≥75 years, compared with those aged <75 years.
Methods
Study design
The rationale, design, and study management of the HONEST study have already been reported (Citation13). The HONEST study population comprised patients receiving olmesartan-based treatment for hypertension. The study is registered at the UMIN Clinical Trials Registry (http://www.umin.ac.jp/ctr/index.htm) with the unique trial number of UMIN000002567.
The study protocol was approved by the Ethical Committee of Daiichi Sankyo Co., Ltd and the research ethics committees of the participating institutions, at their discretion. The study was approved by the Ministry of Health, Labour, and Welfare of Japan before it began and conforms to the country’s pharmaceutical affairs laws. In compliance with Good Post-marketing Study Practices in Japan, the study was performed at registered medical institutions.
The study was conducted between October 2009 and September 2012.
Patients
Olmesartan-naive patients with essential hypertension who had their morning HBP recorded for ≥ 2 days and their CBP recorded for ≥ 1 day in the 28 days before starting olmesartan were eligible to participate. The patients seen in the outpatient clinic were enrolled in the study after providing written informed consent and being prescribed olmesartan. Those who had had a CV event in the previous 6 months were excluded, as well as those with a planned cardiovascular intervention or serious hepatic or renal dysfunction. The selection of CBP and HBP targets was at the discretion of individual physicians. No restrictions were placed on prior antihypertensive drug treatment, except for prior use of olmesartan, or on the use of combination antihypertensive drug treatment during the study. Follow-up was maintained for patients who discontinued treatment with olmesartan.
Measurement of home blood pressure
Patients who already possessed a cuff-oscillometric device were enrolled in the study. All such devices available in Japan have been validated and approved by the Ministry of Health, Labour and Welfare. The devices also comply with the Association for the Advancement of Medical Instrumentation (AAMI) (Citation14) or European standards (Citation15). At the time that informed consent was obtained, the patients were asked to measure their HBP twice in the morning (within 1 h of waking, after urination, before taking any morning medications, before breakfast, and after 1‒2 min of rest in a sitting position) and twice at bedtime (after 1‒2 min of rest in a sitting position), according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009) (Citation16). Participating patients in the present study received a pocket diary to record their HBP. Physicians instructed the patients to report their HBP obtained on the pre-specified measurement day and the day before (when this was not possible, on 2 days in the week prior to or after the pre-specified measurement day). Details of the timing and frequency of measurements were indicated in the informed consent form and the pocket diary.
During the follow-up, the HBP was measured at 1, 4, and 16 weeks and at 6, 12, 18, and 24 months. In principle, patients reported their HBP that were measured twice in the morning and twice at bedtime on 2 different days for each of these measurement points. We calculated the average of the 2 HBP measurements at each time. Then, for each measurement point, we used the average of the HBP over 2 days. For patients who had CV events, the average of BP values obtained until the first occurrence of such events was used for the analysis. HBP was measured both in the morning and in the evening. However, we used only morning HBP (MHBP) in the present analysis. CV events tend to occur frequently in the morning, along with a rise in blood pressure (Citation17).
Measurement of clinic blood pressure
CBP was measured at 4 and 16 weeks and 6, 12, 18, and 24 months. For each measurement point, 1 measurement was reported. CBP was measured according to the usual methods of each study institution. Analyses of the relationship of CBP with the incidence of CV events were performed using the same method used for HBP.
Endpoints and adverse events
The primary endpoint of the HONEST study was a composite of cerebrovascular events (cerebral infarction, intracerebral hemorrhage, subarachnoid hemorrhage, unclassified stroke), cardiac events (myocardial infarction, coronary revascularization procedures for angina pectoris), or sudden death. All CV events and suspected CV events were adjudicated by three Event Review Committees.
Adverse events were monitored throughout the follow-up period.
Statistical analysis
The analysis population comprised eligible patients who received olmesartan at least once during the treatment period. We divided the population into two groups by age at baseline: <75 years and ≥75 years.
Patient characteristics were reported as the mean ± standard deviation (SD) or percentage. Chi-squared test for the categorical data and Student’s t-test for the continuous data were used to compare the patient characteristics between two groups. The incidence rates for CV events in two groups were estimated using the person-years method and compared with Poisson regression. The Cox proportional hazards model was used to compare the relationship between BP during follow-up and CV risk in each age group. The models included BP, age, and interaction between BP and age as factors, sex, family history of CV disease, dyslipidemia, diabetes mellitus, chronic kidney disease, and history of CV diseases and smoking status as adjustment factors. The proportions of patients with adverse drug reactions were compared using the chi-squared test. All statistical tests were 2-sided, using a significance level of 0.05. SAS release 9.2 software (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Baseline characteristics and changes in BP
The baseline characteristics of the patients are shown in . The HONEST study included 21591 patients, of whom 16799 patients were aged <75 years and 4792 patients were aged ≥75 years. Baseline characteristics were different between patients aged <75 years and patients aged ≥75 years as expected with some exceptions such as dyslipidemia, diabetes, and MHSBP.
Table 1. Baseline characteristics of the study patients.
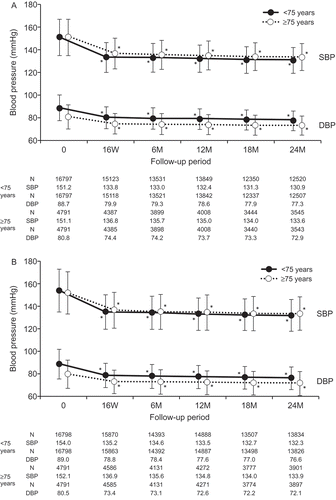
shows the changes in MHBP (A) and CBP (B) in patients aged <75 years and in patients aged ≥75 years. During the follow-up period, MHBP (mean±SD) values were 134.6 ± 10.7/80.2 ± 8.2 mmHg and 137.2 ± 10.7/74.7 ± 7.7 mmHg and CBP values were 134.9 ± 11.5/78.6 ± 8.3 mmHg and 136.2 ± 11.6/73.2 ± 8.1 mmHg in patients aged <75 years and in patients aged ≥75 years, respectively. The numbers of antihypertensive drugs used at baseline and at the study completion were 1.5 ± 0.7 and 1.5 ± 0.9, respectively, in patients aged <75 years, and 1.7 ± 0.8 and 1.6 ± 1.0, respectively, in patients aged ≥75 years.
Cardiovascular outcomes and adverse events
shows the number and incidence of CV events. Primary endpoint occurred in 280 patients (incidence, 6.46/1000 person years). The incidence of the primary endpoint in patients aged <75 years was lower than in patients aged ≥75 years (5.08 vs. 11.38 per 1000 person years, P < 0.0001).
Table 2. Cardiovascular events during follow-up period.
shows the relationship between MHSBP (A) or clinic systolic BP (CSBP) (B) during the follow-up period and the primary endpoint. The risk for primary events increased along with increased MHSBP during follow-up (P for trend: P < 0.0001 for aged <75 years and P < 0.0001 for aged ≥75 years) and CSBP during follow-up (P for trend: P < 0.0001 for aged <75 years and P < 0.0001 for aged ≥75 years) in both age groups without difference in trend between patients aged <75 years and those aged ≥75 years (interaction P = 0.9917 for MHSBP; P = 0.9032 for CSBP).
Figure 2. Relationship between the major cardiovascular event and morning home systolic blood pressure (A; reference, <125 mmHg) or clinic systolic blood pressure (B; reference, <130 mmHg) during follow-up period in patients aged <75 years and ≥75 years. The Cox proportional hazards model was used, adjusting for sex, family history of cardiovascular disease, dyslipidemia, diabetes mellitus, chronic kidney disease, history of cardiovascular disease, and smoking status. *P< 0.05 (vs reference). HR: hazard ratio; CI: confidence interval.
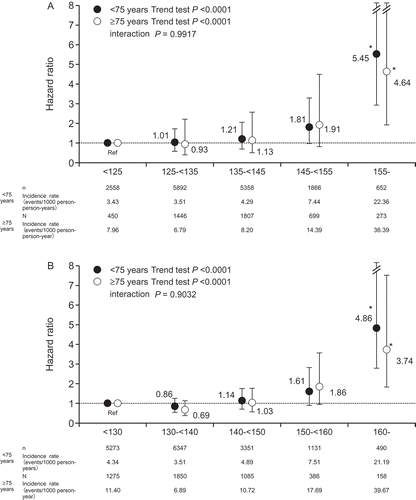
When MHSBP <125 mmHg and CSBP <130 mmHg was defined as reference value, the risk for the primary endpoint tended to increase from MHSBP ≥145 mmHg and CSBP ≥150 mmHg, and a significant increase was observed at MHSBP ≥155 mmHg and CSBP ≥160 mmHg in both patients aged <75 years and patients aged ≥75 years.
In Cox proportional hazards model adjusted for other CV risk factors, hazard ratios (HRs) for CV events for 1-mmHg increase in MHSBP and CSBP were similar in patients aged <75 years and in patients aged ≥75 years (MHSBP 1.040 (95% confidence interval (CI) 1.027–1.054) and CSBP 1.025 (95%CI 1.012–1.038) vs MHSBP 1.038 (95%CI 1.021–1.054) and CSBP 1.027 (95%CI 1.012–1.043) without difference in trend between patients aged <75 years and those aged ≥75 years (interaction P = 0.8265 for MHSBP; P = 0.8279 for CSBP).
To examine the potential influence of patients’ sex, we conducted additional analyses stratified by sex regarding the relationship between BP during the follow-up period and the CV events as well as the HRs for CV events for 1-mmHg increase. Consequently, there was no interaction between BP and age in both men and women (data not shown).
The incidence of adverse drug reactions (ADRs) related to excessive BP lowering (i.e., dizziness, postural dizziness, hypotension, orthostatic hypotension and decreased BP) was significantly lower in patients aged <75 years than in patients aged ≥75 years. (0.73% vs. 1.02%, P = 0.0461). ADRs with a significantly higher incidence in patients aged ≥75 years than those aged <75 years were orthostatic hypotension (0.02% vs. 0.08%, P = 0.0261) and decreased BP (0.15% vs. 0.29%, P = 0.0393) ().
Table 3. Adverse drug reactions associated with excessive blood pressure lowering by age (reported by doctors in charge).
Discussion
We investigated the relationship between MHSBP or CSBP during follow-up and risk for major CV events in patients aged ≥75 years compared with those aged <75 years. The CV risk increased along with increased MHSBP and CSBP in both groups without a significant difference between patients aged <75 years and those aged ≥75 years. In further analyses, HRs for CV events for 1-mmHg increase in MHSBP and CSBP were similar in patients aged <75 years and patients aged ≥75 years.
The relationship between baseline HBP and CV events in subjects aged 80 years or older was studied in the International Database on Home Blood Pressure in Relation to Cardiovascular Outcome (IDHOCO) (Citation18). They stratified subjects according to treatment status at baseline and found that systolic HBP ≥152.4 mmHg was associated with increased CV risk in untreated subjects. In treated subjects, CV risk was quadratically associated with systolic HBP with a nadir at 148.6 mmHg. Out of clinic BP was evaluated in the Hypertension in the Very Elderly Trial (HYVET) study (Citation1). Active treatment was associated with a reduction in total mortality and CV events. Ambulatory blood pressure (ABP) was measured in 284 participants in the HYVET study (Citation19). During follow-up, the 24-hour ABP was 131 ± 17/77 ± 10 mmHg in the placebo and 123 ± 18/72 ± 10 mmHg in the active treatment groups, respectively.
Our result that CV risk increased with increased MHSBP in treated patients aged ≥75 years is in line with the HYVET substudy (Citation19).
The Japanese Society of Hypertension Guidelines for the Management of Hypertension 2014 (JSH 2014) recommends target HBP<145/85 mmHg and CBP <150/90 mmHg in patients aged ≥75 years, and when treatment is well tolerated the same target HBP and CBP for patients aged <75 years is recommended (Citation2). Parati et al. (Citation20) suggested a target HBP of 135/85 mmHg irrespective of age.
The results of additional analyses stratified by sex regarding the relationship between BP during follow-up period and the CV events in patients aged <75 years and in patients aged ≥75 years showed no interaction between BP and age in both men and women, which were consistent with the results of the previous studies (Citation21–Citation23).
Some limitations of the present study should be considered. Firstly, this study was a prospective observational study without a comparator group and was not a randomized, controlled study. Selection of the target BP level, use of concomitant drugs, etc., were left to the discretion of individual physicians in charge. Second, the present study enrolled patients who had already owned cuff-oscillometric devices to measure their HBP, and no specific types of device were used. Third, in the present study, only 2 days of HBP values were used for each measurement point to reduce the burden on patients and physicians in collecting these measurements. The possibility of biased HBP reporting cannot be excluded completely, although potential selection bias is reduced with many patients. Fourth, the follow-up period was relatively short. If the follow-up period was longer, a significant difference in the risk of CV events at the MHSBP ≥ 145 to <155 mmHg and the CSBP ≥150 to 160 mmHg may have been observed. Populations of elderly individuals are often highly heterogeneous and vary widely in functional ability (Citation24). Finally, the generalizability of the HONEST study data that enrolled only patients in the outpatient clinic to clinical practice remains problematic and controversial. Despite such limitations, there has been no previous study that involved about 4800 elderly patients aged ≥75 years receiving antihypertensive treatment, and followed for 2 years to investigate the relationship between on-treatment HBP and CV risk by age groups. The results of the HONEST study provide valuable real-world information about the olmesartan-based treatment of very elderly hypertensive patients in ordinary clinical practice.
In conclusion, the study suggests even in patients ≥75 years antihypertensive treatment targeting the same CSBP and MHSBP levels in patients <75 years may be beneficial in reducing CV risk when treatment is tolerated.
Declaration of Interest
IS, KK, TK, ST, and KS have received lecture fees and honoraria for writing the manuscript from Daiichi Sankyo Co., Ltd. MY, NZ, YM, and YO are employees of Daiichi Sankyo Co., Ltd.
Acknowledgments
We gratefully acknowledge the numerous study investigators, fellows, nurses, and research coordinators at each of the study institutions, who have participated in the HONEST Study. We also gratefully acknowledge the contribution of the patients to the study.
Additional information
Funding
References
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98.
- Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–392.
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–357.
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20.
- Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American college of cardiology foundation task force on clinical expert consensus documents developed in collaboration with the American academy of neurology, American geriatrics society, American society for preventive cardiology, American society of hypertension, American society of nephrology, association of black cardiologists, and European society of hypertension. J Am Soc Hypertens. 2011;5:259–352.
- Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–82.
- Imai Y, Obara T, Asayama K, Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36:661–72.
- Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–75.
- Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–83.
- Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55:1346–51.
- Shimada K, Kario K, Kushiro T, Teramukai S, Zenimura N, Ishikawa Y, et al. Prognostic significance of on-treatment home and clinic blood pressure for predicting cardiovascular events in hypertensive patients in the HONEST study. J Hypertens. 2016;34:1520–27.
- Kario K, Saito I, Kushiro T, Teramukai S, Ishikawa Y, Mori Y, et al. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy. Primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension. 2014;64:989–96.
- Saito I, Kario K, Kushiro T, Teramukai S, Zenimura N, Hiramatsu K, et al. Rationale, study design, baseline characteristics and blood pressure at 16 weeks in the HONEST Study. Hypertens Res. 2013;36:177–82.
- Association for the Advancement of Medical Instrumentation. American National Standard. Electronic or Automated Sphygmomanometers. ANSI/AAMI SP 10-1992. Arlington, VA: AAMI; 1993. 40p
- European Committee for Standardization. Non-invasive sphygmomanometers. Part 3. Supplementary requirements for electromechanical blood pressure measuring systems. British Standard BS EN 1060-3: 1997. European Standard EN 1060-3 1997. Brussels, Belgium: European Committee for Standardization; 1997.
- Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res. 2009;32:3–107.
- Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–43.
- Aparicio LS, Thijs L, Boggia J, Jacobs L, Barochiner J, Odili AN, et al. Defining thresholds for home blood pressure monitoring in octogenarians. Hypertension. 2015;66:865–73.
- Bulpitt CJ, Beckett N, Peters R, Staessen JA, Wang JG, Comsa M, et al. Does white coat hypertension require treatment over age 80? Hypertension. 2013;61:89–94.
- Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al. European society of hypertension guidelines for blood pressure monitoring at home: a summary report of the second international consensus conference on home blood pressure monitoring. J Hypertens. 2008;26:1505–26.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
- Murakami Y, Hozawa A, Okamura T, Ueshima H; Evidence for Cardiovascular Prevention From Observational Cohorts in Japan Research Group (EPOCH-JAPAN). Relation of blood pressure and all-cause mortality in 180000 Japanese participants: pooled analysis of 13 cohort studies. Hypertension. 2008;51:1483–91.
- Oparil S, Davis BR, Cushman WC, Ford CE, Furberg CD, Habib GB, et al. Mortality and morbidity during and after antihypertensive and lipid-lowering treatment to prevent heart attack trial: results by sex. Hypertension. 2013;61:977–86.
- Muller M, Smulders YM, De Leeuw PW, Stehouwer CDA. Treatment of hypertension in the oldest old. A critical role for frailty? Hypertension. 2014;63:433–41.