ABSTRACT
Objective: Cerebral microbleeds (CMBs), which appear as small dot-like hypointense lesions, are strongly associated with cerebrovascular disease. Recently, numerous investigations have suggested that hypertension and age are risk factors for CMBs; however, whether blood pressure grade and age rank are related to the severity of CMBs remains unclear. The purpose of this research was to assess the association between cerebral microbleeds and blood pressure levels.
Methods: In total, 460 consecutive hypertension patients (214 males and 246 females; aged 44–96 years, mean age 60.95 ± 6.82 years) from Lishui Central Hospital were enrolled and classified as CMB or non-CMB patients according to magnetic resonance imaging (MRI). Gradient echo T2*-weighted MRI was used to detect CMBs. Differences in blood pressure, CMB severity, and other patient characteristics were compared between the two groups. Multifactorial logistic regression was used to analyze the correlation between blood pressure and microbleeds.
Results: In our study, CMB lesions were identified in 123 patients (26.7%), including 39 patients with CMB lesions located deep in the brain. In the hypertensive population, smoking is an independent risk factor for CMBs. Additionally, systolic blood pressure (SBP), diastolic blood pressure (DBP) and age are also independent risk factors for CMBs. Furthermore, a modest correlation was noted between the number of microbleeds and grade of hypertension.
Conclusions: This study provides novel evidence that microbleed severity is associated with hypertension grade. This conclusion emphasizes the importance of antihypertensive therapy in hypertension patients to avoid an increase in CMBs.
Introduction
Cerebral microbleeds (CMBs) are subclinical brain lesions with the primary characteristic of minor hemorrhage in tiny blood vessels. Patients with CMBs have no distinctive clinical signs or symptoms. CMBs are well-defined, MRI-detectable brain lesions consisting of small perivascular hemosiderin deposits that are observable using T2*-weighted gradient echo or susceptibility- weighted imaging (Citation1). In 1868, when CMBs were first confirmed by histopathological evidence, doctors mistakenly believed that CMBs caused intracranial hemorrhage (ICH), given the lack of detection methods. Gradient echo T2-weighted MRI exhibits high sensitivity in detecting CMBs. In 1996, a study based on gradient-echo T2-weighted imaging (GRE-T2*WI) reported that 33% (39 in 120 cases) of primary intracerebral hemorrhage patients exhibited focal hemosiderin deposition (Citation2).
CMB is also associated with intracerebral hemorrhage (ICH), which accounts for 20–30% of strokes in Asian countries such as Korea and Japan (Citation3). In one study, 139 patients with CMB lesions or hemorrhage that occurred in the region of CMBs were found among 2019 idiopathic ICH patients, including four patients (2.9%) who experienced symptomatic ICH (Citation4). The latest research suggests that CMB is an independent risk factor for symptomatic cerebral stroke and that asymptomatic CMBs might predict secondary prevention of cerebral infarction (Citation5,Citation6). CMBs occur easily in patients who have cerebral microvascular diseases such as arteriosclerosis, vascular fiber hyaline degeneration or cerebral amyloid angiopathy (Citation7,Citation8). The results of Akoudad (Citation9) and Hilar (Citation10) show that the number and distribution of CMB were closely related to cognitive impairment and dementia. CMB lesions had no significant effect on cognitive function, and their correlation was independent of other cerebrovascular diseases, such as lacunar infarction, Death and white matter lesions.
Moreover, CMBs are often found around injured arterioles or microaneurysms, so we hypothesized that small vascular lesions might play an essential role in CMBs. As reported, CMBs are closely related to high blood pressure, high levels of blood glucose and a history of smoking, which are also risk factors of arteriole lesions (Citation11). In addition, CMBs are associated with age and fibrinogen level (Citation12,Citation13). However, a Framingham study including 472 subjects suggested that CMBs are associated with advanced age and sex but not with blood pressure when the impacts of age and sex are removed. Moreover, no significant relationships were noted between CMBs and cholesterol levels, smoking and diabetes (Citation14). These results remain contradictory, possibly due to population differences. One report indicated that cerebral infarction patients are more prone to CMBs after taking anticoagulant drugs or antiplatelet medicine (Citation15). In one study, 41 patients were treated with thrombolytic therapy, and the researchers suggested that CMBs might be a risk factor for ICH after thrombolytic therapy (Citation16). The risk and fatality rate of cerebral hemorrhage secondary to the number of CMB lesions has been proposed to increase, particularly in patients who have greater than five CMB lesions (Citation17). Further studies of CMBs may play an essential role in guiding the selection of appropriate therapy programs, such as anticoagulation or thrombolytic therapy, for acute stroke and CMBs.
CMBs have been studied for decades, but controversy still exists regarding the risk factors for CMBs, as limited samples from different populations are available. To date, few researchers have investigated the correlation between CMB severity and different hypertension grades. In our study, we classified hypertension patients according to the number of CMB lesions, blood pressure level, and age. Then, we explored the correlation of CMB severity with BP grade and age rank. Moreover, we investigated multiple factors that impact CMB occurrence and severity, and we further examined essential CMB factors.
Materials and methods
Study design and subjects
A total of 460 hypertension patients (214 males and 246 females; aged 44–96 years, mean age 60.95 ± 6.82 years) were recruited from March 2009 to February 2013 from Lishui Central Hospital. Participants were considered to have hypertension if they had a self-reported history of hypertension, a history of antihypertensive medication use, or a BP ≥140/90 mmHg at the visit time. All patients underwent GRE-T2*WI. Patients were divided into two groups based on MRI results: 123 patients had CMBs, and 337 patients were classified as non-CMBs. The following exclusion criteria were employed: bleeding-prone disease, brain tumor, trauma, cavernous hemangioma, brain aneurysm and arteriovenous fistula. The ethics committee of the hospital approved this study, and all the patients or their relatives signed informed consent. All patients were scanned by Signa HD 1.5 T magnetic resonance imaging (Siemens, Germany) with a GRE-T2*WI gradient echo sequence based on the following parameters: 800 ms repetition time, 20–50 ms echo time, 20–30 flip angles, 256 × 256 matrix, 240 × 100 vision, 7-mm scan slice thickness, and 2.5 mm spacing. All raw data are imported into MR Systems Achieva Release 2.6.3.6 workstation for image post-processing. Cerebral microhemorrhages are loss of circular signal with a uniform diameter of 2–5 mm, with clear margin and no edema around the circular punctate non-sulcus area.
Blood pressure measurement
The blood pressure in our study was measured after taking hypertensive drugs in the morning, three times in succession, three times on average. After the first measurement, the subjects were asked to lift their right arm for 5 to 6 seconds. After 0.5–1 minutes, the same arm was taken for the second and third blood pressure measurements. If the blood pressure of the subjects was higher, such as systolic pressure over 180 mmHg, the sphygmomanometer needs to be inflated twice. The start button of the blood pressure meter should be pressed continuously (for more than 3 seconds) and the maximum inflatable pressure should be 30–40 mmHg higher than the expected systolic pressure. Smoking should be stopped 30 minutes before measurement, the mind relaxed, the bladder was empty and rested for 5 minutes.
For all patients, we recorded the history of cerebrovascular diseases, high blood pressure, diabetes, hyperlipidemia, and stroke, as well as smoking and drinking history. Patients were diagnosed according to the World Health Organization criteria for hypertension, diabetes, and hyperlipidemia. Blood pressure was divided into the following grades: BP grade 1 hypertension (SBP: 140~159 mmHg, DBP: 90~99 mmHg), BP grade 2 hypertension (SBP: 160~179 mmHg, DBP: 100~109 mmHg), and BP grade 3 hypertension (SBP: ≥180 mmHg. DBP: ≥110 mmHg). The highest blood pressure values were recorded before medicine was administered. In total, 62 patients were classified as BP grade 1, 30 patients were classified as BP grade 2, and the remaining 28 patients were classified as BP grade 3. Patients were stratified into four age groups: age grade 1 (younger than 60 years), age grade 2 (60–69 years), age grade 3 (70–79 years), and age grade 4 (older than 80 years). Confirmation of stroke was based on definitive diagnosis and treatment from the hospital or imaging findings of obsolete cerebral lesions. Most of the patients in this study could not recall whether they had consecutively consumed aspirin for longer than three months. Therefore, aspirin intake was not considered as one of the observation indexes.
Brain MRI
Two highly qualified radiologists assessed the MRI imaging results. On GRE-T2*WI, CMBs appear as small, homogeneous, round foci of low signal intensity with a diameter less than 10 mm without peripheral edema. Vascular gap, hemosiderin deposition of pia mater, cavernous hemangioma, calcification of the globus pallidus, calcified plaque of atherosclerosis, or blood flow empty shadow should be excluded. The numbers of CMBs located in the cortex-subcortex, basal ganglia, thalamus, brainstem, and cerebellum were recorded separately. Patients were classified into four groups (Citation18): non-CMBs (0), mild (1~2), moderate (3–10) and severe (>10) as grades 0–4.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (America, IBM). Measurement data was recorded as x ± s (x = mean value, s = standard deviation). The comparison of data from a Gaussian distribution was performed using Student’s t-test, whereas data with a non-Gaussian distribution were assessed using the Mann-Whitney U test. For comparisons of enumeration data, we used the χ2 test. We used Spearman’s rank sum tests for correlation analysis between CMB severity and hypertension grade or age. Multifactorial logistic regression analysis was performed to evaluate the possible risk factors of CMBs, such as age, gender, SBP, DBP, diabetes, hyperlipidemia, and history of smoking and drinking. P < .05 was considered to represent statistical significance, and P < .001 was considered to represent a significant difference.
Results
Patient characteristics
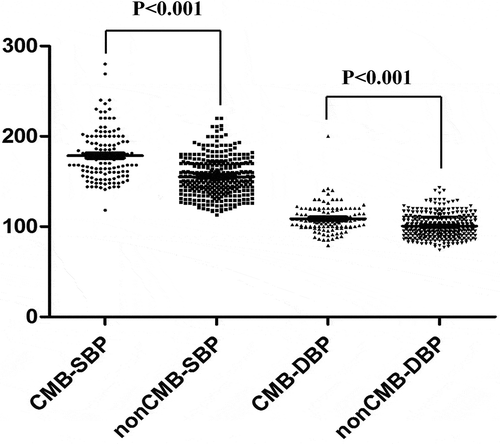
Patient information is summarized in . In total, 123 patients exhibited CMBs under GRE-T2*WI scanning among all 460 hypertension patients. No significant differences in gender or were noted between the CMB group and the non-CMB group. But there were significant differences in age between the two groups. As shown in , intracerebral hemorrhage, cerebral infarction, and smoking and drinking history were more frequently noted in the CMB group than in the non-CMB group. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were considerably increased in the CMB group compared with those in the non-CMB group (). The patient’s medication use information is shown in Table S1. There was no statistical difference between the two groups in Antiplatelet drugs and Hypoglycemic agents use. (Table S1) The results indicate that blood pressure is a risk factor related to CMBs, although no significant difference in glucose or lipid metabolism was noted between the two groups.
Table 1. Characteristics of subjects with and without CMBs.
Characteristics of the distribution of cerebral microbleed lesions
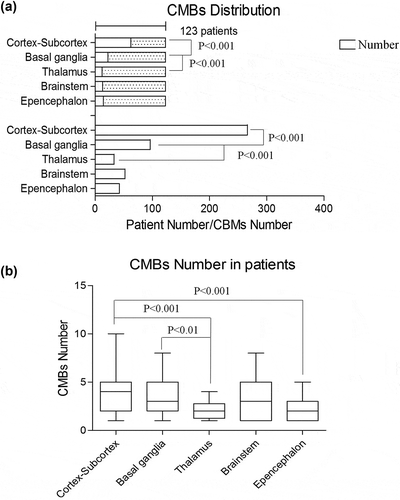
Microbleeds were observed in 123 hypertensive patients (26.7%), and the total number of CMB lesions was 489. This finding suggests that each patient had 4 ~ 6 lesions on average. In fact, the distribution of lesions in CMBs was not uniform: 489 CMB lesions, located in multiple parts of the brain, were observed in 123 CMB patients, with 1 to 10 CMB lesions per patient. As noted in , CMBs in the cortex-subcortex or basal ganglia were noted in 50.4% and 17.8% of all 123 patients, respectively. By comparing the number of CMB lesions, the predilection sites included the cortex-subcortex (266, 54.3%) and basal ganglia (96, 19.6%). Fewer patients had CMBs in the thalamus and epencephalon compared with the cortex-subcortex. Moreover, the severity of these CMBs was also lower. Interestingly, CMBs rarely appeared in the brainstem, as shown in ). However, when CMBs occurred in the brainstem, the severity was similar to that observed in the cortex-subcortex and basal ganglia ()).
Table 2. Distribution of CMBs.
Correlational analysis of hypertension grade, CMB severity, and age
According to the highest blood pressure values, all 460 patients were diagnosed with hypertension. In total, 230 patients were classified as hypertension grade 1 (50%), 125 as grade 2 (27.1%) and 105 (22.8%) as grade 3. Regarding age, 77 patients (16.7%) were younger than 60 years, 199 patients (43.2%) were 60 to 69 years, 153 patients (33.2%) were 70 to 79 years, and 31 patients (6.7%) were older than 80 years. In total, 337 patients (73.2%) did not have CMBs. A total of 63 patients (13.6%) were classified as CMB grade 1, 34 patients (7.3%) were classified as CMB grade 2, and 26 patients (5.6%) were classified as CMB grade 3. Then, we calculated the correlation among hypertension grade, age rank and CMB severity using Spearman’s rank test. The results are presented in . As noted in , no rank correlation was noted between hypertension grade and age (r = 0.039, P = .304). also suggests that age exhibited no rank correlation with the severity of CMBs (r = 0.061, P = .523). Thus, in this selected group of hypertension patients, age was not a risk factor for hypertension grade and CMB severity; however, age was reported as a risk factor for hypertension and CMBs. The results in indicate a significant and progressive increase in CMB severity and hypertension grade in patients (r = 0.685, P < .001).
Table 3. Correlation between hypertension and age.
Table 4. Correlation between age and CMBs.
Table 5. Correlation between hypertension and CMBs.
Logistic regression analysis of CMBs, HBP, and other risk factors
We performed logistic regression analyzes to assess the relationship between blood pressure and microbleeds. Models were adjusted for age, gender, and cardiovascular risk factors, including smoking, drinking, diabetes, and hyperlipidemia. Odds ratios quantifying the relation between CMBs and other risk factors are summarized in . Age, SBP and DBP were significantly associated with the presence of CMBs (P < .05). The results also demonstrated that SBP and DBP were independent risk factors for CMBs (age, adjusted odds ratio = 1.180, 95% confidence interval 0.45~2.545; SBP, adjusted odds ratio = 1.559, 95% confidence interval 1.559~3.328, P = .006; DBP, adjusted odds ratio = 1.236, 95% confidence interval 0.86~1.896, P = .034).
Table 6. Multi-factor logistic regression analysis.
Discussion
The distribution of CMBs differed among patients, and the results from multiple different CMB factors potentially suggest an increased risk of bleeding. Therefore, it is necessary to classify the severity of the CMBs and analyze the distribution characteristics to screen patients with an increased risk of bleeding (Citation19).
The incidence of CMBs differed among cardiovascular disease patients and a healthy elderly population based on different MRI inspection technologies. The use of different MRI inspection technologies leads to different enrollment criteria. By scanning 460 patients using GRE-T2*WI, we demonstrated that the incidence of CMBs was 26.7% in the hypertension group and 58.3% in the cerebral infarction group (Table S2). Interestingly, CMBs were regularly distributed in the brain, and lesions occurred in multiple parts of the brain in most patients. The cortex-subcortex and basal ganglia regions were the most common sites of occurrence, and lesions were rarely observed in the brain stem and cerebellum. There were 489 lesions in CMB patients, with approximately 1 ~ 10 CMB lesions in each patient, and this finding is consistent with past studies (18 ~ 68% in the infarction group (Citation7), 33%~80% in the cerebral hemorrhage group (Citation4)). However, the incidence of CMBs in the hypertension groups was reduced compared with the 56% reported by Lee et al. This difference is potentially attributable to increased proportions (52%) of HBP grade 1 patients and patients younger than 75 years (57.5%). Moreover, the increased incidence of CMBs in the cerebral infarction and cerebral hemorrhage groups suggests that CMBs are associated with cerebral infarction and hemorrhage.
Remote hemorrhages are noted in CMBs, and studies have demonstrated an apparent correlation between CMBs and ICH (Citation20). ICH patients often have brain white matter ischemia and basal ganglia lacunar infarction, indicating that microvascular lesions can cause hemorrhagic cerebral and ischemic damage (Citation4). A strong correlation between ICH location and distribution of microhemorrhage has been noted (Citation7). The position of CMBs might represent the site of ICH (Citation21,Citation22). Chen et al. reported that CMB lesions in the basal ganglia and thalamus were associated with ICH in cerebral ischemic stroke patients.
A study in 106 ICH patients reported that 50.7% of CMBs were located in the cortex subcortex, 34.1% in the basal ganglia region and thalamus, 9.0% in the brainstem, and 6.2% in the cerebellum. These results are consistent with our results regarding CMB distribution. However, the cortex-subcortex and basal ganglia region represent predilection sites of CMBs, suggesting a particular relationship between CMBs and symptomatic ICH. Perhaps a similar mechanism is involved, such as hypertension cerebral hemorrhage. Numerous conductive fibers and functional units are present in the basal ganglia region, and CMBs may damage specific cellular functions. Werring et al. (Citation23) suggested that dysfunction in cognitive and executive ability was related to CMBs in the frontal area and basal ganglia region and potentially resulted from sub-frontal cortex fiber damage. Thus, we proposed that CMBs may be a potential microvascular marker of cognitive impairment.
Igase et al. (Citation24) reported that CMBs exhibit a positive correlation with SBP, and Fanet et al. (Citation21) suggested that CMBs exhibit a positive correlation with DBP. Using Spearman’s analysis of BP grade based on CMB severity, we confirmed a rank correlation between BP grade and CMB severity (r = 0.685, P < .001). Combined with the results of logistic regression analysis, both diastolic and systolic blood pressure were found to be independent risk factors for CMBs.
Long-term high blood pressure-induced arterial lesions manifest an increased wall-cavity ratio and narrowed lumen diameter, leading to tissue ischemia in essential target organs, such as the heart, brain, and kidney. High blood pressure can also cause capillaries to be sparse and twisted, leading to reduced venous compliance. Hypertension prompts atherosclerosis, plaque formation and plaque fracture, which may further develop into cerebral thrombosis. Given the weakening of the small vessel walls, especially with the replacement of smooth muscle by fibrous tissue or necrotic tissue, vascular rupture can occur in hypertension patients with or without the formation of aneurysms (Citation25). Indeed, CMBs can be interpreted as a type of target organ damage due to chronic hypertension. Multiple CMB foci indicate a terminal microvascular lesion that is prone to bleeding (Citation26).
Atherosclerosis is also a risk factor for cerebral microhemorrhage. Arvanitakis et al (Citation27) found that the incidence of small artery atherosclerosis was as high as 54%, which was highly correlated with vascular risk factors. The severity of small artery atherosclerosis is positively correlated with the severity of cognitive impairment. Charidimou et al (Citation28) found that 75% of patients with cerebrovascular disease in the relevant study had arteriosclerosis in the autopsy results. Age greater than 75 years is one of the independent risk factors for CMBs among older adults without cerebrovascular disease (Citation29). Through multifactorial logistic regression analysis, we also confirmed that advanced age is an independent risk factor for CMBs. In a study of 197 primary ICH patients divided into three groups (group A, 60 years old or younger; group B, 61 ~ 69 years old, group C, 70 years old or older), Imaizumi et al. (Citation30) reported CMB morbidity values of 65.6%, 79.4%, and 86.8%, respectively, and the CMB incidence increased with age. In our study, we classified patients into four groups based on age: 16.7% of patients were younger than 60 years; 43.2%, 60–69 years; 33.2%, 70–79 years; and 6.7%, 80 years or older. The CMB morbidity values in these groups were 13.69%, 7.39%, and 5.65%, respectively. However, no significant differences were noted among the groups. In addition, no rank correlation was noted between age and CMB severity (r = 0.061, P = .523, nonsignificant) or between age and hypertension grade. This difference could be attributed to the fact that our study included a small number of patients over 75 years and that different stratification standards were applied.
In conclusion, CMBs occurred in different populations of patients with hypertension, cerebral infarction and cerebral hemorrhage based on GRE-T2*WI scanning, and an increased incidence of CMBs was noted in patients with cerebral hemorrhage. CMBs were regularly distributed in the brain, although they were more likely to occur in the cortex–subcortex and basal ganglia regions and rarely occurred in the brainstem and cerebellum. The number of CMBs was positively correlated with blood pressure levels. SBP, DBP, age, and smoking were independent risk factors of CMBs. No significant correlation was identified between CMBs and high cholesterol, diabetes, gender or history of drinking.
Author Contributions
All authors contributed to the development of the study framework, interpretation of the results, and revisions of successive drafts of the manuscript and approved the version submitted for publication. LL, SJY and ZCL conducted the data analyses. LL, WTM, and MW drafted the manuscript. JJ and HW checked the paper. WTM and MW finalized the manuscript with inputs from all authors.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of Interest
The authors declare that they have no competing interests.
Supplemental Material
Download Zip (21.1 KB)Acknowledgments
None.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Buch S, Cheng YN, Hu J, Liu S, Beaver J, Rajagovindan R, Haacke EM. Determination of detection sensitivity for cerebral microbleeds using susceptibility- weighted imaging. NMR Biomed. 2017;30(4):Apr. doi:10.1002/nbm.3551.
- Offenbacher H, Fazekas F, Schmidt R, Koch M, Fazekas G, Kapeller P. MR of cerebral abnormalities concomitant with primary intracerebral hematomas. AJNR Am J Neuroradiol. 1996;17(3):573–78.
- Cerebral Microbleeds FM. Thrombolysis: clinical consequences and mechanistic implications. JAMA Neurol. 2016;73(6):632–35. doi:10.1001/jamaneurol.2016.0576.
- Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. Am J Neuroradiol. 2003;24(1):88–96.
- Nishikawa T, Ueba T, Kajiwara M, Fujisawa I, Miyamatsu N, Yamashita K. Cerebral microbleeds predict first-ever symptomatic cerebrovascular events. Clin Neurol Neurosurg. 2009;111(10):825–28. doi:10.1016/j.clineuro.2009.08.011.
- Charidimou A, Shoamanesh A. International META-MICROBLEEDS initiative clinical relevance of microbleeds in acute stroke thrombolysis: comprehensive meta-analysis. Neurology. 2016;87(15):1534–41.
- Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 2006;37(2):550–55. doi:10.1161/01.STR.0000199847.96188.12.
- Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35(6):1415–20. doi:10.1161/01.STR.0000126807.69758.0e.
- Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73(8):934–43. doi:10.1001/jamaneurol.2016.1017.
- Hilal S1, Saini M, CS T, JA C, WI K, WJ N, HA V, TY W, Chen C, MK I, et al. Cerebral microbleeds and cognition: the epidemiology of dementia in Singapore study. Alzheimer Dis Assoc Disord. 2014;28(2):106–12. doi:10.1097/WAD.0000000000000015.
- Vernooij M, van der Lugt A, Ikram MA, Wielopolski P, Niessen W, Hofman A, Krestin G, Breteler M. Prevalence and risk factors of cerebral microbleeds The Rotterdam scan study. Neurology. 2008;70(14):1208–14. doi:10.1212/01.wnl.0000307750.41970.d9.
- Oberstein SL, Van Den Boom R, Van Buchem M, Van Houwelingen H, Bakker E, Vollebregt E, Ferrari M, Breuning M, Haan J. Cerebral microbleeds in CADASIL. Neurology. 2001;57(6):1066–70. doi:10.1212/wnl.57.6.1066.
- Henskens LH, Van Oostenbrugge RJ, Kroon AA, De Leeuw PW, Lodder J. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension. 2008;51(1):62–68. doi:10.1161/HYPERTENSIONAHA.107.100610.
- Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds prevalence and associations with cardiovascular risk factors in the Framingham study. Stroke. 2004;35(8):1831–35. doi:10.1161/01.STR.0000131809.35202.1b.
- Lee S-H, Ryu W-S, Roh J-K. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. 2009;72(2):171–76. doi:10.1212/01.wnl.0000339060.11702.dd.
- Kidwell CS, Saver JL, Villablanca JP, Duckwiler G, Fredieu A, Gough K, Leary MC, Starkman S, Gobin YP, Jahan R. Magnetic resonance imaging detection of microbleeds before thrombolysis an emerging application. Stroke. 2002;33(1):95–98. doi:10.1161/hs0102.101792.
- Soo YO, Yang SR, Lam WW, Wong A, Fan YH, Leung HH, Chan AY, Leung C, Leung TW, Wong LK. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255(11):1679–86. doi:10.1007/s00415-008-0967-7.
- Lee S-H, Bae H-J, Yoon B-W, Kim H, Kim D-E, Roh J-K. Low concentration of serum total cholesterol is associated with multifocal signal loss lesions on gradient-echo magnetic resonance imaging analysis of risk factors for multifocal signal loss lesions. Stroke. 2002;33(12):2845–49. doi:10.1161/01.str.0000036092.23649.2e.
- Chen Y-F, Chang -Y-Y, Liu J-S, Lui -C-C, Kao Y-F, Lan M-Y. Association between cerebral microbleeds and prior primary intracerebral hemorrhage in ischemic stroke patients. Clin Neurol Neurosurg. 2008;110(10):988–91. doi:10.1016/j.clineuro.2008.06.003.
- Nighoghossian N, Hermier M, Adeleine P, Blanc-Lasserre K, Derex L, Honnorat J, Philippeau F, Dugor J, Froment J, Trouillas P. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke a gradient-echo T2*-weighted brain MRI study. Stroke. 2002;33(3):735–42. doi:10.1161/hs0302.104615.
- Fan YH, Zhang L, Lam WW, Mok VC, Wong KS. Cerebral microbleeds as a risk factor for subsequent intracerebral hemorrhages among patients with acute ischemic stroke. Stroke. 2003;34(10):2459–62. doi:10.1161/01.STR.0000090841.90286.81.
- Koennecke H-C. Cerebral microbleeds on MRI prevalence, associations, and potential clinical implications. Neurology. 2006;66(2):165–71. doi:10.1212/01.wnl.0000194266.55694.1e.
- Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, Brown MM, Jäger HR. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127(10):2265–75. doi:10.1093/brain/awh253.
- Igase M, Tabara Y, Igase K, Nagai T, Ochi N, Kido T, Nakura J, Sadamoto K, Kohara K, Miki T. Asymptomatic cerebral microbleeds seen in healthy subjects have a strong association with asymptomatic lacunar infarction. Circ J. 2009;73(3):530–33. doi:10.1253/circj.cj-08-0764.
- Lee S-H, Kim BJ, Roh J-K. Silent microbleeds are associated with volume of primary intracerebral hemorrhage. Neurology. 2006;66(3):430–32. doi:10.1212/01.wnl.0000196471.04165.2b.
- Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33(6):1536–40. doi:10.1161/01.str.0000018012.65108.86.
- Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly People: a cross- sectional study. Lancet Neurol. 2016;15(9):934–43. doi:10.1016/S1474-4422(16)30029-1.
- Charidimou A, Martinez-Ramirez S, Reijmer YD, Oliveira-Filho J, Lauer A, Roongpiboonsopit D, Frosch M, Vashkevich A, Ayres A, Rosand J, et al. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy: an imaging- pathologic study of concept validation. JAMA Neurol. 2016;73(8):994–1001. doi:10.1001/jamaneurol.2016.0832.
- Horita Y, Imaizumi T, Niwa J, Yoshikawa J, Miyata K, Makabe T, Moriyama R, Kurokawa K, Mikami M, Nakamura M. Analysis of dot-like hemosiderin spots using brain dock system. No Shinkei Geka Neurol Surg. 2003;31(3):263–67.
- Imaizumi T, Honma T, Horita Y, Kawamura M, Kohama I, Miyata K, Nyon KS, Niwa J. The number of microbleeds on gradient T2*-weighted magnetic resonance image at the onset of intracerebral hemorrhage. J Stroke Cerebrovascular Dis. 2008;17(1):30–34. doi:10.1016/j.jstrokecerebrovasdis.2007.11.001.