ABSTRACT
Purpose
Hypertensive disorder complicating pregnancy (HDCP) is a serious clinical disorder syndrome during pregnancy. This study aims at finding novel targets for HDCP therapy.
Methods
HDCP-related mRNAs were firstly screened out and subjected to gene enrichment analysis. We chose protein kinase AMP-activated catalytic subunit alpha 2 (PRKAA2) as the research object. Thirty-nine HDCP patients at 32 to 40 weeks of gestation were selected as the HDCP group, and 39 normal controls who received cesarean section delivery at 37–42 weeks of pregnancy were enrolled in this study. Chorionic villi samples were collected within 30 min of delivery. The apoptosis of isolated placental trophoblasts was monitored to investigate the regulatory role of PRKAA2.
Results
PRKAA2 expression was further proven to be enhanced in the placental tissues of HDCP patients compared with that of normal puerpera. Subsequently, the results of flow cytometry analysis and western blot indicated that PRKAA2 overexpression accelerated primary placental cell apoptosis, while its knockdown attenuated cell apoptosis. Mechanistically, we determined that the level of PRKAA2 succinylation was elevated in the placental tissue of HDCP patients. Through in vitro succinylation assay and mutagenesis, we confirmed that sirtuin 5 (SIRT5) interacts with PRKAA2 at K69 and K260 to induce PRKAA2 desuccinylation. SIRT5 regulated primary HDCP cell apoptosis through PRKAA2. Finally, the animal study revealed that PRKAA2 elevates the systolic blood pressure of HDCP rat model.
Conclusion
Our findings indicated that SIRT5-mediated PRKAA2 succinylation modulates placental cell apoptosis in HDCP, suggesting that PRKAA2 is a potential therapeutic target for HDCP treatment.
KEYWORDS:
Introduction
Hypertensive disorders of pregnancy (HDCP) affect about 5–10% of pregnancies, impacting maternal, fetal, and neonatal outcomes. HDCP presents as hypertension, edema, proteinuria and even heart and kidney failureCitation1. HDCP has a severe adverse impact on the health and life of pregnant women and their babiesCitation2–4. The lack of effective methods to prevent and treat HDCP leads to the result that the lives of pregnant patients with HDCP and their fetuses have been threatened. To date, the pathogenesis of HDCP has not been well understood. After the expulsion of the placenta, the symptoms of HDCP would relieve and then gradually disappearCitation5. Studies have demonstrated that the placenta is an important factor in the occurrence and progression of HDCP. Therefore, it is imperative to explore the molecular mechanisms associated with the functions of placental cells in HDCP to find new therapeutic methods.
Protein kinase AMP-activated catalytic subunit alpha 2 (PRKAA2) gene expression is a crucial modulator in various human diseases as a cellular energy sensorCitation6–8. PRKAA2 is located at chromosome 1p31 in regions 56 645 317 to 56 715 335 and consists of 12 exons, it encodes AMPKα2 and has been reported as a modulator for gene expressionCitation9. It is known that AMPK is necessary for embryonic development in mice, as the null mutation of genes encoding the alpha 1 and alpha 2 subunits (i.e., PRKAA1 and PRKAA2), results in embryonic lethality around DOP 10.5Citation10. p53 has been shown to regulate uterine AMPK activity, thereby controlling the timing of delivery in preterm mouse modelsCitation11. AAMPK activity mediated by PRKAA2 also plays a regulatory role during pregnancyCitation12. Importantly, PRKAA2 can affect the biological functions of various cells, including proliferation and apoptosis through participating in cell energy metabolismCitation9,Citation13–18. Therefore, it is worthy to investigate the role of PRKAA2 in HDCP.
Succinylation belongs to posttranslational modification, which has received more and more attention due to its important role in the disease progressionCitation19–21. As demonstrated previously, sirtuin 5 (SIRT5) is a desuccinylase, which can catalyze the removal of succinylationCitation22–24. Up to now, more and more targets of SIRT5 have been identified, including amino acid catabolism, glycolysis, ketone body synthesis, and ATP synthesisCitation25,Citation26. Nevertheless, whether SIRT5 regulates PRKAA2 succinylation remains to be investigated.
In summary, the current study aims to investigate the functional role of PRKAA2 in HDCP and explore its succinylation modulated by SIRT5.
Materials and methods
HDCP samples
Thirty-nine HDCP patients who were delivered by cesarean section in our hospital at 32 to 40 weeks of gestation were selected as the HDCP group. Among all patients, 16 cases were gestational hypertension, 14 cases were mild pre-eclampsia, and the rest 9 cases were severe pre-eclampsia. All patients were diagnosed in accordance with the guidelines published by the Chinese Medical Association in 2012 for the diagnosis and treatment of HDCP. Patients enrolled in this study had no other disease history before cesarean section delivery. As for the control group, 39 normal puerperae who received cesarean section delivery in our hospital at 37 ~ 42 weeks of pregnancy were enrolled in this study. The clinical characteristics of pregnant patients are shown in . Samples were collected from two central and two peripheral portions of the placenta within 30 min of delivery. The central samples were collected within a 5 cm radius from the umbilical cord insertion site, and the peripheral samples were collected 2–3 cm from the edge of the placenta. The full depth of 1 cm × 1 cm tissue samples were excised, and the maternal decidua was separated from the chorionic villi using gross dissection. In this study, the maternal and fetal components were separated, and only the fetal components (chorionic villi) were used for analysis. The tissue samples were divided into fresh ones for cell isolation.
Table 1. Clinical characteristics of pregnant patients with hypertensive disorders complicating pregnancy (HDCP) and normal pregnancy (Normal).
Bioinformatic and gene enrichment analysis
Total RNA was isolated from the chorionic villi of HDCP patients or normal puerperal using RNeasy Mini Kit (Qiagen, Germany). RNA quality and integrity were then determined using Agilent Bioanalyzer 2100. Next, mRNA libraries were generated and sequenced by the WuXiNextCODE Genomics Co., Ltd. (Shanghai, China). mRNAs were defined to be upregulated in HDCP samples when logFC ≥2 and adjusted p value <0.05, while mRNAs were determined to be downregulated in HDCP samples when logFC ≤-2 and adjusted p value <0.05. Raw data downloaded from the Gene Expression Omnibus (GEO) Database (the accession number: GSE12767). Subsequently, all upregulated mRNAs were collected for gene enrichment analysis using Funrich softwareCitation27. The potential succinylation sites of PRKAA2 were predicted by the online database Generic and Species-specific Succinylation Sites Prediction Server (http://kurata14.bio.kyutech.ac.jp/GPSuc/index.php).
Cell lines
293T cell line purchased from ATCC (CA, USA) was cultured in DMEM (Invitrogen) supplemented with 10% FBS (Gibco) and 1% P/S (Gibco). It was then maintained in an incubator set at 37°C with 5% CO2.
The human placental trophoblasts were isolated. Briefly, the freshly collected chorionic villi were obtained from the placenta of HDCP patients in an aseptic environment and then cut into pieces at the same size of 1 mm3 using ophthalmic scissors. Then, DMEM containing 1% penicillin–streptomycin–neomycin (PSN) solution, 0.25% trypsin and DNase I was added, and the mixture was placed at 37°C for the first digestion for 20 min. The digested tissue was filtered with a 350 mesh screen, the first digestive fluid was discarded, the filter residue was retained, and 150 mL of the above digestive fluid was added for the second digestion (20 min). The digestive fluid was then filtered with a 350 mesh screen, and the digestive fluid was retained and calf serum was added to terminate digestion. The digestive fluid was centrifuged at room temperature at 1260 × g for 15 min, the supernatant was discarded, and DMEM containing 1% PSN and 15% fetal bovine serum (FBS) were added to the suspension cells. The cells were separated by Percoll continuous density gradient separation method, and 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60% and 65% Percoll solutions were successively added into a 50 mL centrifuge tube with a concentration of 3 mL each. The cell suspension was slowly added to the Percoll separation solution and centrifuged at room temperature at 1340 × g for 20 min. The nebulous cells (i.e. human placental trophoblasts) removed from the cardiac tube were transferred to a new centrifuge tube, and an appropriate amount of complete medium was added for re-suspension. Next, the bottle was added with 3 mL of DMEM complete medium containing 15% FBS and put into an incubator set as 37°C with 5% CO2 for 6 h. When cell confluence reached 80–90%, cells were purified and detached with 0.25% trypsin. Subsequently, cells were centrifuged at 114 × g for 8 min. After removing the supernatant, cells were rinsed, re-suspended, and centrifuged again at 257 × g for 20 min. The purification of cells was made through differential adhesion. Cells were cultured in DMEM containing 15% FBS, and the medium was replaced every 3 days. Cells in the third generation were collected for subsequent experiments.
Cell transfection
The whole sequence of PRKAA2 was inserted into the pcDNA3.1 vector to obtain the PRKAA2 overexpression vector, and the empty vector was taken as the negative control group. Small interfering RNAs specifically targeting PRKAA2 (si-1 GUCAUCCUCAUAUUAUCAAAC and si-2 CAACUUUACCUGGUUGAUAAC) or SIRT5 (si-1 TCTCATTCTGTAGGTTGCCTGTTTA and si-2 CATAGTTGTATAGCCAGCACCATAA) were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) along with their negative control non-specific siRNA (si-NC). According to the user guide of Lipofectamine 2000 (Invitrogen Inc., Carlsbad, CA, USA), cell transfection was carried out.
Cell counting kit-8 assay
Cells were counted at 0, 24, 48, and 72 h after adhering to 96-well plates. The cells were washed twice with phosphate buffered saline (PBS, Servicebio, China). Thereafter, fresh culture medium containing 10 μL of Cell Counting Kit-8 solution (CCK-8; Beyotime, Shanghai, China) was added to each well. After incubating for 4 h at 37°C, the optical density (OD) at a wavelength of 450 nm was measured to determine cell proliferation, using a microplate reader (Bio-Rad, USA).
qPCR
Total RNA extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was treated with the reverse transcription kit (Takara Biotechnology, Beijing, China) to be reversely transcribed into cDNA. Next, real-time qPCR was conducted using SYBR Green PCR Master mix (Roche Applied Science, Mannheim, Germany) on a StepOnePlus™ real-time PCR System (Applied Biosystems, Foster City, CA, USA). With GAPDH as the internal control, relative target gene expression was calculated using 2−ΔΔCt. The primer sequences of PCR are listed as follows (5“→3”): PRKAA2 F ACCAGCTTGCAGTGGCTTAT, R CAGTGCATCCAATGGACATC; KAT2A F GCAAGGCCAATGAAACCTGTA, R TCCAAGTGGGATACGTGGTCA; KAT3B F AGCCAAGCGGCCTAAACTC, R TCACCACCATTGGTTAGTCCC; CPT1A F TCCAGTTGGCTTATCGTGGTG, R TCCAGAGTCCGATTGATTTTTGC; SIRT5 F GCCATAGCCGAGTGTGAGAC, R CAACTCCACAAGAGGTACATCG; SIRT7 F GACCTGGTAACGGAGCTGC, R CGACCAAGTATTTGGCGTTCC; GAPDH F AGTCAGCCGCATCTTCTTTT, R CGCCCAATACGACCAAAT.
In vitro succinylation assay and mutagenesis
The PRKAA2 peptides were succinylated in a 15 μL system by mixing 50 ng/μl peptides and 0.5 mM succinyl-CoA (Sigma) in 30 mM HEPES (pH 7.4). After being incubated at 37°C for 3 h, the peptides were subjected to desalting with C18 ZipTips (Millipore).
The mutant for K6, K69, K224, K255, K260, K398, K470 was generated by utilizing a QuikChange site-directed mutagenesis kit (#200518, Agilent Technologies, Santa Clara, CA, USA). The method is performed using PfuUltra high-fidelity (HF) DNA polymerase for mutagenic primer-directed replication of both plasmid strands with the highest fidelity. The basic procedure utilizes a supercoiled double-stranded DNA (dsDNA) vector with an insert of interest and two synthetic oligonucleotide primers, both containing the desired mutation. Specifically, two complementary oligonucleotide primers were synthesized to prepare the reaction mixture (5 μL 10X reaction buffer, 50 ng template DNA, 1 μL 10 mM dNTP mixture, 1 μL PfDNA polymerase). The cycle reaction: 95°C 30 s (cycle once), 95°C 30 s, 55°C 60 s, 68°C 2 min (cycle 12–18 times). Place the reactants on the ice and cool to <37°C. One microliter DpnI restriction enzyme was added to each amplification reaction after the reactants were cooled to <37°C. The reaction mixture was centrifuged and incubated at 37°C for 1 h. The cells were transformed using the 1 μL reaction mixture.
Immunoprecipitation (IP)
Proteins extracted from indicated cultured cells using a modified buffer were subjected to immunoprecipitation as described previouslyCitation28. Briefly, harvested HEK-293T cells were lysed with lysis buffer (Beyotime) together with protease and phosphatase inhibitors on ice for 15 min. The lysate was then sonicated on ice at 10% power for 2 mi. After centrifugation at 12 000 g for 20 min at 4°C, the supernatant was precleared by incubation with protein A+G magnetic beads (Millipore) and IgG for 1 h at 4°C. The samples were then placed in a magnetic separator for 1 min. The supernatant was incubated with an indicated antibody overnight at 4°C on a rotating platform. Protein A+G magnetic beads were then added to the supernatants and incubated for 2 h at room temperature. The immunocomplexes were washed three times with the lysis buffer, boiled at 95°C for 10 min with 2 × SDS sample buffer, and analyzed by Western blot.
Western blot
Total protein extraction was performed using RIPA lysis buffer (Applied Biosystems). After being mixed with SDS loading buffer and resolved by SDS-PAGE, the lysates were transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were then blocked with 5% nonfat milk, followed by incubation with the primary antibodies: anti-PRKAA2 (ab214425, Abcam), anti-Bax (ab182733, Abcam), anti-Bcl-2 (ab32124, Abcam) and anti-GAPDH (ab8245, Abcam). After washing in TBST, the membranes were further incubated with the secondary antibody (ab7063, Abcam). Ultimately, protein blots were visualized using an ECL detection kit (Abcam).
Flow cytometry analysis
For apoptotic analysis, cells were stained with the Annexin V-FITC apoptosis detection kit (BD Biosciences) and then measured by flow cytometry (BD Biosciences).
Establishment of HDCP rat model
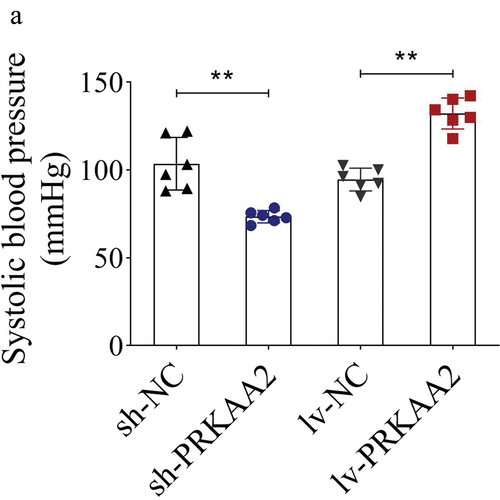
Twenty-four pairs of male and female Sprague Dawley (SD) rats (averagely weighed 220–250 g and aged 9–10 weeks) mated separately overnight. The female rat was defined to be on the day 0 of gestation when the spermatozoa were found in the female rat using vaginal smear. Next, each pregnant rat was kept in a metabolic cage individually. The HDCP of each rat was induced by intraperitoneally injecting with L-NAME (50 mg/kg per day) on day 10, followed by duplicate injection on day 13, day 15 and day 18Citation29. The HDCP rats were randomly divided into four groups: sh-NC (the placental tissues were treated with 20 μL of sh-NC plasmid through local microinjection), sh-PRKAA2 (the placental tissues were treated with 20 μL of sh-PRKAA2 plasmid through local microinjection), lentivirus (lv)-NC (the placental tissues were treated with 20 μL of lv-NC plasmid through local microinjection), and lv-PRKAA2 (the placental tissues were treated with 20 μL of lv-PRKAA2 plasmid through local microinjection). The sequences of vectors are listed in the Supplementary material 1. There were six rats in each group. After successful establishment of four groups of model rats, giving 2 days adaptive feeding to each rat. Finally, the systolic blood pressure (SBP) was measured using a tail-cuff method with IITC Model 229 Blood Pressure Analyzer (Linton Instruments, Harvard Apparatus, UK).
Statistical analysis
All the data measured in this study are shown mean ± standard deviation (SD) and plotted using GraphPad Prism v5.01 (San Diego, CA, USA). The significance of the data was analyzed and defined using SPSS v18.0 (Armonk, NY, USA). Comparisons between two groups or among multiple groups were made by using Student’s t-test or one-way analysis of variance (ANOVA). Defining p < .05 as the threshold of statistical significance.
Results
PRKAA2 is upregulated in HDCP patient samples
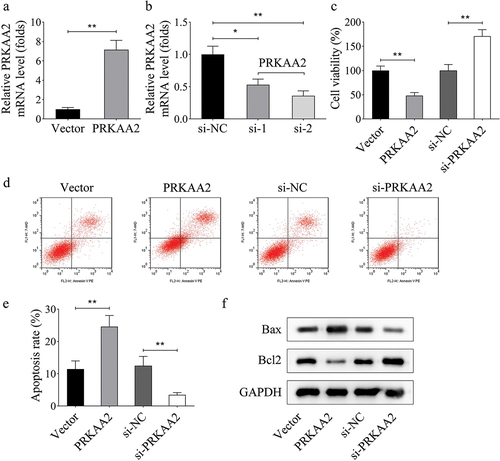
At first, HDCP-related genes were screened out through bioinformatic analysis. The aberrant highly expressed mRNAs in HDCP samples were shown in a volcano plot, and the red blots represented that mRNAs were upregulated in HDCP samples (logFC ≥ 2 and adjusted p value < 0.05), while the blue blots represented that mRNAs were downregulated in HDCP samples (logFC ≤ −2 and adjusted p value < 0.05), as illustrated in . Subsequently, all upregulated mRNAs were collected for gene enrichment analysis. The result indicated that these upregulated mRNAs were closely correlated with metabolism, according to the percentage of genes and the p-value (). Among all upregulated mRNAs, PRKAA2 has been proven to be a regulator of cellular energy metabolism by acting as an energy sensor protein kinaseCitation30,Citation31, and is mentioned in several biological pathways indicated in . Here, we chose PRKAA2 for further investigation, and we measured its mRNA and protein levels in the placental tissues of HDCP patients and normal puerpera collected by ourselves. The mRNA level of PRKAA2 was expressed higher in HDCP samples than that in normal samples, as presented in qPCR results (). Consistently, the level of PRKAA2 protein exhibited high expression in HDCP samples by comparing with normal samples (). Therefore, we summarize that PRKAA2 is upregulated in HDCP patient samples.
Figure 1. PRKAA2 is upregulated in HDCP patient samples a. the aberrant highly expressed mRnas in HDCP samples were screened out through bioinformatic analysis. b. All upregulated mRnas were subjected to gene enrichment analysis using the Funrich software. c. the mRNA level of PRKAA2 in the placental tissues of HDCP patients and normal puerpera was measured by qPCR. d. the level of PRKAA2 protein in the placental tissues of HDCP patients and normal puerpera was measured by western blot. **P < .01 indicated statistically significant difference between groups.
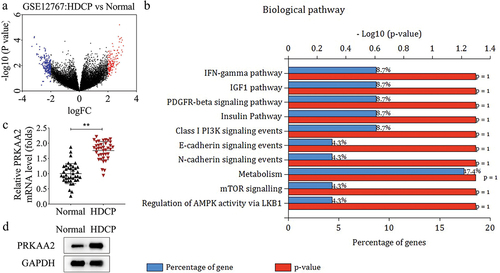
PRKAA2 induces apoptosis of primary placental cells in HDCP
To clarify the role of PRKAA2 in HDCP, we isolated primary placental cells from the placental tissues of HDCP patients and conducted gain- or loss-of-function assays. Before functional assays, we overexpressed and knocked down PRKAA2 in primary placental cells, respectively (). Cell viability was inhibited by overexpression of PRKAA2 but promoted by inhibition of PRKAA2 (). Through flow cytometry analysis, we found that PRKAA2 overexpression accelerated cell apoptosis, while its knockdown attenuated cell apoptosis (). Meanwhile, the apoptosis-proteins were detected in primary placental cells after transfections. The level of pro-apoptotic protein (Bax) was positively regulated by PRKAA2, while that of anti-apoptotic protein (Bcl-2) was negatively modulated by PRKAA2 (). These data suggest that PRKAA2 promotes apoptosis of primary placental cells.
Figure 2. PRKAA2 induces apoptosis of primary placental cells in HDCP a-b. PRKAA2 was overexpressed and knocked down in primary placental cells, respectively, and the efficiency of overexpression or knockdown was identified by qPCR. c. Cell viability of primary placental cells with PRKAA2 overexpression or knockdown was measured by CCK-8 analysis. d-e. the apoptotic rate of primary placental cells with PRKAA2 overexpression or knockdown was measured by flow cytometry analysis. f. the levels of apoptosis-related proteins (Bax and Bcl-2) was measured by western blot in primary placental cells after transfected with PRKAA2 overexpression vector or PRKAA2-specific siRnas. **P < .01 indicated statistically significant difference between groups.
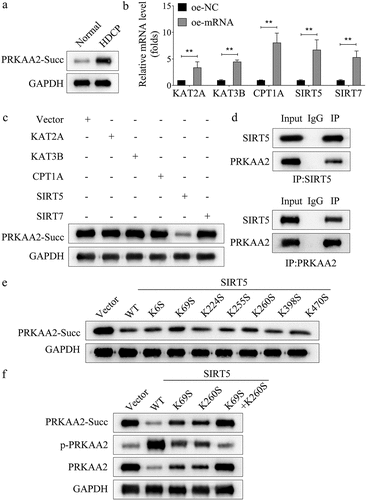
SIRT5 interacts with PRKAA2 to inhibit PRKAA2 succinylation
Succinylation belongs to posttranslational modification, which has received more and more attention due to its essential role in the disease progression [16–18]. Here, we explored whether the succinylation of PRKAA2 protein was started up in HDCP. Moreover, we determined that the level of PRKAA2 succinylation was elevated in the placental tissue of HDCP patients compared with that of normal puerpera (). To investigate the mechanism modulating PRKAA2 succinylation, we overexpressed known succinyltransferase, including P300 (KAT3B), KAT2A, and CPT1A, as well as desuccinylase, including SIRT5 and SIRT7 in primary HDCP cells (). We further detected and found that the overexpression of SIRT5 could efficiently decrease the level of PRKAA2 succinylation (). Therefore, we chose SIRT5 for further validation. The interaction between SIRT5 and PRKAA2 was then proven by Co-IP assay (). Subsequently, we identified the sites that contributed to the PRKAA2 desuccinylation induced by SIRT5. We mutated the individual site, including K6, K69, K224, K255, K260, K398, K470. Next, we detected that mutation of K69 or K260 could partially attenuate the effect of SIRT5 overexpression on the desuccinylation of PRKAA2 (). PRKAA2 is also known as AMPKalpha2, which is often inactivated through phosphorylationCitation32. We then mutated K69, K260, respectively, or both K69 and K260. It was uncovered that mutation of both K69 and K260 abolished the effects of SIRT5 overexpression on the desuccinylation, phosphorylation, and protein levels of PRKAA2 (), indicating that both K69 and K260 were responsible for the SIRT5-induced desuccinylation and phosphorylation of PRKAA2 protein. In summary, SIRT5 increases the phosphorylation of PRKAA2 to downregulate PRKAA2 protein through mediating its succinylation.
Figure 3. SIRT5 interacts with PRKAA2 to inhibit PRKAA2 succinylation a. the level of PRKAA2 succinylation in the placental tissue of HDCP patients or normal puerpera was measured through western blot. b. Succinylation regulators, including P300 (KAT3B), KAT2A, CPT1A, SIRT5 and SIRT7 were overexpressed in primary placental cells, and the overexpression efficiency was identified using qPCR. c. the effects of overexpression of succinylation-related mRnas on the level of PRKAA2 succinylation were analyzed by western blot analysis. d. the interaction between SIRT5 and PRKAA2 was then proven by Co-IP assay. e. the sites of PRKAA2 succinylation induced by SIRT5 were identified through western blot analysis. f. the effects of SIRT5 overexpression on the succinylation, phosphorylation, and protein levels of PRKAA2 were detected using western blot analysis in each group. **P < .01 indicated statistically significant difference between groups.
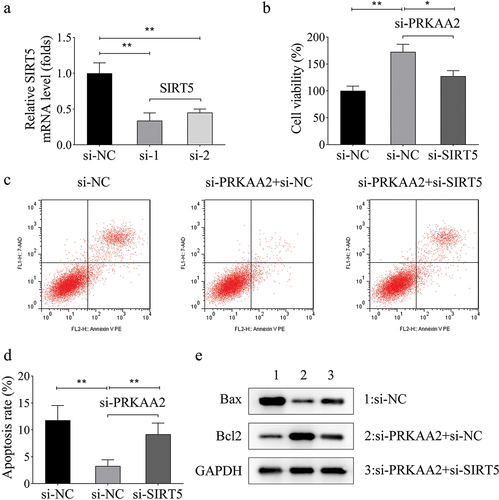
SIRT5 regulates primary placental cell apoptosis through PRKAA2
Rescue assays were then carried out to confirm the synergistical regulation of SIRT5 and PRKAA2 on primary placental cell apoptosis. SIRT5 mRNA and protein expression were significantly downregulated in HDCP samples (Figure S1A-B). SIRT5 was knocked down by using two specific siRNAs targeting SIRT5 (). The results of CCK-8 assay suggested that cell viability promoted by silenced PRKAA2 was partly inhibited by SIRT5 inhibition (). According to the data obtained using flow cytometry analysis, the apoptosis rate of primary placental cells reduced by silenced PRKAA2 was restored by knockdown of SIRT5 (). Additionally, the decreased level of Bax and the increased level of Bcl-2 caused by PRKAA2 silencing were reset after SIRT5 knockdown (). The above data demonstrate that SIRT5 interacts with PRKAA2 to regulate the apoptosis of primary placental cells.
Figure 4. SIRT5 regulates primary placental cell apoptosis through PRKAA2 a. SIRT5 was knocked down by using two specific siRnas targeting SIRT5. The knockdown efficiency was proven by qPCR. c. Cell viability of primary placental cells transfected with si-NC, si-PRKAA2 and si-PRKAA2+si-SIRT5 was measured by CCK-8 analysis. c-d. the apoptosis of primary placental cells transfected with si-NC, si-PRKAA2 and si-PRKAA2+si-SIRT5 was measured by flow cytometry analysis. e. the levels of apoptosis-related proteins (Bax and Bcl-2) was measured by western blot in primary placental cells transfected with si-NC, si-PRKAA2 and si-PRKAA2+si-SIRT5. **P < .01 indicated statistically significant difference between groups.
PRKAA2 elevates the systolic blood pressure of HDCP rat model
Finally, we established an HDCP animal model to validate the effect of PRKAA2 silencing or overexpression on the systolic blood pressure. Based on the results, the HDCP rat model injected with sh-PRKAA2 plasmids presented lower systolic blood pressure than those with sh-NC. However, overexpression of PRKAA2 led to the elevation of the systolic blood pressure ().
Discussion
HDCP is a frequent complication during pregnancy, which is also a major menace to pregnant women and perinatal fetusesCitation33. Studies have unveiled the involvement of placental apoptosis in the pathophysiology of preeclamptic pregnanciesCitation34 and HDCPCitation35. In this study, we initially found that the aberrant highly expressed mRNAs in HDCP samples in accordance with the results of bioinformatic analysis. Gene enrichment analysis indicated that upregulated mRNAs were enriched in metabolism. Since the upregulated mRNA PRKAA2 has been proven to be a regulator in cellular energy metabolism by acting as an energy sensor protein kinaseCitation30,Citation31, we chose PRKAA2 for further investigation. We also collected the placental tissues from HDCP patients and normal puerpera for the detection of PRKAA2 expression. Consistently, PRKAA2 was expressed higher in HDCP samples compared with normal samples at both mRNA and protein levels. These results implied the potential involvement of PRKAA2 in HDCP.
Studies have demonstrated that the apoptosis of placental cells is an important factor in the progression of HDCPCitation35–37 The ability of PRKAA2 to affect apoptosis-related disorders has been reported in multiple studiesCitation38–40. Here, we isolated primary placental cells from the placental tissues of HDCP patients and conducted gain- or loss-of-function assays after overexpressing or silencing PRKAA2 in primary placental cells. According to the results, PRKAA2 promoted apoptosis of primary placental cells. Hereto, we confirmed that PRKAA2 modulated the apoptosis of placental cells in HDCP.
Subsequently, we tried to elucidate the mechanism of PRKAA2-related apoptosis in HDCP. Succinylation is a posttranslational modification, playing crucial role in the metabolismCitation41–43. Here, we explored whether the succinylation of PRKAA2 protein was started up in HDCP. We determined that the level of PRKAA2 succinylation was elevated in the placental tissue of HDCP patients compared with that of normal puerpera. According to previous studies, succinyltransferase including P300 (KAT3B)Citation44, KAT2ACitation45, and CPT1ACitation46 as well as desuccinylase, including SIRT5Citation20 and SIRT7Citation47 are essential regulators for protein succinylation. Our present study detected and found that overexpression of SIRT5 could efficiently decrease the level of PRKAA2 succinylation. Moreover, we proved the direct interaction between SIRT5 and PRKAA2. Importantly, we identified the sites that contributed to the PRKAA2 desuccinylation induced by SIRT5. More importantly, we uncovered that mutation of both K69 and K260 abolished the effects of SIRT5 overexpression on the desuccinylation of PRKAA2. Considering that PRKAA2 (AMPKalpha2) is often inactivated through phosphorylation [29], we further detected and confirmed that mutation of both K69 and K260 abolished the effects of SIRT5 overexpression on the phosphorylation of PRKAA2 and PRKAA2 protein expression, indicating that both K69 and K260 were responsible for the SIRT5-induced desuccinylation and phosphorylation of PRKAA2 protein. Furthermore, data from rescue assays indicated that SIRT5 regulated primary placental cell apoptosis through PRKAA2. Finally, we established an HDCP animal model and validated that PRKAA2 could elevate the systolic blood pressure of HDCP rats.
Overall, our study reveals that PRKAA2 regulated by the desuccinylase SIRT5 is upregulated in HDCP and induces the apoptosis of placental cells. Our research findings may contribute to enriching the therapeutic strategies for HDCP patients.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. F R and M Y drafted the work and revised it critically for important intellectual content; G L, Y Q, A L and J L were responsible for the acquisition, analysis, or interpretation of data for the work; L Z made substantial contributions to the conception or design of the work. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Second Affiliated Hospital of Shandong First Medical University. Informed consent was obtained from all individual participants included in the study.
Supplemental Material
Download Zip (167.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641963.2024.2358030.
Additional information
Funding
References
- Jaafar J, Pechere-Bertschi A, Ditisheim A. Hypertension in pregnancy: practical considerations. Rev Med Suisse. 2014;10(441):1645–10. doi:10.53738/REVMED.2014.10.441.1645.
- Agrawal A, Wenger NK. Hypertension During Pregnancy. Curr Hypertens Rep. 2020;22(9):64. doi:10.1007/s11906-020-01070-0.
- Li LX, Liu YL, Wen JG, Li ZZ, Zhao YP. Expression and significance of aquaporin 1 in placenta, placental membranes and peritoneum of patients with hypertensive disorder complicating pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2008;43(7):497–501.
- Zhu J, Li M, Li L. Expression and significance of heat shock protein 70 in maternal serum, umbilical cord blood and placenta of patients with hypertensive disorders complicating pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2014;49(9):676–80.
- Wang RG, You ZL, Liu XL. Effect of ingredients of Astragalus-Salvia compound on vascular endothelial cell in placenta and vascular endothelial growth factor mRNA expression in trophocyte in pregnant rats with inhibited nitric oxide synthesis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25(6):516–19.
- Vara-Ciruelos D, Russell FM, Hardie DG. The strange case of AMPK and cancer: dr Jekyll or mr Hyde? Open Biol. 2019;9(7):190099. doi:10.1098/rsob.190099.
- Virginia DM, Dwiprahasto I, Wahyuningsih M, Nugrahaningsih D. The Effect of PRKAA2 Variation on Type 2 Diabetes Mellitus in the Asian Population: A Systematic Review and Meta-Analysis. Malays J Med Sci. 2022;29(3):5–16. doi:10.21315/mjms2022.29.3.2.
- Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P, Mao X, Huang K, Xie Z, Zou MH. Hyperglycemia-driven inhibition of amp-activated protein kinase alpha2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation. 2019;139(16):1913–36. doi:10.1161/CIRCULATIONAHA.118.033552.
- Okamoto S, Asgar NF, Yokota S, Saito K, Minokoshi Y. Role of the α2 subunit of AMP-activated protein kinase and its nuclear localization in mitochondria and energy metabolism-related gene expressions in C2C12 cells. Metabolism. 2019;90:52–68. doi:10.1016/j.metabol.2018.10.003.
- McCallum ML, Pru CA, Smith AR, Kelp NC, Foretz M, Viollet B, Du M, Pru JK. A functional role for AMPK in female fertility and endometrial regeneration. Reproduction. 2018;156(6):501–13. doi:10.1530/REP-18-0372.
- Deng W, Cha J, Yuan J, Haraguchi H, Bartos A, Leishman E, Viollet B, Bradshaw HB, Hirota Y, Dey SK. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest. 2016;126(8):2941–54. doi:10.1172/JCI87715.
- Griffiths RM, Pru CA, Behura SK, Cronrath AR, Ml M, Kelp NC, Winuthayanon W, Spencer TE, Pru JK. AMPK is required for uterine receptivity and normal responses to steroid hormones. Reproduction. 2020;159(6):707–17. doi:10.1530/REP-19-0402.
- Kopsiaftis S, Sullivan KL, Garg I, Taylor JR, Claffey KP. AMPKalpha2 regulates bladder cancer growth through skp2-mediated degradation of p27. Mol Cancer Res. 2016;14(12):1182–94. doi:10.1158/1541-7786.MCR-16-0111.
- Qiu S, Liu T, Piao C, Wang Y, Wang K, Zhou Y, Cai L, Zheng S, Lan F, Du J. AMPKalpha2 knockout enhances tumour inflammation through exacerbated liver injury and energy deprivation-associated AMPKalpha1 activation. J Cell Mol Med. 2019;23(3):1687–97. doi:10.1111/jcmm.13978.
- Das B, Neilsen BK, Fisher KW, Gehring D, Hu Y, Volle DJ, Kim HS, McCall JL, Kelly DL, MacMillan JB, et al. A Functional Signature Ontology (FUSION) screen detects an AMPK inhibitor with selective toxicity toward human colon tumor cells. Sci Rep-Uk. 2018;8(1):3770. doi:10.1038/s41598-018-22090-6.
- Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5’-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Bioph Res Co. 2001;287(2):562–67. doi:10.1006/bbrc.2001.5627.
- Vila IK, Yao Y, Kim G, Xia W, Kim H, Kim S, Park M, Hwang JP, González-Billalabeitia E, Hung M, et al. A UBE2O-AMPKα2 axis that promotes tumor initiation and progression offers opportunities for therapy. Cancer Cell. 2017;31(2):208–24. doi:10.1016/j.ccell.2017.01.003.
- Xu Q, Wu N, Li X, Guo C, Li C, Jiang B, Wang H, Shi D. Inhibition of PTP1B blocks pancreatic cancer progression by targeting the PKM2/AMPK/mTOC1 pathway. Cell Death Disease. 2019;10(12):874. doi:10.1038/s41419-019-2073-4.
- Tong Y, Guo D, Lin S, Liang J, Yang D, Ma C, Shao F, Li M, Yu Q, Jiang Y, et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol Cell. 2021;81(11):2303–16. doi:10.1016/j.molcel.2021.04.002.
- Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, Chen W, Zhang S, Weiss RS, Locasale JW, et al. Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. P Natl Acad Sci Usa. 2016;113(16):4320–25. doi:10.1073/pnas.1519858113.
- Wang X, Shi X, Lu H, Zhang C, Li X, Zhang T, Shen J, Wen J. Succinylation inhibits the enzymatic hydrolysis of the extracellular matrix protein fibrillin 1 and promotes gastric cancer progression. Adv Sci. 2022;9(27):e2200546. doi:10.1002/advs.202200546.
- Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18(6):920–33. doi:10.1016/j.cmet.2013.11.013.
- Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BMM, Skinner ME, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50(6):919–30. doi:10.1016/j.molcel.2013.06.001.
- Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7(1):58–63. doi:10.1038/nchembio.495.
- Hirschey MD, Zhao Y. Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation*. Molecular & Cellular Proteomics: MCP. 2015;14(9):2308–15. doi:10.1074/mcp.R114.046664.
- Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19(4):605–17. doi:10.1016/j.cmet.2014.03.014.
- Pathan M, Keerthikumar S, Chisanga D, Alessandro R, Ang CS, Askenase P, Batagov AO, Benito-Martin A, Camussi G, Clayton A, et al. A novel community driven software for functional enrichment analysis of extracellular vesicles data. J Extracell Vesicles. 2017;6(1):1321455. doi:10.1080/20013078.2017.1321455.
- Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18(2):839–45. doi:10.1128/MCB.18.2.839.
- Guo L, Liu M, Duan T. Hydrogen suppresses oxidative stress by inhibiting the p38 MAPK signaling pathway in preeclampsia. Advances in clinical and experimental medicine: official organ Wroclaw Medical University. Adv Clin Exp Med. 2023;32(3):357–67. doi:10.17219/acem/154623.
- Towler MC, Hardie DG. AMP-Activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–41. doi:10.1161/01.RES.0000256090.42690.05.
- Fang LD. Partial endocardial cushion defect associated with left superior vena cava drainage into left atrium (author’s transl). Zhonghua Xin Xue Guan Bing Za Zhi. 1980;8(4):289–91.
- Feng L, Gao L, Guan Q, Hou X, Wan Q, Wang X, Zhao J. Long-term moderate ethanol consumption restores insulin sensitivity in high-fat-fed rats by increasing SLC2A4 (GLUT4) in the adipose tissue by AMP-activated protein kinase activation. J Endocrinol. 2008;199(1):95–104. doi:10.1677/JOE-08-0026.
- Karthikeyan VJ, Lip GY. Hypertension in pregnancy: pathophysiology and management strategies. Curr Pharm Design. 2007;13(25):2567–79. doi:10.2174/138161207781663019.
- Naicker T, Dorsamy E, Ramsuran D, Burton GJ, Moodley J. The role of apoptosis on trophoblast cell invasion in the placental bed of normotensive and preeclamptic pregnancies. Hypertens Pregnancy. 2013;32(3):245–56. doi:10.3109/10641955.2013.796969.
- Wang Y, Liu L, Tian Y, Chen Y, Zha W, Li Y, Wu F. Upregulation of DAPK2 ameliorates oxidative damage and apoptosis of placental cells in hypertensive disorder complicating pregnancy by suppressing human placental microvascular endothelial cell autophagy through the mTOR signaling pathway. Int J Biol Macromol. 2019;121:488–97. doi:10.1016/j.ijbiomac.2018.09.111.
- Guo L, Guan Z, Li H, Yang X. Hydrogen inhibits cytotrophoblast cells apoptosis in hypertensive disorders complicating pregnancy. Cell Mol Biol (Noisy-le-Grand). 2016;62(6):59–64.
- Zhao L, Xiong M, Liu Y. Baicalin enhances the proliferation and invasion of trophoblasts and suppresses vascular endothelial damage by modulating long non‐coding RNA NEAT1/miRNA‐205‐5p in hypertensive disorder complicating pregnancy. J Obstet Gynaecol Res. 2021;47(9):3060–70. doi:10.1111/jog.14789.
- Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, Xie Y, Liu J, Klionsky DJ, Kroemer G, et al. AMPK-Mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc–activity. Curr Biol. 2018;28(15):2388–99. doi:10.1016/j.cub.2018.05.094.
- Yue C, Chen J, Li Z, Li L, Chen J, Guo Y. microRNA-96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKalpha2-FTO-m6A/MYC axis. J Exp Clin Canc Res. 2020;39(1):240. doi:10.1186/s13046-020-01731-7.
- Hallstrom TC, Mori S, Nevins JR. An E2F1-Dependent Gene Expression Program that Determines the Balance between Proliferation and Cell Death. Cancer Cell. 2008;13(1):11–22. doi:10.1016/j.ccr.2007.11.031.
- Yang Y, Gibson GE. Succinylation Links Metabolism to Protein Functions. Neurochem Res. 2019;44(10):2346–59. doi:10.1007/s11064-019-02780-x.
- Trefely S, Lovell CD, Snyder NW, Wellen KE. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol Metab. 2020;38:100941. doi:10.1016/j.molmet.2020.01.005.
- Wang G, Meyer JG, Cai W, Softic S, Li ME, Verdin E, Newgard C, Schilling B, Kahn CR. Regulation of UCP1 and mitochondrial metabolism in brown adipose tissue by reversible succinylation. Mol Cell. 2019;74(4):844–57. doi:10.1016/j.molcel.2019.03.021.
- Kelly R, Chandru A, Watson PJ, Song Y, Blades M, Robertson NS, Jamieson AG, Schwabe J, Cowley SM. Histone deacetylase (HDAC) 1 and 2 complexes regulate both histone acetylation and crotonylation in vivo. Sci Rep-Uk. 2018;8(1):14690. doi:10.1038/s41598-018-32927-9.
- Wang Y, Guo YR, Liu K, Yin Z, Liu R, Xia Y, Tan L, Yang P, Lee JH, Li XJ, et al. KAT2A coupled with the alpha-KGDH complex acts as a histone H3 succinyltransferase. Nature. 2017;552(7684):273–77. doi:10.1038/nature25003.
- Wang C, Zhang C, Li X, Shen J, Xu Y, Shi H, Mu X, Pan J, Zhao T, Li M, et al. CPT1A-mediated succinylation of S100A10 increases human gastric cancer invasion. J Cell Mol Med. 2019;23(1):293–305. doi:10.1111/jcmm.13920.
- Yuan HF, Zhao M, Zhao LN, Yun HL, Yang G, Geng Y, Wang YF, Zheng W, Yuan Y, Song TQ, et al. PRMT5 confers lipid metabolism reprogramming, tumour growth and metastasis depending on the SIRT7-mediated desuccinylation of PRMT5 K387 in tumours. Acta Pharmacol Sin. 2022;43(9):2373–85. doi:10.1038/s41401-021-00841-y.