ABSTRACT
Background
Aortic endothelial diastolic dysfunction is an early complication of diabetes and the abnormal differentiation of Th17 cells is involved in the development of diabetes. However, the exact role of exercise on regulating the Th17 cells differentiation and the underlying molecular mechanisms remain to be elucidated in diabetic mice.
Methods
db/db and db/m+ mice were randomly divided into exercise and sedentary groups. Mice in exercise group were exercised daily, 6 days/week, for 6 weeks and mice in sedentary groups were placed on a nonmoving treadmill for 6 weeks. Vascular endothelial function was measured via wire myograph and the frequencies of Th17 from peripheral blood in mice were assessed via flow cytometry.
Results
Our data showed that exercise improved insulin resistance and aortic endothelial diastolic function in db/db mice. In addition, the proportion of Th17 cells and IL-17A level in peripheral blood of db/db mice were significantly increased, and exercise could promote Th17 cell differentiation and reduce IL-17A level. More importantly, STAT3 or ROR-γt inhibitors could promote Th17 cell differentiation in db/db mice, while exercise significantly down-regulated p-STAT3/ROR-γt signaling in db/db mice, suggesting that exercise regulated Th17 differentiation through STAT3/ROR-γt signaling.
Conclusions
This study demonstrated that exercise improved vascular endothelial function in diabetic mice via reducing Th17 cell differentiation through p-STAT3/ROR-γt pathway, suggesting exercise may be an important non-pharmacological intervention strategy for the treatment of diabetes-related vascular complications.
At present, the global incidence of diabetes shows a progressive increase and younger trend (Citation1). Diabetes can lead to a series of vital organ complications, including diabetic nephropathy, diabetic cardiomyopathy, diabetic retinopathy and diabetic vasculopathy, which cause extremely serious adverse effects on the body and mind of patients, but also bring heavy economic burden to society (Citation2). How to reduce the complications caused by chronic diseases, especially diabetes, is the focus of medical basic and clinical research.
Vascular endothelial dysfunction caused by diabetes is an early pathophysiological change that causes the dysfunction of vital organs (Citation3). Previous studies have shown that oxidative stress, inflammation, endoplasmic reticulum stress, mitochondrial dysfunction and other factors are involved in diabetes-related vascular endothelial dysfunction (Citation4). Controlling hyperglycemia and improving insulin resistance in diabetic patients is the basis of restoring vascular endothelial function (Citation5). Lifestyle changes, especially exercise, play a key role in the treatment of diabetes (Citation6). Studies have found that exercise can improve insulin resistance, restore vascular endothelial function and renal dopamine D1-mediated sodium excretion, which is involved in the regulation of atherosclerosis and hypertension (Citation7,Citation8). Therefore, exercise has been recommended as an important strategy for the non-drug treatment of cardiovascular disease (Citation9). However, the mechanism by which exercise improves vascular endothelial function of diabetes remains to be further clarified.
In recent years, it has been found that T helper type 17 (Th17) cells are involved in the occurrence and development of diabetes and its related complications (Citation10). In the peripheral blood of diabetic animal models and patients, Th17/Treg balance is disregulated, mainly manifested as Th17 level is significantly increased, while Treg is decreased (Citation11,Citation12). Restoration of Th17/Treg balance suppresses diabetic nephropathy in db/db mice via SGLT2 knockdown (Citation13). Whether exercise could improve vascular endothelial function in diabetic mice by modulating Th17 cell signaling is unclear. In the present study, we observed that exercise improved vascular endothelial function in diabetic mice by modulating Th17 differentiation through STAT3/ROR-γt pathway.
Materials and methods
Animals and experimental design
10 week old male leptin-receptor-deficient diabetic mice (db/db) and non-diabetic heterozygous littermates (db/m+) were purchased from Beijing Vital River Laboratory Animal Technology. Mice were housed in an environmentally controlled room with 12 : 12 hour light/dark cycle, 70% humidity, and 24°C temperature and had free access to standard laboratory food and water. Mice were randomly divided into exercise and sedentary groups. Mice in exercise group were exercised daily, 6 days/week, for 6 weeks with the speed at 8 m/min and mice in sedentary groups were placed on a nonmoving treadmill for 6 weeks. Body weight and heart rate in exercise and sedentary groups were measured during the intervention. Animal experimental procedures were approved by the Animal Care and Use Committee of Chongqing Medical University and were carried out in accordance with the NIH Guide for the Care Use of Laboratory Animals.
Biochemical analysis
After 6 weeks of intervention, blood samples were collected for measurement of biochemical indicators. Fasting glucose were measured with a glucose analyzer (Sinocare, Hunan, China) and plasma insulin was measured by radioimmunoassay using a rat insulin kit (Beyotime Biotechnology, Shanghai, China). For the measurement of glucose tolerance test (GTT) and insulin tolerance test (ITT), mice were injected with glucose (1 g/kg body weight) and 1.5 U/kg insulin intraperitoneally after fasting for 6 h.
IL-17A, IL-6 and IL-10 measurement
The levels of IL-17A, IL-6 and IL-10 in plasma were quantified using mouse ELISA kits (Nanjing Jiancheng Bioengineering institute, Nanjing, China) and the detailed detection methods were carried out according to the kit instructions.
Thoracic aortas preparation and functional study
The reactivity of thoracic aortas was determined as previously described (Citation14). Briefly, segments of mouse aortas were mounted to a myograph (DMT, Aarhus, Denmark) and isometric tension were measured. Endothelium-dependent relaxation (EDR) was determined by acetylcholine chloride (Ach, 3 nM-10 μM, Sigma-Aldrich, MO, USA) and endothelium-independent relaxation was determined by sodium nitroprusside (SNP, 1 nM-10 μM, Sigma-Aldrich) in phenylephrine (Phe) (3 μM) precontracted segments. Some segments were incubated with IL-17A inhibitor (LY3509754, 1 mM, MCE) or IL-17A protein (20 ng/mL, MCE) for 30 min before EDR measurement. The possible role of eNOS in Ach-mediated endothelium-dependent relaxation was investigated by preincubation with L-NAME (100 µM, Sigma-Aldrich) for 30 minutes.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were harvested from the peripheral blood of mice and cultured in 1640 complete medium at a concentration of 1 × 106 cells/mL. Cells were stimulated with phorbol myristate acetate, ionomycin, and monensin in 5% CO2 at 37°C for 4 h. Subsequently, the cells were then stained with live/dead dye and BV605-anti-mouse CD4, followed by fixation and permeabilization. Finally, cells were stained with anti-IL-17A antibody. Flow cytometric analysis was used to detect the Th17 according to the proportions of CD4+/IL-17+ cells.
Cell culture
CD4+ T cells were obtained from spleens of mice and were precoated with anti-mouse CD3 mAb and anti-mouse CD28 mAb (5 µg/mL, Abcam, Cambridge, UK). Cells were cultured at 37°C and 5% CO2 in RPMI-1640 medium supplemented with 10% fetal bovine serum. Experiments were performed with cells at 80 ~ 90% of confluence and the cells were stimulated by STAT3 inhibitor (Stattic, 10 µM, MCE) or ROR-γt inhibitor (GSK805, 1 mM, MCE) for 12 h. After treatment with the reagent or vehicle, the cells were harvested for western blotting.
Western blotting
Western blotting was performed according to standard protocols described previously (Citation15). Briefly, thoracic aortas and CD4+ T cells were homogenized using ice-cold RIPA lysis buffer. The proteins lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked using 8% fat-free milk in TBS (Tris-buffered saline) with 0.5% Tween-20 for 2 h at room temperature and then probed with primary antibodies against phosphorylated eNOS at Ser1177 (1:500; Cell Signaling Technology, Danvers, MA, USA), t-eNOS (1:1000; Cell Signaling Technology), p-STAT3 (1:400, Abcam), t-STAT3 (1:400, Abcam), ROR-γt (1:400, Abcam) and GAPDH (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies at 4°C overnight. The anti-rabbit secondary antibody (1:10000, Li-Cor Bioscience, Bad Homburg, Germany) was incubated for 2 h at room temperature. The protein bands were detected by Odyssey Western Blot Detection System (LI-COR) and the band intensities were evaluated with ImageJ.
Statistical analysis
The data were expressed as mean ± SEM. The relaxation was presented as percentage reduction of the phenylephrine contraction. Concentration-response curves were analyzed using the Graphpad Prism 5.0 software. Data were analyzed by one-way ANOVA followed by Student-Newman-Keuls test whenever appropriate. Value of p < .05 was considered significant.
Results
Exercise improved insulin resistance in diabetic mice
Consistent with previous studies, exercise, as one of the important forms of non-drug treatment for diabetes, can improve insulin resistance and lower blood glucose levels to a certain extent (Citation16). Regarding the effect of exercise on insulin resistance, we observed that diabetic mice exhibited higher fasting glucose levels, fasting insulin levels and impaired glucose tolerance, determined by IPGTT, and impaired insulin tolerance, determined by ITT, which were markedly reversed by regular running exercise (). In addition, exercise reduced body weight in diabetic mice, but had no effect on heart rate ().
Figure 1. The effects of exercise on insulin resistance in diabetic mice. db/db and db/m+ mice were randomly divided into exercise and sedentary groups. Mice in exercise group were exercised daily, 6 days/week, for 6 weeks and mice in sedentary groups were placed on a nonmoving treadmill for 6 weeks. (a and b) fasting blood glucose and serum insulin levels. (c and d) glucose tolerance test (GTT) and insulin tolerance test (ITT). (e and f) body weight and heart rate. Values are represented as mean ± SEM, n = 6. *p < 0.05 vs control in db/m+, #p < 0.05 vs control in db/db.
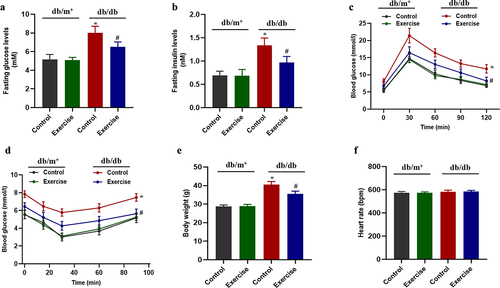
Exercise could improve vascular endothelial function in diabetic mice
Previous studies have shown that exercise can improve vascular endothelial function in diabetic and obese mice (Citation17). Our present study also found that vascular endothelium-dependent relaxation function was impaired in diabetic mice compared with non-diabetic mice, while there was no significant difference in the endothelium-independent relaxation (). In diabetic mice, exercise could significantly improve endothelium-dependent diastolic function, but have no effect on endothelium-independent relaxation (). In addition, in order to further confirm the role of endothelial NO production in impaired vascular endothelial dilation function, the production of endothelial NO was inhibited by preincubation of L-NAME. The results showed that there was no significant difference in Ach-induced vasodilation between the exercise group and the non-exercise group of diabetic mice after adding to L-NAME (). More importantly, the present results suggested that exercise could significantly improve the expression level of p-eNOS in diabetic mice, suggesting that the production of NO is significantly increased ().
Figure 2. The effects of exercise on vascular endothelial function in diabetic mice. db/db and db/m+ mice were randomly divided into exercise and sedentary groups. Mice in exercise group were exercised daily, 6 days/week, for 6 weeks and mice in sedentary groups were placed on a nonmoving treadmill for 6 weeks. (a-b) vascular endothelial function included Ach-induced relaxation and SNP-induced relaxation in thoracic aortas from diabetic mice and non-diabetic mice. *p < 0.05 vs control in db/m+, #p < 0.05 vs control in db/db. (c) Ach-induced relaxation with L-NAME pretreatment in thoracic aortas from diabetic mice. *p < 0.05 vs others. (d) Phosphorylation of eNOS in thoracic aortas. *p < 0.05 vs control in db/m+, #p < 0.05 vs control in db/db. Data were expressed as the means ± SEM (n = 6/group).
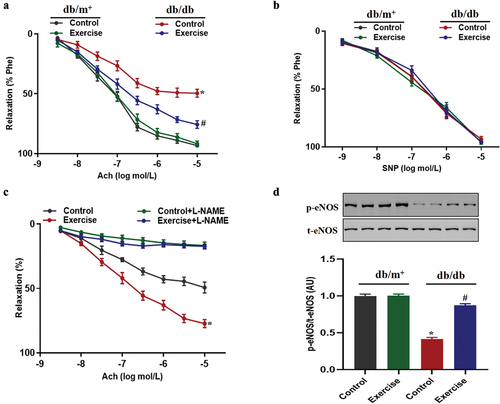
Exercise could reduce the proportion of Th17 cells in peripheral blood of diabetic mice
An increased level of Th17 cells in peripheral blood is closely related to the occurrence and development of diabetes (Citation18). Our present results showed that the proportion of Th17 cells in peripheral blood of diabetic mice in the exercise group was significantly lower than that in non-exercise diabetic mice (). In addition, exercise also reduced the levels of Th17 cell effector (IL-17A) and IL-6, and increased the level of anti-inflammatory cytokine IL-10 in peripheral blood of diabetic mice (). In order to further investigate the relationship between IL-17A and vascular endothelial diastolic function, it was found that pre-incubation of IL-17A protein inhibited endothelial diastolic function in non-diabetic mice and pre-incubation of IL-17A inhibitors significantly improved the impaired endothelial diastolic function in diabetic mice (), suggesting that the increased level of IL-17A is an important cause of impaired vascular endothelial diastolic function of diabetic mice.
Figure 3. The effects of exercise on Th17 cell differentiation in diabetic mice. db/db and db/m+ mice were randomly divided into exercise and sedentary groups. Mice in exercise group were exercised daily, 6 days/week, for 6 weeks and mice in sedentary groups were placed on a nonmoving treadmill for 6 weeks. (a) The proportion of Th17 cells in peripheral blood of mice was measured by flow cytometry. (b-d) the IL-17A, IL-6 and IL-10 levels in peripheral blood of mice were measured by ELISA. (e) Ach-induced relaxation with IL-17A protein or IL-17 inhibitor pretreatment in thoracic aortas from diabetic or non-diabetic mice. Data were expressed as the means ± SEM (n = 6/group). *p < 0.05 vs control in db/m+, #p < 0.05 vs control in db/db.
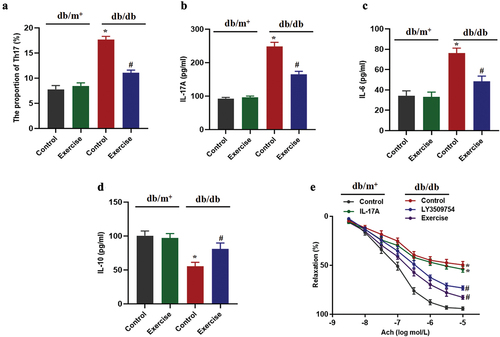
Exercise reduces Th17 cell differentiation by inhibiting ROR-γt
ROR-γt is a key factor in promoting Th17 cell differentiation (Citation19). The expression level of ROR-γt in diabetic exercise group was significantly lower than that in diabetic non-exercise group (). However, ROR-γt inhibitor could significantly reduce the secretion of IL-17 in Th17 cells extracted from diabetic mice (), suggesting the role of ROR-γt in Th17 cell differentiation.
Figure 4. Exercise reduces Th17 cell differentiation by inhibiting ROR-γt. db/db and db/m+ mice were randomly divided into exercise and sedentary groups. Mice in exercise group were exercised daily, 6 days/week, for 6 weeks and mice in sedentary groups were placed on a nonmoving treadmill for 6 weeks. (a) The ROR-γt expression in Th17 cells was analysed by western blotting. Th17 cells in diabetic mice were pretreated with ROR-γt inhibitor for 12 h. (b) The IL-17 levels were measured by ELISA. Data were expressed as the means ± SEM (n = 6/group). *p < 0.05 vs control in db/m+, #p < 0.05 vs control in db/db.
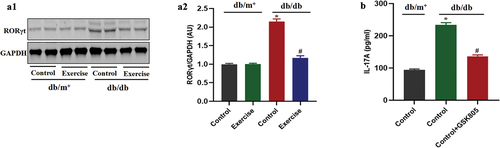
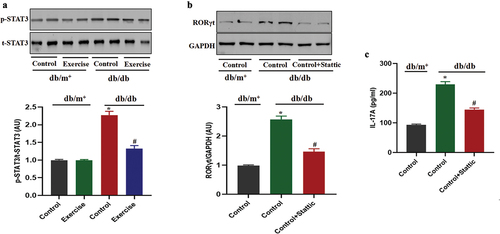
STAT3 is involved in the regulation of exercise on ROR-γt in diabetic mice
Previous studies have shown that STAT3, as the upstream of ROR-γt, is involved in the regulation of Th17 cell differentiation (Citation20). The present results showed that the p-STAT3 level of Th17 cells from diabetic mice in the exercise group was significantly decreased compared with that in the non-exercise group (). However, STAT3 inhibitor could significantly reduce ROR-γt expression and the secretion of IL-17 in Th17 cells extracted from diabetic mice (), indicating that STAT3 is involved in the differentiation of Th17 cells through regulation of ROR-γt.
Figure 5. STAT3 is involved in the regulation of exercise on ROR-γt in diabetic mice. db/db and db/m+ mice were randomly divided into exercise and sedentary groups. Mice in exercise group were exercised daily, 6 days/week, for 6 weeks and mice in sedentary groups were placed on a nonmoving treadmill for 6 weeks. (a) The p-STAT3 levels in Th17 cells was analysed by western blotting. Th17 cells in diabetic mice were pretreated with STAT3 inhibitor for 12 h. (b) ROR-γt expression in Th17 cells was analysed by western blotting. (c) The IL-17 levels were measured by ELISA. Data were expressed as the means ± SEM (n = 6/group). *p < 0.05 vs control in db/m+, #p < 0.05 vs control in db/db.
Discussion
Diabetes mellitus is an important risk factor for coronary heart disease and peripheral vascular atherosclerosis. How to restrain the vascular disease caused by diabetes is a hot issue in the field of chronic disease research. In our current study, we provided the first evidence that exercise as a non-drug intervention could significantly improve impaired vascular endothelial function in diabetic mice, through mechanisms related to down-regulating STAT3/ROR-γt signaling, inhibiting Th17 differentiation, and specifically reducing IL-17A secretion.
A large number of studies have shown that exercise has a large number of beneficial effects as an intervention strategy for the non-drug treatment and prevention of cardiovascular diseases (Citation21). Endoplasmic reticulum stress and autophagy are important pathogenesis of myocardial ischemia-reperfusion injury (Citation22). Exercise attenuates myocardial ischemia-reperfusion injury and improves impaired cardiac function by regulating endoplasmic reticulum stress and mitophagy (Citation22). Mitochondrial fragmentation was found in both animal models and patients with hypertension (Citation23). Swimming exercise improves vascular function in hypertension via alleviating endothelial mitochondrial fragmentation (Citation23). A decrease of endothelial NO synthase activity is an early characteristic of diabetic vasculopathy (Citation24). Exercise improves endothelium-dependent relaxation in diabetic mice via increasing NO bioavailability through restoring the endothelial expression of Krüppel-like factor 2 (Citation25). Consistent with previous studies (Citation25), our present results also showed that exercise intervention for 6 weeks could significantly improve aortic endothelial diastolic function in diabetic mice. Therefore, exercise plays a protective role against cardiovascular disease through multiple mechanisms. Although it has been reported that exercise can improve vascular endothelial function in diabetes mellitus, the more comprehensive protective mechanism needs to be further elucidated.
A large number of studies have shown that the body’s immune system is closely related to the occurrence and development of diabetes mellitus and its related complications (Citation26). Among them, Th17 cells, as a subgroup of helper T cells that specifically secrete IL-17A, are significantly increased in the peripheral blood of obese and diabetic patients (Citation27). Previous studies have shown that glucagon-like peptide-1 receptor agonist exenatide can promote Th17 cell differentiation by regulating FoxO1 pathway, thereby reducing islet inflammation (Citation28). Reduction of Th17 cell differentiation is associated with endothelial dysfunction in spontaneously hypertensive rat and Dieckol obtained from Ecklonia cava and E. cava extract improves endothelial function through the Th17/NF-κB/IL-6 pathway (Citation29). In order to observe the relationship between exercise and Th17 differentiation in peripheral blood of diabetic mice, the proportion of Th17 cells and IL-17A level in peripheral blood were detected. The results showed that exercise intervention for 6 weeks could significantly promote Th17 cell differentiation and reduce IL-17A level in peripheral blood of diabetic mice. In addition, in order to further observe the direct effect of specific IL-17A secreted by Th17 cells on vascular endothelial function, it was found that inhibition of IL-17 could improve the aortic endothelial function of diabetic mice after incubation of IL-17 inhibitors, suggesting that exercise can improve the aortic endothelial function of diabetic mice by decreasing IL-17. However, the specific mechanism of exercise promoting Th17 cell differentiation in diabetic mice remains to be further elucidated.
RORγt is specifically expressed in Th17 cells and is a key transcription factor regulating the differentiation of CD4+ T cells into Th17 cells and the secretion of pro-inflammatory factor IL-17A (Citation30). High-salt intake is known to induce hypertension, endothelial dysfunction and proinflammatory Th17 cell differentiation, and the enrichment of RORγt in the IL-17A promoter region is a key mechanism that promotes the abnormal differentiation of Th17 cells (Citation31). In our present study, it was found that exercise significantly reduced the expression level of RORγt in Th17 cells of diabetic mice, and inhibition of RORγt promoted the differentiation of Th17 cells in diabetic mice, suggesting that exercise could affect the differentiation of Th17 cells by regulating the expression of RORγt. It has been found that STAT3 initiates Th17 cell differentiation and IL-17A expression through facilitating RORγt in multiple sclerosis (Citation32) and autoimmune encephalomyelitis (Citation33). Previous studies have shown that exercise significantly down-regulates p-STAT3 levels to improve type 2 diabetes mellitus-related cognitive impairment (Citation34) and attenuate neuronal apoptosis in ischemic stroke (Citation35). Our present study also found that exercise could significantly down-regulate p-STAT3 expression in Th17 cells of diabetic mice, and treatment with STAT3 inhibitors could down-regulate RORγt and promote Th17 cell differentiation, suggesting that exercise promote Th17 cell differentiation depending on STAT3/RORγt signaling. The mechanism of exercise improving vascular endothelial function is complex, in addition to promoting Th17 cell differentiation, reducing the production of ROS and increasing the bioavailability of NO in the endothelium, which are also involved (Citation36).
In conclusion, our study found that exercise could improve aortic endothelial function in diabetic mice by promoting Th17 cell differentiation through STAT3/RORγt signaling. Therefore, exercise could be recommended as an important non-pharmacological intervention strategy for the treatment of diabetes-related vascular complications.
Author contribution
YL conceived and designed the experiments and wrote the manuscript; LL and DW performed the experiments and analyzed the data; LZ contributed reagents analysis tools; QZ approved the final version of the manuscript.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tomkins M, Lawless S, Martin-Grace J, Sherlock M, Thompson CJ. Diagnosis and management of central diabetes insipidus in adults. J Clin Endocrinol Metab. 2022;107(10):2701–8. doi:10.1210/clinem/dgac381.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151.
- Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med. 2007;262(2):173–83. doi:10.1111/j.1365-2796.2007.01830.x.
- Odegaard AO, Jacobs DR Jr., Sanchez OA, Goff DC Jr., Reiner AP, Gross MD. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016;15(1):51. doi:10.1186/s12933-016-0369-6.
- Hamilton SJ, Chew GT, Watts GF. Therapeutic regulation of endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res. 2007;4(2):89–102. doi:10.3132/dvdr.2007.026.
- Balducci S, Sacchetti M, Haxhi J, Orlando G, D’Errico V, Fallucca S, Menini S, Pugliese G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab Res. 2014;30(Suppl S1):13–23. doi:10.1002/dmrr.2514.
- Lee JH, Lee R, Hwang MH, Hamilton MT, Park Y. The effects of exercise on vascular endothelial function in type 2 diabetes: a systematic review and meta-analysis. Diabetol Metab Syndr. 2018;10(1):15. doi:10.1186/s13098-018-0316-7.
- Tao Y, Luo W, Chen Y, Chen C, Chen S, Li X, Chen K, Zeng C. Exercise ameliorates skeletal muscle insulin resistance by modulating GRK4-mediated D1R expression. Clin Sci (Lond). 2023;137(17):1391–407. doi:10.1042/CS20230664.
- Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, Earnest CP, Church TS, O’Keefe JH, Milani RV, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117(2):207–19. doi:10.1161/CIRCRESAHA.117.305205.
- Guzman-Flores JM, Ramirez-Emiliano J, Perez-Vazquez V, Lopez-Briones S. Th17 and regulatory T cells in patients with different time of progression of type 2 diabetes mellitus. Cent Eur J Immunol. 2020;45(1):29–36. doi:10.5114/ceji.2020.94670.
- Tao L, Liu H, Gong Y. Role and mechanism of the Th17/Treg cell balance in the development and progression of insulin resistance. Mol Cell Biochem. 2019;459(1–2):183–88. doi:10.1007/s11010-019-03561-4.
- Duan W, Ding Y, Yu X, Ma D, Yang B, Li Y, Huang L, Chen Z, Zheng J, Yang C. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting treg production. Am J Transl Res. 2019;11(4):2393–402.
- Wang D, Zhang Q, Dong W, Ren S, Wang X, Su C, Lin X, Zheng Z, Xue Y. SGLT2 knockdown restores the Th17/Treg balance and suppresses diabetic nephropathy in db/db mice by regulating SGK1 via Na+. Mol Cell Endocrinol. 2024;584:112156. doi:10.1016/j.mce.2024.112156.
- Liu H, Li Y, Li M, Xie L, Li F, Pan R, Pei F. Follistatin-like 1 protects endothelial function in the spontaneously hypertensive rat by inhibition of endoplasmic reticulum stress through AMPK-dependent mechanism. Clin Exp Hypertens. 2023;45(1):2277654. doi:10.1080/10641963.2023.2277654.
- Xu C, Zhao Z, Yuan W, Fengping Z, Zhiqiang Y, Xiaoqin Z. Effect of allisartan on blood pressure and left ventricular hypertrophy through Kv1.5 channels in hypertensive rats. Clin Exp Hypertens. 2022;44(3):199–207. doi:10.1080/10641963.2021.2018597.
- Li Z, Calhoun P, Rickels MR, Gal RL, Beck RW, Jacobs PG, Clements MA, Patton SR, Castle JR, Martin CK, et al. Factors affecting reproducibility of change in glucose during exercise: results from the type 1 diabetes and EXercise initiative. J Diabetes Sci Technol. 2024:19322968241234687. doi:10.1177/19322968241234687.
- Cheang WS, Wong WT, Zhao L, Xu J, Wang L, Lau CW, Chen ZY, Ma RCW, Xu A, Wang N, et al. PPARδ is required for exercise to attenuate endoplasmic reticulum stress and endothelial dysfunction in diabetic mice. Diabetes. 2017;66(2):519–28. doi:10.2337/db15-1657.
- Mendez-Frausto G, Romero-Aguilera G, Sanchez-Gutierrez R, Garcia-Jacobo RE, Lara-Ramirez EE, Uresti-Rivera EE, Gonzalez-Amaro R, Enciso-Moreno JA, Garcia-Hernandez MH. B regulatory cells associated with changes in biochemical and inflammatory parameters in normal-glycemic individuals, pre-diabetes and T2DM patients. Diabetes Res Clin Pract. 2021;173:108692. doi:10.1016/j.diabres.2021.108692.
- Kumar R, Theiss AL, Venuprasad K. RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol. 2021;42(11):1037–50. doi:10.1016/j.it.2021.09.005.
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151(2):289–303. doi:10.1016/j.cell.2012.09.016.
- Villella M, Villella A. Exercise and cardiovascular diseases. Kidney Blood Press Res. 2014;39(2–3):147–53. doi:10.1159/000355790.
- Chen W, Ma M, Song Y, Hua Y, Jia H, Liu J, Wang Y. Exercise attenuates myocardial ischemia-reperfusion injury by regulating endoplasmic reticulum stress and mitophagy through M 2 acetylcholine receptor. Antioxid Redox Signal. 2024;40(4–6):209–21. doi:10.1089/ars.2022.0168.
- Li G, Xu K, Xing W, Yang H, Li Y, Wang X, Zhou J, An J, Dong L, Zhang X, et al. Swimming exercise alleviates endothelial mitochondrial fragmentation via inhibiting dynamin-related protein-1 to improve vascular function in hypertension. Hypertension. 2022;79(10):e116–28. doi:10.1161/HYPERTENSIONAHA.122.19126.
- Jia G, Bai H, Mather B, Hill MA, Jia G, Sowers JR. Diabetic vasculopathy: molecular mechanisms and clinical insights. Int J Mol Sci. 2024;25(2):804. doi:10.3390/ijms25020804.
- Luo JY, Cheng CK, Gou L, He L, Zhao L, Zhang Y, Wang L, Lau CW, Xu A, Chen AF, et al. Induction of KLF2 by exercise activates eNOS to improve vasodilatation in diabetic mice. Diabetes. 2023;72(9):1330–42. doi:10.2337/db23-0070.
- Mincu I, Cheta D. Diabetes mellitus and immunity. Med Interne. 1983;21(4):257–66.
- Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother. 2018;101:287–92. doi:10.1016/j.biopha.2018.02.103.
- Xu Q, Zhang X, Li T, Shao S. Exenatide regulates Th17/Treg balance via PI3K/Akt/FoxO1 pathway in db/db mice. Mol Med. 2022;28(1):144. doi:10.1186/s10020-022-00574-6.
- Oh S, Shim M, Son M, Jang JT, Son KH, Byun K. Attenuating effects of dieckol on endothelial cell dysfunction via modulation of Th17/Treg balance in the intestine and aorta of spontaneously hypertensive rats. Antioxid (Basel). 2021;10(2):298. doi:10.3390/antiox10020298.
- Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, Deng Z, Yao Z, Tsun A, Li B. FOXP3 and RORgammat: transcriptional regulation of treg and Th17. Int Immunopharmacol. 2011;11(5):536–42. doi:10.1016/j.intimp.2010.11.008.
- Kim JY, Lee S, Jang S, Kim CW, Gu BH, Kim M, Kim I. T helper cell polarity determines salt sensitivity and hypertension development. Hypertens Res. 2023;46(9):2168–2178. doi:10.1038/s41440-023-01365-0.
- Chen JY, Tian XY, Wei SS, Xu W, Pan RR, Chen LL, Chen LD, Nan LH, Yao L, Shan D, et al. Magnolol as STAT3 inhibitor for treating multiple sclerosis by restricting Th17 cells. Phytomedicine. 2023;117:154917. doi: 10.1016/j.phymed.2023.154917.
- Gharibi T, Barpour N, Hosseini A, Mohammadzadeh A, Marofi F, Ebrahimi-Kalan A, Nejati-Koshki K, Abdollahpour-Alitappeh M, Safaei S, Baghbani E, et al. STA-21, a small molecule STAT3 inhibitor, ameliorates experimental autoimmune encephalomyelitis by altering Th-17/Treg balance. Int Immunopharmacol. 2023;119:110160. doi: 10.1016/j.intimp.2023.110160.
- Lin L, Wang Y, Xu W, Huang C, Hu J, Chen X, Lv X, Qin Y, Zhao X, Li H. Aerobic exercise improves type 2 diabetes mellitus-related cognitive impairment by inhibiting JAK2/STAT3 and enhancing AMPK/SIRT1 pathways in mice. Dis Markers. 2022;2022:6010504. doi:10.1155/2022/6010504.
- Shan Y, Wang L, Sun J, Chang S, Di W, Lv H. Exercise preconditioning attenuates cerebral ischemia-induced neuronal apoptosis, Th17/Treg imbalance, and inflammation in rats by inhibiting the JAK2/STAT3 pathway. Brain Behav. 2023;13(6):e3030. doi: 10.1002/brb3.3030.
- Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol. 1996;28(7):1652–60. doi:10.1016/S0735-1097(96)00393-2.
