Abstract
The need for better nutrient management has spurred efforts towards more comprehensive recycling of nutrients contained in human excreta to agriculture. Research in this direction has intensified throughout the past years, continuously unfolding new knowledge and technologies. The present review aspires to provide a systematic synthesis of the field by providing an accessible overview of terminology, recovery pathways and treatment options, and products rendered by treatment. Our synthesis suggests that, rather than focusing on a specific recovery pathway or product and on a limited set of nutrients, there is scope for exploring how to maximize nutrient recovery by combining individual pathways and products and including a broader range of nutrients. To this end, finding ways to more effectively share and consolidate knowledge and information on recovery pathways and products would be beneficial. The present review aims to provide a template that aims to facilitate designing human excreta management for maximum nutrient recovery, and that can serve as foundation for organizing and categorizing information for more effective sharing and consolidation.
1. Introduction
Growing concern about future fertilizer availability has re-emphasized the need for better nutrient management, including comprehensive recycling of nutrients contained in human excreta to agriculture (Elser & Bennett, Citation2009; Dawson & Hilton, Citation2011; McConville et al., Citation2015). Human excreta have a long history of being used as fertilizer and organic soil amendment but urbanization, the introduction of water closets and sewer networks, and the growing and nowadays widespread use of synthetic fertilizers has contributed to a significant departure from this practice (Rockefeller, Citation1998; Ferguson, Citation2014).
In urban areas in industrialized countries, water is used to convey human excreta through extensive sewer networks to municipal sewage treatment plants (STPs). Treatment renders a treated effluent, gaseous emissions, and a solid residual referred to as sewage sludge (in European regulations) or biosolids (in North American regulations). Land application of sewage sludge is a common practice in many countries and allows for partial recycling of nutrients to agriculture. The practice has been heavily debated for a long time, however, due to concerns about contaminants such as pathogens, organic pollutants, and heavy metals in the sludge (Petrik, Citation1954; Renner, Citation2000; McBride, Citation2003; Bengtsson & Tillman, Citation2004; Singh & Agrawal, Citation2008; Öberg & Mason-Renton, Citation2018). As a result, there is a trend towards incineration of a larger portion of the sludge (Kelessidis & Stasinakis, Citation2012; Kirchmann et al., Citation2017).
The adequacy and long-term sustainability of conventional urban water and sanitation systems has increasingly been called into question. For low-income countries, the high infrastructure costs are prohibitive for widespread adoption (Larsen et al., Citation2016). In the context of high-income countries, issues of concern include high energy and water demand, sludge disposal problems, and limited nutrient recycling (Brands, Citation2014). Some scholars hold on to the idea of municipal sewers and call for more comprehensive resource recovery at municipal STPs (Peccia & Westerhoff, Citation2015; Verstraete et al., Citation2016; Puyol et al., Citation2017). Other scholars hold that source separation and control provide greater opportunities for resource recovery, as it minimizes dilution and contamination of human excreta (Larsen & Gujer, Citation1997; Otterpohl et al., Citation1997; Wilsenach et al., Citation2003; Larsen et al., Citation2009). Approaches based on source separation and control are commonly referred to as new, ecological, resource-oriented, source-separating, or decentralized sanitation or wastewater management.
Overall, significant research and development has taken place in recent decades to enable more comprehensive recovery of nutrients contained in human excreta. New knowledge and technologies are continuously unfolding, as evidenced by the number and scope of recent reviews published in the scientific literature, see . These reviews provide detailed insights into certain aspects of nutrient recovery. It is, however, challenging to identify broad patterns and opportunities in the field as a whole, when technical details or certain technologies are studied in isolation.
Table 1. Examples of previous reviews on the recovery of nutrients found in human excreta and streams containing human excreta.
The present review aims to provide a rigorously informed and systematic synthesis of available and proposed recovery pathways designed to facilitate recycling of nutrients contained in human excreta to agriculture, covering treatment processes as well as products rendered by treatment. Our aspiration is to present the material in a way that is accessible across diverse yet relevant fields of expertise. The focus is on highlighting broad patterns and opportunities in the field as a whole, and to point to literature that specifically describes certain selected aspects, technologies, or products in more detail.
Most importantly, we hope to facilitate communication and cross-fertilization not only among the various engineering groups that work on the recovery of nutrients found in human excreta, but also between these groups and research communities active in the fields of soil sciences and food and farming systems, as well as other related fields such as industrial ecology, urban metabolism, circular economy, and environmental systems analysis.
2. Human excreta
As our intention is to write for a diverse audience, we start by providing a short description of human excreta and how they may get mixed with other streams prior to treatment. To clarify what it is that treatment aims at recovering or removing, we also describe factors that impact the composition of different streams that consist of, or contain human excreta, and can form the starting point for the recovery of resources contained in human excreta.
2.1. Carbon and nutrient content of human urine and faeces
Human urine consists of more than 90% water (H2O) by weight, the remainder being inorganic salts and organic compounds (Rose et al., Citation2015). The dried solids contain about 13% carbon (C), 14–18% nitrogen (N), 3.7% phosphorus (P), and 3.7% potassium (K) (Rose et al., Citation2015). Urea (CH4N2O) is the dominant solute in fresh urine, making up over 50% of the organic compounds (Rose et al., Citation2015). About 85% of N is fixed in urea and about 5% as total ammonia (NH3 and NH4+) (Udert, Larsen, & Gujer, Citation2003, Udert, Larsen, & Gujer, Citation2006). Shortly after urination, the nonvolatile urea is broken down into bicarbonate (HCO3−) and carbonate (CO32−) as well as nonvolatile ammonium (NH4+) and volatile ammonia (NH3) (Udert, Larsen, & Gujer, Citation2003). Urea hydrolysis is a spontaneous process because the bacteria that produce the urea hydrolyzing enzyme urease are ubiquitous (Udert, Larsen, Biebow, & Gujer, Citation2003). After urea hydrolysis, about 90% of total N in urine is present as ammonia or ammonium (Udert et al., Citation2006). Urea hydrolysis implies the potential for ammonia volatilization during collection, storage, transport and application of urine, especially because the pH can increase up to 9 during the process, shifting the equilibrium from nonvolatile ammonium to volatile ammonia (Hellström et al., Citation1999; Chang et al., Citation2015). Human feces consist of about 75% H2O by weight and 25% solid material, mainly organic matter (Rose et al., Citation2015). C is a major constituent of the dried solids as approximately half of organic matter generally is C (Vassilev et al., Citation2010) and this is also true for feces (Rose et al., Citation2015). N, P, and K make up 5–7%, 3–5.4%, and 1–2.5% of the dried solids respectively (Rose et al., Citation2015). Both urine and feces also contain a range of micronutrients such as magnesium (Mg) and selenium (Se). The amount of excreted nutrients depends on dietary intake, while the digestibility of the diet determines the partitioning of nutrients between urine (digested) and feces (undigested) (Jonsson et al., Citation2004). Generally, urine contains the majority of N and about half of P and K contained in human excreta, while feces are rich in P and K and contain the majority of C (Heinonen-Tanski & Van Wijk-Sijbesma, Citation2005).
2.2. Contaminants of concern in human urine and faeces
Human excreta commonly contain pathogens. Faeces always contain high numbers of enteric bacteria (e.g. Campylobacter, Salmonella) and may also contain high numbers of viruses (e.g. Norovirus, Rotavirus), protozoa (e.g. Cryptosporidium, Giardia), and parasitic worm eggs (e.g. Ascaris) (Heinonen-Tanski & Van Wijk-Sijbesma, Citation2005). Fresh urine, especially from healthy persons, contains few pathogens (Heinonen-Tanski & Van Wijk-Sijbesma, Citation2005; Udert et al., Citation2006). Faecal cross-contamination of urine during and after excretion, however, can increase the number of pathogens in urine (Jönsson et al., Citation1997; Schönning et al., Citation2002). Human excreta can also contain heavy metals and organic pollutants, notably pharmaceutically active substances such as pharmaceuticals, pharmaceutical residues, and (synthetic) hormones. Heavy metal concentrations in urine are generally very low in relation to the nutrients; feces constitute a much higher heavy metal load compared to urine (Jönsson et al., Citation1997; Tervahauta et al., Citation2014). Some of the organic pollutants are mainly excreted with urine, while others are excreted mostly with feces (Lienert et al., Citation2007).
2.3. Mixing of human excreta with other streams
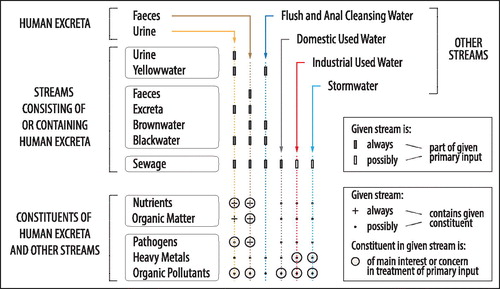
Collection of human excreta often involves mixing with other streams (see ). Separate collection of urine, depending on the type of toilet or urinal, may involve mixing with flush water and the respective stream is commonly referred to as source-separated urine or yellowwater. Separate collection of human feces, depending on the type of toilet, may involve mixing with urine, flush water, anal cleansing water, toilet paper, and additives such as ash, lime, or dried soil. The respective stream is commonly referred to as source-separated feces, brownwater, excreta, or blackwater. In conventional urban water and sanitation infrastructures, human excreta generally become mixed with flush water, anal cleansing water, toilet paper, domestic used water, industrial used water, and possibly even stormwater. The stream resulting from this form of collecting human excreta is commonly referred to as domestic or municipal sewage or wastewater.
Figure 1. Overview of: (1) how human excreta and other streams are combined into a range of primary inputs that form the starting point for recovery pathways reported in peer-reviewed studies dealing with recovery of resources from human excreta; and (2) constituents of interest or concern in human excreta and other streams. Note that used diapers are not considered in the present review, even though they also represent a stream containing human excreta.
2.4. Sources of carbon, nutrients, and contaminants in mixed streams
Human excreta normally are the major contributor of nutrients and organic matter in any of the streams containing human excreta, although the amount of nutrients and organic matter will increase if organic kitchen refuse is collected through the same collection system or added to treatment as supplemental feedstock (Kujawa-Roeleveld et al., Citation2006; Friedler et al., Citation2013). Flushwater can add heavy metals and organic pollutants originating from the water supply system, as for example copper (Cu) and lead (Pb) can be released from metal pipes (Renner, Citation2008; Schock et al., Citation2008) or organic compounds from polymeric pipes (Zhang & Liu, Citation2014). Contamination levels are further increased following mixing with used water from households, hospitals, industry, and the commercial sector, and with stormwater where it is also discharged to the same sewer. Pathogens mainly originate from human excreta (Dumontet et al., Citation2001), but can also originate from meat preparation in domestic kitchens (e.g. Salmonella and Campylobacter during the preparation of chicken) (Cogan et al., Citation1999) or commercial processing of animal products (e.g. in tanneries, meat markets, abattoirs) (Dumontet et al., Citation2001). Organic pollutants include substances such as pharmaceutically active coumpounds and hormones, personal care products, and detergents (Kümmerer, Citation2013). Pharmaceutically active compounds and hormones mainly originate from human excreta whereas other organic pollutants mainly originate from other sources. Heavy metals originate from different sources (Sörme & Lagerkvist, Citation2002). Compared with other sources of heavy metals, those contained in human excreta typically account for less than a tenth of total load in sewage (Tervahauta et al., Citation2014).
3. Recovery pathways
Efforts to recover resources from human excreta or streams containing human excreta have typically targeted water, energy, carbon, nutrients, metals, or a combination of these resources.
Here, we compile and describe recovery pathways that facilitate nutrient recovery. Given the iterative nature of the literature search and analysis, information about what we found is presented along with how we found it.
3.1. Conceptual model and terminology
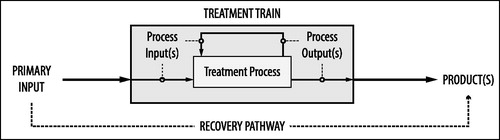
A simple input-output model () guided our literature analysis and is useful to explain central terms. We define as primary input any stream that contains human urine and/or feces and that forms the starting point for resource recovery. Treatment aims to facilitate recovery and recycling of resources found in the primary input. Where treatment comprises more than one treatment process, the output from one process can become the input to another. Products are defined as outputs that do not become the input to another treatment process. We refer to a specific sequence of treatment processes as a treatment train. A treatment train either transforms a primary input into one single product, or into a number of different products. The combination of a certain primary input, a certain treatment train, and a certain product we refer to as a recovery pathway. Where multiple products are obtained from the same primary input and treatment train, each product comprises a separate recovery pathway.
3.2. Recovery pathways facilitating nutrient recovery
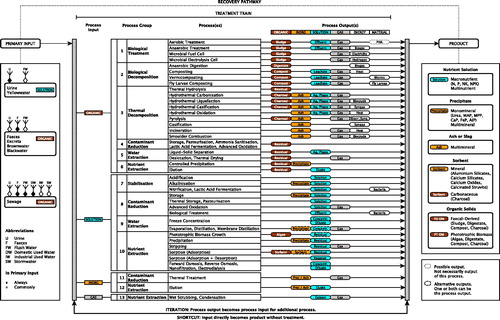
We searched the scientific literature for documents describing recovery pathways specifically targeted towards nutrient recovery through the search strategies described in Supporting Information 1. In doing so, we iteratively identified and developed categories for treatment processes and products rendered by treatment. Recovery pathways were arranged into clusters of pathways that start from similar primary inputs, feature similar treatment processes, and/or render similar products. These clusters as well as variations within clusters and a list of documents constituting each cluster are detailed in Supporting Information 1. A simplified representation of common recovery pathways is shown in and explained in the remainder of this section.
Figure 3. Simplified representation of recovery pathways targeted towards nutrient recovery from human excreta and streams containing human excreta, as reported in the peer-reviewed scientific studies included in the present review. Abbreviations: BIO.TREAT = biological treatment; BIO.DEC = biological decomposition; TH.DEC = thermal decomposition; STAB = stabilization; W.EXT = water extraction; CONT.RED = contaminant reduction; NUT.EXT = nutrient extraction. A more comprehensive representation can be found in Figure S1.1 in Supporting Information 1. For further explanation of product groups and specifications the reader is referred to chapter 5.
Treatment trains starting from urine or yellowwater represent two broad strategies. The first strategy applies treatment processes that aim at prevention of ammonia volatilization, separation of water from nutrients, and/or contaminant reduction (through separation of contaminants from nutrients and/or the pathogen inactivation and/or organic pollutant degradation). The second strategy is characterized by selective nutrient extraction. Treatment processes applied to this end often also imply volume reduction (through separation of nutrients from water) and contaminant reduction (through separation of nutrients from contaminants) (Maurer et al., Citation2006).
Treatment trains starting from brownwater or blackwater commonly begin with (anaerobic) biological treatment followed by liquid-solid separation. Biological treatment can be designed such as to enable simultaneous nutrient extraction, for instance through precipitation or granulation. The liquid fraction can be the input to processes aimed at depollution (notably pathogen inactivation) or nutrient extraction. The solid fraction, or the dryer primary inputs feces and excreta, can be the input to depollution (notably pathogen inactivation), or to biological decomposition (possibly enhanced by additional pathogen inactivation) or thermal decomposition of organic matter.
Treatment trains starting from sewage commonly involve liquid-solid separation, usually preceded by or as a part of (aerobic) biological treatment. The liquid fraction or effluent can be the input to processes aimed at contaminant reduction or nutrient extraction. The solid fraction (sewage sludge) can be the input to processes aimed at contaminant reduction (pathogen inactivation), nutrient extraction, and/or biological or thermal decomposition of organic matter. Ash, the inorganic residual rendered by some thermal decomposition processes, can be the input to processes aimed at nutrient extraction or contaminant reduction (heavy metal removal).
Note that there are certain thermal decomposition processes that have generally been targeted towards recovering carbon in the form of energy carriers rather than nutrient recovery. Processes of this kind include hydrothermal carbonization (HTC) (Danso-Boateng et al., Citation2013; Danso-Boateng, Holdich, Martin, Shama, & Wheatley, Citation2015; Danso-Boateng, Shama, Wheatley, Martin, & Holdich, Citation2015), hydrothermal gasification (HTG) (Afif, Azadi, & Farnood, Citation2011; He, Chen, Giannis, Yang, & Wang, Citation2014), and gasification (Rong et al., Citation2015) with feces or sewage sludge as feedstock. We have included these processes because they can facilitate nutrient recovery, in principle.
3.3. Developing the option space for nutrient recovery
Mapping recovery pathways quickly becomes subject to redundancy, even in a simplified representation like , because different primary inputs can be subjected to similar treatment trains, and different treatment trains can feature similar treatment processes and render similar products. Our aspiration was to produce a map of primary inputs, treatment processes, products rendered by treatment, and their relationships. This map we refer to as the ‘option space’ for nutrient recovery. To avoid redundancy, we identified four broad categories of similar process inputs and outputs (including primary inputs and products). We mapped these four categories of process inputs and outputs, indicating how treatment processes can convert an input belonging to one category to an output belonging to the same or a different category. This resulted in a refined input-output model that forms the backbone of the option space for nutrient recovery. The step-wise process leading to the option space is illustrated in Figure S2.1 in Supporting Information 2. The actual option space is shown in . Note that the option space allows for the output from one process to become the input to a following process. The option space is a generic representation that can map any recovery pathway that builds on the primary inputs, treatment processes, and products featured as building blocks of the option space. Treatment processes are described in more detail in Section 4, products in Section 5.
4. Treatment processes
A brief description of each treatment process featured in the option space, a simple input-output diagram, and details regarding the fate of constituents (nutrients, organic matter, pathogens, organic pollutants, and heavy metals) during treatment are provided in Supporting Information 3. Here, we summarize treatment processes and the fate of constituents during treatment.
4.1. Decomposition of organic matter
Decomposition refers to the breakdown of organic matter into smaller and more stable molecules, which can be achieved via biotic (biological) or abiotic (mechanical, thermal, chemical, or thermo-chemical) processes (Atay & Akbal, Citation2016). As for biotic processes, we here distinguish biological treatment and biological decomposition. Biological treatment refers to processes where the solid retention time (SRT) is larger than the hydraulic retention time (HRT), that is, the solid fraction of the input stays in the system longer than the liquid fraction. Biological decomposition refers to processes where solid and liquid fraction stay in the system for the same period of time (SRT equals HRT).
4.1.1. Biological treatment
The activated sludge process, invented roughly a century ago, is still at the core of many contemporary municipal STPs and was originally designed to remove organic matter from municipal sewage or industrial wastewaters (Orhon, Citation2015). Over the years, biological N and biological or chemical P removal processes have been incorporated into overall process design to meet ever stricter effluent standards aimed at minimizing the release of N and P to the aquatic environment (Cooper, Citation2001). In tropical climates, anaerobic treatment of sewage is a frequently applied alternative to the activated sludge process (Seghezzo et al., Citation1998). The upflow anaerobic sludge blanket (UASB) reactor, developed in the 1980s (Lettinga et al., Citation1980), is the most applied anaerobic system for treatment of sewage and industrial wastewaters. Also blackwater is succesfully treated applying UASB technology (de Graaff et al., Citation2010; Hernández Leal et al., Citation2017). Bioelectrochemical systems such as microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) represent an emerging technology for treating wastewater under anaerobic conditions and can be applied to urine, low-strength wastewater such as municipal sewage, as well as high-strength industrial wastewaters (Gude, Citation2016). While initially designed for efficient wastewater treatment, nutrient recovery has become an integral part of process design (Kelly & He, Citation2014; Nancharaiah et al., Citation2016; Goglio et al., Citation2019). More recently, treatment configurations that enable the formation of polyhydroxyalkanoates (PHA) or other precursors for high-value products have received increased attention (Morgan-Sagastume et al., Citation2014; Modin et al., Citation2016; Pittmann & Steinmetz, Citation2017; Puyol et al., Citation2017). The decomposition of organic matter releases nutrients from the organic matter to the liquid phase, adding to those nutrients already present in their dissolved form. Process designs based on the activated sludge process can achieve much lower concentrations of N and P in the effluent compared to anaerobic treatment (Seghezzo et al., Citation1998). Retaining nutrients in the effluent is beneficial if the effluent is used for fertigation in agriculture. Treatment under anaerobic conditions avoids consuming energy for aeration. Instead, energy is recovered in the form of biogas (in anaerobic treatment such as UASB) (Seghezzo et al., Citation1998), electricity (in microbial fuel cells) or biofuels such as ethanol, methane, or hydrogen (in microbial electrolysis cells). Pathogens tend to only partly accumulate in the sludge (Wen et al., Citation2009; Li et al., Citation2015; Huang et al., Citation2018). In aerobic treatment, heavy metals partition fairly equally between effluent and sludge (Karvelas et al., Citation2003). In anaerobic treatment, heavy metal precipitation to the sludge is generally higher due to sulfide precipitation (Cowling et al., Citation1992; De la Varga et al., Citation2013). The partitioning behavior of organic pollutants depends on the compound, with a tendency towards sorbing to the sludge (Katsoyiannis & Samara, Citation2005). In addition, both aerobic and anaerobic treatment schemes have the potential to inactivate some pathogens and degrade some organic pollutants (Butkovskyi, Rijnaarts, Zeeman, & Hernandez Leal, Citation2016). Bioelectrochemical systems in particular have been shown to have the potential for high removal of recalcitrant pollutants (Huang et al., Citation2011).
4.1.2. Biological decomposition
Anaerobic digestion enables recovery of energy in the form of biogas and nutrients in the form of digestate. Composting renders a soil amendment while vermicomposting and fly larvae composting render a soil amendment as well as worms or fly larvae for potential use as animal feed. These processes are commonly applied to more concentrated streams such as feces or excreta, or fecal, blackwater, or sewage sludge.
As in biological treatment, nutrients are released from organic matter upon its decomposition. If biological treatment takes place in an open system, as often the case for composting and usually the case for vermicomposting and fly larvae composting, volatile forms of N can be lost to the atmosphere, and soluble nutrients to a liquid leachate (Ulén, Citation1997; Jonsson et al., Citation2004; Lalander et al., Citation2015; Nigussie et al., Citation2016). Biological decomposition can inactivate some pathogens (especially when temperatures above 60 °C are achieved) (Gajurel et al., Citation2007; Lalander et al., Citation2013), decompose some organic pollutants (Butkovskyi, Ni, Hernandez Leal, Rijnaarts, & Zeeman, Citation2016), and influence heavy metal speciation (He et al., Citation2016).
4.1.3. Thermal decomposition
Thermal decomposition processes can be geared towards facilitating further treatment or safe disposal of an organic feedstock, but can also be designed to facilitate the recovery of resources such as energy, carbon, nutrients, and/or metals. Thermal hydrolysis and advanced oxidation processes (AOPs) such as ozonation aim to make wet organic matter, usually sewage sludge, more biodegradable and are commonly applied as pretreatment to anaerobic digestion (Barber, Citation2016). Hydrothermal carbonization (HTC) (Danso-Boateng et al., Citation2013; Danso-Boateng, Holdich, et al., Citation2015; Danso-Boateng, Shama, et al., Citation2015), liquefaction (HTL) (Aida et al., Citation2016; Lu et al., Citation2017), and gasification (HTG) (Afif et al., Citation2011; He et al., Citation2014) aim to convert wet organic matter into charcoal, biocrude, or syngas, respectively. Hydrothermal oxidation (HTO) processes such as low pressure wet oxidation (LOPROX) (Blöcher et al., Citation2012) and supercritical water oxidation (SCWO) (Stendahl & Jäfverström, Citation2004) aim at complete destruction and conversion of wet organic matter to carbon dioxide. Pyrolysis (Bridle & Pritchard, Citation2004; Shepherd et al., Citation2016; Bai et al., Citation2017) aims to convert dry organic matter into charcoal and/or biocrude. Gasification (Rong et al., Citation2015) aims to convert dry organic matter into syngas. Incineration (Li et al., Citation2017) usually involves complete decomposition of organic matter by means of oxidation to carbon dioxide. Smoulder combustion (Yermán et al., Citation2015, Citation2017; Fabris et al., Citation2017) also aims at complete decomposition of organic matter but can be designed to yield pyrolysis products such as biocrude. Pyrolysis and HTL have been investigated with recovery of both nutrients and energy in mind, while the other processes have typically been targeted primarily towards recovering carbon in the form of charcoal or energy carriers.
Hydrothermal processes (i.e. HTC, HTL, HTG, and HTO) generally yield an inorganic residual in addition to the target product(s). This residual generally consists of a liquid fraction and a solid fraction, which can be separated from one another by means of liquid-solid separation. Nutrients are partitioned to the carbonaceous target product as well as the inorganic (liquid or solid) residual (Stendahl & Jäfverström, Citation2004; Blöcher et al., Citation2012; Kruse et al., Citation2016; Yao et al., Citation2016; Lu et al., Citation2017). Monovalent ions (e.g. NH4+ and K+) tend to partition to the liquid fraction of the inorganic residual, multivalent ions (e.g. PO43− and most metal ions) to the solid fraction (Toufiq Reza et al., Citation2016). Pyrolysis, gasification, and incineration are subject to N volatilization, while P and K as well as most metals are retained in the char or ashes, respectively (Bridle & Pritchard, Citation2004; Hossain et al., Citation2011; Gorazda et al., Citation2018). Pathogens are generally fully inactivated while organic pollutants are partly or fully decomposed depending on process conditions and type of compound (Libra et al., Citation2011).
4.2. Stabilisation processes
Stabilisation of urine and other liquid streams such as treated effluent is specifically directed to prevent volatilization of ammonia as this can help avoid N losses and negative impacts associated with released ammonia gas such as odor nuisance (Hellström et al., Citation1999) and acidification of soils and water bodies (Hunter et al., Citation2011). Stabilisation of urea-rich solutions (e.g. fresh urine) aims at preventing urea hydrolysis and hence preserving N in the form of nonvolatile urea. Stabilisation of ammonia-rich solutions (e.g. urine after urea hydrolysis) aims at converting volatile ammonia to ammonium and other nonvolatile forms of N. While stabilization can be applied as standalone process, it is typically applied in combination with other processes such as storage (e.g. Hellström et al., Citation1999), or as pretreatment to other processes, notably evaporation (e.g. Senecal & Vinnerås, Citation2017), distillation (e.g. Fumasoli et al., Citation2016), membrane distillation (e.g. Tun et al., Citation2016) and phototrophic biomass growth (e.g. Coppens et al., Citation2016). Processes geared towards stabilization of liquid streams that have gained most traction include chemical processes such as acidification (e.g. Hellström et al., Citation1999) and alkalinisation (e.g. Randall et al., Citation2016) as well as biological processes such as partial nitrification (e.g. Sun et al., Citation2012) and lactic acid fermentation (e.g. Andreev et al., Citation2017). Stabilisation processes generally have some potential to inactivate some pathogens (Hellström et al., Citation1999; Bischel et al., Citation2015; Randall et al., Citation2016). Biological processes in addition also have the potential to degrade some organic pollutants (Fumasoli et al., Citation2016; Andreev et al., Citation2017).
4.3. Separation processes
The main purpose of separation processes is to separate various constituents in the process input from one another. Several treatment processes have been investigated for liquid streams such as urine or treated effluent, aiming at separating water and/or contaminants from nutrients, or nutrients from water and/or contaminants.
4.3.1. Freeze concentration
Processes geared towards water extraction from liquid streams include freeze concentration, that is, the concentration of a solution through freezing and melting. Freeze concentration has the potential to retain most nutrients in the concentrate (Lind et al., Citation2000; Gulyas et al., Citation2004).
4.3.2. Vaporisation and membrane separation
Processes based on vaporization and/or membrane separation include passive evaporation (e.g. Pahore et al., Citation2010; Bethune et al., Citation2014, Bethune, Chu, & Ryan, Citation2016; Dutta & Vinnerås, Citation2016), thermal (e.g. Ek et al., Citation2006; Senecal & Vinnerås, Citation2017) or solar thermal evaporation (e.g. Antonini et al., Citation2012), high temperature (Jiang et al., Citation2017) or low pressure distillation (e.g. Udert & Wächter, Citation2012; Fumasoli et al., Citation2016), membrane distillation (MD) (e.g. Tun et al., Citation2016), forward osmosis (FO) (e.g. Ek et al., Citation2006; Zhang et al., Citation2014; Liu et al., Citation2016), reverse osmosis (RO) (e.g. Ek et al., Citation2006), and nanofiltration (NF) (e.g. Pronk, Palmquist, Biebow, & Boller, Citation2006; Lazarova & Spendlingwimmer, Citation2008). These processes can enable the separation of water from nutrients in liquids as well as liquid-solid separation in slurries that also contain particulate organic matter or minerals. In thermal drying of sewage sludge (e.g. Horttanainen et al., Citation2017), for example, water is extracted from a slurry through evaporation. Separation of the liquid and solid fractions can also be achieved through membrane separation processes such as FO, RO and NF, as well as a number of other processes such as centrifugation or sedimentation.
N losses can occur due to ammonia volatilization during vaporization (Bethune et al., Citation2016; Dutta & Vinnerås, Citation2016; Tun et al., Citation2016; Jiang et al., Citation2017), and due to low rejection of urea, ammonia, nitrite and nitrate in membrane separation (Ek et al., Citation2006; Pronk, Palmquist, et al., Citation2006; Zhang et al., Citation2014). Pathogens and heavy metals are generally retained in the concentrate, both in case of vaporization and membrane separation (Pronk, Palmquist, et al., Citation2006; Liu et al., Citation2016). The fate of organic pollutants depends on their volatility during vaporization (Wijekoon et al., Citation2014), and upon membrane and pollutant properties during membrane separation (Alturki et al., Citation2013). Electrodialysis (ED) is another membrane separation process that has been investigated and enables the transfer of nutrients from a liquid stream (such as urine) or the liquid fraction of a slurry (such as sewage sludge) to another liquid stream (Pronk, Biebow, & Boller, Citation2006; Pronk et al., Citation2007; Tice & Kim, Citation2014). Desalination degrees of up to 99% have been achieved (Pronk, Biebow, et al., Citation2006). Retention is high for pathogens while organic pollutants and heavy metals permeate through the membrane to some extent (Pronk, Biebow, et al., Citation2006; Pronk et al., Citation2007).
4.3.3. Phototrophic biomass growth
Through phototrophic biomass growth in aquatic or terrestrial systems, nutrients can be extracted from liquid streams, notably urine and treated effluent, and incorporated into phototrophic biomass. Algal systems have been found to have the potential to simultaneously extract N and P (as well as K and micronutrients) (Shilton et al., Citation2012; Vasconcelos Fernandes et al., Citation2015; Sukačová & Červený, Citation2017), but have also been found to extract organic pollutants (de Wilt et al., Citation2016) and heavy metals (Zeraatkar et al., Citation2016; Demey et al., Citation2018) through sorption. Moreover, algae were found to have the potential to degrade some organic pollutants (de Wilt et al., Citation2016; Wang et al., Citation2017).
4.3.4. Sorption
Sorption processes have been investigated to transfer nutrients from liquid streams (notably urine and treated effluent) to a range of carbonaceous or mineral sorbents. The sorbent itself can be the target product, or it can be an intermediary nutrient carrier from which nutrients can be transferred back to a desorption solution or regenerant. In the latter case, also synthetic sorbents/resins have been investigated.
Charcoal has been shown to have the potential to adsorb urea (Kameda et al., Citation2017), NH4+ (Cai, Qi, Liu, & He, Citation2016), and PO43− (Takaya et al., Citation2016; Trazzi et al., Citation2016). Mineral sorbents generally have good cation exchange properties and good affinity for NH4+ and K+ (Hedström, Citation2006; Jaskūnas, Citation2015), and have also been shown to act as precipitation nuclei for the surface precipitation of phosphates, for instance as calcium phosphate, notably if Ca2+ is released in exchange for NH4+ and K+ (Hedström, Citation2006; Gustafsson et al., Citation2008; Karapinar, Citation2009; Köse & Kivanç, Citation2011; Guaya et al., Citation2016; Wan et al., Citation2017). Charcoal and mineral sorbents do not only remove nutrients from aqueous solutions. Charcoal, notably in the form of activated carbon, has the potential to remove some waterborne pathogens (Busscher et al., Citation2006), organic pollutants (Nam et al., Citation2014; Tong et al., Citation2016), and heavy metals (Kołodyńska et al., Citation2012) from solutions. Mineral sorbents have the potential to remove organic pollutants (Tsai et al., Citation2008; De Ridder et al., Citation2012; Chraibi et al., Citation2016) and heavy metals (Zorpas et al., Citation2000; Babel & Kurniawan, Citation2003; Shaheen et al., Citation2012; Choi & Lee, Citation2015) from solutions. There are indications, however, that sorbents (e.g. some types of activated carbon) can be designed to remove either nutrients or pollutants but not both.
4.3.5. Controlled precipitation
Through precipitation, crystallisation, or granulation, nutrients can be transferred from a liquid stream or the liquid fraction of a slurry to a mineral in amorphous or crystalline form. Precipitation in the Mg-Ca-NH3-PO4 system has been explored thoroughly (Ronteltap et al., Citation2007a, Ronteltap, Maurer, Hausherr, & Gujer, Citation2010; Marti et al., Citation2008; Triger et al., Citation2012; Liu et al., Citation2013; Muster et al., Citation2013; Vasenko & Qu, Citation2017). In the absence of ammonium, it is possible to precipitate the struvite analog magnesium potassium phosphate (MPP, also referred to as potassium struvite) (Wilsenach et al., Citation2007; Xu, Wang, Wang, & Qian, Citation2012; Xu et al., Citation2015; Nakao et al., Citation2017). Other studies have targeted calcium phosphate (Cunha et al., Citation2018), aluminum phosphate (Huang et al., Citation2015), ferric phosphate (Lin et al., Citation2017), or magnesium and sodium phosphates (Huang et al., Citation2015). Key mineral precipitates are described in Table S2.1 in Supporting Information 2. Pathogens may accumulate in the precipitate (Udert et al., Citation2006; Decrey et al., Citation2011; Lahr et al., Citation2016). Pharmaceuticals have been found to attach to the surface of precipitates rather than being incorporated in the crystal structure (Escher et al., Citation2006; Ronteltap et al., Citation2007b) and can be removed by washing (Schürmann et al., Citation2012).
4.3.6. Ammonia volatilisation and capture
The transfer of volatile components from a liquid to a gas stream is referred to as stripping and has long been known to be useful to remove ammonia from concentrated streams such as urine (e.g. Maurer et al., Citation2003; Liu et al., Citation2015) or ammonia-rich wastewater (e.g. Siegrist, Citation1996; Yuan et al., Citation2016). Process variations include stripping columns (e.g. Katehis et al., Citation1998; Antonini et al., Citation2011; Morales et al., Citation2013; Huang et al., Citation2015) as well as a range of setups where ammonia release is facilitated by (bio)electrochemical systems such as microbial fuel cells (e.g. Kuntke et al., Citation2012; Zhou et al., Citation2015), microbial electrolysis cells (e.g. Wu & Modin, Citation2013; Kuntke et al., Citation2014), or electrochemical cells (e.g. Desloover et al., Citation2012; Luther et al., Citation2015). Ammonia release from a liquid to a gas stream can also occur as side-effect of water extraction processes and in these cases often turns into a nitrogen loss, although the released ammonia can be captured, in principle (e.g. Horttanainen et al., Citation2017). Ammonia is commonly captured by wet scrubbing (i.e. absorbtion in an acid such as sulfuric acid) which renders an ammonia-rich solution (for example ammonium sulfate). Pathogens, organic pollutants, and heavy metals can be expected to largely remain in the stream from which ammonia has been released.
4.3.7. Mobilisation - separation
Prior to liquid-solid separation of slurry-like organics, release of P from the solid to the liquid fraction can be facilitated by processes such as bioelectrochemical systems (e.g. Fischer et al., Citation2011; Happe et al., Citation2016), ozonation (e.g. Suzuki et al., Citation2006), additional anaerobic tanks or zones in enhanced biological phosphorus removal (EBPR) schemes (e.g. Heinzmann, Citation2005), or acid elution (e.g. Güney et al., Citation2008; Niewersch et al., Citation2008; Antakyali et al., Citation2013). Elution has also been investigated for the extraction of P from ashes. Alkaline elution dissolves phosphorus and aluminum to some extent but not iron and heavy metals; acid elution dissolves phosphorus as well as metals. Elution is often followed by processes such as membrane separation, sorption, or solvent extraction in order to separate P from heavy metals, and possibly by processes aiming at the precipitation of phosphates (Egle et al., Citation2015).
4.3.8. Thermal ash treatment
Separation of P and heavy metals found in ashes can be achieved through thermal ash treatment designed to release heavy metals or P from the ash, or both (sequentially) (e.g. Adam et al., Citation2009; Nowak et al., Citation2012; Schönberg et al., Citation2014).
4.4. Pathogen inactivation and degradation of organic pollutants
Pathogen inactivation and removal aim to ensure hygienic safety of the fertilizer product while organic pollutant degradation and removal target pharmaceuticals, pharmaceutical residues, and (synthetic) hormones. Some of the separation processes above have aimed to separate desired products (i.e. nutrients, organic matter) from those not desired (i.e. pathogens, organic pollutants, heavy metals). Treatment can also be geared towards the inactivation of pathogens or degradation of organic pollutants. Storage (e.g. Tilley et al., Citation2008), thermal storage (e.g. Zhou et al., Citation2017), and pasteurization (e.g. Lahr et al., Citation2016) are processes that focus on pathogen inactivation in liquid streams. These processses need concentrated streams to limit storage capacity and/or energy use. Advanced oxidation processes (Pronk et al., Citation2007; Lazarova & Spendlingwimmer, Citation2008) have the potential to achieve inactivation of pathogens (Deng & Zhao, Citation2015; Giannakis et al., Citation2017) as well as degradation of organic pollutants (Deng & Zhao, Citation2015). Biological treatment of liquid streams such as urine has been targeted mainly towards the degradation of organic pollutants (e.g. Abdel-Shafy & Mansour, Citation2016). Pathogen inactivation in slurry-like organic matter has been investigated by means of storage (e.g. Fidjeland et al., Citation2013), pasteurization (e.g. Forbis-Stokes et al., Citation2016), ammonia sanitization (e.g. Fidjeland et al., Citation2015), lime stabilization (e.g. Anderson et al., Citation2015), and desiccation (e.g. Magri et al., Citation2013).
5. Products
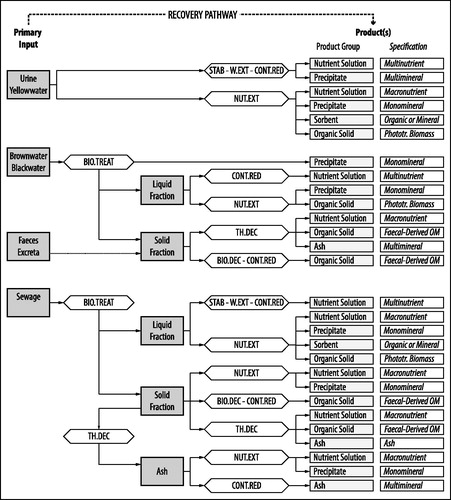
Products rendered by treatment of human excreta and streams containing human excreta can be in the form of for example energy carriers, fertilizers, or feed, while in other cases the products can be utilized for the production of biopolymers, biofuels or other high-value chemicals (Puyol et al., Citation2017; Chen et al., Citation2018). Here, we focus on products that are useful as fertilizers or for the production of synthetic fertilizers. The composition and quality of these products vary widely within the same product category. This is in part because the quality of the primary input varies between locations as a result of for example the type of industries or habits of the population, in part because different treatment trains can yield similar products (see and Figure S2.2 in Supporting Information 2). Below we describe different products with respect to composition and usability ().
Figure 5. Characteristics, application potential, and fertilizing type of different product subcategories.
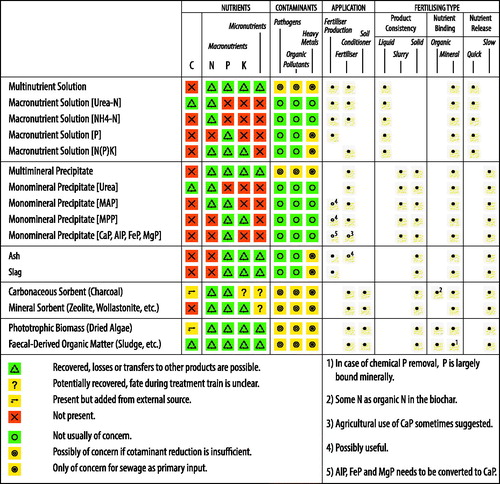
5.1. Nutrient solutions
Excreta-derived nutrient solutions contain nutrients derived from human excreta but are devoid of suspended organic matter. Given the wide variety of combinations of primary inputs and treatment trains that render nutrient solutions, there is considerable variation within this product category. If used as fertilizers, nutrient solutions generally are considered quick-release fertilizers as the nutrients are present as dissolved ionic species and thus directly available for plant uptake. Alternatively, some of the nutrient solutions can be used as input for the production of fertilizers. Broadly, nutrient solutions fall into two subgroups we refer to as multinutrient and macronutrient solutions. The term multinutrient solution here is used to refer to nutrient solutions that generally contain both macro- and micronutrients. The term macronutrient solution here is used to refer to nutrient solutions that contain one or several of the macronutrients NPK but no or only traces of micronutrients.
5.1.1. Multinutrient solutions
One set of recovery pathways that has received considerable attention is based on treatment trains starting from urine or yellowwater and featuring (a combination of) stabilization, contaminant reduction, and water extraction processes, or nutrient extraction processes; but the same (combinations of) processes have also been applied to other liquid streams, notably treated effluent and liquid process side streams rendered during treatment of primary inputs that contain feces. Human urine has long been known for its usefulness as fertilizer particularly rich in N (and that also contains P, K and micronutrients) (Heinonen-Tanski & Van Wijk-Sijbesma, Citation2005). Urine-based liquid fertilizers can be expected to be similarly useful (e.g. Bonvin et al., Citation2015). Aurin is an example of a marketable urine-based liquid fertilizer that is obtained through nitrification-distillation of hydrolyzed urine (Eawag, Citation2018a). Human urine has also been shown to be useful as liquid fertilizer for aquaculture (e.g. Jana et al., Citation2012; Rana et al., Citation2017), and has been investigated as input for the production of methylene urea, a slow-release synthetic nitrogen fertilizer (e.g. Ito et al., Citation2013). Treated effluent is useful for fertigation.
5.1.2. Macronutrient solutions (Urea-N)
Solutions rich in urea-N have been obtained, starting from unhydrolyzed urine, through sorption to and desorption from activated carbon (e.g. Ganesapillai et al., Citation2016; Simha et al., Citation2018), and through membrane separation processes, notably nanofiltration (e.g. Pronk, Palmquist, et al., Citation2006; Lazarova & Spendlingwimmer, Citation2008). In membrane separation, pathogens and organic pollutants are retained by the membrane (Pronk, Palmquist, et al., Citation2006) and heavy metals are not of concern for urine as primary input. For sorption, the fate of contaminants is less reported. Also solutions rich in urea-N can be expected to be useful as fertilizer rich in nitrogen (Pronk, Palmquist, et al., Citation2006) and possibly as feedstock for the production of synthetic fertilizers such as methylene urea.
5.1.3. Macronutrient solutions (Ammonia-N)
Solutions rich in ammonia-N have been obtained, starting from hydrolyzed urine, through membrane separation, notably nanofiltration (e.g. Pronk, Palmquist, et al., Citation2006; Lazarova & Spendlingwimmer, Citation2008). A more widely researched approach to obtaining a solution rich in ammonia-N is the release of ammonia from liquid streams (e.g. through air stripping from urine or treated effluent) (e.g. Desloover et al., Citation2012; Luther et al., Citation2015) or organics (e.g. during thermal drying of sewage sludge) (e.g. Horttanainen et al., Citation2017) followed by absorption in an acid trap. Depending on the acid trap used, the respective product is ammonium sulfate ((NH4)2SO4) (e.g. Desloover et al., Citation2012), ammonium borate ((NH4)3BO3) (e.g. Kuntke et al., Citation2014), ammonium chloride (NH4Cl) (e.g. Wu & Modin, Citation2013), ammonium nitrate (NH4NO3) (e.g. Horttanainen et al., Citation2017), or diammonium phosphate ((NH4)2HPO4) (e.g. Licon Bernal et al., Citation2016). These products are generally free of pathogens, organic pollutants, and heavy metals. Yet other studies have used sorption followed by desorption to render ammonia water, starting from urine (e.g. Tarpeh et al., Citation2017) or treated effluent (e.g. Sancho et al., Citation2017; You et al., Citation2017). The fate of contaminants is less reported. Ammonium nitrate and ammonium sulfate are common fertilizer products applied for instance in combination with CULTAN (controlled uptake long-term ammonia nutrition) fertilization (Deppe et al., Citation2016). Ammonium nitrate is also a common ingredient in the production of synthetic fertilizers.
5.1.4. Macronutrient solutions (NK or NPK)
Sorption followed by desorption has also been investigated for the simultaneous recovery of NH4+ and K+ from urine and their separation from Na+ (e.g. Casadellà et al., Citation2016). If two or more sorbent materials are combined, sorption can also render a solution rich in in N, P and K. A less explored pathway to render a solution rich in N, P an K is HTL of wet organic matter such as feces (e.g. Lu et al., Citation2017). HTL transfers N, P, and K to a liquid residue while most metals (e.g. Ca, Mg, Zn, Al, Fe) are transferred to a solid residue (Lu et al., Citation2017). While the respective studies do not point towards a specific end use, it seems likely the macronutrient solutions would be useful as liquid fertilizers.
5.1.5. Macronutrient solutions (P)
Solutions rich in P have been obtained through a broad range of treatment trains designed to extract P from organics (e.g. sewage sludge) or inorganics (e.g. sewage sludge ash) where sewage is the primary input, as revieved extensively in Egle et al. (Citation2015). These treatment trains generally yield a phosphoric acid, ranging from rather diluted to very pure and concentrated. Pathogens and organic pollutants are not usually of concern. Depending on the treatment train, heavy metals can be of concern, but several efforts are under way to reduce heavy metal contamination by subsequent processes such as membrane separation (e.g. Schaum et al., Citation2007; Parés Viader et al., Citation2017), sorption (e.g. Xu, He, Gu, Wang, & Shao, Citation2012), or solvent extraction (e.g. Hong et al., Citation2005). Another way to obtain a solution rich in P is through adsorption from treated effluent or other liquid process side-streams followed by desorption (e.g. Ohura et al., Citation2011). Phosphoric acid is rarely used for direct agricultural application but is instead commonly used in the production of synthetic fertilizers. P-rich desorption solutions seem to be most useful as starting point for the precipitation of phosphate minerals such as struvite (e.g. O’Neal & Boyer, Citation2013).
5.2. Precipitates
Precipitates are rendered by a wide range of processes, either as non-target (e.g. during storage) or target product (e.g. in crystallisation reactors). Common precipitates include struvite (MAP) and potassium struvite (MPP) as well as calcium, aluminum, and iron phosphates. Precipitates range from slurries consisting of individual precipitated nuclei, which can be filtered and dried to obtain a powder, to larger crystals or granules. Broadly, precipitates fall into two subgroups we refer to as multimineral and monomineral precipitates. The term multimineral precipitate here refers to precipitates that contain a range of different minerals. The term monomineral precipitate here refers to precipitates that only contain one mineral, or at least where only one mineral is the target mineral.
5.2.1. Multimineral precipitates
Treatment trains involving dehydration of urine, for instance, usually yield an inhomogeneous slurry or powder containing most of the nutrients found in the original solution, incorporated into a broad range of minerals (Antonini et al., Citation2012; Bethune et al., Citation2016; Jiang et al., Citation2017); where applicable also minerals originating from drying agents such as ash or lime (Dutta & Vinnerås, Citation2016; Senecal & Vinnerås, Citation2017). Multimineral precipitates have also been obtained through dehydration of anaerobic digester liquor (Ek et al., Citation2006). These multimineral precipitates are commonly held to be directly useful as fertilizers (Lemming et al., Citation2017), although their usefulness can be hampered by high salt contents (Jiang et al., Citation2017).
5.2.2. Monomineral precipitates
Spontaneous precipitation of MAP or calcium phosphate (CaP) is a common phenomenon in urine collection systems (Tilley et al., Citation2008; Udert et al., Citation2003) and pipes returning anaerobic digester supernatant to the STP inflow. Treatment trains that induce precipitation through pH adjustment and/or the addition of metal ions most commonly target MAP or CaP (notably hydroxylapatite) (Melia et al., Citation2017), but co-precipitation of a wide variety of non-target minerals may occur (Muster et al., Citation2013). As amorphous precipitates may easily be overlooked, what is believed to be mostly struvite may in fact contain more other precipitates than thought, particularly in higher pH ranges (Hao et al., Citation2008, Hao, Wang, Loosdrecht, Van, & Hu, Citation2013). Other precipitates that have been targeted include MPP, AlP, FeP, and MgP. Slurries and powders tend to be less homogeneous and more prone to contain non-target minerals as well as pathogens, organic pollutants, and heavy metals. Crystals and granules can have a very high purity and homogeneity, and their quality is rather independent of the primary input and treatment train (Antonini et al., Citation2012); even if sewage is the primary input (and e.g. anaerobic digester supernatant the process input), heavy metal concentrations are generally lower than in commercial fertilizers (Krüger et al., Citation2016). The usefulness of struvite as slow-release fertilizer has long been known (Bridger et al., Citation1962; Johnston & Richards, Citation2003; Rahman et al., Citation2014; Talboys et al., Citation2016; Degryse et al., Citation2017). Marketed struvite pellets include Ostara Crystal Green (Ostara, Citation2018) and Berliner Pflanze (Berliner Wasserbetriebe, Citation2018). Calcium phosphate in the form of hydroxylapatite, and to a lesser extent also aluminum and ferric phosphate are held to be more useful to produce synthetic fertilizers (Melia et al., Citation2017).
5.3. Ashes and slags
Ashes and slags are rendered by thermal decomposition of organic matter. Ashes and slags contain nonvolatile nutrients and heavy metals incorporated in a range of minerals. These minerals are not normally further specified in the respective studies. Ashes and slags are free of pathogens and organic pollutants but heavy metals can be of concern, notably for primary inputs with higher heavy metal loads, such as sewage. Several treatment processes are under development that aim to separate P from heavy metals contained in ashes or slags, and render a decontaminated ash or slag, as described in detail in Egle et al. (Citation2015). Ashes and slags generally are not considered a product of direct use for agriculture unless subjected to additional treatment (Lemming et al., Citation2017; Melia et al., Citation2017). Thermo-chemical ash treatment, for example, has been shown to increase the bioavailability of P in the ash, making the product (calcined ash) potentially useful for direct agricultural application (Adam et al., Citation2008, Adam et al., Citation2009; Herzel et al., Citation2016). More commonly, however, ashes and slags are the starting point for the recovery of fertilizer products such as struvite or the production of synthetic fertilizers (Cabeza et al., Citation2011).
5.4. Sorbents
A wide range of sorbents has been investigated to extract one or several of the macronutrients NPK from liquid streams. Sorbents can be broadly divided into two subgroups: carbonaceous and mineral sorbents. The main carbonaceous sorbent is charcoal. Key mineral sorbents include calcinated struvite as well as aluminum silicates, calcium silicates, or calcium oxides. These sorbents are described in Table S2.2 in Supporting Information 2. Sorbents can be applied as a combined soil amendment and fertilizer (Zhang et al., Citation2015; Bai et al., Citation2017; Nakhli et al., Citation2017). They are generally considered slow-release fertilizers, as nutrients are released from the sorbent to soil pore water over time. Salinity potentially present in the feed solution can be reduced as sorbents have a higher affinity for desired nutrient cations (i.e. NH4+ and K+) than for undesired salts cations (e.g. Na+) (Beler-Baykal et al., Citation2011). Sorbents, however, are also commonly applied to remove organic micropollutants and heavy metals from aqueous solutions (Zorpas et al., Citation2000; Babel & Kurniawan, Citation2003; Kołodyńska et al., Citation2012; Shaheen et al., Citation2012; Choi & Lee, Citation2015). The respective bodies of literature are largely separate and studies using sorbents for selective nutrient extraction remain largely silent about potential sorption of micropollutants and heavy metals along with nutrients, as well as desorption characteristics of these contaminants. Some sorbents may also contain heavy metals to start with, for example charcoal where the feedstock is sewage sludge or sewage-derived algal biomass.
5.5. Organic solids
Organic solids include a wide variety of products that contain organic matter originating from human excreta or biomass produced during treatment of human excreta or streams containing human excreta. We here distinguish between phototrophic biomass and excreta-derived organic matter.
5.5.1. Phototrophic biomass
Phototrophic algae and cyanobacteria have received much attention in recent years and have been grown in urine and yellowater but also in liquid streams rendered by treatment of primary inputs containing faces, such as treated effluent, anaerobic digester supernatant, or the aqueous phase after HTL. High removal of N and P from the substrate have generally been achieved (Shilton et al., Citation2012; Sukačová & Červený, Citation2017). Also heterotrophic or mixotrophic growth of microalgae has received some attention, but mostly in combination with algal biofuel production (Perez-Garcia et al., Citation2011). Contaminants may be of concern as algae have been shown to extract not only nutrients but also micropollutants and heavy metals from liquid streams through uptake or sorption (de Wilt et al., Citation2016; Zeraatkar et al., Citation2016; Demey et al., Citation2018). Algal biomass is a promising product potentially useful as plant fertilizer or animal feed (Cole et al., Citation2017; Wells et al., Citation2017). The nutrient-rich biomass is usually dried before application as a soil conditioner and fertilizer (Mulbry et al., Citation2005). Alternatively, it can be used as feedstock for biological decomposition (e.g. composting) or thermal decomposition (e.g. HTL).
5.5.2. Fecal-derived organic matter
Fecal-derived organic matter includes a wide variety of products that contain organic matter originating from feces or biomass produced during treatment of fecal-derived organic matter. This product type includes products that closely resemble the primary input (e.g. hygienized feces), products rendered after collection and treatment of the primary input (e.g. blackwater sludge or sewage sludge), as well as products rendered after further decomposition of aforementioned fecal-based feedstocks. These feedstocks can possibly be supplemented by other organic feedstocks (e.g. organic kitchen, yard, or wood waste) and additives (e.g. charcoal, lime, or ash) prior to (biological or thermal) decomposition. Biological decomposition renders digestate or compost (including vermicompost and fly larvae compost), whereas charcoal is the result of thermal decomposition.
These products are useful as combined soil amendments and fertilizers (Grigatti et al., Citation2014; Sangare et al., Citation2015; Kathijotes et al., Citation2016; Horta, Citation2017; Liu et al., Citation2018). When treatment takes place in a closed system, the product can contain N both in the form of inorganic and organic N. Treatment in open systems, however, is prone to N losses through volatilization and/or leaching. Similarly, if liquid-solid separation is applied, inorganic N can be transferred to the liquid fraction. Most N in sewage sludge and in compost in fact is organically bound and not immediately available to plants (Cogger et al., Citation2006; Horttanainen et al., Citation2017). The majority of P in fecal-derived organics is bound in mineral form. In feces, for instance, P is mainly present as calcium and iron phosphate (Rose et al., Citation2015). In sewage sludge, P can be present as polyphosphate incorporated in microbial biomass (biological P removal), or as aluminum or iron phosphate (chemical P removal). P availability is variable and strongly depends on the treatment train. Sludge from biological P removal was found to be superior to precipitation with high Fe/P ratios regarding P availability and recycling (Römer, Citation2006; Kahiluoto et al., Citation2015; Lemming et al., Citation2017). There are indications, however, that phosphorus recovery from iron phosphate can be substantially improved by a better understanding of iron–phosphorus chemistry (Wilfert et al., Citation2015).
It is commonly held that satisfactory pathogen inactivation can be achieved in processes that involve exposure to elevated temperatures (Jonsson et al., Citation2004). Decomposition processes have the potential to fully or partially decompose organic pollutants. Heavy metals are generally of concern for (derivatives of) sewage (Tervahauta et al., Citation2014). As heavy metals present in human excreta were found to primarily originate from dietary sources, agricultural use of human excreta would not increase the amount of heavy metals in the food cycle (Tervahauta et al., Citation2014). Therefore, the importance of distinguishing black water sludge from municipal sewage sludge in sludge reuse regulations has been emphasized (Tervahauta et al., Citation2014). Heavy metals present in sewage sludge are mainly adsorbed to the cell surfaces of the microorganisms in the sludge (Yoshizaki & Tomida, Citation2000). Several studies have shown at the laboratory scale the possibility of extracting heavy metals from sewage sludge by means of acid leaching (e.g. Yoshizaki & Tomida, Citation2000; Naoum et al., Citation2001; Stylianou et al., Citation2007; Usharani & Vasudevan, Citation2016). Acid leaching, however, also dissolves phosphorus (Guilayn et al., Citation2017) and thus would leave a product in the form of fecal-derived organic matter depleted of heavy metals as well as phosphorus and other nutrients.
6. Patterns and trends
The synthesis presented in this review was informed by a rigorous process of organizing and and exctracting information from the pertinent literature. There is clearly no shortage of proposed recovery pathways, treatment processes, and products rendered by treatment. Here we outline a number of broader trends and patterns regarding efforts to facilitate recycling of nutrients contained in human excreta to agriculture.
6.1. Trends in process technology
For a long time, agricultural use of human excreta and streams containing human excreta was the dominant way to recycle nutrients and organic matter found in human excreta and wastewater back to agriculture (Petrik, Citation1954; Rockefeller, Citation1998; Ferguson, Citation2014). Beginning in the 1970s, the extraction of nutrients started to complement recycling of the streams themselves. Early efforts include: extraction of nutrients from liquid streams through precipitation (e.g. Salutsky et al., Citation1972), algae growth (e.g. Mcgarry et al., Citation1971), or sorption (e.g. Liberti et al., Citation1981); and extraction of phosphorus from sewage sludge ash (e.g. Hino et al., Citation1998). Roughly since the mid 2000s, efforts towards nutrient extraction have intensified. Approaches that have been investigated include: extraction of nutrients from liquid streams and wet organic matter through precipitation, sorption, membrane processes, or phototrophic biomass growth; extraction of P from sewage sludge or ash; and extraction of N through various forms of ammonia release and capture (see Supporting Information 1). Also, bioelectrochemical systems have gained currency, among others to support the extraction of nutrients through electrodialysis (e.g. Zhang et al., Citation2013), ammonia release (e.g. Desloover et al., Citation2012; Wu & Modin, Citation2013), or precipitation (e.g. Hug & Udert, Citation2013). Continued research and development is taking place. For source-separated primary inputs, recent developments range from simple (e.g. struvite precipitation from urine in a simple sedimentation reactor) to more complicated approaches (e.g. bioelectrochemical systems) and include pathways that decontaminate and concentrate nutrients (to a liquid or solid product) as well as pathways based on selective nutrient extraction (notably of NPK), or a combination thereof. For sewage as primary input, recent developments are predominantly technology-intensive approaches based on selective nutrient extraction, notably of P (e.g. P leaching from sewage sludge incineration ashes). These approaches are reviewed extensively in Egle et al. (Citation2015). The great variety of recovery pathways that involve extraction of nutrients (notably P) from sewage sludge or sewage sludge ash aligns with the trend towards incineration of a larger portion of the sludge (Kelessidis & Stasinakis, Citation2012; Kirchmann et al., Citation2017) and the anticipation of more stringent future regulation for pathogens, heavy metals, organic pollutants, and other emerging contaminants in sludge intended for land application (Mininni et al., Citation2015; Peccia & Westerhoff, Citation2015).
6.2. Focus on macronutrients NPK
The trend towards nutrient extraction coincides with a focus on the macronutrients NPK. There is no single recovery pathway that captures all nutrients and carbon in human excreta in a single product free of contamination. We see a clear divide between recovery pathways that target the recovery of (some of the) macronutrients NPK and those that more broadly target a wider selection of nutrients, and possibly also organic matter. Products containing a broader spectrum of nutrients as well as organic matter generally are less polluted, notably regarding heavy metals, when obtained from source-separated primary inputs such as urine, feces, or blackwater. When sewage is the primary input, products containing a broader spectrum of nutrients as well as organic matter generally are prone to contain higher levels of contamination. Recovery pathways that render a product of high purity and homogeneity (e.g. macronutrient solutions, monomineral precipitates) achieve this through selective extraction of only some of the nutrients, notably macronutrients N and P. Many studies in fact do not even investigate or report the fate of K and micronutrients. For conventional urban water management and sanitation systems, and often also for new sanitation systems, the discourse generally focuses even more narrowly on P extraction and recovery. P extraction and recovery in fact is expected to become an established process in the coming decades in industrialized countries (Sartorius, von Horn, & Tettenborn, Citation2012).
6.3. Multiple uses for carbon
Human excreta, notably feces, contain carbon that can be valuable to improve soil quality. In conventional sewage treatment, the more readily biodegradable fraction of this carbon is usually converted into biogas and carbon dioxide through microbial metabolism. Unless sewage sludge is incinerated, the less readily biodegradable fraction of the carbon is preserved in the organic residual and potentially available to improve soil quality. But carbon may increasingly be appropriated for other purposes. Human feces and streams containing human feces can potentially serve as feedstock for the production of biocrude, bioethanol, biodiesel, biohydrogen, and syngas (Gomaa & Abed, Citation2017; Puyol et al., Citation2017; Manyuchi et al., Citation2018).
Likewise, feces and streams containing feces can serve as feedstock for the production of higher-value industrial chemicals, for example precursors for biopolymer synthesis and bioplastic production (Pittmann & Steinmetz, Citation2017; Puyol et al., Citation2017). These different uses of carbon do not necessarily exclude one another. But appropriation of a larger fraction of the carbon for the production of energy carriers or higher-value chemicals means that less carbon is available for the improvement of soils. The appropriation of organic matter for the production of energy or chemicals thus may to some extent compete with the recovery of organic matter to improve soil quality.
6.4. Synergies and opportunities for combining recovery pathways
While many of the studies covered in the present review target a single product with agricultural value, some studies report on a combination of recovery pathways leading to multiple products, or at least point to the possibility for a combination of recovery pathways. For example, NF of unhydrolysed urine followed by precipitation yields a solution rich in urea and a precipitate containing N and P (e.g. Pronk, Palmquist, et al., Citation2006). Likewise, ammonia stripping from urine can be complemented by struvite precipitation, yielding a solution rich in ammonium and a precipitate containing N and P (e.g. Antonini et al., Citation2011; Wei et al., Citation2018). Evaporation in a vertical evaporation pipe preceded by alkalinisation yields one precipitate rich in P and a one precipitate containing the other nutrients (e.g. Eawag, Citation2018b). The combination of ammonia stripping, struvite precipitation, and biomass growth in a hydroponic system to treat source-separated urine even yields three products useful for agriculture: a solution rich in ammonia, a precipitate containing N and P, and a residual solution used as input to the hydroponic system (e.g. Yang et al., Citation2015). Other possible combinations are: pyrolysis of for instance sewage sludge and use of the char thus obtained as sorbent (e.g. Shepherd et al., Citation2016), or urea extraction from unhydrolysed urine through sorption in order to facilitate MPP (magnesium potassium phosphate) precipitation in the absence of N (e.g. Simha et al., Citation2018). Similarly, CaP granulation during anaerobic digestion of blackwater would yield three products from one reactor system: CaP, digestate, and a concentrated liquid with N, K and micronutrients.
The combination of recovery pathways can enhance overall nutrient recovery and recycling. What might be nutrient losses in a single pathway might well be captured in another product if several pathways are combined to target more than one product. This means that individual recovery pathways or products should not be judged in isolation. For example, one could argue that recovery pathways based on urine separation fail to capture about half of the P and most of the C in human excreta. Urine separation, however, does not prevent recovery of nutrients and organic matter from the stream containing the feces. On the contrary, the fact that most of the N is in the urine means that any recovery pathway starting from the stream containing the feces will be subject to lesser N losses than would be the case if urine were in this stream. Similarly, one could argue that struvite precipitation usually only captures a fraction of the P if sewage is the primary input (and anaerobic digester reject water the process input). Struvite precipitation during sewage treatment, however, does not prevent the subsequent recovery of additional P from sewage sludge or sewage sludge ashes; though systems become more complicated. Finally, individual products can also be applied in combination, for example mineral sorbents and precipitates (Lind et al., Citation2000; Xu et al., Citation2001), or compost and precipitates (Karak et al., Citation2015).
7. Discussion and outlook
As outlined in this review and elsewhere in the literature, a broad range of recovery technologies and pathways to facilitate recovery of nutrients and organic matter contained in human excreta is available or under development. The two currently most mature recovery pathways are struvite crystallisation from anaerobic digester supernatant and incineration of sewage sludge with subsequent P recovery from incineration ashes. While further development and refinement of these and other recovery technologies and pathways is valuable in its own right, we believe that there is scope to ask questions that go beyond individual recovery technologies and pathways, and that better integrate end-user needs and the bigger picture.
The call for further development of technologies that recover N and K in addition to P (Mehta et al., Citation2015) is a step in the right direction. But in light of soil nutrient stripping (Jones et al., Citation2013) and soil carbon losses (Amundson et al., Citation2015), we think the scope of nutrient recovery should be even broader and also include micronutrients and organic matter. This will ultimately require a shift away from thinking in terms of individual recovery pathways, towards thinking in terms of sensible combinations of recovery pathways that maximize recovery of nutrients and organic matter while minimizing risks associated with contaminants.
Recognising that comprehensive nutrient recovery will again have to become a key function of human excreta management in order to help reinvigorate soil and food security, it becomes evident that a conceptual change towards framing human excreta management as part of the food cycle rather than the urban water cycle might be productive. We believe that broadening the discourse along these lines would strongly benefit from the integration of perspectives and considerations from food and farming systems with those from managing human excreta.
In other words, we argue that it is not sufficient to ask: how to recover (some of the) nutrients from (streams containing) human excreta? It is also necessary to ask: which kind of production system is envisioned as recipient of the nutrients and organic matter? How can the products best support a given production system and the achievement of specific goals such as food and soil security? Are there specific functions that need to be fulfilled by recycling from human excreta and that cannot be fulfilled by other ways of (re)cycling nutrients and organic matter? Still, there is only little research into how various recovered products fit the needs of soils and farmers (e.g. Wielemaker et al., Citation2018). While the present review briefly touches upon general product characteristics, further research on the effects of nutrients and contaminants from products recovered from human excreta on soil health, plant growth and human well-being would be helpful.
We recognize that recycling nutrients (and organic matter) from human excreta and streams containing human excreta to food production is only one dimension of establishing a circular nutrient metabolism where nutrients from food are recycled back to the production of food. Establishing such a circular nutrient metabolism requires action along the entire food chain from agriculture and food processing to consumers and Waste Managementement; this includes proper management of harvest residues, animal manure, food processing residuals and waste, and human excreta (McConville et al., Citation2015). But nutrient recycling is currently constrained by spatial disconnects between livestock intensive areas and areas where feed is produced, and between rural areas where food is produced and urban areas where food is consumed and human excreta produced (Jones et al., Citation2013; Nesme et al., Citation2018).
Other factors that are critical for a smooth and effective transition to the widespread use of recovered fertilizer products but were not considered in the present review include legislation (Hukari et al., Citation2016) and social acceptance (Dahlin et al., Citation2016), as well as technological maturity, environmental performance, and costs (Egle et al., Citation2016).
We agree with Trimmer et al. (Citation2017) that sanitation systems could become an inspirational component of societal infrastructure and an amplifiying force for sustainable development. We hope that the present review can make valuable contributions to this end, by providing inspiration to look upon the recovery of nutrients and organic matter from a broader perspective, and to better integrate perspectives from food and farming systems, the recipients of the recovered fertilizer products. The organization and classification of recovery pathways that underpins the present review could also serve as a foundation to more effectively share and consolidate what we already know about various aspects of human excreta management, and to keep track of further technological advancements.
supporting_imformation.pdf
Download Zip (3.8 MB)Additional information
Funding
References
- Abdel-Shafy, H. I., & Mansour, M. S. M. (2016). Integration of effective microorganisms and membrane bioreactor for the elimination of pharmaceutical active compounds from urine for safe reuse. Journal of Water Reuse and Desalination, 6, 495–504.
- Abinandan, S., Subashchandrabose, S. R., Venkateswarlu, K., & Megharaj, M. (2018). Nutrient removal and biomass production: advances in microalgal biotechnology for wastewater treatment. Critical Reviews in Biotechnology, 38, 1–17.
- Adam, C., Peplinski, B., Kley, G., Kratz, S., Schick, J., & Schnug, E. (2008). Phosphorrückgewinnung aus Klärschlammaschen – Ergebnisse aus dem EU-Projekt SUSAN. Österreichische Wasser- und Abfallwirtschaft, 60, 55–64.
- Adam, C., Peplinski, B., Michaelis, M., Kley, G., & Simon, F. G. (2009). Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Management, 29, 1122–1128.
- Afif, E., Azadi, P., & Farnood, R. (2011). Catalytic hydrothermal gasification of activated sludge. Applied Catalysis B: Environmental, 105(1–2), 136–143.
- Aida, T. M., Nonaka, T., Fukuda, S., Kujiraoka, H., Kumagai, Y., Maruta, R., … Inomata, H. (2016). Nutrient recovery from municipal sludge for microalgae cultivation with two-step hydrothermal liquefaction. Algal Research, 18, 61–68.
- Alturki, A. A., McDonald, J. A., Khan, S. J., Price, W. E., Nghiem, L. D., & Elimelech, M. (2013). Removal of trace organic contaminants by the forward osmosis process. Separation and Purification Technology, 103, 258–266.
- Amundson, R., Berhe, A. A., Hopmans, J. W., Olson, C., Sztein, A. E., & Sparks, D. L. (2015). Soil and human security in the 21st century. Science, 348, 1261071.
- Anderson, C., Malambo, D. H., Perez, M. E. G., Nobela, H. N., Pooter, L., de, Spit, J., … Thole, B. (2015). Lactic acid fermentation, urea and lime addition: Promising faecal sludge sanitizing methods for emergency sanitation. International Journal of Environmental Research and Public Health, 12, 13871–13885.
- Andreev, N., Ronteltap, M., Boincean, B., Wernli, M., Zubcov, E., Bagrin, N., … Lens, P. N. L. (2017). Lactic acid fermentation of human urine to improve its fertilizing value and reduce odour emissions. Journal of Environmental Management, 198(Pt 1), 63–69.
- Ansari, A. J., Hai, F. I., Price, W. E., Drewes, J. E., & Nghiem, L. D. (2017). Forward osmosis as a platform for resource recovery from municipal wastewater – A critical assessment of the literature. Journal of Membrane Science, 529, 195–206.
- Antakyali, D., Meyer, C., Preyl, V., Maier, W., & Steinmetz, H. (2013). Large-scale application of nutrient recovery from digested sludge as struvite. Water Practice and Technology, 8, 256–262.
- Antonini, S., Nguyen, P. T., Arnold, U., Eichert, T., & Clemens, J. (2012). Solar thermal evaporation of human urine for nitrogen and phosphorus recovery in Vietnam. Science of the Total Environment, 414, 592–599.
- Antonini, S., Paris, S., Eichert, T., & Clemens, J. (2011). Nitrogen and phosphorus recovery from human urine by struvite precipitation and air stripping in Vietnam. CLEAN - Soil, Air, Water, 39, 1099–1104.
- Atay, Ş., & Akbal, F. (2016). Classification and Effects of sludge disintegration technologies integrated into sludge handling units: An overview. CLEAN - Soil, Air, Water, 44, 1198–1213.
- Babel, S., & Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: A review. Journal of Hazardous Materials, 97(1–3), 219–243.
- Bai, X., Li, Z., Zhang, Y., Ni, J., Wang, X., & Zhou, X. (2017). Recovery of ammonium in urine by biochar derived from faecal sludge and its application as soil conditioner. Waste and Biomass Valorization, 9, 1–10.
- Balmér, P. (2004). Phosphorus recovery-an overview of potentials and possibilities. Water Science and Technology, 49, 185–190.
- Barber, W. P. F. (2016). Thermal hydrolysis for sewage treatment: A critical review. Water Research, 104, 53–71.
- Batstone, D. J., Hülsen, T., Mehta, C. M., & Keller, J. (2015). Platforms for energy and nutrient recovery from domestic wastewater: A review. Chemosphere, 140, 2–11.
- Beler-Baykal, B., Allar, A. D., & Bayram, S. (2011). Nitrogen recovery from source-separated human urine using clinoptilolite and preliminary results of its use as fertilizer. Water Science and Technology, 63, 811–817.
- Bengtsson, M., & Tillman, A. M. (2004). Actors and interpretations in an environmental controversy: The Swedish debate on sewage sludge use in agriculture. Resources, Conservation and Recycling, 42(1), 65–82.
- Berliner Wasserbetriebe. (2018). Retrieved from http://www.bwb.de/berlinerpflanze
- Bethune, D. N., Chu, A., & Ryan, M. C. (2014). Passive evaporation of source-separated urine from dry toilets: A lab study. Journal of Water, Sanitation and Hygiene for Development, 4, 654.
- Bethune, D. N., Chu, A., & Ryan, M. C. (2016). Passive evaporation of source-separated urine from dry toilets: UES optimization and dry product accumulation over time. Journal of Water, Sanitation and Hygiene for Development, 6(1), 96–103.
- Bischel, H. N., Schertenleib, A., Fumasoli, A., Udert, K. M., & Kohn, T. (2015). Inactivation kinetics and mechanisms of viral and bacterial pathogen surrogates during urine nitrification. Environmental Science: Water Research & Technology, 1(1), 65–76.
- Blöcher, C., Niewersch, C., & Melin, T. (2012). Phosphorus recovery from sewage sludge with a hybrid process of low pressure wet oxidation and nanofiltration. Water Research, 46, 2009–2019.
- Bonvin, C., Etter, B., Udert, K. M., Frossard, E., Nanzer, S., Tamburini, F., & Oberson, A. (2015). Plant uptake of phosphorus and nitrogen recycled from synthetic source-separated urine. AMBIO, 44, 217–227.
- Brands, E. (2014). Prospects and challenges for sustainable sanitation in developed nations: A critical review. Environmental Reviews, 22, 346–363.
- Bridger, G. L., Salutsky, M. L., & Starostka, R. W. (1962). Micronutrient sources, metal ammonium phosphates as fertilizers. Journal of Agricultural and Food Chemistry, 10, 181–188.
- Bridle, T., & Pritchard, D. (2004). Energy and nutrient recovery from sewage sludge via pyrolysis. Water Science and Technology , 50, 169–175.
- Busscher, H. J., Dijkstra, R. J. B., Engels, E., Langworthy, D. E., Collias, D. I., Bjorkquist, D. W. … Van Der Mei, H.C. (2006). Removal of two waterborne pathogenic bacterial strains by activated carbon particles prior to and after charge modification. Environmental Science & Technology, 40, 6799–6804.
- Butkovskyi, A., Ni, G., Hernandez Leal, L., Rijnaarts, H. H. M., & Zeeman, G. (2016). Mitigation of micropollutants for black water application in agriculture via composting of anaerobic sludge. Journal of Hazardous Materials, 303, 41–47.
- Butkovskyi, A., Rijnaarts, H. H. M., Zeeman, G., & Hernandez Leal, L. (2016). Fate of personal care and household products in source separated sanitation. Journal of Hazardous Materials, 320, 427–434.
- Cabeza, R., Steingrobe, B., Römer, W., & Claassen, N. (2011). Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutrient Cycling in Agroecosystems, 91, 173–184.
- Cai, Y., Qi, H., Liu, Y., & He, X. (2016). Sorption/desorption behavior and mechanism of NH4+ by biochar as a nitrogen fertilizer sustained-release material. Journal of Agricultural and Food Chemistry, 64, 4958–4964.
- Casadellà, A., Kuntke, P., Schaetzle, O., & Loos, K. (2016). Clinoptilolite-based mixed matrix membranes for the selective recovery of potassium and ammonium. Water Research, 90, 62–70.
- Chang, Y., Deng, C., Dore, A. J., & Zhuang, G. (2015). Human excreta as a stable and important source of atmospheric ammonia in the megacity of Shanghai. PLoS One, 10, 1–13.
- Chen, P., Anderson, E., Addy, M., Zhang, R., Cheng, Y., Peng, P., … Lu, Q. (2018). Breakthrough technologies for the biorefining of organic solid and liquid wastes. Engineering, 4, 574–580.
- Choi, H. J., & Lee, S. M. (2015). Heavy metal removal from acid mine drainage by calcined eggshell and microalgae hybrid system. Environmental Science and Pollution Research, 22, 13404–13411.
- Chraibi, S., Moussout, H., Boukhlifi, F., Ahlafi, H., & Alami, M. (2016). Utilization of calcined eggshell waste as an adsorbent for the removal of phenol from aqueous solution. Journal of Encapsulation and Adsorption Sciences, 6, 132–146.
- Cieślik, B., & Konieczka, P. (2017). A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. Journal of Cleaner Production, 142, 1728–1740.
- Cogan, T. A., Bloomfield, S. F., & Humphrey, T. J. (1999). The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcases in the domestic kitchen. Letters in Applied Microbiology, 29, 354–358.
- Cogger, C. G., Forge, T. A., & Neilsen, G. H. (2006). Biosolids recycling: Nitrogen management and soil ecology. Canadian Journal of Soil Science, 86, 613–620.
- Cole, A. J., Paul, N. A., Nys, R., de., & Roberts, D. A. (2017). Good for sewage treatment and good for agriculture: Algal based compost and biochar. Journal of Environmental Management, 200, 105–113.
- Cooper, P. F. (2001). Historical aspects of wastewater treatment. In: Lens, P. N. L., G. Zeeman, and G. Lettinga (Eds.), Decentralised sanitation and reuse: Concepts, systems and implementation (pp. 11–38). London, UK: IWA.
- Coppens, J., Lindeboom, R., Muys, M., Coessens, W., Alloul, A., Meerbergen, K., … Vlaeminck, S. E. (2016). Nitrification and microalgae cultivation for two-stage biological nutrient valorization from source separated urine. Bioresource Technology, 211, 41–50.
- Cornel, P., & Schaum, C. (2009). Phosphorus recovery from wastewater: Needs, technologies and costs. Water Science and Technology, 59, 1069–1076.
- Cowling, S. J., Gardner, M. J., & Hunt, D. T. E. (1992). Removal of heavy metals from sewage by sulphide precipitation: Thermodynamic calculations and tests on a pilot‐scale anaerobic reactor. Environmental Technology , 13, 281–291.
- Cunha, J. R., Schott, C., Weijden, R. D., van der, Leal, L. H., Zeeman, G., & Buisman, C. (2018). Calcium addition to increase the production of phosphate granules in anaerobic treatment of black water. Water Research, 130, 333–342.
- Dahlin, J., Halbherr, V., Kurz, P., Nelles, M., & Herbes, C. (2016). Marketing green fertilizers: Insights into consumer preferences. Sustain, 8, 1–15.
- Danso-Boateng, E., Holdich, R. G., Martin, S. J., Shama, G., & Wheatley, A. D. (2015). Process energetics for the hydrothermal carbonisation of human faecal wastes. Energy Conversion and Management, 105, 1115–1124.
- Danso-Boateng, E., Holdich, R. G., Shama, G., Wheatley, A. D., Sohail, M., & Martin, S. J. (2013). Kinetics of faecal biomass hydrothermal carbonisation for hydrochar production. Applied Energy, 111, 351–357.
- Danso-Boateng, E., Shama, G., Wheatley, A. D., Martin, S. J., & Holdich, R. G. (2015). Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresource Technology, 177, 318–327.
- Darwish, M., Aris, A., Puteh, M. H., Abideen, M. Z., & Othman, M. N. (2016). Ammonium-nitrogen recovery from wastewater by struvite crystallization technology. Separation & Purification Reviews, 45, 261–274.
- Dawson, C. J., & Hilton, J. (2011). Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy, 36, S14–S22.
- Decrey, L., Udert, K. M., Tilley, E., Pecson, B. M., & Kohn, T. (2011). Fate of the pathogen indicators phage ΦX174 and Ascaris suum eggs during the production of struvite fertilizer from source-separated urine. Water Research, 45, 4960–4972.
- Degryse, F., Baird, R., Silva, R. C., da., & McLaughlin, M. J. (2017). Dissolution rate and agronomic effectiveness of struvite fertilizers – Effect of soil pH, granulation and base excess. Plant and Soil, 410(1–2), 139–152.
- Demey, H., Vincent, T., & Guibal, E. (2018). A novel algal-based sorbent for heavy metal removal. Chemical Engineering Journal, 332, 582–595.
- Deng, Y., & Zhao, R. (2015). Advanced Oxidation Processes (AOPs) in wastewater treatment. Current Pollution Reports, 1, 167–176.
- Deppe, M., Well, R., Kücke, M., Fuß, R., Giesemann, A., & Flessa, H. (2016). Impact of CULTAN fertilization with ammonium sulfate on field emissions of nitrous oxide. Agriculture, Ecosystems & Environment, 219, 138–151.
- Desloover, J., Abate Woldeyohannis, A., Verstraete, W., Boon, N., & Rabaey, K. (2012). Electrochemical resource recovery from digestate to prevent ammonia toxicity during anaerobic digestion. Environmental Science & Technology, 46, 12209–12216.
- Desmidt, E., Ghyselbrecht, K., Zhang, Y., Pinoy, L., Bruggen, B., Van Der, Verstraete, W., … Meesschaert, B. (2015). Global phosphorus scarcity and full-scale P-recovery techniques: A review. Critical Reviews in Environmental Science and Technology, 45, 336–384.
- Donatello, S., & Cheeseman, C. R. (2013). Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review. Waste Managementement, 33, 2328–2340.
- Doyle, J. D., & Parsons, S. A. (2002). Struvite formation, control and recovery. Water Research, 36, 3925–3940.
- Dumontet, S., Scopa, A., Kerje, S., & Krovacek, K. (2001). The importance of pathogenic organisms in sewage and sewage sludge. Journal of the Air & Waste Managementement Association (1995), 51, 848–860.
- Dutta, S., & Vinnerås, B. (2016). Fertilizer from dried human urine added to ash and lime – A potential product from eco-sanitation system. Water Science and Technology, 74, 1436–1445.
- Eawag. (2018a). Retrieved from https://www.eawag.ch/en/department/eng/projects/aurin-fertilisers-from-urine/
- Eawag. (2018b). Retrieved from https://www.eawag.ch/en/research/humanwelfare/wastewater/projekte/autarky/
- Egle, L., Rechberger, H., Krampe, J., & Zessner, M. (2016). Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Science of the Total Environment, 571, 522–542.
- Egle, L., Rechberger, H., & Zessner, M. (2015). Overview and description of technologies for recovering phosphorus from municipal wastewater. Resources, Conservation and Recycling, 105, 325–346.
- Ek, M., Bergström, R., Bjurhem, J. E., Björlenius, B., & Hellström, D. (2006). Concentration of nutrients from urine and reject water from anaerobically digested sludge. Water Science and Technology, 54, 437–444.
- Elser, J., & Bennett, E. M. (2009). A broken geochemical cycle. Nature, 478, 2009–2011.
- Escher, B. I., Pronk, W., Suter, M. J. F., & Maurer, M. (2006). Monitoring the removal efficiency of pharmaceuticals and hormones in different treatment processes of source-separated urine with bioassays. Environmental Science & Technology, 40, 5095–5101.
- Fabris, I., Cormier, D., Gerhard, J. I., Bartczak, T., Kortschot, M., Torero, J. L., & Cheng, Y. L. (2017). Continuous, self-sustaining smouldering destruction of simulated faeces. Fuel, 190, 58–66.
- Ferguson, D. T. (2014). Nightsoil and the “Great Divergence”: Human waste, the urban economy, and economic productivity, 1500-1900. Journal of Global History, 9, 379–402.
- Fidjeland, J., Magri, M. E., Jönsson, H., Albihn, A., & Vinnerås, B. (2013). The potential for self-sanitisation of faecal sludge by intrinsic ammonia. Water Research, 47, 6014–6023.
- Fidjeland, J., Svensson, S. E., & Vinnerås, B. (2015). Ammonia sanitization of blackwater for safe use as fertilizer. Water Science and Technology, 71, 795–800.
- Fischer, F., Bastian, C., Happe, M., Mabillard, E., & Schmidt, N. (2011). Microbial fuel cell enables phosphate recovery from digested sewage sludge as struvite. Bioresource Technology, 102, 5824–5830.
- Forbis-Stokes, A. A., O'Meara, P. F., Mugo, W., Simiyu, G. M., & Deshusses, M. A. (2016). On-site fecal sludge treatment with the anaerobic digestion pasteurization latrine. Journal of Environmental Engineering and Science, 33, 898–906.
- Friedler, E., Butler, D., & Alfiya, Y. (2013). Wastewater composition In: Larsen, T.A., K.M. Udert, and J. Lienert, Eds., Source Separation and Decentralization for Wastewater Management, pp. 241–257. London, UK: IWA.
- Fumasoli, A., Etter, B., Sterkele, B., Morgenroth, E., & Udert, K. M. (2016). Operating a pilot-scale nitrification/distillation plant for complete nutrient recovery from urine. Water Science and Technology, 73(1), 215–222.
- Gajurel, D., Deegener, S., Shalabi, M., & Otterpohl, R. (2007). Potential of filter-vermicomposter for household wastewater pre-treatment and sludge sanitisation on site In. Water Science and Technology, 55, 65–70.
- Ganesapillai, M., Simha, P., Gupta, K., & Jayan, M. (2016). Nutrient recovery and recycling from human urine: A circular perspective on sanitation and food security. Procedia Engineering, 148, 346–353.
- Giannakis, S., Rtimi, S., & Pulgarin, C. (2017). Light-assisted advanced oxidation processes for the elimination of chemical and microbiological pollution of wastewaters in developed and developing countries. Molecules, 22, 1–21.
- Goglio, A., Tucci, M., Rizzi, B., Colombo, A., Cristiani, P., & Schievano, A. (2019). Microbial recycling cells (MRCs): A new platform of microbial electrochemical technologies based on biocompatible materials, aimed at cycling carbon and nutrients in agro-food systems. Science of the Total Environment, 649, 1349–1361.
- Gomaa, M. A., & Abed, R. M. M. (2017). Potential of fecal waste for the production of biomethane, bioethanol and biodiesel. Journal of Biotechnology, 253, 14–22.
- Gorazda, K., Tarko, B., Werle, S., & Wzorek, Z. (2018). Sewage sludge as a fuel and raw material for phosphorus recovery: Combined process of gasification and P extraction. Waste Management, 73, 404–415.
- Graaff, M. S., de, Temmink, H., Zeeman, G., & Buisman, C. J. N. (2010). Anaerobic treatment of concentrated black water in a UASB reactor at a short HRT. Water, 2, 101–119.
- Grigatti, M., Cavani, L., Marzadori, C., & Ciavatta, C. (2014). Recycling of dry-batch digestate as amendment: Soil C and N dynamics and ryegrass nitrogen utilization efficiency. Waste and Biomass Valorization, 5, 823–833.
- Guaya, D., Hermassi, M., Valderrama, C., Farran, A., & Cortina, J. L. (2016). Recovery of ammonium and phosphate from treated urban wastewater by using potassium clinoptilolite impregnated hydrated metal oxides as N-P-K fertilizer. Journal of Environmental Chemical Engineering, 4, 3519–3526.
- Gude, V. G. (2016). Wastewater treatment in microbial fuel cells – An overview. Journal of Cleaner Production, 122, 287–307.
- Guilayn, F., Braak, E., Piveteau, S., & Daumer, M. L. (2017). Sequencing biological acidification of waste-activated sludge aiming to optimize phosphorus dissolution and recovery. Environmental Technology , 38, 1399–1407.
- Gulyas, H., Bruhn, P., Furmanska, M., Hartrampf, K., Kot, K., Lüttenberg, B., … Otterpohl, R. (2004). Freeze concentration for enrichment of nutrients in yellow water from no-mix toilets. Water Science and Technology, 50, 61–68.
- Güney, K., Weidelener, A., & Krampe, J. (2008). Phosphorus recovery from digested sewage sludge as MAP by the help of metal ion separation. Water Research, 42, 4692–4698.
- Gustafsson, J. P., Renman, A., Renman, G., & Poll, K. (2008). Phosphate removal by mineral-based sorbents used in filters for small-scale wastewater treatment. Water Research, 42(1–2), 189–197.
- Hao, X., Wang, C., Loosdrecht, M. C. M., Van., & Hu, Y. (2013). Looking beyond struvite for P ‑ recovery. Environmental Science & Technology, 47, 4965–4966.
- Hao, X. D., Wang, C. C., Lan, L., & Van Loosdrecht, M. C. M. (2008). Struvite formation, analytical methods and effects of pH and Ca2++. Water Science and Technology, 58, 1687–1692.
- Happe, M., Sugnaux, M., Cachelin, C. P., Stauffer, M., Zufferey, G., Kahoun, T., … Grogg, A. F. (2016). Scale-up of phosphate remobilization from sewage sludge in a microbial fuel cell. Bioresource Technology, 200, 435–443.
- Haq, G., & Cambridge, H. (2012). Exploiting the co-benefits of ecological sanitation. Current Opinion in Environmental Sustainability, 4, 431–435.
- He, C., Chen, C. L., Giannis, A., Yang, Y., & Wang, J. Y. (2014). Hydrothermal gasification of sewage sludge and model compounds for renewable hydrogen production: A review. Renewable & Sustainable Energy Reviews, 39, 1127–1142.
- He, X., Zhang, Y., Shen, M., Zeng, G., Zhou, M., & Li, M. (2016). Effect of vermicomposting on concentration and speciation of heavy metals in sewage sludge with additive materials. Bioresource Technology, 218, 867–873.
- Hedström, A. (2006). Wollastonite as reactive filter medium for sorption of wastewater ammonium and phosphorus. Environmental Technology, 27, 801–809.
- Heinonen-Tanski, H., & Van Wijk-Sijbesma, C. (2005). Human excreta for plant production. Bioresource Technology, 96, 403–411.
- Heinzmann, B. (2005). Phosphorus recycling in sewage treatment plants with biological phosphorus removal. Water Science and Technology, 52, 543–548.
- Hellström, D., Johansson, E., & Grennberg, K. (1999). Storage of human urine: Acidification as a method to inhibit decomposition of urea. Ecological Engineering, 12, 253–269.
- Hernández Leal, L., Tervahauta, T., & Zeeman, G. (2017). Resource recovery from source separated domestic wastewater; energy, water, nutrients, and organics. In J. M. Lema & S. Suarez (Eds.), Innovative Wastewater Treatment & Resource Recovery Technology: Impacts on Energy, Economy and Environment. London, UK: IWA.
- Herzel, H., KrüGer, O., Hermann, L., & Adam, C. (2016). Sewage sludge ash – A promising secondary phosphorus source for fertilizer production. Science of the Total Environment, 542, 1136–1143.
- Hino, A., Hirai, T., & Komasawa, I. (1998). Te recovery of phosphorus value from incineration ashes of sewage sludge using solvent extraction. Kagaku Kogaku Ronbunshu, 24, 277–278.
- Hong, K. J., Tarutani, N., Shinya, Y., & Kajiuchi, T. (2005). Study on the recovery of phosphorus from waste-activated sludge incinerator ash. Journal of Environmental Science and Health Part A Toxic/Hazardous Substances & Environmental Engineering, 40, 617–631.
- Horta, C. (2017). Bioavailability of phosphorus from composts and struvite in acid soils. Revista Brasileira de Engenharia Agrícola e Ambiental, 21, 459–464.
- Horttanainen, M., Deviatkin, I., & Havukainen, J. (2017). Nitrogen release from mechanically dewatered sewage sludge during thermal drying and potential for recovery. Journal of Cleaner Production, 142, 1819–1826.
- Hossain, M. K., Strezov, V., Chan, K. Y., Ziolkowski, A., & Nelson, P. F. (2011). Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. Journal of Environmental Management, 92(1), 223–228.
- Huang, H., Liu, J., & Ding, L. (2015). Recovery of phosphate and ammonia nitrogen from the anaerobic digestion supernatant of activated sludge by chemical precipitation. Journal of Cleaner Production, 102, 437–446.
- Huang, K., Mao, Y., Zhao, F., Zhang, X.-X., Ju, F., Ye, L., … Zhang, T. (2018). Free-living bacteria and potential bacterial pathogens in sewage treatment plants. Applied Microbiology and Biotechnology, 102, 2455–2464.
- Huang, L., Cheng, S., & Chen, G. (2011). Bioelectrochemical systems for efficient recalcitrant wastes treatment. Journal of Chemical Technology & Biotechnology, 86, 481–491.
- Hug, A., & Udert, K. M. (2013). Struvite precipitation from urine with electrochemical magnesium dosage. Water Research, 47(1), 289–299.
- Hukari, S., Hermann, L., & Nättorp, A. (2016). From wastewater to fertilisers – Technical overview and critical review of European legislation governing phosphorus recycling. Science of the Total Environment, 542, 1127–1135.
- Hülsen, T., Batstone, D. J., & Keller, J. (2014). Phototrophic bacteria for nutrient recovery from domestic wastewater. Water Research, 50, 18–26.
- Hunter, K. A., Liss, P. S., Surapipith, V., Dentener, F., Duce, R., Kanakidou, M., … Sarin, M. (2011). Impacts of anthropogenic SO x, NO x and NH 3 on acidification of coastal waters and shipping lanes. Geophysical Research Letters, 38, 1–6.
- Ito, R., Takahashi, E., & Funamizu, N. (2013). Production of slow-released nitrogen fertilizer from urine. Environmental Technology, 34, 2813–2819.
- Jana, B., Bag, S. K., & Rana, S. (2012). urine, cow manure and their mix for the production. Aquaculture International, 20, 735–749.
- Jaskūnas, A. (2015). Adsorption of potassium ions on natural zeolite: Kinetic and equilibrium studies. Chemija, 26, 69–78.
- Jiang, S., Wang, X., Yang, S., & Shi, H. (2017). Effect of initial pH and pH-adjusted acid on nutrient recovery from hydrolysis urine by combining acidification with evaporation-crystallization. Environmental Science and Pollution Research, 24, 3872–3881.
- Johnston, A. E., & Richards, I. R. (2003). Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil Use and Management, 19(1), 45–49.
- Jones, D. L., Cross, P., Withers, P. J. A., Deluca, T. H., Robinson, D. A., Quilliam, R. S., … Edwards-Jones, G. (2013). REVIEW: Nutrient stripping: The global disparity between food security and soil nutrient stocks. Journal of Applied Ecology, 50, 851–862.
- Jönsson, H., Stenström, T., Svensson, J., & Sundin, A. (1997). Source separated urine-nutrient and heavy metal content, water saving and fecal contamination. Water Science and Technology, 35, 145–152.
- Jonsson, H., Stinzing, A. R., Vinneras, B., & Salomon, E. (2004). Guidelines on the use of urine and faeces in crop production. EcoSanRes, 2, 1–35.
- Kahiluoto, H., Kuisma, M., Ketoja, E., Salo, T., & Heikkinen, J. (2015). Phosphorus in manure and sewage sludge more recyclable than in soluble inorganic fertilizer. Environmental Science & Technology, 49, 2115–2122.
- Kameda, T., Ito, S., & Yoshioka, T. (2017). Kinetic and equilibrium studies of urea adsorption onto activated carbon: Adsorption mechanism. Journal of Dispersion Science and Technology, 38, 1063–1066.
- Karak, T., Sonar, I., Nath, J. R., Paul, R. K., Das, S., Boruah, R. K., … Das, K. (2015). Struvite for composting of agricultural wastes with termite mound: Utilizing the unutilized. Bioresource Technology, 187, 49–59.
- Karapinar, N. (2009). Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. Journal of Hazardous Materials, 170, 1186–1191.
- Karunanithi, R., Szogi, A. A., Bolan, N., Naidu, R., Loganathan, P., Hunt, P. G., … Krishnamoorthy, S. (2015). Phosphorus recovery and reuse from waste streams. Advances in Agronomy, 131, 173–250. Elsevier Ltd.
- Karvelas, M., Katsoyiannis, A., & Samara, C. (2003). Occurrence and fate of heavy metals in the wastewater treatment process. Chemosphere, 53, 1201–1210.
- Katehis, D., Diyamandoglu, V., & Fillos, J. (1998). Stripping and recovery of ammonia from centrate of anaerobically digested biosolids at elevated temperatures. Water Environment Research, 70, 231–240.
- Kathijotes, N., Zlatareva, E., Marinova, S., & Petrova, V. (2016). Soil fertilization with wastewater biosolids -monitoring changes in the “soil-fertilizer-plant” system and phosphorus recovery options. Water Science and Technology, 74, 1499–1508.
- Katsoyiannis, A., & Samara, C. (2005). Persistent organic pollutants (POPs) in the conventional activated sludge treatment process: Fate and mass balance. Environmental Research, 97, 245–257.
- Kelessidis, A., & Stasinakis, A. S. (2012). Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Management, 32, 1186–1195.
- Kelly, P. T., & He, Z. (2014). Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresource Technology, 153, 351–360.
- Kirchmann, H., Börjesson, G., Kätterer, T., & Cohen, Y. (2017). From agricultural use of sewage sludge to nutrient extraction: A soil science outlook. AMBIO, 46, 143–154.
- Kołodyńska, D., Wnętrzak, R., Leahy, J. J., Hayes, M. H. B., Kwapiński, W., & Hubicki, Z. (2012). Kinetic and adsorptive characterization of biochar in metal ions removal. Chemical Engineering Journal, 197, 295–305.
- Köse, T. E., & Kivanç, B. (2011). Adsorption of phosphate from aqueous solutions using calcined waste eggshell. Chemical Engineering Journal, 178, 34–39.
- Krüger, O., Fattah, K. P., & Adam, C. (2016). Phosphorus recovery from the wastewater stream—necessity and possibilities. Desalination and Water Treatment, 57, 15619–15627.
- Kruse, A., Koch, F., Stelzl, K., Wüst, D., & Zeller, M. (2016). Fate of nitrogen during hydrothermal carbonization. Energy & Fuels, 30, 8037–8042.
- Kujawa-Roeleveld, K., Elmitwalli, T., & Zeeman, G. (2006). Enhanced primary treatment of concentrated black water and kitchen residues within DESAR concept using two types of anaerobic digesters. Water Science and Technology, 53, 159–168.
- Kumar, R., & Pal, P. (2015). Assessing the feasibility of N and P recovery by struvite precipitation from nutrient-rich wastewater: A review. Environmental Science and Pollution Research International, 22, 17453–17464.
- Kümmerer, K. (2013). The issue of micropollutants in urban water management In: Larsen, T.A., Udert, K. M., and J. Lienert, (Eds.), Source separation and decentralization for wastewater management. pp. 71–84. London, UK: IWA.
- Kuntke, P., Sleutels, T. H. J. A., Saakes, M., & Buisman, C. J. N. (2014). Hydrogen production and ammonium recovery from urine by a Microbial Electrolysis Cell. International Journal of Hydrogen Energy, 39, 4771–4778.
- Kuntke, P., Smiech, K. M., Bruning, H., Zeeman, G., Saakes, M., Sleutels, T. H. J. A., … Buisman, C. J. N. (2012). Ammonium recovery and energy production from urine by a microbial fuel cell. Water Research, 46, 2627–2636.
- De la Varga, D., Díaz, M. A., Ruiz, I., & Soto, M. (2013). Heavy metal removal in an UASB-CW system treating municipal wastewater. Chemosphere, 93, 1317–1323.
- Lahr, R. H., Goetsch, H. E., Haig, S. J., Noe-Hays, A., Love, N. G., Aga, D. S., … Luo, T. (2016). Urine bacterial community convergence through fertilizer production: Storage, pasteurization, and struvite precipitation. Environmental Science & Technology, 50, 11619–11626.
- Lalander, C., Fidjeland, J., Diener, S., Eriksson, S., & Vinnerås, B. (2015). High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agronomy for Sustainable Development, 35(1), 261–271.
- Lalander, C., Hill, G. B., & Vinnerås, B. (2013). Hygienic quality of faeces treated in urine diverting vermicomposting toilets. Waste Management, 33, 2204–2210.
- Larsen, T. A., Alder, A. C., Eggen, R. I. L., Maurer, M., & Lienert, J. (2009). Source separation: Will we see a paradigm shift in wastewater handling? Environmental Science & Technology, 43, 6121–6125.
- Larsen, T. A., & Gujer, W. (1997). The concept of sustainable urban water management. Water Science and Technology, 35, 3–10.
- Larsen, T. A., Hoffmann, S., Lüthi, C., Truffer, B., & Maurer, M. (2016). Emerging solutions to the water challenges of an urbanizing world. Science, 352, 928–933.
- Lazarova, Z., & Spendlingwimmer, R. (2008). Treatment of yellow water by membrane separations and advanced oxidation methods. Water Science and Technology, 58, 419–426.
- Le Corre, K. S., Valsami-Jones, E., Hobbs, P., & Parsons, S. A. (2009). Phosphorus recovery from wastewater by struvite crystallization: A review. Critical Reviews in Environmental Science and Technology, 39, 433–477.
- Ledezma, P., Kuntke, P., Buisman, C. J. N., Keller, J., & Freguia, S. (2015). Source-separated urine opens golden opportunities for microbial electrochemical technologies. Trends in Biotechnology, 33, 214–220.
- Lemming, C., Bruun, S., Jensen, L. S., & Magid, J. (2017). Plant availability of phosphorus from dewatered sewage sludge, untreated incineration ashes, and other products recovered from a wastewater treatment system. Journal of Plant Nutrition and Soil Science, 180, 779–787.
- Lettinga, G., Velsen, A. F. M., van, Hobma, S. W., Zeeuw, W. J., de., & Klapwijk, A. (1980). Use of Upflox Anaerobic Sludge Blanket (UASB) reactor concept for biological wastewater especially for anaerobic treatment. Biotechnology and Bioengineering, 22, 699–734.
- Li, B., Ju, F., Cai, L., & Zhang, T. (2015). Profile and fate of bacterial pathogens in sewage treatment plants revealed by high-throughput metagenomic approach. Environmental Science & Technology, 49, 10492–10502.
- Li, R., Teng, W., Li, Y., Wang, W., Cui, R., & Yang, T. (2017). Potential recovery of phosphorus during the fluidized bed incineration of sewage sludge. Journal of Cleaner Production, 140, 964–970.
- Liberti, L., Boari, G., Petruzzelli, D., & Passino, R. (1981). Nutrient removal and recovery from wastewater by ion exchange. Water Research, 15, 337–342.
- Libra, J. A., Ro, K. S., Kammann, C., Funke, A., Berge, N. D., Neubauer, Y., … Kern, J. (2011). Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels, 2(1), 71–106.
- Licon Bernal, E. E., Maya, C., Valderrama, C., & Cortina, J. L. (2016). Valorization of ammonia concentrates from treated urban wastewater using liquid-liquid membrane contactors. Chemical Engineering Journal, 302, 641–649.
- Lienert, J., Bürki, T., & Escher, B. I. (2007). Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine In. Water Science and Technology, 56, 87–96.
- Lin, L., Li, R., Hong, Li, Y., Xu, J., & Li, X. Y. (2017). Recovery of organic carbon and phosphorus from wastewater by Fe-enhanced primary sedimentation and sludge fermentation. Process Biochemistry, 54, 135–139.
- Lind, B. B., Ban, Z., & Bydén, S. (2000). Nutrient recovery from human urine by struvite crystallization with ammonia adsorption on zeolite and wollastonite. Bioresource Technology, 73, 169–174.
- Liu, B., Giannis, A., Zhang, J., Chang, V. W. C., & Wang, J. Y. (2015). Air stripping process for ammonia recovery from source-separated urine: Modeling and optimization. Journal of Chemical Technology & Biotechnology, 90, 2208–2217.
- Liu, D., Xie, B., Dong, C., Liu, G., Hu, D., Qin, Y., … Liu, H. (2018). Effect of fertilizer prepared from human feces and straw on germination, growth and development of wheat. Acta Astronautica, 145, 76–82.
- Liu, Q., Liu, C., Zhao, L., Ma, W., Liu, H., & Ma, J. (2016). Integrated forward osmosis-membrane distillation process for human urine treatment. Water Research, 91, 45–54.
- Liu, X., Hu, Z., Zhu, C., Wen, G., Meng, X., & Lu, J. (2013). Influence of process parameters on phosphorus recovery by struvite formation from urine. Water Science and Technology, 68, 2434–2440.
- Loganathan, P., Vigneswaran, S., Kandasamy, J., & Bolan, N. S. (2014). Removal and recovery of phosphate from water using sorption. Critical Reviews in Environmental Science and Technology, 44, 847–907.
- Lu, J., Zhang, J., Zhu, Z., Zhang, Y., Zhao, Y., Li, R., … Liu, Z. (2017). Simultaneous production of biocrude oil and recovery of nutrients and metals from human feces via hydrothermal liquefaction. Energy Conversion and Management, 134, 340–346.
- Lutchmiah, K., Verliefde, A. R. D., Roest, K., Rietveld, L. C., & Cornelissen, E. R. (2014). Forward osmosis for application in wastewater treatment: A review. Water Research, 58, 179–197.
- Luther, A. K., Desloover, J., Fennell, D. E., & Rabaey, K. (2015). Electrochemically driven extraction and recovery of ammonia from human urine. Water Research, 87, 367–377.
- Magri, M. E., Philippi, L. S., & Vinnerås, B. (2013). Inactivation of pathogens in feces by desiccation and urea treatment for application in urine-diverting dry toilets. Applied and Environmental Microbiology, 79, 2156–2163.
- Manyuchi, M. M., Chiutsi, P., Mbohwa, C., Muzenda, E., & Mutusva, T. (2018). Bio ethanol from sewage sludge: A bio fuel alternative. South African Journal of Chemical Engineering, 25, 123–127.
- Marti, N., Ferrer, J., Seco, A., & Bouzas, A. (2008). Optimisation of sludge line management to enhance phosphorus recovery in WWTP. Water Research, 42, 4609–4618.
- Maurer, M., Pronk, W., & Larsen, T. A. (2006). Treatment processes for source-separated urine. Water Research, 40, 3151–3166.
- Maurer, M., Schwegler, P., & Larsen, T. A. (2003). Nutrients in urine: Energetic aspects of removal and recovery. Water Science and Technology, 48(1), 37–46.
- McBride, M. B. (2003). Toxic metals in sewage sludge-amended soils: Has promotion of beneficial use discounted the risks? Advances in Environmental Research, 8(1), 5–19.
- McConville, J., Drangert, J.-O., Tidåker, P., Neset, T.-S., Rauch, S., Strid, I., & Tonderski, K. (2015). Closing the food loops: Guidelines and criteria for improving nutrient management. Sustainability: Science, Practice, & Policy, 11, 33–43.
- Mcgarry, M. G., Tongkasame, C., & Mcgarry, A. G. (1971). Water reclamation and algae harvesting. Journal of the Water Pollution Control Federation, 43, 824–835.
- Mehta, C. M., Khunjar, W. O., Nguyen, V., Tait, S., & Batstone, D. J. (2015). Technologies to recover nutrients from waste streams: A critical review. Critical Reviews in Environmental Science Technology, 45, 385–427.
- Melia, P. M., Cundy, A. B., Sohi, S. P., Hooda, P. S., & Busquets, R. (2017). Trends in the recovery of phosphorus in bioavailable forms from wastewater. Chemosphere, 186, 381–395.
- Mininni, G., Blanch, A. R., Lucena, F., & Berselli, S. (2015). EU policy on sewage sludge utilization and perspectives on new approaches of sludge management. Environmental Science and Pollution Research International, 22, 7361–7374.
- Modin, O., Persson, F., Wilén, B. M., & Hermansson, M. (2016). Nonoxidative removal of organics in the activated sludge process. Critical Reviews in Environmental Science and Technology, 46, 635–672.
- Monfet, E., Aubry, G., & Ramirez, A. A. (2017). Nutrient removal and recovery from digestate: A review of the technology. Biofuels, 7269, 1–16.
- Morales, N., Boehler, M. A., Buettner, S., Liebi, C., & Siegrist, H. (2013). Recovery of N and P from urine by struvite precipitation followed by combined stripping with digester sludge liquid at full scale. Water, 5, 1262–1278.
- Morgan-Sagastume, F., Valentino, F., Hjort, M., Cirne, D., Karabegovic, L., Gerardin, F., … Alexandersson, T. (2014). Polyhydroxyalkanoate (PHA) production from sludge and municipal wastewater treatment. Water Science and Technology, 69(1), 177–184.
- Mulbry, W., Westhead, E. K., Pizarro, C., & Sikora, L. (2005). Recycling of manure nutrients: Use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresource Technology, 96, 451–458.
- Muster, T. H., Douglas, G. B., Sherman, N., Seeber, A., Wright, N., & Güzükara, Y. (2013). Towards effective phosphorus recycling from wastewater: Quantity and quality. Chemosphere, 91, 676–684.
- Nakao, S., Nishio, T., & Kanjo, Y. (2017). Simultaneous recovery of phosphorus and potassium as magnesium potassium phosphate from synthetic sewage sludge effluent. Environmental Technology , 38, 2416–2426.
- Nakhli, S. A. A., Delkash, M., Bakhshayesh, B. E., & Kazemian, H. (2017). Application of zeolites for sustainable agriculture: A review on water and nutrient retention water, air, & soil pollution. Water Air and Soil Pollution, 228, 464.
- Nam, S.-W., Choi, D.-J., Kim, S.-K., Her, N., & Zoh, K.-D. (2014). Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. Journal of Hazardous Materials, 270, 144–152.
- Nancharaiah, Y. V., Venkata Mohan, S., & Lens, P. N. L. (2016). Recent advances in nutrient removal and recovery in biological and bioelectrochemical systems. Bioresource Technology, 215, 173–185.
- Naoum, C., Fatta, D., Haralambous, K. J., & Loizidou, M. (2001). Removal of heavy metals from sewage sludge by acid treatment. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 36, 873–881.
- Nesme, T., Metson, G. S., & Bennett, E. M. (2018). Global phosphorus flows through agricultural trade. Global Environmental Change, 50, 133–141.
- Niewersch, C., Koh, C. N., Wintgens, T., Melin, T., Schaum, C., & Cornel, P. (2008). Potentials of using nanofiltration to recover phosphorus from sewage sludge. Water Science and Technology, 57, 707–714.
- Nigussie, A., Kuyper, T. W., Bruun, S., & de Neergaard, A. (2016). Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. Journal of Cleaner Production, 139, 429–439.
- Nowak, B., Wegerer, H., Aschenbrenner, P., Rechberger, H., & Winter, F. (2012). Sewage sludge ash to phosphate fertilizer by chlorination and thermal treatment: Residence time requirements for heavy metal removal. Environmental Technology, 33, 2375–2381.
- O’Neal, J. A., & Boyer, T. H. (2013). Phosphate recovery using hybrid anion exchange: Applications to source-separated urine and combined wastewater streams. Water Research, 47, 5003–5017.
- Öberg, G., & Mason-Renton, S. A. (2018). On the limitation of evidence-based policy: Regulatory narratives and land application of biosolids/sewage sludge in BC, Canada and Sweden. Environmental Science & Policy, 84, 88–96.
- Ohura, S., Harada, H., Biswas, B. K., Kondo, M., Ishikawa, S., Kawakita, H., … Inoue, K. (2011). Phosphorus recovery from secondary effluent and side-stream liquid in a sewage treatment plant using zirconium-loaded saponified orange waste. Journal of Material Cycles and Waste Managementement, 13, 293–297.
- Orhon, D. (2015). Evolution of the activated sludge process: the first 50 years. Journal of Chemical Technology & Biotechnology, 90, 608–640.
- Ostara. (2018). Retrieved from http://crystalgreen.com/
- Otterpohl, R., Grottker, M., & Lange, J. (1997). Sustainable water and waste managementement in urban areas. Water Science and Technology, 35, 121–133.
- Pahore, M. M., Ito, R., & Funamizu, N. (2010). Rational design of an on-site volume reduction system for source-separated urine. Environmental Technology , 31, 399–408.
- Parés Viader, R., Jensen, P. E., Ottosen, L. M., Ahrenfeldt, J., & Hauggaard-Nielsen, H. (2017). Sequential electrodialytic recovery of phosphorus from low-temperature gasification ashes of chemically precipitated sewage sludge. Waste Management, 60, 211–218.
- Peccia, J., & Westerhoff, P. (2015). We should expect more out of our sewage sludge. Environmental Science & Technology, 49, 8271–8276.
- Perez-Garcia, O., Escalante, F. M. E., de-Bashan, L. E., & Bashan, Y. (2011). Heterotrophic cultures of microalgae: Metabolism and potential products. Water Research, 45(1), 11–36.
- Petrik, M. (1954). Utilization of night-soil, sewage, and sewage sludge in agriculture. Bulletin of the World Health Organization, 10, 207–228.
- Petzet, S., & Cornel, P. (2011). Towards a complete recycling of phosphorus in wastewater treatment-options in Germany. Water Science and Technology, 64(1), 29–35.
- Pittmann, T., & Steinmetz, H. (2017). Polyhydroxyalkanoate production on waste water treatment plants: process scheme, operating conditions and potential analysis for German and European Municipal Waste Water Treatment Plants. Bioengineering, 4, 54.
- Pronk, W., Biebow, M., & Boller, M. (2006). Electrodialysis for recovering salts from a urine solution containing micropollutants. Environmental Science & Technology, 40, 2414–2420.
- Pronk, W., & Koné, D. (2009). Options for urine treatment in developing countries. Desalination, 248(1–3), 360–368.
- Pronk, W., Palmquist, H., Biebow, M., & Boller, M. (2006). Nanofiltration for the separation of pharmaceuticals from nutrients in source-separated urine. Water Research, 40, 1405–1412.
- Pronk, W., Zuleeg, S., Lienert, J., Escher, B., Koller, M., Berner, A., … Boller, M. (2007). Pilot experiments with electrodialysis and ozonation for the production of a fertiliser from urine. Water Science and Technology, 56, 219–227.
- Puyol, D., Batstone, D. J., Hülsen, T., Astals, S., Peces, M., & Krömer, J. O. (2017). Resource recovery from wastewater by biological technologies: Opportunities, challenges, and prospects. Front. Microbiol, 7, 1–23.
- Rahman, M. M., Salleh, M. A. M., Rashid, U., Ahsan, A., Hossain, M. M., & Ra, C. S. (2014). Production of slow release crystal fertilizer from wastewaters through struvite crystallization – A review. Arabian Journal of Chemistry, 7(1), 139–155.
- Rana, S., Biswas, J. K., Rinklebe, J., Meers, E., & Bolan, N. (2017). Harnessing fertilizer potential of human urine in a mesocosm system: a novel test case for linking the loop between sanitation and aquaculture. Environmental Geochemistry and Health, 39, 1545–1561.
- Randall, D. G., Krähenbühl, M., Köpping, I., Larsen, T. A., & Udert, K. M. (2016). A novel approach for stabilizing fresh urine by calcium hydroxide addition. Water Research, 95, 361–369.
- Renner, R. (2000). Sewage sludge – Pros & cons. Environmental Science & Technology, 34, 431A–435A.
- Renner, R. (2008). Pipe scales release hazardous metals into drinking water. Environmental Science & Technology, 42, 4241.
- Ridder, D. J., De, Verberk, J. Q. J. C., Heijman, S. G. J., Amy, G. L., & Van Dijk, J. C. (2012). Zeolites for nitrosamine and pharmaceutical removal from demineralised and surface water: Mechanisms and efficacy. Separation and Purification Technology, 89, 71–77.
- Rittmann, B. E., Mayer, B., Westerhoff, P., & Edwards, M. (2011). Capturing the lost phosphorus. Chemosphere, 84, 846–853.
- Rockefeller, A. A. (1998). Civilization and sludge: Notes on the history of the management of human excreta. Capitalism Nature Socialism, 9, 3–18.
- Rodríguez Arredondo, M., Kuntke, P., Jeremiasse, A. W., Sleutels, T. H. J. A., Buisman, C. J. N., & ter Heijne, A., (2015). Bioelectrochemical systems for nitrogen removal and recovery from wastewater. Environmental Science: Water Research & Technology, 1(1), 22–33.
- Römer, W. (2006). Vergleichende untersuchungen zur pflanzenverfügbarkeit von phosphat aus verschiedenen P-recycling-produkten im keimpflanzenversuch. Journal of Plant Nutrition and Soil Science, 169, 826–832.
- Rong, L., Maneerung, T., Ng, J. C., Neoh, K. G., Bay, B. H., Tong, Y. W., … Wang, C. H. (2015). Co-gasification of sewage sludge and woody biomass in a fixed-bed downdraft gasifier: Toxicity assessment of solid residues. Waste Management, 36, 241–255.
- Ronteltap, M., Maurer, M., & Gujer, W. (2007a). Struvite precipitation thermodynamics in source-separated urine. Water Research, 41, 977–984.
- Ronteltap, M., Maurer, M., & Gujer, W. (2007b). The behaviour of pharmaceuticals and heavy metals during struvite precipitation in urine. Water Research, 41, 1859–1868.
- Ronteltap, M., Maurer, M., Hausherr, R., & Gujer, W. (2010). Struvite precipitation from urine – Influencing factors on particle size. Water Research, 44, 2038–2046.
- Rose, C., Parker, A., Jefferson, B., & Cartmell, E. (2015). The characterization of feces and urine: A review of the literature to inform advanced treatment technology. Critical Reviews in Environmental Science and Technology, 45, 1827–1879.
- Roy, E. D. (2017). Phosphorus recovery and recycling with ecological engineering: A review. Ecological Engineering, 98, 213–227.
- Salutsky, M. L., Dunseth, M., Ries, K., & Shapiro, J. (1972). Ultimate disposal of phosphate from wastewater by recovery as fertilizer. Effluent and Water Treatment Journal, 12, 509–519.
- Sancho, I., Licon, E., Valderrama, C., Arespacochaga, N., de, López-Palau, S., & Cortina, J. L. (2017). Recovery of ammonia from domestic wastewater effluents as liquid fertilizers by integration of natural zeolites and hollow fibre membrane contactors. Science of the Total Environment, 584–585, 244–251.
- Sangare, D., Soudakoure, M., Hijikata, N., Lahmar, R., Yacouba, H., Coulibaly, L., & Funamizu, N. (2015). Toilet compost and human urine used in agriculture: Fertilizer value assessment and effect on cultivated soil properties. Environmental Technology , 36, 1291–1298.
- Santos, F. M., & Pires, J. C. M. (2018). Nutrient recovery from wastewaters by microalgae and its potential application as bio-char. Bioresource Technology, 267, 725–731.
- Sartorius, C., von Horn, J., & Tettenborn, F. (2012). Phosphorus recovery from wastewater-expert survey on present use and future potential. Water Environment Research, 84, 313–322.
- Schaum, C., Cornel, P., Jardin, N. (2007). Phosphorus recovery from sewage sludge ash–a wet chemical approach. Proceeding IWA Conf. 583–590.
- Schock, M. R., Hyland, R. N., & Welch, M. M. (2008). Occurrence of contaminant accumulation in lead pipe scales from domestic drinking-water distribution systems. Environmental Science & Technology, 42, 4285–4291.
- Schönberg, A., Samiei, K., Kern, H., & Raupenstrauch, H. (2014). Der RecoPhos-Prozess – Rückgewinnung von Phosphor aus KIärschlammasche. Österreichische Wasser- und Abfallwirtschaft, 66, 403–407.
- Schönning, C., Leeming, R., & Stenström, T. A. (2002). Faecal contamination of source-separated human urine based on the content of faecal sterols. Water Research, 36, 1965–1972.
- Schürmann, B., Everding, W., Montag, D., & Pinnekamp, J. (2012). Fate of pharmaceuticals and bacteria in stored urine during precipitation and drying of struvite. Water Science and Technology, 65, 1774–1780.
- Seghezzo, L., Zeeman, G., Lier, J. B., Van, Hamelers, H. V. M., & Lettinga, G. (1998). A review: The anaerobic treatment of sewage in UASB and EGSB reactors. Bioresource Technology, 65, 175–190.
- Senecal, J., & Vinnerås, B. (2017). Urea stabilisation and concentration for urine-diverting dry toilets: Urine dehydration in ash. Science of the Total Environment, 586, 650–657.
- Shaheen, S. M., Derbalah, A. S., & Moghanm, F. S. (2012). Removal of heavy metals from aqueous solution by zeolite in competitive sorption system. International Journal of Environmental Science and Development, 3: 362–367.
- Shepherd, J. G., Sohi, S. P., & Heal, K. V. (2016). Optimising the recovery and re-use of phosphorus from wastewater effluent for sustainable fertiliser development. Water Research, 94, 155–165.
- Shilton, A. N., Powell, N., & Guieysse, B. (2012). Plant based phosphorus recovery from wastewater via algae and macrophytes. Current Opinion in Biotechnology, 23, 884–889.
- Siegrist, H. (1996). Nitrogen removal from digester supernatant – Comparison of chemical and biological methods. Water Science and Technology, 34, 399–406.
- Simha, P., Zabaniotou, A., & Ganesapillai, M. (2018). Continuous urea-nitrogen recycling from human urine: A step towards creating a human excreta based bio-economy. Journal of Cleaner Production, 172, 4152–4162.
- Singh, R. P., & Agrawal, M. (2008). Potential benefits and risks of land application of sewage sludge. Waste Managementement, 28, 347–358.
- Sörme, L., & Lagerkvist, R. (2002). Sources of heavy metals in urban wastewater in Stockholm. Science of the Total Environment, 298(1–3), 131–145.
- Stendahl, K., & Jäfverström, S. (2004). Recycling of sludge with the Aqua Reci process. Water Science and Technology, 49, 233–240.
- Stylianou, M. A., Kollia, D., Haralambous, K.-J., Inglezakis, V. J., Moustakas, K. G., & Loizidou, M. D. (2007). Effect of acid treatment on the removal of heavy metals from sewage sludge. Desalination, 215(1–3), 73–81.
- Sukačová, K., & Červený, J. (2017). Can algal biotechnology bring effective solution for closing the phosphorus cycle? Use of algae for nutrient removal: Review of past trends and future perspectives in the context of nutrient recovery. European Journal of Environmental Sciences, 7(1), 63–72.
- Sun, F. Y., Dong, W. Y., Shao, M. F., Li, J., & Peng, L. Y. (2012). Stabilization of source-separated urine by biological nitrification process: Treatment performance and nitrite accumulation. Water Science and Technology, 66, 1491–1497.
- Suzuki, Y., Kondo, T., Nakagawa, K., Tsuneda, S., Hirata, A., Shimizu, Y., & Inamori, Y. (2006). Evaluation of sludge reduction and phosphorus recovery efficiencies in a new advanced wastewater treatment system using denitrifying polyphosphate accumulating organisms. Water Science and Technology, 53, 107–113.
- Takaya, C. A., Fletcher, L. A., Singh, S., Anyikude, K. U., & Ross, A. B. (2016). Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere, 145, 518–527.
- Talboys, P. J., Heppell, J., Roose, T., Healey, J. R., Jones, D. L., & Withers, P. J. A. (2016). Struvite: a slow-release fertiliser for sustainable phosphorus management? Plant and Soil, 401(1–2), 109–123.
- Tarpeh, W. A., Udert, K. M., & Nelson, K. L. (2017). Comparing ion exchange adsorbents for nitrogen recovery from source-separated urine. Environmental Science & Technology, 51, 2373–2381.
- Tervahauta, T., Rani, S., Hernández Leal, L., Buisman, C. J. N., & Zeeman, G. (2014). Black water sludge reuse in agriculture: Are heavy metals a problem?. Journal of Hazardous Materials, 274, 229–236.
- Tice, R. C., & Kim, Y. (2014). Energy efficient reconcentration of diluted human urine using ion exchange membranes in bioelectrochemical systems. Water Research, 64, 61–72.
- Tilley, E., Atwater, J., & Mavinic, D. (2008). Effects of storage on phosphorous recovery from urine. Environmental Technology , 29, 807–816.
- Tong, Y., Mayer, B. K., & McNamara, P. J. (2016). Triclosan adsorption using wastewater biosolids-derived biochar. Environmental Science: Water Research & Technology, 2, 761–768.
- Toufiq Reza, M., Freitas, A., Yang, X., Hiibel, S., Lin, H., & Coronella, C. J. (2016). Hydrothermal Carbonization (HTC) of cow manure: Carbon and nitrogen distributions in HTC Products. Environmental Progress & Sustainable Energy, 35, 1002–1011.
- Trazzi, P. A., Leahy, J. J., Hayes, M. H. B., & Kwapinski, W. (2016). Adsorption and desorption of phosphate on biochars. Journal of Environmental Chemical Engineering, 4(1), 37–46.
- Triger, A., Pic, J. S., & Cabassud, C. (2012). Determination of struvite crystallization mechanisms in urine using turbidity measurement. Water Research, 46, 6084–6094.
- Trimmer, J. T., Cusick, R. D., & Guest, J. S. (2017). Amplifying progress toward multiple development goals through resource recovery from sanitation. Environmental Science & Technology, 51, 10765–10776.
- Tsai, W. T., Hsien, K. J., Hsu, H. C., Lin, C. M., Lin, K. Y., & Chiu, C. H. (2008). Utilization of ground eggshell waste as an adsorbent for the removal of dyes from aqueous solution. Bioresource Technology, 99, 1623–1629.
- Tun, L. L., Jeong, D., Jeong, S., Cho, K., Lee, S., & Bae, H. (2016). Dewatering of source-separated human urine for nitrogen recovery by membrane distillation. Journal of Membrane Science, 512, 13–20.
- Udert, K. M., Larsen, T. A., Biebow, M., & Gujer, W. (2003). Urea hydrolysis and precipitation dynamics in a urine-collecting system. Water Research, 37, 2571–2582.
- Udert, K. M., Larsen, T. A., & Gujer, W. (2003). Biologically induced precipitation in urine-collecting systems. Water Science and Technology: Water Supply, 3, 71–78.
- Udert, K. M., Larsen, T. A., & Gujer, W. (2006). Fate of major compounds in source-separated urine. Water Science and Technology, 54, 413–420.
- Udert, K. M., & Wächter, M. (2012). Complete nutrient recovery from source-separated urine by nitrification and distillation. Water Research, 46, 453–464.
- Ulén, B. (1997). Leaching of plant nutrients and heavy metals during the composting of household wastes and chemical characterization of the final product. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science, 47, 142–148.
- Usharani, B., & Vasudevan, N. (2016). Eco-friendly approach for leaching out heavy metals from sewage sludge. Journal of Chemical Ecology, 32, 507–519.
- Vaneeckhaute, C., Lebuf, V., Michels, E., Belia, E., Vanrolleghem, P. A., Tack, F. M. G., & Meers, E. (2017). Nutrient recovery from digestate: Systematic technology review and product classification. Waste and Biomass Valorization, 8(1), 21–40.
- Vasconcelos Fernandes, T., Shrestha, R., Sui, Y., Papini, G., Zeeman, G., Vet, L. E. M., … Lamers, P. (2015). Closing domestic nutrient cycles using microalgae. Environmental Science & Technology, 49, 12450–12456.
- Vasenko, L., & Qu, H. (2017). Effect of NH4-N/P and Ca/P molar ratios on the reactive crystallization of calcium phosphates for phosphorus recovery from wastewater. Journal of Crystal Growth., 459, 61–66.
- Vassilev, S. V., Baxter, D., Andersen, L. K., & Vassileva, C. G. (2010). An overview of the chemical composition of biomass. Fuel, 89, 913–933.
- Verstraete, W., Clauwaert, P., & Vlaeminck, S. E. (2016). Used water and nutrients: Recovery perspectives in a “panta rhei” context. Bioresource Technology, 215, 199–208.
- Wan, C., Ding, S., Zhang, C., Tan, X., Zou, W., Liu, X., & Yang, X. (2017). Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application. Separation and Purification Technology, 180, 1–12.
- Wang, S., & Peng, Y. (2010). Natural zeolites as effective adsorbents in water and wastewater treatment. Chemical Engineering Journal, 156(1), 11–24.
- Wang, Y., Liu, J., Kang, D., Wu, C., & Wu, Y. (2017). Removal of pharmaceuticals and personal care products from wastewater using algae-based technologies: A review. Reviews in Environmental Science and Bio/Technology, 16, 717–735.
- Wei, S. P., Rossum, F., Van, Jan, G., Pol, V., De., & Winkler, M. H. (2018). Recovery of phosphorus and nitrogen from human urine by struvite precipitation, air stripping and acid scrubbing: A pilot study. Chemosphere, 212, 1030–1037.
- Wells, M. L., Potin, P., Craigie, J. S., Raven, J. A., Merchant, S. S., Helliwell, K. E., … Brawley, S. H. (2017). Algae as nutritional and functional food sources: revisiting our understanding. Journal of Applied Phycology, 29, 949–982.
- Wen, Q., Tutuka, C., Keegan, A., & Jin, B. (2009). Fate of pathogenic microorganisms and indicators in secondary activated sludge wastewater treatment plants. Journal of Environmental Management, 90, 1442–1447.
- Wielemaker, R. C., Weijma, J., & Zeeman, G. (2018). Harvest to harvest: Recovering nutrients with New Sanitation systems for reuse in Urban Agriculture. Resources, Conservation and Recycling, 128, 426–437.
- Wijekoon, K. C., Hai, F. I., Kang, J., Price, W. E., Cath, T. Y., & Nghiem, L. D. (2014). Rejection and fate of trace organic compounds (TrOCs) during membrane distillation. Journal of Membrane Science, 453, 636–642.
- Wilfert, P., Kumar, P. S., Korving, L., Witkamp, G. J., & Van Loosdrecht, M. C. M. (2015). The Relevance of Phosphorus and Iron Chemistry to the Recovery of Phosphorus from Wastewater: A Review. Environmental Science & Technology, 49, 9400–9414.
- Wilsenach, J. A., Maurer, M., Larsen, T. A., & van Loosdrecht, M. C. M. (2003). From waste treatment to integrated resource management. Water Science and Technology, 48(1), 1–9.
- Wilsenach, J. A., Schuurbiers, C. A. H., & van Loosdrecht, M. C. M. (2007). Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Research, 41, 458–466.
- Wilt, A., de, Butkovskyi, A., Tuantet, K., Leal, L. H., Fernandes, T. V., Langenhoff, A., … Eeman, G. (2016). Micropollutant removal in an algal treatment system fed with source separated wastewater streams. Journal of Hazardous Materials, 304, 84–92.
- Winker, M., Vinnerås, B., Muskolus, A., Arnold, U., & Clemens, J. (2009). Bioresource Technology Fertiliser products from new sanitation systems: Their potential values and risks. Bioresource Technology, 100, 4090–4096.
- Wu, X., & Modin, O. (2013). Ammonium recovery from reject water combined with hydrogen production in a bioelectrochemical reactor. Bioresource Technology, 146, 530–536.
- Xie, M., Shon, H. K., Gray, S. R., & Elimelech, M. (2016). Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Research, 89, 210–221.
- Xu, H., He, P., Gu, W., Wang, G., & Shao, L. (2012). Recovery of phosphorus as struvite from sewage sludge ash. Journal of Environmental Sciences (China)), 24, 1533–1538.
- Xu, K., Li, J., Zheng, M., Zhang, C., Xie, T., & Wang, C. (2015). The precipitation of magnesium potassium phosphate hexahydrate for P and K recovery from synthetic urine. Water Research, 80, 71–79.
- Xu, K., Wang, C., Wang, X., & Qian, Y. (2012). Laboratory experiments on simultaneous removal of K and P from synthetic and real urine for nutrient recycle by crystallization of magnesium-potassium-phosphate-hexahydrate in a draft tube and baffle reactor. Chemosphere, 88, 219–223.
- Xu, Y., Wang, J., Xu, Z., & Xu, H. (2001). A study on the PP hollow fiber membrane contactor and its performances for removing ammonia from wastewater or mixed gas: I. Removal and recovery of ammonia from wastewater. Water Science and Technology: Water Supply, 1, 185–194.
- Yang, L., Giannis, A., Chang, V. W. C., Liu, B., Zhang, J., & Wang, J. Y. (2015). Application of hydroponic systems for the treatment of source-separated human urine. Ecological Engineering, 81, 182–191.
- Yao, C., Pan, Y., Lu, H., Wu, P., Meng, Y., Cao, X., & Xue, S. (2016). Utilization of recovered nitrogen from hydrothermal carbonization process by Arthrospira platensis. Bioresource Technology, 212, 26–34.
- Yermán, L., Cormier, D., Fabris, I., Carrascal, J., Torero, J. L., Gerhard, J. I., & Cheng, Y. L. (2017). Potential bio-oil production from smouldering combustion of faeces. Waste and Biomass Valorization, 8, 329–338.
- Yermán, L., Hadden, R. M., Carrascal, J., Fabris, I., Cormier, D., Torero, J. L., … Cheng, Y. L. (2015). Smouldering combustion as a treatment technology for faeces: Exploring the parameter space. Fuel, 147, 108–116.
- Yoshizaki, S., & Tomida, T. (2000). Principle and process of heavy metal removal from sewage sludge. Environmental Science & Technology, 34, 1572–1575.
- You, X., Valderrama, C., Querol, X., & Cortina, J. L. (2017). Recovery of ammonium by powder synthetic zeolites from wastewater effluents: Optimization of the regeneration step. Water, Air, & Soil Pollution, 228, 396.
- Yuan, M. H., Chen, Y. H., Tsai, J. Y., & Chang, C. Y. (2016). Ammonia removal from ammonia-rich wastewater by air stripping using a rotating packed bed. Process Safety and Environmental Protection, 102, 777–785.
- Zeraatkar, A. K., Ahmadzadeh, H., Talebi, A. F., Moheimani, N. R., & McHenry, M. P. (2016). Potential use of algae for heavy metal bioremediation, a critical review. Journal of Environmental Management, 181, 817–831.
- Zhang, J., She, Q., Chang, V., Tang, C., & Webster, R. (2014). Mining nutrients (N, P, K) from urban source seprated urine by forward osmosis. Environmental Science & Technology, 48, 3386–3394.
- Zhang, L., & Liu, S. (2014). Investigation of organic compounds migration from polymeric pipes into drinking water under long retention times. Procedia Engineering, 70, 1753–1761.
- Zhang, Y., Desmidt, E., Looveren, A., Van Pinoy, L., Meesschaert, B.,. & Van Der Bruggen, B. (2013). Phosphate separation and recovery from wastewater by novel electrodialysis. Environmental Science & Technology, 47, 5888–5895.
- Zhang, Y., Li, Z., & Mahmood, I. B. (2015). Effects of corn cob produced biochars on urea recovery from human urine and their application as soil conditioners. CLEAN – Soil, Air, Water, 43, 1167–1173.
- Zhou, X., Li, Y., Li, Z., Xi, Y., Nazim Uddin, S. M., & Zhang, Y. (2017). Investigation on microbial inactivation and urea decomposition in human urine during thermal storage. Journal of Water Sanitation and Hygiene for Development, 7, 378–386.
- Zhou, X., Qu, Y., Kim, B. H., Du, Y., Wang, H., Li, H., … Feng, Y. (2015). Simultaneous current generation and ammonia recovery from real urine using nitrogen-purged bioelectrochemical systems. RSC Advances, 5, 70371–70378.
- Zorpas, A. A., Constantinides, T., Vlyssides, A. G., Haralambous, I., & Loizidou, M. (2000). Heavy metal uptake by natural zeolite and metals partitioning in sewage sludge compost. Bioresource Technology, 72, 113–119.