 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Diarrheal illnesses claim the lives of hundreds of thousands of children each year, most of whom live in rural and low-income communities. Ceramic Water Filters (CWF) are widely regarded as one water treatment technology with the potential to increase access to safe drinking water. While physical filtration mechanisms are a key contributor to improving the water safety, silver is commonly added to improve disinfection performance. Therefore, a thorough review of silver disinfection efficacy and disinfection mechanisms in relation to CWFs are critically important. This paper reviews filter mechanisms and efficacy for bacteria removal for cases with and without silver addition. Method of silver application (dipping, painting, or co-firing) is assessed. Silver release and retention is discussed. The findings from this paper illustrate that eluted silver contributes to filter bacterial disinfection. However, more research is needed on the impact of silver on preventing a “slime layer” on the filter surface and receptacle. Silver application method, water quality and particle characteristics were demonstrated to impact release. For instance, co-firing results in the most consistent elution over time but at lower concentrations than other methods. Finally, research into alternative metals to silver for enhanced disinfection present emerging opportunities within the CWF field.
Graphical Abstract
Keywords:
1. Introduction
Access to safe water is not a universal challenge, but is concentrated among the world’s poorest and most vulnerable who are often disadvantaged by compounding economic, social, geographic, ethnic and gender-based marginalization (UNESCO, Citation2015). Low-income communities are thus disproportionately burdened by water-related illness and child mortality rates. Furthermore, water-related illnesses can lead to a feedback loop where regular and/or chronic diarrhea can cause malnutrition and child stunting, which causes more diarrhea and hampers the child’s long term development, subsequently impairing socioeconomic development within that community (Brown, Citation2003; Heltberg, Citation2009). Therefore, as diarrhea is typically caused by a viral, parasitic or bacterial infection in the intestinal tract, which is spread through contact with food or water that has been contaminated by fecal matter (World Health Organization, Citation2017), increasing access to safe water is integral to mitigating this pervasive challenge (World Health Organization; UNICEF, Citation2014).
Methods of addressing such needs require alternative approaches than are typical in Western Nations though. Specifically, centralized treatment and distribution infrastructure have practical and economic challenges associated with short-term capital investment and long-term maintenance costs, as well as geographic limitations in the rural environment where safe water access is most limited. Therefore, implementation and use of decentralized, point-of-use water treatment solutions (POUWTS) to increase access to improved drinking water is in many cases, a more practical treatment option (Sobsey et al., Citation2008; World Health Organization; UNICEF, Citation2014).
Many POUWTS, such as biosand filters, chemical disinfectants, natural and chemical coagulants, solar disinfection technologies (SoDIS), and others exist (see Murphy et al., Citation2009; Pagsuyoin et al., Citation2015; Santos et al., Citation2016), however this article is focused on ceramic water filters (CWF). Importantly, the objective of the work presented herein is not to proclaim the value of this technology over the others, as each possesses benefits and drawbacks which make them appropriate for different contexts. Rather, the intention is to review the literature for the CWF, for which there were approximately 700,000 CWFs estimated to be in use in 2014, serving 4 million people (van der Laan et al., Citation2014). This review is therefore intended to facilitate and improve upon CWF applications to ensure its long-term robustness and sustainable use.
Ceramic Water Filters, which utilize a filtration mechanism to treat the water that passes through them, are a widely recommended POUWTS for implementation in Low- and Low-Middle Income contexts (World Health Organization; UNICEF, Citation2017). CWFs are easily manufacturable in the countries of intended use, as they are comprised primarily of a mixture of clay, firing material (FM; most commonly sawdust, rice husks or flour), water and often, a silver additive. The silver additive is the only component which may need to be imported. The wet mixture is typically pressed into a mold to form a pot shape, and after air drying, is heated to a high temperature in a kiln. During the heating process, the firing material burns away and leaves a porous network through which water may pass and subsequently be treated by size exclusion (Rayner, Citation2009; van Halem et al., Citation2009).
The Ceramics Manufacturing Working Group (CMWG), a long-standing supporter of, and respected authority on CWFs, highlights silver as a key feature of the technology for the purpose of improving microbial disinfection performance and inhibiting bacterial growth along the interior filter barrier (Ceramics Manufacturing Working Group, Citation2010). Due, in part, to silver’s high cost of approximately $3 USD per gram (Argenol Laboratories, Citation2017), CWFs have been notably more expensive than the financial means of its target market (Burt et al., Citation2017; Francis, Citation2015; Luoto et al., Citation2012), indicating a thorough understanding of its disinfection contribution within a CWF context, as well as its mode of action, is imperative for the field.
This review paper focuses on the impact of silver as an additive to CWFs to highlight areas of interest for CWF manufacturers and researchers. The paper examines filter material ratio and particle size and discusses firing temperatures. In all areas, the paper attempts to highlight where further research is also needed. In particular, the paper addresses the state of understanding regarding microbial removal resulting from silver introduction into contaminated water, silver elution from a CWF into a filtrate receptacle during use, and how water quality influences both of those processes. A final section notes alternative emerging metal technologies with potential to progress the field.
2. Methods
Searches on SCOPUS and Web of Science were conducted independently for the three core themes reviewed in this article: Ceramic Water Filters, Silver Disinfection and Non-Silver Disinfection. For Ceramic Water Filters, the search string was (“Ceramic Filt*” OR “Ceramic Water Filt*” OR “CWF”). For Silver Disinfection, the search string was (Silver AND (disinfect* OR Bacteri*) AND Nanoparticle AND Water). For Non-Silver Disinfection, the string was (“Nanoparticle” AND (Disinfect* OR Bacteri*) AND Water). The results procured a total 1227, 3187 and 8068 documents, respectively, from the two databases combined; upon review the list was narrowed to the 134 references in this paper. These searches were originally conducted in Spring 2019, meaning articles published after this time were excluded. Some later papers were, however, included later in the writing process when authors felt appropriate. Moreover, titles were then reviewed for relevance, as per the exclusion criteria for each category shown in . Any paper written in a language other than English was excluded from all categories, and duplicate papers were ignored during this review process.
Table 1. Exclusion criteria for review.
3. Microbial removal mechanisms
As the primary aim of a POUWTS is to reduce the prevalence and impacts of diarrheal illnesses, the microbial removal efficiency of a CWF is of utmost importance. The removal of pathogenic microorganisms is mostly facilitated through filtration, which can be defined as “any process for the removal of solid particles from a suspension by passage of the suspension through a porous medium” (Crittenden, Citation2012a). Furthermore, it is well established that size-exclusion plays a fundamental role in CWF effectiveness (Bielefeldt et al., Citation2009; Clark & Elmore, Citation2011; Panasewicz, Citation2011).
CMWG and other CWF manufacturing authorities also suggest silver significantly improves filter microbe removal efficacy (Innovation for Development and South-South Cooperation, Citationn.d.; Murray et al., Citation2011; Ceramics Manufacturing Working Group, Citation2010; van Halem et al., Citation2009). However, as will be demonstrated, the literature supporting the efficacy of the silver addition in both field and laboratory studies is quite variable and indicates that the understanding of how the silver acts with regards to disinfection requires more attention (Bielefeldt et al., Citation2009; Brown, Citation2007; Kowalski, Citation2008; Lucier et al., Citation2017). For instance, van der Laan et al. (Citation2014) conducted a study to examine bacterial and virus removal from filters painted with colloidal silver, as well as ones without any added metals. The study results found that there was no statistically significant difference between filters with and without silver, as they yielded LRVs between 0.6 and 2.5 for filters with no silver and between 0.5 and 3.1 for filters with silver, when evaluating E. coli. The study also found LRVs between 0.2 and 1 for filters without silver and between 0 and 1.4 for filters with silver, when evaluating MS2 Bacteriophages (van der Laan et al., Citation2014). The removals were thus associated with the physical removal mechanisms of the CWF itself versus any disinfection action from the silver. An earlier study by Lantagne et al. (Citation2010) also found that when silver was painted on the CWF surface, coliform removals were similar to filters with no silver addition (Lantagne et al., Citation2010). Finally, Rayner et al. (Citation2013) even found both significant and negligible changes to CWF microbe removal between disk filters with no silver, with 0.003 mg/g of silver, and 0.3 mg/g, depending on the clay and firing materials (Rayner et al., Citation2013). A comprehensive discussion of CWF microbe removal mechanisms may therefore assist in understanding why such discrepancies in data are being observed (see Section 3.2). This section reviews literature on CWF microbial removal behavior when absent of silver, followed by silver disinfection mechanisms and water quality impacts when acting independently in an aqueous environment.
3.1. Physical microbe removal
Size exclusion is the dominant mechanism for bacterial removal in a CWF, meaning filter performance is closely tied with pore size and porosity (Rayner et al., Citation2017). Therefore, differences in the material parameters that lead to filter pore size and porosity also greatly impact filter performance. This section breaks down the bacterial removal in CWF relying on physical removal alone by examining the most commonly discussed CWF characteristics that impact microbe removal: material ratios, firing material particle size, filter thickness and final firing temperature.
From and , one may see that pots without silver addition have LRVs ranging from 0.4 to 8.17 (n = 25), pots with silver have LRVs ranging from 0.5 to 6.7 (n = 12), disks without silver have LRVs ranging from 0.3 to 5.9 (n = 36) and disks with silver have LRVs ranging from 2.2 to 6.3 (n = 4). Thus, from a broad overview perspective it appears as though there is no significant difference between filters with and without silver. Disk filters also appear to act as representative samples for lab-scale experimentation. And finally, it appears that the variety of input material parameters used across the literature may create the observed ranges in filter performance; this concept is examined further in Sections 3.1.1 to 3.1.4.
Table 2. Summary of CWF pot parameters and performance outcomes.
Table 3. Summary of CWF disk parameters and performance outcomes.
3.1.1. Filtration theory
CWFs operate through a combination of media layer surface removal (dominant) and depth filtration (secondary). Specifically, the majority of contaminants/microbes within the influent water are removed along the filter surface, as the majority are larger than the pore matrix throughout the ceramic filter body. However, removal also occurs through a combination of straining within the randomly oriented pathways throughout the filter matrix, and by getting caught within small “dead-end” pores. Thus, contaminants, are either blocked entry into the CWF body, or if able to enter, adhere to the ceramic pore walls or get trapped within pores that do not connect to the outer filter surface.
As contaminants are removed at the filter surface or trapped within the filter body, the water flowrate through the filter declines over time, while the contaminant removal increases. How much change is observed thus acts as a proxy for measuring how much of the contaminant load has been removed. Van Halem et al. (Citation2017) demonstrated that in high flowrate filters without silver (approx. 12–20 L/h initially), in both falling head filtration and continuous loading experiments, initial E. coli LRV values increased over filtration time as the filter became clogged with contaminants; specifically at initial water collection volumes of 60–85 L E. coli LRV was <1, while at filter volumes of 240–320 L for the falling head filtration experiments and >1700 L for the continuous loading experiments, the E. coli LRV improved to approximately 2 in all cases. It was further noted that flowrates dropped upwards of 78% over the duration of filtered water collection, even with the inner surface of the filter having been scrubbed 5 times intermittently (van Halem et al., Citation2017). These results together illustrate that the vast majority of contaminants are either captured on the filter surface or trapped within the filter matrix itself and accumulate over time. The functioning of a CWF may thereby be defined by the physical properties and component parts that dictate water’s ability to pass through the filter matrix.
Filter size influences flowrate and antibacterial performance by governing hydraulic head, and thus the pressure exerted by the water on the filters. Flow conditions through the pores, as well as the resulting shear force between the water and the pores, are therefore functions of the filter height, and by extension, size (Annan et al., Citation2014; Schweitzer et al., Citation2013), among other parameters. CWF shape, however, has shown no significant impact on filter performance, as observed when comparing rounded- and frustum-bottomed filters, the most commonly utilized designs (Schweitzer et al., Citation2013). Clay minerology influences CWF performance by both dictating the proclivity of bacteria to attach to pore walls (Asadishad et al., Citation2013; Unuabonah et al., Citation2018), and by governing the amount of shrinkage experienced during the firing process (Oyanedel-Craver & Smith, Citation2008). Further discussion of these parameters is, however, excluded from this article, as the impacts of optimizing it are believed to be superseded by the importance of using local materials/resources to the sustainability of the product (Ceramics Manufacturing Working Group, Citation2010).
Other manufacturing parameters are, however, easier to manipulate, and have been shown to closely relate with pore size and porosity (Rayner et al., Citation2017; Yakub et al., Citation2013). Specifically, the most commonly discussed CWF characteristics that impact microbe removal are material ratios, firing material particle size, filter thickness and final firing temperature, which are further discussed in this section.
3.1.2. Impact of material ratios
CWFs (without silver) are fabricated by first creating a homogeneous clay-FM mixture, where the clay is the ingredient which allows for the filter to be molded and subsequently maintain its shape after firing, and the FM burns away during the firing process to create a porous network throughout the clay matrix. Therefore, the ratio of these ingredients theoretically influences both bacterial removal and flowrate, as with more firing material added to the mixture, the more space is available for water, and consequently, bacteria to pass. A reduction in removal and an increase in filtration rate are thus anticipated. However, as may be observed in and , there is significant variability in the data within the literature, raising uncertainty in such theory.
Yakub et al. (Citation2013) tested filter pots with the same sawdust particle size and material ratios of 35% and 50% (by volume). The study found that permeability increased, and tortuosity decreased as the percentage of sawdust content was increased, which corresponded to average Log Removal Values (LRV) of 6.36 ± 0.54 and 5.67 ± 2.50 for filters with 35% and 50% sawdust, respectively (Yakub et al., Citation2013). The high level of variance for the filter with 50% sawdust, however, indicates that filters with this material ratio were more heterogeneous, making the data difficult to compare.
Rayner et al (Citation2017) conducted a comprehensive study on the impacts of firing material ratios, and their data was also inconclusive as to its impacts on bacteria removal. For example, disks with a sawdust particle size range of 0.250 mm – 0.595 mm and material ratios of 13.7%, 17% and 24% sawdust (by weight) yielded average LRV of 2.06 ± 1.33, 4.00 ± 0.285 and 2.78 ± 0.156 which shows no trend (Rayner et al., Citation2017). Similarly variable results are found when the researchers tested filters with material ratios (by weight) of 18%, 19% and 25% milled rice husks of the same size range, yielding average LRVs of 1.93 ± 0.110, 1.26 ± 0.166 and 1.26 ± 0.097 (Rayner et al., Citation2017). These findings illustrate that material ratios, regardless of the FM, are not controlling this filter performance metric. It is also noteworthy that variability decreased in the study conducted by Rayner et al (Citation2017), which sits contrary to the findings by Yakub et al. (Citation2013). This point suggests compounding factors contribute to CWF bacteria removal by size exclusion.
In a third study, Soppe et al. (Citation2015) reported no significant differences in LRV between filters with 24% and 31% rice husks (by weight), which yielded mean LRVs of 2.1 and 2.2, respectively (Soppe et al., Citation2015). Interestingly, however, these researchers found weather influenced their results. For instance, the same filters made with 31% rice husks yielded an interquartile range for LRV of 3.6–3.9 in the dry season and 1.3–2.9 in the wet season, showing more variance and a lower average LRV during the wet season when compared with the dry. Thus, humidity or moisture content may need to be factored into the CWF production process.
Furthermore, the data presented herein suggests material ratios do not contribute to average LRV, and thus should not be considered a key parameter for this performance measure. Having said that, the same studies presented all demonstrate that there is indeed a linear relationship between material ratios and flowrate (Rayner et al., Citation2017; Soppe et al., Citation2015), however analysis of this data lies beyond the scope of this review. These findings suggest that CWF flowrate may be increased without compromising microbe removal, though more research is necessary.
3.1.3. Impact of firing material particle size
From and , only 5 of 21 studies investigated FM particle size, even though it is considered a key design parameter by leading organizations in the field (Rayner, Citation2009). As such, more research is certainly necessary in this domain to improve the field’s understanding of this input parameter’s impact.
Soppe et al. (Citation2015) found a significant decrease in LRV with increasing particle size, with a median of 2.8 ± 0.7 (with an average flow rate of 3 L/h) for filters with <1mm FM size and 0.7 ± 0.2 (with an average flowrate of 10.1 L/h) for filters with 0.5-1mm FM size (Soppe et al., Citation2015). A less controlled particle range thus led to a higher LRV because smaller particles were included within the mixture, but it yielded larger variance. Conversely, the controlled, larger particle sizes had a smaller LRV with less variance, and a larger flowrate. Such results importantly indicate smaller particles may improve bacteria removal, but also highlight the challenges with reproducibility in the field.
Servi et al. (Citation2013) also observed particle sizes of 388, 505 and 650 µm had very similar bacteria removal performances of 3.05 ± 0.8, 2.04 ± 0.5, and 2.77 ± 0.8, respectively, while a steep drop-off in LRV was observed after average size was increased to 780 µm and 925 µm, with average LRVs of 0.59 ± 0.2 and 0.83 ± 0.1, respectively (Servi et al., Citation2013). Rayner et al. (Citation2017), conversely, found that filters with 13.7% sawdust (by weight) and sawdust particle size ranges of 0.250–0.595 mm, 0.595–1.19 mm and 1.19–2.38 mm yielded average LRVs of 2.06 ± 1.33, 4.43 ± 0.402 and 1.87 ± 0.261, respectively, showing no discernable relationship between FM size and LRV (Rayner et al., Citation2017). Research from Varkey and Dlamini (Citation2012) and Scannell (Citation2016) also found no discernable trend in LRV with changing FM size (see and , respectively) (Scannell, Citation2016; Varkey & Dlamini, Citation2012), demonstrating the need for more detailed analyses into its contribution to microbe removal by size exclusion.
3.1.4. Filter thickness and firing temperature
Two other parameters that are considered to contribute to LRV are filter thickness and kiln firing temperature. Although multiple studies were found to discuss the importance of thickness as a design parameter (Ceramics Manufacturing Working Group, Citation2010; Rayner et al., Citation2017; Schweitzer et al., Citation2013; Yakub et al., Citation2013), only Servi et al. (Citation2013) investigated its impact on LRV. The researchers found that LRV increased linearly from 0 to 0.6 as filter thickness increased from 3 to 20 mm with a particle size range of 500–1000 µm, and 0 to 2.1 when made with a particle size range of 400–500 µm (Servi et al., Citation2013). The parameter interaction is logically consistent, as a thicker matrix provides more space for bacteria to be trapped within the pores, and smaller pores reduce space available for which the bacteria may pass, creating a compounding effect; a significant interaction between FM size and material ratios may also be expected as per the same reasoning, though such an investigation was not discovered in the literature.
Similarly, though many studies report the maximum firing temperature reached during filter fabrication (see and ), Soppe et al. (Citation2015) is the only study to investigate an impact of firing temperature on LRV. In their study, the researchers found the mean LRV of filters fired at 800, 885 and 950 0C were 2.3, 2.1 and 1.9, respectively, demonstrating a slight decrease with increasing temperature (Soppe et al., Citation2015). In addition, the mean pore diameters were reported to marginally increase from 27.8 µm to 28.9 µm to 30.6 µm with respective increases firing temperature (Soppe et al., Citation2015). The interquartile LRV range (1.7–2.2, 1.5–2.6 and 1.1–2.8 with respective increased firing temperature) demonstrated an increase in data variability that also makes it difficult to confirm any significant difference between filters. Further to that end, the clay is vitrified at the peak temperature of the firing process, but sawdust burns away much earlier in the firing process; the final temperature reached is thus not the best measure of firing’s influence on pore sizes, and consequently, LRV (Ceramics Manufacturing Working Group, Citation2010). Research into the firing process itself, and specifically the rate of temperature increase, is therefore needed to fully elucidate this design parameter’s influence on filter performance.
3.2. Silver disinfection
To understand the impact of silver on bactericidal/bacteriostatic effectiveness in a CWF system, one must first elucidate the mechanisms by which silver inhibits bacterial growth, or further causes cell lysis. This section reviews the disinfection mechanisms discussed in literature, followed by a discussion of the impacts that various nanoparticle and water quality characteristics have on bactericidal efficacy. Results on silver’s disinfection efficacy from literature are also presented.
Silver disinfection is theorized to occur through two possible mechanisms, namely (1) Ag - Bacterial Interaction, and (2) Reactive Oxygen Species (ROS) generation (Fauss et al., Citation2014; Le Ouay & Stellacci, Citation2015; Lemire et al., Citation2013). Both mechanisms have demonstrated toxicity effects, as well as DNA replication inhibition (Duran, Citation2016). However, the respective individual contributions or potential synergistic impacts are still under investigation.
3.2.1. Ag – bacteria interaction
One of the most commonly sourced mechanisms for silver-induced bactericide is the interaction between silver and the bacterial species, seen both in ionic (Ag+) and nanoparticle (Ag-NP) forms. This interaction is hypothesized to result from electrostatic interactions and/or ionic bonding with sulfur or phosphate containing groups on the cell wall and within the membrane, resulting in bactericidal and bacteriostatic effects (Dror-Ehre et al., Citation2009; Le Ouay & Stellacci, Citation2015). However, there is a lack of consensus on their respective roles. Please note that bactericidal effects are defined here as effects which entirely kill the bacteria cell, whereas bacteriostatic effects are those which inhibit a cells ability to replicate DNA.
3.2.1.1. Silver and cell attachment
In terms of ionic silver, studies by Yamanaka et al. (Citation2005) and others suggested that a monovalent cation like Ag+ attaches to the negatively charged cell walls of the bacterium by electrostatic attraction, which leads to cell death as per the mechanisms explained in Section 3.2.1.2 (Dror-Ehre et al., Citation2009; Stoimenov et al., Citation2002; Yamanaka et al., Citation2005). Other researchers, however, have reported negatively charged Ag-NPs attachment to the cell walls, suggesting electrostatic attraction is not necessarily the governing mechanism (Morones et al., Citation2005; Sondi & Salopek-Sondi, Citation2004; Yakub & Soboyejo, Citation2012). Rather, attachment of this nature is generally attributed to the bonding between silver nanoparticles and the sulfuric thiol groups on the cell membrane, which subsequently leads to toxicity effects or ROS generation and eventual cell death (Matsumura et al., Citation2003; Shuang et al., Citation2014). Therefore it may be understood at the current juncture that electrostatic forces may play a role in silver-bacteria attachment, however silver bonding with sulfuric groups (primarily thiols (-SH)) on the cell wall is also central to initiating cell degradation or death (Shuang et al., Citation2014).
3.2.1.2. Cell lysis
After bonding has occurred and Ag+ and/or Ag-NP accumulation develops along the cell wall, bacteriostatic and bactericidal processes ensue (Feng et al., Citation2000; Morones et al., Citation2005). Bacteriostasis results from a clustering of DNA in the center of the cell known as the electron light region, which creates stress within the cell and inhibits its replication (Feng et al., Citation2000). For E. coli, Ruparelia et al. (Citation2008) found a minimum of 40–180 µg/mL of Ag-NPs (strain dependent) was required to inhibit growth of 99.9% of cells after 24 h depending on size and morphology (Ruparelia et al., Citation2008). Bactericidal processes occur upon greater accumulation of Ag+ or Ag-NPs, which can both form pits in the cell wall, allowing for silver to infiltrate the cytoplasm and exhibit toxicity effects by bonding with DNA directly (Dror-Ehre et al., Citation2009), deactivating cellular enzymes (Choi et al., Citation2008) and releasing cytoplasm from within the cell, leading to degradation (El-Badawy, Citation2011). For complete bactericide to occur in 99.9% of E. coli cells, Ruparelia et al. (Citation2008) found a minimum concentration of 60–220 µg/mL of silver nanoparticles was required depending on the E. coli strain (Ruparelia et al., Citation2008). Wu et al. (Citation2018) found the same definitions of bacteriostasis and bactericide as outlined by Ruparelia et al. (Citation2008) for E. coli required 10–60 µg/L and 20–140 µg/L, though the nanoparticles used by these researchers were of different size and morphology than Ruparelia et al. (Citation2008), possibly explaining the observed differences (Wu et al., Citation2018).
3.2.1.3 Reactive Oxygen Species (ROS) generation
Although Ag-NP can contribute directly to bactericidal impacts, they can also react with oxygen in the water and release Reactive Oxygen species (ROS) as well as silver ions, as shown in Reaction 1 (Duran, Citation2016; Fauss et al., Citation2014; Lemire et al., Citation2013). Both silver ions and ROS also contribute to disinfection efficacy, though the relative contribution of Ag-NP, Ag + and ROS is still unclear.
[Reaction 1]
[Reaction 1]
In this case, ROS generation occurs before the nanoparticles interact with the cell, and thus ROS-based bacterial toxicity of this variety is an indirect result of silver presence. Other ROS, however, may be generated via electrochemical interactions with silver (McEvoy & Zhang, Citation2014; Slavin et al., Citation2017). For example, under photocatalytic conditions, the influx of energy from the light displaces one or more electrons from the valence band, which are subsequently pushed to the conductor band below (i.e. closer to the molecule). Resulting from this change, a positive hole (h+) is created in the valence band and a free electron (e-) in the conductor band, which makes the molecule subject to interactions with the aquatic environment as per Reactions 2–6 (McEvoy & Zhang, Citation2014; Padmavathy & Vijayaraghavan, Citation2008; Sirelkhatim et al., Citation2015):
[Reaction 2]
[Reaction 2]
[Reaction 3]
[Reaction 3]
[Reaction 4]
[Reaction 4]
[Reaction 5]
[Reaction 5]
[Reaction 6]
[Reaction 6]
Interestingly, these reactions have been shown to occur under dark conditions as well, however the instigation of the electron transfer and subsequent generation of ROS is still not well understood due to the fast rate at which the reactions occur (Fauss et al., Citation2014). Having said that, both gram-positive and gram-negative bacteria have been shown to export electrons through their membrane when in contact with metals, suggesting the nanoparticles may be reduced by electrons on the bacterial surface, as per Reaction 7 (Ehrlich, Citation2008):
[Reaction 7]
[Reaction 7]
This particle-cell reaction is believed to occur because of silver bonding with sulfuric thiol groups and phosphoric DNA, which act as electron donors to initiate the reaction series of Reactions 3–6 (Choi et al., Citation2008; Kashida, Citation2003; Matsumura et al., Citation2003). The kinetics with which silver and thiols/DNA react to form ROS has not been entirely elucidated, however Shuang et al. (Citation2014) posit that ROS result as intermediates in the process of ionic silver bonding with thiols, eventually becoming Ag2S (Shuang et al., Citation2014). Silver-thiol bonding can lead to disinfection resulting from both ROS (Shuang et al., Citation2014) and silver directly (Le Ouay & Stellacci, Citation2015), although it is still unclear which mechanism dominates (Fauss et al., Citation2014).
Furthermore, it has been observed that the small size and unstable nature of ROS allows them to penetrate the bacterial cell wall and enter the cytoplasm, accumulating inside and destroying DNA and stopping it from replicating cells from within (Choi et al., Citation2008). Carlson et al. (Citation2008) found that Ag-NPs in concentrations of 10, 25 and 50 µg/mL generated an increase in ROS concentration, measured as fluorescence intensity fold increase (unitless), from 4 to 7 to 15, respectively, after 24 h of incubation, which led to approximately 38, 65 and 80% reductions in cell viability, respectively (Carlson et al., Citation2008). Park et al. (Citation2009) even found that 40 and 60 min after AgNO3 was added to water at 0.5 mg/L, a total LRV of 1.4 and 2.2 was achieved, respectively, to which ROS contributed 78% and 77% of the removal relative to Ag+, respectively. Further, at a concentration of 1 mg/L, ROS contributed to 61% and 42% of total LRVs of 2.3 and 3.3 after 40 and 60 min, respectively. These findings suggest that concentration impacts the mechanics of disinfection (Park et al., Citation2009).
3.2.2. Impacts of water quality parameters
As is expected for any water treatment application, water quality parameters play a fundamental role in the efficacy of a given system. With CWFs in particular, researchers must be conscious of water quality to ensure the results will translate from laboratory waters into the field. This section therefore reviews the impact of pH, dissolved oxygen content (DO), charged species, and organic matter on silver disinfection.
3.2.2.1. pH Effect
The pH of water has a variety of effects on Ag-NP bactericide and/or bacteriostasis. Specifically, Ag+ and ROS generation increase as pH decreases (Fabrega et al., Citation2009; Fauss et al., Citation2014; He et al., Citation2012), which leads to a subsequent improvement in the silver’s bacterial disinfection efficacy. In terms of ion generation, Liu and Hurt (Citation2010) demonstrated that ion release results primarily from Reaction 8 (Liu & Hurt, Citation2010), which indicates that with a lower pH (4–6), greater Ag+ generation is observed than at more neutral pH (7–9) values. Specifically, as may be observed in , Ag+ release increases from approximately 0.05–0.6 mg/L when pH decreases from pH of 9 to 4 (Liu & Hurt, Citation2010). ROS was not measured within this study, and thus its rate of generation under their study conditions is unknown.
Table 4. Dissolved silver (Ag+) concentration resulting from Ag-NP conversion at various pH and dissolved oxygen levels. (Data adapted from Liu and Hurt (Citation2010)).
Further, the impact of pH on Ag+ release is hypothesized to improve bactericide as per two mechanisms. First, as the Ag-NP becomes oxidized, the oxide layer surrounding the nanoparticle becomes more soluble, thus making Ag+ more available for disinfection. Second, nanoparticles are often stabilized with negatively charged capping agents, which when undissolved, aggregate Ag+ ions. The resulting positively charged surface is consequently more attracted toward bacteria, improving disinfection (Lok et al., Citation2007).
[Reaction 8]
[Reaction 8]
At the current juncture, no studies have been found to date that detail the mechanisms behind the increase in ROS generation in the lower end of the environmental pH range (i.e. pH of 4–6). However, it is believed that this improvement results from an increase in Ag-NP oxidation, similar to the increase in Ag+ generation (Fauss et al., Citation2014). Moreover, as water chemistry, and particularly pH, inevitably differs across geographies and with changes in climate, it is important for CWF researchers and practitioners to understand how and why such changes may influence filter performance.
3.2.2.2. Dissolved oxygen content
Dissolved Oxygen (DO) has been proven to be a critical parameter in the disinfection properties of Ag-NP. , adapted from Liu and Hurt (Citation2010), shows how the researchers demonstrated that the Ag+ concentration in water was nearly ten times greater in natural water than “deoxygenated” water at a pH of 4 (Liu & Hurt, Citation2010). Further, their study found almost no Ag+ generation in “deoxygenated” water, even after 24 h of incubation (Liu & Hurt, Citation2010). Fauss et al. (Citation2014) also found that both Ag+ and ROS generation dropped to zero after their test water was deoxygenated with a nitrogen purge, further reinforcing the necessity of DO in Ag-NP bactericidal effectiveness (Fauss et al., Citation2014). The importance of oxygen in disinfection is understood from Reactions 1 and 8, where Ag-NP reacts directly with oxygen to release Ag+ and/or ROS, which attack bacteria. More oxygen in the water therefore leads to faster conversion of nanoparticles into Ag+ and/or ROS, increasing the concentration of these species and thereby improving the rate at which bacteria is killed (Xiu et al., Citation2011).
In terms of application to CWFs, these observations are important because DO concentrations will vary depending on the water source. For example, heavily fecal-contaminated surface waters or groundwaters may have very low concentrations of DO, which could lead to a reduction in CWF effectiveness or ineffectiveness in terms of silver disinfection (Shwarzenbach et al., Citation2003). DO is also known to fluctuate with temperature and at different periods of the year, meaning CWF behavior may change depending on a certain geography. To date, no specific oxygen concentration has been established as the cutoff point when silver becomes ineffective, and no CWF studies have directly investigated the impacts of oxygen on removal performance, demonstrating a need for more research.
3.2.2.3. Charged species
Research has demonstrated that divalent cations and halide ions can significantly impact silver bactericidal effectiveness, and are therefore the topics of discussion herein (Bielefeldt et al., Citation2013; Jin et al., Citation2010).
Zhang et al. (Citation2012) illustrated that water containing 1000 mg/L of divalent cations (Mg2+ or Ca2+) and 11.5 mg/L of Ag-NPs led to an ionic silver release of 12–17 µg/L, whereas water containing silver and monovalent cations (Na+) in the same concentrations saw an ionic silver release of 22–25 µg/L. These conditions correspond with disinfection performances of 72–73% and 81–82% after 20 h of incubation, respectively (Zhang et al., Citation2012). Jin et al. (Citation2010) found similar results, with the cell viability for the gram-negative bacterial species Pseudomonas Putida (p. putida), which has similar size and biological characteristics to E. coli, increasing from 45% to 90% once the cations were added to the water (Jin et al., Citation2010). These reductions in effectiveness have been attributed to the aggregation of the positive ions on the surface of Ag-NPs, increasing the hydrodynamic diameter of the nanoparticles and reducing their ability to penetrate the cell wall of the bacterium (See Section 3.1.3) (Jin et al., Citation2010; Zhang et al., Citation2012). Thus, it is important to recognize that bacterial disinfection may be reduced in groundwaters or other waters with high divalent ions concentrations.
Chloride (Cl-) is the most commonly discussed anionic species due to its high prevalence in nature and its common presence in water resulting from the use of chlorine as a disinfectant in municipal-scale water treatment facilities (Bielefeldt et al., Citation2013; Crittenden, Citation2012b). As such, Cl- may be expected in influent water for a CWF if one is used in an urban environment or receives groundwater (Crittenden, Citation2012b). Moreover, due to the high affinity of silver nanoparticles and silver ions to Cl- (pKsp = 9.75) (Jin et al., Citation2010), AgCl forms in an aqueous environment quite quickly. Choi et al. (Citation2008) found this reaction and the formation of AgCl decreased the silver’s disinfection effectiveness, as AgCl colloids inhibited E. coli growth by 66% compared with 100% by Ag+ when added to water in a concentration of 4.2 µg/L (Choi et al., Citation2008). Similar results were also exhibited by Levard et al. (Citation2013), who found molar Cl/Ag ratios of 535 resulted in solid precipitates of AgCl to form, which, after 30 h, resulted in the proportion of silver within their sample to decreased to 2% from approximately 8% when Ag-NPs were released into DI water only. Interestingly however, when the Cl/Ag ratio was increased to 2675 and further, 26750, Ag+ release exponentially increased to approximately 6.5% and 17% dissolved content, respectively. These results also correlate closely with bacteriostatic efficacy, as the authors showed complete bacterial growth inhibition after 24 h with 2 × 10-3 mol/L of Ag+, Ag-NPs and Ag-NPs added to a 0.5 M NaCl solution, and only approximately 10% and 5% inhibition when the same concentrations were added to 0.1 M and 0.01 M NaCl solutions, respectively (Levard et al., Citation2013). The reductions in bactericidal efficacy are therefore understood to occur as a result of the amount of dissolved species that are able to form. In other words, when Cl- concentration is less than 3 orders of magnitude greater than the Ag+ concentration (Levard et al., Citation2013), Ag+ and Cl− form AgCl and this compound aggregates onto Ag-NPs to form a solid precipitate which inhibits bactericide by increasing the particle size, reducing its affinity toward the cell wall, and reducing the amount of release of Ag+ from the AgNP (Baalousha et al., Citation2013; Liu & Hurt, Citation2010). However, at much higher concentrations of Cl−, solid AgCl no longer forms, but rather Ag+ and Cl− form dissolved AgCl2− or AgCl43−, which do not as significantly reduce disinfection efficacy (Levard et al., Citation2013). Similar effects have been found with other monovalent anions like hydroxide (OH-), however the impacts are not as significant as those of Cl− (Jin et al., Citation2010).
3.2.2.4 Organic matter
Organic matter is very commonly found in surface waters and has demonstrated to have a profound influence on silver nanoparticle disinfection (Zhang et al., Citation2012). Fabrega et al. (Citation2009) showed that at a pH of 9, 2000 ppb of Ag-NP led to 85% bacterial growth inhibition, however essentially no growth inhibition was observed after 10 mg/L of Suwanee River humic acid was added to the water (Fabrega et al., Citation2009). The reduction in efficacy is understood to result from the Ag-NP being unable to release Ag+, as was clearly demonstrated by Liu and Hurt (Citation2010). The researchers showed that at the same concentration of humic acid used by Fabrega et al. (Citation2009), Ag+ release dropped by 50%, and it continued to decrease along an exponential trend as natural organic matter (NOM) increased to 50 mg/L, at which point it was enumerated at nearly 0 mg/L of Ag+ (Liu & Hurt, Citation2010). The reduction in ion release has been ascribed to NOM sorption onto the nanoparticle, which creates a physical barrier through which the ionic silver cannot pass (Fabrega et al., Citation2009). No literature was discovered on the impact of organic matter on ROS generation, however the complete failure of bacterial growth inhibition resulting from the presence of humic acid in the feed water demonstrated by Fabrega et al. (Citation2009) suggests similar effects likely occur (Fabrega et al., Citation2009). This impact is of particular importance to the CWF field, as the technology is often implemented in rural and remote communities that rely on surface water sources for drinking. As such, NOM can be expected in water sources, potentially impacting filter effectiveness in terms of silver disinfection.
3.2.3. Ag-NP size and shape
Physical nanoparticle characteristics have also been shown to impact disinfection efficacy; specifically, their size and shape are demonstrably important (Duran, Citation2016; Morones et al., Citation2005), because of the vastly increased specific surface area (i.e. surface area to mass/volume ratio) of particles sized on the nanometer scale, relative to those of larger size (Lok et al., Citation2007; Rai et al., Citation2009). Furthermore, Lok et al. (Citation2007) found that 9.2 nm and 62 nm Ag-NP achieved the same bactericidal effectiveness (values unreported) when in concentrations of 12 µg/L and 108 µg/L, respectively. The researchers explained that the larger specific surface area of the smaller nanoparticle provided greater space for Ag+ to be chemisorbed, which increased the nanoparticles’ affinity toward the cell and therefore its bactericidal and bacteriostatic effectiveness in a lower concentration than its larger-sized counterpart (Lok et al., Citation2007). Additionally, Helmlinger et al. (Citation2016) found a direct correlation between specific surface area and nanoparticle dissolution, where after approximately 100 h, particles of different shapes/morphologies with specific surface areas of 0.234, 0.100, 0.040 and 0.038 nm-1 dissolved such that the silver ion concentration was, on average, 40%, 27%, 15% and 10% of the silver-ultrapure water solution, respectively (Helmlinger et al., Citation2016). These findings indicate that smaller particles with greater surface areas produce and adsorb more Ag+, compounding to improve disinfection (Helmlinger et al., Citation2016; Lok et al., Citation2007).
Carlson et al. (Citation2008) also showed that in a concentration of 25 µg/L, fluorescence intensity increased as sizes decreased, with reported values of (measured as fold increases) 2, 3 and 7.5 for Ag-NPs sized 55 nm, 30 nm and 15 nm, respectively. Smaller particles are therefore more easily oxidized by constituents in the aqueous environment than larger ones, leading to greater reductions of oxygen and subsequently vast differences in ROS generation (Carlson et al., Citation2008).
Aside from size, nanoparticle morphology has also been shown to contribute significantly to disinfection efficacy. For example, Pal et al. (Citation2007) showed that truncated-triangular nanoparticles achieved complete bacterial growth inhibition with only 10 µg/100 mL of silver in the culture medium after 26 h, while after the same time period, spherical particles of the same size in a concentration of 12 µg/100 mL achieved only 40% growth inhibition (Pal et al., Citation2007). This result is particularly relevant because Collargol nanoparticles produced and sold by Argenol Laboratories, the most commonly used AgNPs in CWF manufacturing and research, are primarily spherical in shape (Oyanedel-Craver & Smith, Citation2008; Panasewicz, Citation2011; Rayner, Citation2009). Further research into differently shaped AgNPs may therefore offer an opportunity to reduce the number of particles needed per filter, ultimately reducing the cost per unit.
3.3. Combined physical and silver disinfection for bacterial removal in CWF
Within CWF research, the traditional understanding of the silver’s effectiveness has been based largely on studies such as Oyanedel-Craver and Smith (2008), where after 75–85 min of filtration, filters painted with, and submerged in, a 600 mg/L colloidal silver solution yielded LRVs of 6 and 6.5, respectively, whereas filters without any silver yielded LRVs of 4.6 and 5.5 (Oyanedel-Craver & Smith, Citation2008). And because of findings like these, Potters-for-Peace (PfP; the largest organization in this domain) states that “Silver is applied to the filter to achieve two objectives: (1) to take advantage of the bactericidal quality of silver in the purification of water as it is filtered; and (2) to prevent the growth of the “slime layer” of bacteria that can form on the filter wall” (p. 91) (Ceramics Manufacturing Working Group, Citation2010). This section therefore addresses both of these assertations, beginning with the latter.
3.3.1. Silver impacts on “slime layer” growth
Larimer’s (Citation2013) PhD thesis was the only document found to directly investigate this phenomenon, even though it is touted as one of two primary reasons to add silver to a filter (Ceramics Manufacturing Working Group, Citation2010; Larimer, Citation2013). In it, the author shows that only 0.03% and 0.003% of Mycobacterium smegmatis remained on microporous track etched polycarbonate membranes (used to simulate a CWF surface) that were coated with an Ag-NP solution of 1.1 or 2.2 g/L, respectively, whereas the species grew by 1000% when no silver was deposited. Similarly, he found that 84% and 60% of Mycobacterium avium remained on the same membranes when 1.1 and 2.2 g/L of Ag-NP solution was deposited, respectively, whereas the species grew by 637% without any silver presence (Larimer, Citation2013). The silver concentrations selected, however, are orders of magnitude greater than what is typically added to a CWF. Additionally, Ag-NPs have been shown to disinfect gram-positive Actinobacteria (the phylum of the Mycobacteria genus) more than gram-negative Enterobacteriaceae (the phylum of the Escherichia genus) like E. coli (Yoon et al., Citation2007), which makes these results difficult to generalize. This point is of particular importance as well, as diseases like cholera and typhoid are quite common causes of diarrhea in rural LIC and LMIC communities, which are gram-negative bacterial species. Thus, additional research that considers gram-negative bacterial species and at silver concentration more reflective of field conditions is necessary.
3.3.2. Silver impacts during the filtering process
With filters, on average, flowing at a rate of approximately 1–4 L/hr through, on average, about 2.5 cm (1 inch) of filter material, the actual contact time between any single silver nanoparticle and bacterium is very brief. However, previous research (see Section 3.2.1.1) clearly indicates that silver disinfection is a somewhat slow process that requires time upwards of hours to inhibit DNA replication or exhibit toxicity effects (Pal et al., Citation2007; Rai et al., Citation2009; Yoon et al., Citation2007). Therefore, the generalized hypothesis that that silver disinfects during filtration is questionable. Rather, it seems like a more likely that silver disinfects bacteria in the receptacle after filtration has already occurred.
Furthermore, Oyanedel-Craver and Smith (Citation2008) reported initial differences in LRV between filters with and without silver of approximately 0.8. However, after about 20 min of filtration, the LRV difference between filter types became more prevalent, increasing to approximately 2 after 84 min (Oyanedel-Craver & Smith, Citation2008). Figure S5 in their Supplementary Materials shows an initial flush of 0.5 mg/L of silver in the effluent after 20 min, which explains why the growing difference in LRV is observed (Oyanedel-Craver & Smith, Citation2008). That is, impregnated filters introduced a lot of silver into the effluent initially, which reduced the bacterial concentration over time through disinfection action. Comparatively, the filter with no metal maintained a relatively constant effluent bacterial concentration, leading to an increasing difference between the two types of filters with time.
Abebe et al. (Citation2015) reached a similar conundrum when attempting to remove Cryptosporidium from a variety of source waters – though silver was shown to effectively deactivate the oocysts in a batch reactor system, as observed through High-Resolution Differential Interference Contrast Imagery, the researchers were not able to identify whether it was filtration or disinfection that was responsible for their removal after passage through a ceramic disk in a separate experiment (Abebe, et al., Citation2015).
Direct evidence demonstrating the prevalence of postfiltration disinfection has also been reported by van der Laan et al. (Citation2014). The researchers found that E. coli LRV values taken less than 5 min after filtration were approximately 0.75 ± 0.25 for filters with no silver, 1.10 ± 0.4 with silver painted on the outside and 1.15 ± 0.5 for filters painted with silver both on the inside and outside, showing no significant difference from silver addition. Conversely, filters painted with silver on both sides (in a separate experiment as above) yielded LRVs of 1.3 ± 0.7 after less than 5 min of storage compared with 3.8 ± 1.0 after 660 min of storage (van der Laan et al., Citation2014).
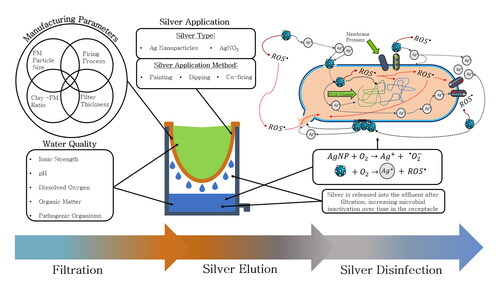
4. Silver elution
Silver elution is a significant feature of CWF performance, as it is necessary to ensure the concentration in the effluent remains below the WHO’s recommended limit of 0.1 mg/L (World Health Organization, Citation2006). Daily ingestion of silver above this concentration may lead a lifetime accumulation greater than 10 g (WHO, Citation2011), which can lead to negative health outcomes such as Argyria or even DNA damage (Fewtrell et al., Citation2017). Guidelines developed by The Canadian Council of Ministers of the Environment (CCME) also stipulate total silver directly released into freshwater and marine environments should be in quantities no greater than 0.25 and 7.5 µg/L, respectively, to ensure the protection of aquatic life (CCME, Citation2015). Conversely, as indicated in Section 3, silver elution also helps ensure sufficient microbe disinfection (van der Laan et al., Citation2014). The release of ions and nanoparticles into the effluent initiates the interaction with bacteria that pass through the ceramic matrix, which improves removal with time (van der Laan et al., Citation2014). Therefore, it is important to illuminate how silver attaches to, and is released from, ceramic to control for its impact in CWF performance.
Silver elution is a function of the particle characteristics, water chemistry and application method of the silver. Furthermore, subsequent disinfection is additionally impacted by whether particulate or dissolved silver is eluted. Thus, it is important to differentiate between particle release and ion release, as they occur via different mechanisms and the different forms of silver will contribute to disinfection differently. As is seen herein however, a significantly greater quantity of research is required to better understand the mechanisms by which these processes occur, as limited studies have investigated elution (see ). This section discusses application method, particle characteristics and water chemistry impacts on elution, and how each may contribute to disinfection.
Table 5. Summary of elution data from CWFs painted or co-fired with silver nanoparticles.
4.1. Impacts of application method
While the addition of silver to CWF via painting and submerging methods are the most widespread in local manufacturing facilities (Ceramics Manufacturing Working Group, Citation2010; Rayner, Citation2009), it appears, as seen in , that the co-firing (addition of a silver solution to the dry mixture before firing) leads to a more consistent and sustained release of silver into the effluent at quantities up to 1000 times less than the alternatives mentioned (Ehdaie et al., Citation2017; Nunnelley et al., Citation2016; Ren & Smith, Citation2013)
Ren and Smith (Citation2013) were the first to study this phenomenon, showing that over 180 min of filtration with synthetic water, filters impregnated with 2.76 mg/disk and 27.6 mg/disk released a consistent amount of total silver averaging 0.004 mg/L and 0.009 mg/L, respectively. When the influent water flowrate doubled, the disks impregnated with 2.76 mg/disk released between 0.003–0.007 mg/L, and those with 27.6 mg/disk released 0.015 mg/L consistently. Furthermore, after 360 min of filtration, the filters lost approximately 0.0045% and 0.001% of the initial silver content, respectively (Ren & Smith, Citation2013). Lyon-Marion et al. (Citation2018) found similar results for the co-firing method, where after 20 pore volumes of filtration with water containing an ionic strength (IS) of 10 mM (NaNO3), co-fired filters released an average of 0.5% of the silver applied, compared with 11.8% and 8.7% for filters painted with AgNO3 and Ag-NPs, respectively (Lyon-Marion et al., Citation2018). These results, as well as others, illustrate the comparatively low-level and consistent release of silver realized when filters are impregnated with the co-firing method (Ehdaie et al., Citation2017; Kahler et al., Citation2016; Lyon-Marion et al., Citation2018; Nunnelley et al., Citation2016; Ren & Smith, Citation2013).
Conversely, studies that have investigated painting and dipping methods illustrate much higher releases, particularly at the beginning of filtration. For example, Ren and Smith (Citation2013) show that disk filters painted and dipped to have an impregnated concentration of 2.76 mg/disk both initially released 11 mg/L of silver, decreasing to 0.4 mg/L and 1 mg/L, respectively, after 180 min of filtration. These values corresponded to a loss of approximately 1.20% and 1.25% of the initial silver content added for painted and submerged filters, respectively (Ren & Smith, Citation2013). Mittelman et al. (Citation2015) found agreeing results with disk filters painted with AgNO3 initially releasing 1 mg/L, averaging just below 0.1 mg/L after about 50 pore volumes of filtration. Filters painted with Ag-NP had an initial release of approximately 0.5 mg/L, decreasing to 0.1 mg/L after 20 pore volumes, and then linearly decreasing to 0.03 mg/L after 160 pore volumes (Mittelman et al., Citation2015). Lyon-Marion et al. (Citation2018) found almost identical results to those of Mittelman et al (2016) (Lyon-Marion et al., Citation2018). Furthermore, when scaled to full filters, Mikelonis et al. (Citation2016) saw initial releases of 0.7 mg/L, 0.12 mg/L, 0.4 mg/L and 0.325 mg/L for filters painted with citrate-, polyvinylpyrollidine- (PVP), branched polyethylenimine- (BPEI) and Casein-stabilized nanoparticles, respectively, after exposure to a real surface water (Mikelonis et al., Citation2016).
When taken together, all of these results, as well as the results presented in , highlight a trend where much more silver is lost when filters are painted with, or submerged in, a silver solution, than when co-fired. These results align with the findings from Yakub and Soboyejo (Citation2012), who enumerated the attachment force between silver and CWF material to be only 125 ± 32 nN after painting/submerging, which was attributed to van der Waals forces (Yakub & Soboyejo, Citation2012). As such, the interfacial energy between the materials is low, which is why co-firing has exhibited more consistent release and less vulnerability to the impacts of water chemistry (Ren & Smith, Citation2013; Lyon-Marion et al., Citation2018). Because the energy between two interfaces ( i.e. silver and ceramic) is a function of enthalpy (Hs), temperature (T), and entropy (Ss), as shown in Equation (1) (Howe, Citation1997). So, as temperature increases, the interfacial energy also decreases as per the second law of thermodynamics, explaining the stronger attachment of silver when filters are co-fired.
[1]
[1]
4.2. Impacts of silver characteristics
Mittelman et al. (Citation2015) demonstrated that between 0.008 and 1 mg/L of total silver from disk filters painted with AgNO3 was released into the effluent, depending on variable water quality characteristics. Comparatively, disk filters painted with the same quantity of silver nanoparticles released between 0.006 and 0.4 mg/L of total silver under the same variable water quality conditions. With 10 mM of ionic strength background and after 10 pore volumes of filtration, AgNO3-painted disks released 0.15 mg/L, whereas Ag-NP painted disks released 0.04 mg/L; after approximately 90 pore volumes of filtration though, the difference became negligible (Mittelman et al., Citation2015). This observation may be due to the faster conversion of AgNO3 into Ag+, and the subsequent displacement of silver ions via cation exchange with ions in the influent, especially since painted silver is more vulnerable to removal (Mittelman et al., Citation2015). No other literature was found to evaluate the difference between Ag-NP and AgNO3.
Mikelonis et al. (Citation2016) demonstrated that negatively charged, electrostatically stabilized nanoparticles had greater attachment to an anodisc when positively charged than when negatively charged, whereas sterically stabilized nanoparticles exhibited the same amount of attachment to the anodisc, regardless of charge (Mikelonis et al., Citation2016). Having said that, Mikelonis et al. (Citation2020) and Sullivan et al (Citation2017) illustrated that elution is impacted by stabilizing agent, as electrostatically stabilized Ag-NPs were eluted from ceramic surfaces in higher quantities than sterically stabilized ones, even if their charges were the same (Mikelonis et al., Citation2020; Sullivan et al., Citation2017) The stabilizing agent of the silver nanoparticle was therefore found to impact elution from a CWF but not initial attachment. Electrostatically stabilized silver nanoparticles, meaning by ion associated adsorption onto the silver particle creating an electric charge that facilitates repulsion, are consequently more influenced by oppositely charged constituents in the surrounding environment. By contrast, sterically stabilized, meaning by polymer associated adsorption onto the silver particles that results in particle-particle repulsion, Ag-NP mobility is less influence by charged constituents in the environment. Surface functionalization is therefore closely related with application method, as certain particle stabilizations may yield more desirable elution levels if the CWF is painted or submerged, versus co-fired; further research into this relationship is highly recommended. Finally, Fauss et al. (Citation2014) further reported improved disinfection with electrostatically stabilized Ag-NPs over steric stabilized particles (Fauss et al., Citation2014), demonstrating the importance of considerations regarding consideration for what type of nanoparticle is utilized in a CWF for disinfection.
4.3. Impacts of water chemistry on elution
Ionic strength was shown by Ren and Smith (Citation2013) to improve silver retention when silver-spiked influent water (10 mg/L) was passed through a filter disk without silver impregnation for 100 min (6 pore volumes). The researchers found that with 50 nm diameter particles in the feed, 21%, 40% and 76% of the silver was retained when the water had a 1 mM, 10 mM and 50 mM IS, established using MgSO4, respectively (pH not reported). More silver was also retained as the particle sizes increased, illustrating a compounding impact of ionic strength and size on retention (Ren & Smith, Citation2013).
Interestingly Mittelman et al. (Citation2015) demonstrated opposing results when disks were painted with a 200 mg/L silver solution and flushed with clear water for 12–15 h, where they released approximately 0.006 mg/L, 0.08 mg/L and 0.8 mg/L with an influent water IS of 1 mM, 10 mM and 50 mM NaNO3, respectively (Mittelman et al., Citation2015). Similar results were reported by Lyon-Marion et al. (Citation2018), where approximately 0.1 mg/L and 0.25 mg/L of silver was released from disk filters exposed to 1 mM and 10 mM IS of NaNO3, respectively (Lyon-Marion et al., Citation2018). The reason for the difference in observations is uncertain (Lyon-Marion et al., Citation2018; Mittelman et al., Citation2015), however is most likely due to MgSO4 releasing divalent cations into the water, which cannot exchange with the monovalent Ag+, relative to NaNO3 releasing monovalent cations into the water, which can engage in ion exchange processes (Crittenden, Citation2012c). It may also be because the former study injected ions and silver into the feed, whereas the silver was only in the disks in the latter. As shown by Huynh and Chen (Citation2011), a higher ionic strength leads to greater conversion of Ag-NPs to Ag+, and divalent cations increase the rate of aggregation regardless of nanoparticle charge (Huynh & Chen, Citation2011). It is thus most likely that the silver nanoparticles in the feed were quickly converted into Ag+, which then aggregated to form neutral agglomerates that were larger in size than the original nanoparticles, explaining why less than 1% ionic silver was enumerated by the researchers (Ren & Smith, Citation2013). These larger agglomerates were therefore more retained because of their size, which is why more of the larger nanoparticles were retained with higher background IS, but size was irrelevant at a lower IS.
Changes in pH consistently created notable changes in silver leaching as well, however the nature of this impact is not completely understood. One explanation is that higher concentrations of hydrogen ions (e.g. pH = 5) creates a greater potential for cation exchange and thus displaces a greater quantity of silver, in comparison to higher pH levels (e.g. pH = 9) (Bielefeldt et al., Citation2013; Mittelman et al., Citation2015). An alternative perspective however is that a step-wise reaction occurs, as shown in Reaction 8, whereby silver solid particles bond with oxygen in the water or on the ceramic surface, forming AgO or Ag2O, which subsequently reacts with hydrogen and dissociates into dissolved silver ions and water (Bielefeldt et al., Citation2013; Fauss et al., Citation2014; Hong et al., Citation1995; Liu & Hurt, Citation2010; Nguyen, Citation2014). Consequently, Mittelman et al. (Citation2015), reported silver release to decrease from 0.6 mg/L to 0.1 mg/L and then 0.008 mg/L as pH increased from 5 to 7 to 9 sequentially (Mittelman et al., Citation2015), demonstrating a clear relationship.
Chlorine concentration has a similar effect as well, as chlorine in the influent water can lead to silver chloride (AgCl) compounds forming as a precipitate (under conditions explained in Section 3.2.2.3), which detach the silver from the ceramic and can limit disinfection in the receptacle (Baalousha et al., Citation2013; Bielefeldt et al., Citation2013; Huynh & Chen, Citation2011; Le Ouay & Stellacci, Citation2015). For example, Lyon-Marion et al. (Citation2018) found essentially no impact of 2 mg/L Cl2 on elution of painted disks over 36 pore volumes, however they did find that when the influent concentration was subsequently increased to 4 mg/L of Cl2, a decrease in elution from 0.1 mg/L to 0.06 mg/L was observed over 24 pore volumes (Lyon-Marion et al., Citation2018).This effect is likely due to the retention of AgCl within the CWF matrix. No literature was discovered that discusses the influence of other monovalent anions (such as fluoride) on silver elution, which highlights an important research gap. Fluoride is, however, of concern, as it is abundant in natural waters, particularly in areas where water is exposed to volcanic rock and soil (Crittenden, Citation2012b). Unlike AgCl, however, AgF does not precipitate (Salt Lake Metals, Citation2017), meaning it is unlikely to be retained within the CWF matrix, and thus more likely to appear in the effluent. As limited literature investigating AgF bactericidal efficacy is available, the impact of fluoride on subsequent disinfection potential remains uncertain. Further research into such effects would therefore be beneficial to the field.
5. Other disinfection enhancing additives
While silver is the most common metal additive to CWFs for disinfection control, research on other metal species has also demonstrated some promise; most notably titanium dioxide (TiO2), cupric oxide (CuO), and zinc oxide (ZnO) (Dimapilis et al., Citation2018; Foster et al., Citation2011; Grass et al., Citation2011).
Like silver, these other metal species are suspected to release ions or ROS as mechanisms for disinfection, however TiO2 seldom releases Ti2+ and is only efficient as a source of ROS generation after photocatalysis (Foster et al., Citation2011), so it is excluded from this discussion. CuO has demonstrated good levels of microbe removal as both ionic copper and as a source of ROS generation (Pandey et al., Citation2012), and has also shown promise within a ceramic system (Drelich et al., Citation2017; Ehdaie et al., Citation2020; Varkey & Dlamini, Citation2012; Yakub & Soboyejo, Citation2012). Copper also has a higher recommended ingestion level of 2 mg/L (10 mg/day) which thus has lower longer term health risks than silver (World Health Organization, Citation2004). CCME, however, stipulates a maximum of 4 µg/L of total copper should be released directly into a receiving waterbody to ensure the protection of aquatic life (CCME, Citation1987), which poses some environmental risk associated with its inclusion in a CWF system. This quantity is, however, significantly higher than the absolute minimum allowable concentration for silver release (see Section 4), suggesting its imposed risk is indeed lower than the current methods employed in the field.
ZnO has also shown to achieve bactericide under both dark and light conditions (Sirelkhatim et al., Citation2015) and there is no WHO recommended limit for consumption of zinc (World Health Organization, Citation1996), unlike the silver limit of 0.1 mg/L (World Health Organization, Citation1996). Zinc has even demonstrated to substantially reduce the incidence and severity of diarrheal episodes when ingested (Gitanjali & Weerasuriya, Citation2011; Malik et al., Citation2013) making it an attractive option to CWF application. CCME, however, states total zinc concentrations released directly into a waterbody with aquatic life should not exceed 9.1 µg/L, though that concentration may increase as water hardness, pH and dissolved organic carbon concentrations increase beyond 13.8 mg/L, 6.5 and 0.3 mg/L, respectively (CCME, Citation2018). Furthermore, though environmental risks associated with zinc do indeed exist, they are significantly lower than those posed by the inclusion of silver or copper in a CWF.
No literature was discovered that discusses a ZnO composite CWF; only an unpublished study by van Halem showed it was retained within a filter (Rayner, Citation2009). The value of using ZnO as a replacement or supplement for AgNPs is therefore unexplored.
5.1. CuO disinfection
CuO disinfection has been a recognized phenomenon dating back to ancient civilizations using copper vessels to ensure safe storage of water (Grass et al., Citation2011). Following a similar mechanistic pathway as both silver and zinc, CuO has shown to generate ROS via Reaction 9 or 10, as well as release copper ions, which also have bactericidal impacts (Grass et al., Citation2011; Thurman et al., Citation1989). Though far less studied within the CWF research field, CuO has demonstrated comparable, and sometimes even greater, bactericidal effectiveness than AgNP, highlighting an area of great potential for manufacturing adjustments. For example, Yoon et al. (Citation2007) found 33.5 µg/mL (0.421 µmol/mL) of CuO nanoparticles were required to achieve an E.coli LRV of 1 after 24 h of incubation compared with 58.4 µg/mL (0.541 µmol/mL) of Ag-NPs (Yoon et al., Citation2007). Conversely, Ananth et al. (Citation2015) found the minimum inhibitory concentration for E. coli after 12 h was 6.25 µg/mL (0.079 µmol/mL) for CuO compared with 1.56 µg/mL (0.014 µmol/mL) of Ag, illustrating opposite results (Ananth et al., Citation2015). Pandey et al. (Citation2012) found LRVs of 2.5 and 4.3 with CuO concentrations of 10 µg/mL (0.126 µmol/mL) and 25 µg/mL (0.314 µmol/mL), respectively, against E. coli, however they did not compare effects with silver (Pandey et al., Citation2012).
[Reaction 9]
[Reaction 9]
[Reaction 10]
[Reaction 10]
In terms of application to the CWF field, only three studies were found within the literature that specifically studied copper addition to a CWF, which were Varkey and Dlamini (Citation2012), Lucier et al (Citation2017) and Jackson et al. (Citation2019). The lattermost researchers found that filters co-fired with 2 and 4 g of Cu(NO3)2 both yielded LRVs of 3.54 and released 3.5 and 9.5 µg/L of copper, respectively, whereas filters painted with 0.4 g AgNP achieved an LRV of 3.76 and released 21.5 µg/L of silver (Jackson et al., Citation2019). Lucier et al. (Citation2017) found that filters without any metals yielded interquartile LRV ranges of 3 to 6, whereas filters co-fired with CuO and Ag (concentrations not reported) yielded LRVs ranging from 3 to 6 and 3.2 to 6, respectively (Lucier et al., Citation2017). The former results indicate better disinfection for AgNP-impregnated filters, whereas the latter illustrates no discernable difference between any of the filters evaluated. Varkey and Dlamini (Citation2012) reported a marginal difference in bacteria removal when adding a copper mesh directly into the receiving receptacle and no copper in the CWF itself; Removals were reported as 100% and 99.4% removal of E. coli with and without the copper mesh, respectively (Varkey & Dlamini, Citation2012). Additional research on copper disinfection within a CWF system would benefit the field, as this alternative metal may offer CWF manufacturers more diversity in their supply chain so there does not have to be such a great reliance on silver for enhanced disinfection.
5.2. ZnO disinfection
As discussed, ZnO nanoparticles (ZnO-NP) disinfect bacteria with similar mechanisms to silver and copper, where zinc ions (Zn2+) and/or ROS inhibit DNA replication within the cell organism or exhibit toxic effects (Dimapilis et al., Citation2018; Yoon et al., Citation2007). Most literature on this compound cites UV and visible light activation as the source of ROS generation via electron-hole pairs, as shown through Reaction 11 and Reaction 3-6. For example, Padmavathy and Vijayaraghavan (Citation2008) found that under these conditions, ZnO had a 90% bactericidal efficiency after 24 h of incubation, which was reportedly due to ROS generation on the nanoparticle surface (Padmavathy & Vijayaraghavan, Citation2008). Hirota et al. (Citation2010) and others, however, have found ROS generated under dark conditions as well (Adams et al., Citation2006; Hirota et al., Citation2010; Sirelkhatim et al., Citation2015). Song et al. (Citation2010) found under these conditions, the nanoparticle interacts with the cell and causes damage to the mitochondria, which releases oxygen that then quickly reacts to become ROS and improve disinfection efficacy over time (Song et al., Citation2010).
[Reaction 11]
[Reaction 11]
[Reaction 3]
[Reaction 3]
[Reaction 4]
[Reaction 4]
[Reaction 5]
[Reaction 5]
[Reaction 6]
[Reaction 6]
The same researchers, however, found that ROS was not as impactful as dissolved, ionic zinc (Zn2+) in disinfection under dark conditions, as they found ionic zinc resulted in a 55% reduction in cell viability, and a zinc oxide suspension resulted in a 65% reduction with “fine-ZnO” particles at a concentration of 100 µg/mL (Song et al., Citation2010). Ionic zinc is believed to disinfect the bacterial cell by attaching to the cell outer membrane by either electrostatic forces or by bonding with the sulfuric thiol- or phosphoric protein groups, causing damage to the cellular wall and eventually infiltrating the cytoplasm, destroying the cell from within (Maret, Citation2004; Qu et al., Citation2013). Contrary to those findings, Joe et al. (Citation2017) found an 87% reduction in cell viability after 6 h of exposure to 2.85 mg/L of ZnO-NPs with only 11% of that solution in ionic form. The researchers therefore concluded that Zn2+ was not in a high enough concentration to be toxic to the cell, and therefore could not control bacterial disinfection. Rather, they hypothesize that ZnO-NPs attach to the cell membrane and initiate the bactericidal process, and in so doing, release Zn2+ onto the cell itself, which improves bactericide even further over time (Joe et al., Citation2017). Regardless of which antibacterial pathway is dominant, the fact that disinfection is observed under both light and dark conditions is important to the CWF field, as filtrate is most often kept under dark conditions. And as discussed in Section 3.3.1.2, metal-based disinfection is predominantly active in the receptacle (i.e. after filtration), meaning those conditions are of particular importance to the field for further research. These findings further highlight the importance of studying the impact of ZnO within a CWF system, as no research has been discovered that studies zinc-impregnated filters.
6. Conclusion
Diarrheal illnesses claim the lives of hundreds of thousands of children each year, meaning access to safe drinking water is a key element of a global strategy to eradicate this challenge. Its disruptive impacts, though, are clearly observed along socioeconomic lines, where those most significantly afflicted are most often low-income groups living in a rural setting. POUWTS are therefore widely regarded as useful technological solutions that may be implemented with immediacy in rural environments, Ceramic Water Filters being one of the most common.
Silver is typically added to a CWF as an antimicrobial agent to improve the bactericidal efficacy of the technology. However, the contribution of silver to the CWF has been, at times, unclear. Importantly, in this review of the literature, it was demonstrated that when evaluating filter bacteria removal immediately after filtration, those with silver do not perform significantly better than those without. Only after storage time do filters with silver reduce the bacterial concentration significantly more than those without, illustrating that silver nanoparticles add to the overall filter performance primarily in the receptacle. Thus, user behavior will impact the level of safety from the CWF – does the user consume water immediately after filtration or wait a certain amount of time?
Furthermore, this review highlights that silver elution is thus critical to the realization of safe drinking water for the technology’s users. Its relationship with disinfection kinetics in the receptacle, however, remains understudied, demonstrating an important research gap. Specific attention should be placed on co-fired filters, as results from literature suggest it is a superior silver application method to painting and submerging in terms of release consistency, concentration, and robustness of performance across diverse water qualities. Considerations of influent water quality, physical filter characteristics and silver type are also critical. Further research is also still required to elucidate the metallic influence on the formation of a microbial “slime” layer along the inner part of the filter, and how the filter performs over its expected lifespan.
Review of literature regarding alternative metals to silver for enhanced disinfection, such as zinc oxide (ZnO) and cupric oxide (CuO), also present emerging opportunities for new areas of research with the potential of reducing cost and increasing metal nanoparticle options without compromising the effectiveness of a CWF. Greater attention to this research area could expand the potential reach and effectiveness of this technology, importantly increasing access for more marginalized individuals and communities.
References
- Abebe, L. A. et al., (2015). Point-of-use removal of cryptosporidium parvus from water: independent effects of disinfection by silver nanoparticles and silver ions and by physical filtration in ceramic porous media. Environmental Science & Technology, 49, 12958–12967.
- Adams, L. K., Lyon, D. Y., & Alvarez, P. J. (2006). Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Research, 40(19), 3527–3532. https://doi.org/https://doi.org/10.1016/j.watres.2006.08.004
- Ananth, A., Dharaneedharan, S., Heo, M.-S., & Mok, Y. S. (2015). Copper oxide nanomaterials: synthesis, characterization and structure-specific antibacterial performance. Chemical Engineering Journal, 262, 179–188. https://doi.org/https://doi.org/10.1016/j.cej.2014.09.083
- Annan, E., Mustapha, K., Odusanya, O. S., Malatesta, K., & Soboyejo, W. O. (2014). Statistics of flow and the scaling of ceramic water filters. Journal of Environmental Engineering, 140(11), 04014039. https://doi.org/https://doi.org/10.1061/(ASCE)EE.1943-7870.0000862
- Argenol Laboratories. (2017). Cost of silver. Personal Communication.
- Asadishad, B., Ghoshal, S., & Tufenkji, N. (2013). Short-term inactivation rates of selected gram-positive and gram-negative bacteria attached to metal oxide mineral surfaces: Role of solution and surface chemistry. Environmental Science & Technology, 47(11), 5729–5737. https://doi.org/https://doi.org/10.1021/es4003923
- Baalousha, M., Nur, Y., Römer, I., Tejamaya, M., & Lead, J. R. (2013). Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles. The Science of the Total Environment, 454-455, 119–131. https://doi.org/https://doi.org/10.1016/j.scitotenv.2013.02.093
- Bielefeldt, A. R., Kowalski, K., & Summers, R. S. (2009). Bacterial treatment effectivenesss of point-of-use ceramic water filters. Water Research, 43(14), 3559–3565. https://doi.org/https://doi.org/10.1016/j.watres.2009.04.047
- Bielefeldt, A. R., Stewart, M. W., Mansfield, E., Scott Summers, R., & Ryan, J. N. (2013). Effects of chlorine and other water quality parameters on the release of silver nanoparticles from a ceramic surface. Water Research, 47(12), 4032–4039. https://doi.org/https://doi.org/10.1016/j.watres.2013.01.058
- Brown, J. (2007). Laboratory and field effectiveness of low-cost ceramic filters for drinking water treatment in Cambodia. In: Effectiveness of ceramic filtration for drinking water treatment in Cambodia. University of North Carolina. (PhD Thesis), pp. 51–110.
- Brown, K. (2003). Diarrhea and malnutrition. New Orleans, LA, s.n.
- Burt, Z., Njee, R. M., Mbatia, Y., Msimbe, V., Brown, J., Clasen, T. F., Malebo, H. M., & Ray, I. (2017). User preferences and willingness to pay for safe drinking water: experimental evidence from rural Tanzania. Social Science & Medicine (1982)), 173, 63–71. https://doi.org/https://doi.org/10.1016/j.socscimed.2016.11.031
- Carlson, C., Hussain, S. M., Schrand, A. M., Braydich-Stolle, L. K., Hess, K. L., Jones, R. L., & Schlager, J. J. (2008). Unique Cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. The Journal of Physical Chemistry. B, 112(43), 13608–13619. https://doi.org/https://doi.org/10.1021/jp712087m
- CCME. (1987). Copper. [Online]. http://st-ts.ccme.ca/en/index.html?lang=en&factsheet=71#aql_fresh_concentration
- CCME. (2015).,Silver. [Online]. http://st-ts.ccme.ca/en/index.html?lang=en&factsheet=198 [Accessed 05 2020].
- CCME. (2018). Zinc. [Online]. http://st-ts.ccme.ca/en/index.html?lang=en&factsheet=229#aql_fresh_concentration
- Ceramics Manufacturing Working Group. (2010). Best practice recommendations for local manufacturing of ceramic pot filters for household water treatment. Ceramics Manufacturing Working Group.
- Choi, O., Deng, K. K., Kim, N.-J., Ross, L., Surampalli, R. Y., & Hu, Z. (2008). The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Research, 42(12), 3066–3074. https://doi.org/https://doi.org/10.1016/j.watres.2008.02.021
- Clark, K. N., & Elmore, A. C. (2011). Bacteria removal effectiveness of ceramic pot filters not applied with colloidal silver. Water Supply, 11(6), 765–772. https://doi.org/https://doi.org/10.2166/ws.2011.012
- Crittenden, J. C. (2012a). Granular filtration (Ch. 11). In: MWH water treatment: principles and design (3rd Ed., pp. 727–818). John Wiley & Sons.
- Crittenden, J. C. (2012b). Physical and chemical quality of water. In: MWH water treatment principles and design (pp. 32–33). MWH; John Wiley and Sons'.
- Crittenden, J. C. (2012c). Ion exchange. In: MWH's water treatment principles and design (pp. 1263–1268). John Wiley and Sons.
- Dimapilis, E. A. S., Hsu, C.-S., Mendoza, R. M. O., & Lu, M.-C. (2018). Zinc oxide nanoparticles for water disinfection. Sustainable Environment Research, 28(2), 47–56. Volume https://doi.org/https://doi.org/10.1016/j.serj.2017.10.001
- Drelich, A. J., Miller, J., Donofrio, R., & Drelich, J. W. (2017). Novel durable antimicrobial ceramic with embedded copper sub-microparticles for a steady-state release of copper ions. Materials, 10(7), 775. https://doi.org/https://doi.org/10.3390/ma10070775
- Dror-Ehre, A., Mamane, H., Belenkova, T., Markovich, G., & Adin, A. (2009). Silver nanoparticle-E. coli colloidal interaction in water and effect on E. coli survival . Journal of Colloid and Interface Science, 339(2), 521–526. https://doi.org/https://doi.org/10.1016/j.jcis.2009.07.052
- Duran, N. (2016). Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine: Nanotechnology, Biology and Medicine, 12(3), 789–799.
- Ehdaie, B., Rento, C. T., Son, V., Turner, S. S., Samie, A., Dillingham, R. A., & Smith, J. A. (2017). Evaluation of silver-embedded ceramic tablet as a primary and secondary point-of-use water purification technology in limpopo province. Plos One, 12 (1), e0169502. https://doi.org/https://doi.org/10.1371/journal.pone.0169502
- Ehdaie, B., Su, Y.-H., Swami, N. S., & Smith, J. A. (2020). Protozoa and virus disinfection by silver- and copper-embedded ceramic tables for water purification. Journal of Environmental Engineering, 146(4), 04020015. https://doi.org/https://doi.org/10.1061/(ASCE)EE.1943-7870.0001664
- Ehrlich, H. L. (2008). Are gram-positive bacteria capable of electron transfer across their cell wall without an externally available electron shuttle? Geobiology, 6(3), 220–224. https://doi.org/https://doi.org/10.1111/j.1472-4669.2007.00135.x
- El-Badawy, A. M. (2011). Surface charge-dependent toxicity of silver nanoparticles. Environmental Science and Technology, 45(1), 283–287.
- Fabrega, J., Fawcett, S. R., Renshaw, J. C., & Lead, J. R. (2009). Silver nanoparticle impact on bacterial growth: Effect of pH, concentration, and organic matter. Environmental Science & Technology, 43(19), 7285–7290. https://doi.org/https://doi.org/10.1021/es803259g
- Fauss, E. K., MacCuspie, R. I., Oyanedel-Craver, V., Smith, J. A., & Swami, N. S. (2014). Disinfection action of electrostatic versus steric-stabilized silver nanoparticles on E. coli under different water chemistries. Colloids and Surfaces B: Biointerfaces, 113, 77–84. https://doi.org/https://doi.org/10.1016/j.colsurfb.2013.08.027
- Feng, Q. L., Wu, J., Chen, G. Q., Cui, F. Z., Kim, T. N., & Kim, J. O. (2000). A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of Biomedical Materials Research, 52(4), 662–668. https://doi.org/https://doi.org/10.1002/1097-4636(20001215)52:4<662::AID-JBM10>3.0.CO;2-3
- Fewtrell, L., Majuru, B., & Hunter, P. R. (2017). A re-assessment of the safety of silver in household water treatment: Rapid systematic review of mammalian in vivo genotoxicity studies. Environmental Health : a Global Access Science Source, 16(1), 66. https://doi.org/https://doi.org/10.1186/s12940-017-0279-4
- Foster, H. A., Ditta, I. B., Varghese, S., & Steele, A. (2011). Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Applied Microbiology and Biotechnology, 90(6), 1847–1868. https://doi.org/https://doi.org/10.1007/s00253-011-3213-7
- Francis, M. R. (2015). Perceptions of drinking water safety and factors influencing acceptance and sustainability of a water quality intervention in rural Southern India. MBC Public Health, 15(731), 1–9.
- Gitanjali, B., & Weerasuriya, K. (2011). The curious case of zinc for diarrhea: Unavailable, unprescribed, and unused. Journal of Pharmacology & Pharmacotherapeutics, 2(4), 225–229. https://doi.org/https://doi.org/10.4103/0976-500X.85933
- Grass, G., Rensing, C., & Solioz, M. (2011). Metallic copper as an antimicrobial surface. Applied and Environmental Microbiology, 77(5), 1541– 1547. https://doi.org/https://doi.org/10.1128/AEM.02766-10
- Guerrero-Latorre, L., Rusiñol, M., Hundesa, A., Garcia-Valles, M., Martinez, S., Joseph, O., Bofill-Mas, S., & Girones, R. (2015). Development of improved low-cost ceramic water filters for viral removal in the Haitian context. Journal of Water, Sanitation and Hygiene for Development, 5(1), 28–39. https://doi.org/https://doi.org/10.2166/washdev.2014.121
- He, D., Garg, S., & Waite, T. D. (2012). H2O2-mediated oxidation of zero-valent silver and resultant interactions among silver nanoparticles, silver ions, and reactive oxygen species. Langmuir : The ACS Journal of Surfaces and Colloids, 28(27), 10266–10275. https://doi.org/https://doi.org/10.1021/la300929g
- Helmlinger, J., Sengstock, C., Groß-Heitfeld, C., Mayer, C., Schildhauer, T. A., Köller, M., & Epple, M. (2016). Silver nanoparticles with different size and shape: Equal cytotoxicity but different antibacterial effects. RSC Advances, 6(22), 18490–18501. https://doi.org/https://doi.org/10.1039/C5RA27836H
- Heltberg, R. (2009). Malnutrition, poverty and economic growth. Health Economics, 18(S1), S77–S88. https://doi.org/https://doi.org/10.1002/hec.1462
- Hirota, K., Sugimoto, M., Kato, M., Tsukagoshi, K., Tanigawa, T., & Sugimoto, H. (2010). Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceramics International, 36(2), 497–506. https://doi.org/https://doi.org/10.1016/j.ceramint.2009.09.026
- Hong, T., Smith, J., & Srolovitz, D. J. (1995). Theory of metal-ceramic adhesion. Acta Metallurgica Et Materialia, 43(7), 2721–2730. https://doi.org/https://doi.org/10.1016/0956-7151(94)00457-S
- Howe, J. M. (1997). Interfaces in materials: Atomic structure, thermodynamics and kintecis of solid-vapor, solid-liquid and solid-solid interfaces. Wiley.
- Huynh, K. A., & Chen, K. L. (2011). Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environmental Science & Technology, 45(13), 5564–5571. https://doi.org/https://doi.org/10.1021/es200157h
- Innovation for Development and South-South Cooperation. n.d. Filtron: Ceramic filter for drinking water. Potters for Peace.
- Jackson, K. N., & Smith, J. A. (2018). A new method for the deposition of metallic silver on porous ceramic water filters. Journal of Nanotechnology, 2018, 1–9. https://doi.org/https://doi.org/10.1155/2018/2573015
- Jackson, K. N., Smith, J. A., & Edokpayi, J. N. (2019). New method for the deposition of metallic silver and metallic copper on full-size porous ceramic water filters. Environmental Engineering Science, 36(1), 2–11. https://doi.org/https://doi.org/10.1089/ees.2018.0149
- Jin, X., Li, M., Wang, J., Marambio-Jones, C., Peng, F., Huang, X., Damoiseaux, R., & Hoek, E. M. V. (2010). High-throughput screening of silver nanoparticle stability and bacterial inactivation in aquatic media: Influence of specific Ions. Environmental Science & Technology, 44(19), 7321–7328. https://doi.org/https://doi.org/10.1021/es100854g
- Joe, A., Park, S.-H., Shim, K.-D., Kim, D.-J., Jhee, K.-H., Lee, H.-W., Heo, C.-H., Kim, H.-M., & Jang, E.-S. (2017). Antibacterial mechanism of ZnO nanoparticles under dark conditions. Journal of Industrial and Engineering Chemistry, 45(25), 430–439. https://doi.org/https://doi.org/10.1016/j.jiec.2016.10.013
- Kahler, D., Koermer, N., Reichl, A., Samie, A., & Smith, J. (2016). Performance and acceptance of novel silver-impregnated ceramic cubes for drinking water treatment in two field sites: Limpopo Provice, South Africa and Dodoma Region. Water, 8(3), 95. https://doi.org/https://doi.org/10.3390/w8030095
- Kashida, S. (2003). Electronic structure of Ag2S, band calculation and photoelectron spectroscopy. Solid State Ionics., 158(1-2), 167–175. https://doi.org/https://doi.org/10.1016/S0167-2738(02)00768-3
- Kowalski, K. (2008). The disinfection efficiency of E. coli by the ceramic water filter. In: Removal of virus-sized particles and E. coli by the potters for peace ceramic water filter (pp. 114–208). Masters of Science.
- Lantagne, D., Klarman, M., Mayer, A., Preston, K., Napotnik, J., & Jellison, K. (2010). Effect of production variables on microbiological removal in locally-produced ceramic filters for Household water treatment. International Journal of Environmental Health Research, 20(3), 171–1619. https://doi.org/https://doi.org/10.1080/09603120903440665
- Larimer, C. J. (2013). Effect of silver nanoparticle coatings on mycobacterial biofilm attachment and growth: Implications for ceramic water filters [PhD Thesis] Swanson School of Engineering.
- Le Ouay, B., & Stellacci, F. (2015). Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today., 10(3), 339–354. https://doi.org/https://doi.org/10.1016/j.nantod.2015.04.002
- Lemire, J. A., Harrison, J. J., & Turner, R. J. (2013). Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nature Reviews. Microbiology, 11(6), 371–384. https://doi.org/https://doi.org/10.1038/nrmicro3028
- Levard, C., Mitra, S., Yang, T., Jew, A. D., Badireddy, A. R., Lowry, G. V., & Brown, G. E. (2013). Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli. Environmental Science & Technology, 47(11), 5738–5745. https://doi.org/https://doi.org/10.1021/es400396f
- Liu, J., & Hurt, R. H. (2010). Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environmental Science & Technology, 44(6), 2169–2175. https://doi.org/https://doi.org/10.1021/es9035557
- Lok, C.-N., Ho, C.-M., Chen, R., He, Q.-Y., Yu, W.-Y., Sun, H., Tam, P. K.-H., Chiu, J.-F., & Che, C.-M. (2007). Silver nanoparticles: Partial oxidation and antibacterial activities. Journal of Biological Inorganic Chemistry : JBIC : a Publication of the Society of Biological Inorganic Chemistry, 12(4), 527–534. https://doi.org/https://doi.org/10.1007/s00775-007-0208-z
- Lucier, K. J., Dickson-Anderson, S. E., & Schuster-Wallace, C. J. (2017). Effectiveness of silver and copper infused ceramic drinking water filters in reducing microbiological contaminants. Journal of Water Supply: Research and Technology, 66(7), 528–536.
- Luoto, J., Mahmud, M., Albert, J., Luby, S., Najnin, N., Unicomb, L., & Levine, D. I. (2012). Learning to dislike safe water products: Results from a randomized controlled trial of the effects of direct and peer experience on willingness to pay. Environmental Science & Technology, 46(11), 6244–6251. Volume https://doi.org/https://doi.org/10.1021/es2027967
- Lyon-Marion, B. A., Mittelman, A. M., Rayner, J., Lantagne, D. S., & Pennell, K. D. (2018). Impact of chlorination on silver elution from ceramic water filters. Water Research, 142, 471–479. Volume https://doi.org/https://doi.org/10.1016/j.watres.2018.06.008
- Malik, A., Taneja, D. K., Devasenapathy, N., & Rajeshwari, K. (2013). Short-course prophylactic zinc supplementation for diarrhea morbidity in infants of 6 to 11 months. Pediatrics, 132(1), e46. https://doi.org/https://doi.org/10.1542/peds.2012-2980
- Maret, W. (2004). Zinc and sulfur: A critical biological partnership. Biochemistry, 43(12), 3301–3309. https://doi.org/https://doi.org/10.1021/bi036340p
- Matsumura, Y., Yoshikata, K., Kunisaki, S-i., & Tsuchido, T. (2003). Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Applied and Environmental Microbiology, 69(7), 4278–4281. https://doi.org/https://doi.org/10.1128/aem.69.7.4278-4281.2003
- McEvoy, J. G., & Zhang, Z. (2014). Antimicrobial and photocatalytic disinfection mechanisms in silver-modified photocatalysts under dark and light conditions. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 19, 62–75. Volume https://doi.org/https://doi.org/10.1016/j.jphotochemrev.2014.01.001
- Mikelonis, A. M., Rowles, III, L. S., & Lawler, D. F. (2020). The effects of water chemistry on the detachment and dissolution of differently stabilized silver nanoparticles for ceramic membranes. Water Research and Technology.
- Mikelonis, A. M., Lawler, D. F., & Passalacqua, P. (2016). Multilevel modeling of retention and disinfection efficacy of silver nanopartiles on ceramic water filters. Science of the Total Environment, 566-567, 368–377. https://doi.org/https://doi.org/10.1016/j.scitotenv.2016.05.076
- Mikelonis, A. M., Youn, S., & Lawler, D. F. (2016). DLVO approximation methods for predicting the attachment of silver nanoparticles to ceramic membranes. Langmuir : The ACS Journal of Surfaces and Colloids, 32(7), 1723–1731. https://doi.org/https://doi.org/10.1021/acs.langmuir.5b04675
- Mittelman, A. M., Lantagne, D. S., Rayner, J., & Pennell, K. D. (2015). Silver dissolution and release from ceramic water filters. Environmental Science & Technology, 49(14), 8515–8522. https://doi.org/https://doi.org/10.1021/acs.est.5b01428
- Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ramírez, J. T., & Yacaman, M. J. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16(10), 2346–2353. https://doi.org/https://doi.org/10.1088/0957-4484/16/10/059
- Murphy, H. M., McBean, E. A., & Farahbakhsh, K. (2009). Appropriate technology - A comprehensive approach forwater and sanitation in the developing world. Technology in Society, 31(2), 158–167. https://doi.org/https://doi.org/10.1016/j.techsoc.2009.03.010
- Murray, M., Simpson, D., & Fox, J. (2011). Newly Designed ceramic water pot for low-income households. Program for Appropriate Technology in Health (PATH).
- Nguyen, T. A. T. (2014). Bactericidal activity and silver release of porous ceramic candle filter prepared by sintering silica with silver nanoparticles/zeolite for water disinfection. Andvances in Natural Science: Nanoscience and Nanotechnology, 5(3), 1–6.
- Nunnelley, K., Smith, J. A., Smith, M. Y., & Samie, A. (2016). A new method for nanosilver application in ceramic water filters [Paper presentation]. s.l., s.n. https://doi.org/https://doi.org/10.1061/9780784479865.031
- Oyanedel-Craver, V. A., & Smith, J. A. (2008). Sustainable colloidal-silver-impregnated ceramic filter for point-of-use water treatment. Environmental Science & Technology, 42(3), 927–933. https://doi.org/https://doi.org/10.1021/es071268u
- Padmavathy, N., & Vijayaraghavan, R. (2008). Enhanced bioactivity of ZnO nanoparticles - An antimicrobial insight. Science and Technology of Advanced Materials, 9(3), 035004. https://doi.org/https://doi.org/10.1088/1468-6996/9/3/035004
- Pagsuyoin, S. A., Santos, J. R., Latayan, J. S., & Barajas, J. R. (2015). A multi-attribute decision-making approach to the selection of point-of-use water treatment. Environment Systems and Decisions, 35(4), 437–452. Volume https://doi.org/https://doi.org/10.1007/s10669-015-9567-0
- Pal, S., Tak, Y. K., & Song, J. M. (2007). Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of gram negative bacterium Escherichia coli. Applied and Environmental Microbiology, 73 (6), 1712–1720. https://doi.org/https://doi.org/10.1128/AEM.02218-06
- Panasewicz, L. (2011). Evaluation of enhanced ceramic water filtration (ECWF) Systems for the removal of turbidity and bacteria for househods in developing countries. Master f Science Thesis at the University of Colorado at Boulder Department of Civil, Environmental and Architectural Engineering.
- Pandey, P., Merwyn, S., Agarwal, G. S., Tripathi, B. K., & Pant, S. C. (2012). Electrochemical synthesis of multi-armed CuO nanoparticles and their remarkable bactericidal potential against waterborne bacteria. Journal of Nanoparticle Research, 14(1), 709. https://doi.org/https://doi.org/10.1007/s11051-011-0709-0
- Park, H.-J., Kim, J. Y., Kim, J., Lee, J.-H., Hahn, J.-S., Gu, M. B., & Yoon, J. (2009). Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Research, 43(4), 1027–1032. https://doi.org/https://doi.org/10.1016/j.watres.2008.12.002
- Qu, X., Alvarez, P. J. J., & Li, Q. (2013). Applications of nanotechnology in water and wastewater treatment. Water Research, 47(12), 3931–3946. https://doi.org/https://doi.org/10.1016/j.watres.2012.09.058
- Rai, M., Yadav, A., & Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances, 27(1), 76–83. https://doi.org/https://doi.org/10.1016/j.biotechadv.2008.09.002
- Rayner, J. (2009). Current practices in manufacturing of ceramic pot filters for water treatment. Department of Civil and Building Engineering, University of Loughborough.
- Rayner, J., Luo, X., Schubert, J., Lennon, P., Jellison, K., & Lantagne, D. (2017). the effects of input materials on ceramic water filter efficacy for household drinking water treatment. Water Supply, 17(3), 859–869. https://doi.org/https://doi.org/10.2166/ws.2016.176
- Rayner, J., Zhang, H., Schubert, J., Lennon, P., Lantagne, D., & Oyanedel-Craver, V. (2013). Laboratory investigation into the effect of silver application on the bacterial removal efficacy of filter material for use on locally produced ceramic water filters for household drinking water treatment. ACS Sustainable Chemistry & Engineering, 1(7), 737–745. https://doi.org/https://doi.org/10.1021/sc400068p
- Ren, D., & Smith, J. A. (2013). Retention and transport of silver nanoparticles in a ceramic porous medium used for point-of-use water treatment. Environmental Science & Technology, 47(8), 3825–3832. Volume https://doi.org/https://doi.org/10.1021/es4000752
- Ruparelia, J. P., Chatterjee, A. K., Duttagupta, S. P., & Mukherji, S. (2008). Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomaterialia, 4(3), 707–716. https://doi.org/https://doi.org/10.1016/j.actbio.2007.11.006
- Salt Lake Metals. (2017). Solubility of silver compounds in water. [Online] https://saltlakemetals.com/solubility_of_silver_compounds/
- Santos, J., Pagsuyoin, S. A., & Latayan, J. (2016). A multi-criteria decision analysis framework for evaluating point-of-use water treatment technologies. Clean Technologies and Environmental Policy, 18(5), 1263–1279. Volume https://doi.org/https://doi.org/10.1007/s10098-015-1066-y
- Scannell, L. W. (2016). Removal of bacteria. In: An analysis of performance criteria of porous ceramic water filter production methods. Proquest Dissertation Publication. (Thesis), pp. 59–75.
- Schweitzer, R. W., Cunningham, J. A., & Mihelcic, J. R. (2013). Hydraulic modeling of clay ceramic water filters for point-of-use water treatment. Environmental Science & Technology, 47(1), 429–435. https://doi.org/https://doi.org/10.1021/es302956f
- Servi, A. T., Kang, P. K., Frey, D., & Murcott, S. (2013). A holistic optimization framework for improving ceramic pot filter performance. San Jose, s.n.
- Shuang, T. H., Batchelor-Mcauley, C., Tschulik, K., & Compton, R. G. (2014). Chemical interactions between silver nanoparticles and thiols: a compatison of mercaptohexanol against cysteine. Science China Chemistry, 57(9), 1199–1210. https://doi.org/https://doi.org/10.1007/s11426-014-5141-8
- Shwarzenbach, R. P., Gschwend, P. M., & Imboden, D. M. (2003). Thermodynamic considerations for redox reactions: Processes determining the redox conditions in the environment. In: Environmental organic chemistry: Second Edition (pp. 569–572). John Wiley & Sons Inc.
- Sirelkhatim, A., Mahmud, S., Seeni, A., Kaus, N. H. M., Ann, L. C., Bakhori, S. K. M., Hasan, H., & Mohamad, D. (2015). Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanisms. Nano-Micro Letters, 7(3), 219–242. https://doi.org/https://doi.org/10.1007/s40820-015-0040-x
- Slavin, Y. N., Asnis, J., Hafeli, U. O., & Bach, H. (2017). Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. Journal of Nanobiotechnology, 15(1), 65–20. https://doi.org/https://doi.org/10.1186/s12951-017-0308-z
- Sobsey, M. D., Stauber, C. E., Casanova, L. M., Brown, J. M., & Elliott, M. A. (2008). Point of use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world. Environmental Science & Technology, 42(12), 4261–4267. Volume https://doi.org/https://doi.org/10.1021/es702746n
- Sondi, I., & Salopek-Sondi, B. (2004). Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria. Journal of Colloid and Interface Science, 275(1), 177–182. https://doi.org/https://doi.org/10.1016/j.jcis.2004.02.012
- Song, W., Zhang, J., Guo, J., Zhang, J., Ding, F., Li, L., & Sun, Z. (2010). Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicology Letters, 199(3), 389–397. https://doi.org/https://doi.org/10.1016/j.toxlet.2010.10.003
- Soppe, A. I. A., Heijman, S. G. J., Gensburger, I., Shantz, A., van Halem, D., Kroesbergen, J., Wubbels, G. H., & Smeets, P. W. M. H. (2015). Critical Parameters in the production of ceramic pot filters for household water treatment in developing countries. Journal of Water and Health, 13(2), 587–599. https://doi.org/https://doi.org/10.2166/wh.2014.090
- Stoimenov, P. K., Klinger, R. L., Marchin, G. L., & Klabunde, K. J. (2002). Metal oxide nanoparticles as bactericidal agents. Langmuir, 18(17), 6679–6686. https://doi.org/https://doi.org/10.1021/la0202374
- Sullivan, R. K., Erickson, M., & Oyanedel-Craver, V. A. (2017). Understanding microbiological, organic and inorganic contaminnt removal capacity of ceramic water filters doped with different silver nanoparticles. Environmental Science: Nano, 4(12), 2348–2355. Volume https://doi.org/https://doi.org/10.1039/C7EN00443E
- Thurman, R. B., Gerba, C. P., & Bitton, G. (1989). The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Critical Reviews in Environmental Control, 18(4), 295–315. https://doi.org/https://doi.org/10.1080/10643388909388351
- UNESCO. (2015). The united nations world water development report 2015: Water for a sustainable world. UNESCO.
- Unuabonah, E. I., Ugwuja, C. G., Omorogie, M. O., Adewuyi, A., & Oladoja, N. A. (2018). Clays for efficient disinfection of bacteria in water. Applied Clay Science, 151, 211–223. Volume https://doi.org/https://doi.org/10.1016/j.clay.2017.10.005
- van der Laan, H., van Halem, D., Smeets, P. W. M. H., Soppe, A. I. A., Kroesbergen, J., Wubbels, G., Nederstigt, J., Gensburger, I., & Heijman, S. G. J. (2014). Bacteria and virus removal effectiveness of ceramic pot filters with different silver applications in a long term experiment. Water Research, 51, 47–54. Volume https://doi.org/https://doi.org/10.1016/j.watres.2013.11.010
- van Halem, D., van der Laan, H., Heijman, S. G. J., van Dijk, J. C., & Amy, G. L. (2009). Assessing the sustainability of the silver-impregnated ceramic pot filter for low cost household drinking water treatment. Physics and Chemistry of the Earth, 34(1-2), 36–42. Volume https://doi.org/https://doi.org/10.1016/j.pce.2008.01.005
- van Halem, D., van der Laan, H., Soppe, A., & Heijman, S. (2017). High flow ceramic pot filters. Water Research, 124, 398–406. https://doi.org/https://doi.org/10.1016/j.watres.2017.07.045
- Varkey, A., & Dlamini, M. D. (2012). Point of use water purification using clay pot water filters and copper mesh. Water Sa, 38(5), 721–726. https://doi.org/https://doi.org/10.4314/wsa.v38i5.10
- WHO. (2011). Guidelines for drinking-water quality. World Health Organization.
- World Health Organization; UNICEF. (2014). Progress on drinking water and sanitation: 2014 update. WHO; UNICEF.
- World Health Organization; UNICEF. (2017). Water and sanitation for health facility improvement tool (WASH FIT). World Health Organization.
- World Health Organization. (1996). Drinking water quality: Zinc in drinking water. World Health Organization.
- World Health Organization. (1996). Silver in drinking water. World Health Organization.
- World Health Organization. (2004). Copper in drinking water. World Health Organization.
- World Health Organization. (2006). Guidelines for Drinking Water Quality: Volume 1. World Health Organization.
- World Health Organization. (2017). Diarrheal disease fact sheet. [Online]. www.who.int
- Wu, Y., Yang, Y., Zhang, Z., Wang, Z., Zhao, Y., & Sun, L. (2018). A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Advanced Powder Technology, 29(2), 407–415. https://doi.org/https://doi.org/10.1016/j.apt.2017.11.028
- Xiu, Z.-M., Ma, J., & Alvarez, P. J. (2011). DIfferential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environmental Science & Technology, 45(20), 9003–9008. https://doi.org/https://doi.org/10.1021/es201918f
- Yakub, I., Plappally, A., Leftwich, M., Malatesta, K., Friedman, K. C., Obwoya, S., Nyongesa, F., Maiga, A. H., Soboyejo, A. B. O., Logothetis, S., & Soboyejo, W. (2013). Porosity, flow and filtration characteristics of frustum-shaped ceramic water filters. Journal of Environmental Engineering, 139(7), 986–1161. https://doi.org/https://doi.org/10.1061/(ASCE)EE.1943-7870.0000669
- Yakub, I., & Soboyejo, W. O. (2012). Adhesion of E. coli to silver- or copper-coated porous clay ceramic surfaces. Journal of Applied Physics, 111(12), 124324. pp. 124324-1 - 124324-9. https://doi.org/https://doi.org/10.1063/1.4722326
- Yamanaka, M., Hara, K., & Kudo, J. (2005). Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis . Applied and Environmental Microbiology, 71(11), 7589–7593. https://doi.org/https://doi.org/10.1128/AEM.71.11.7589-7593.2005
- Yoon, K.-Y., Byeon, J. H., Park, J.-H., & Hwang, J. (2007). Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. The Science of the Total Environment, 373(2-3), 572–575. https://doi.org/https://doi.org/10.1016/j.scitotenv.2006.11.007
- Zhang, H., & Oyanedel-Craver, V. (2013). Comparison of the Bacterial removal performance of silver nanoparticles and a polymer based quaternary amine functiaonalized silsesquioxane coated point-of-use ceramic water filters. Journal of Hazardous Materials, 260, 272–277. https://doi.org/https://doi.org/10.1016/j.jhazmat.2013.05.025
- Zhang, H., Smith, J. A., & Oyanedel-Craver, V. (2012). The effect of natural water conditions on the anti-bacterial performance and stability of silver nanoparticles capped with different polymers. Water Research, 46(3), 691–699. https://doi.org/https://doi.org/10.1016/j.watres.2011.11.037