?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Arsenic and antimony are metalloids that exhibit similar geochemical behavior and are often found in the same mineral associations, co-occurring in mine waste derived from ore exploitation. Due to their adverse effects on ecosystems and human health, techniques to limit their release into the environment from mine wastes and the consequent pollution of surrounding areas are of great interest. This review addresses the geochemical behavior of these two metalloids in mining environments and presents an overview of the main strategies used to stabilize or encapsulate As- or Sb-bearing mine waste, highlighting and comparing their shortcomings and advantages. Several approaches have been investigated, including the 1) establishment of surface covers, 2) application of amendments, 3) solidification using cementing or geopolymer, and 4) application of protecting coatings. Several of these methods have exhibited potential for treating As mine waste. However, the weathering state, composition, and final fate of wastes are determining factors affecting treatment effectiveness. Regarding Sb mine waste, only cementation processes have shown some good performance. Further research effort is required to develop effective passivating coatings or stabilization methods to treat these mine wastes.
Graphical abstract
HANDLING EDITORS:
1. Arsenic and antimony: Sources and toxicity
Arsenic (As) and antimony (Sb) are trace elements with metalloid properties, showing a wide distribution in the environment (Kabata-Pendias & Mukherjee, Citation2007). Both are chalcophile elements and usually co-occur in hydrothermal sulfide ores (Johnston et al., Citation2020). Moreover, both exhibit similar geochemical behavior and tend to coexist (Herath et al., Citation2017).
Arsenic is the 20th most abundant element in the Earth’s crust. It is observed at variable concentrations, usually between 0.5 and 2.5 mg kg−1 (Mandal & Suzuki, Citation2002; Kabata-Pendias & Mukherjee, Citation2007), and it exists in four primary oxidation states: (+V), (+III), (0), and (-III) (Wang & Mulligan, Citation2006). The first two states are the most common in soils and waters (Mandal & Suzuki, Citation2002). Arsenic(V) is the dominant under oxic conditions, whereas As(III) is observed under anaerobic conditions. The As concentration in uncontaminated soils and fresh waters is typically in the ranges of 1–40 mg kg−1 and 1–10 µg L−1, respectively (Mandal & Suzuki, Citation2002). Under the usual pH values of these environmental compartments, the occurring inorganic forms are mainly H2AsO4−, HAsO42−, and H3AsO3 (Mandal & Suzuki, Citation2002). These oxidation states can also form organic compounds via methylation. This process is performed by microorganisms, originating monomethylated, dimethylated, and trimethylated species of As(III) (MMA(III), DMA(III), and TMA(III), respectively) and As(V) (MMA(V), DMA(V), and TMAO(V), respectively) (Mandal & Suzuki, Citation2002; Wang & Mulligan, Citation2006). The toxicity of As largely depends on its chemical form, varying according to the following sequence: MMA(III) ≈ DMA(III) > As(III) > As(V) > MMA(V) ≈ DMAs(V) > TMA(III) ≈ TMAO(V) (Di et al., Citation2019). Its toxic forms can cause many adverse health effects to humans. Particularly, chronic intake via water or food can induce skin lesions (hyperpigmentation, keratosis, and ulceration), cardiovascular diseases, respiratory system problems, nervous system disorders, immunological and hematological alterations, reproductive complications, and cancers (specifically in the skin, bladder, and lungs). Its chronic inhalation primarily affects the skin and respiratory and reproductive systems, and the most adverse effect from this exposure route is the development of skin and lung cancers (Mandal & Suzuki, Citation2002).
Antimony is less abundant than As in the Earth’s crust, ranking 62th, with usual contents varying between 0.1 and 0.9 mg kg−1 (Kabata-Pendias & Mukherjee, Citation2007). Like As, it can exist in four oxidation states: (+V), (+III), (0), and (-III) (Filella et al., Citation2002; Wilson et al., Citation2010; Saerens et al., Citation2019), with the first two states primarily observed in soils and waters (Filella et al., Citation2002). Antimony(V) dominates in oxic environments, while Sb(III) prevails under reducing conditions. Typical Sb concentrations in uncontaminated soils and fresh waters are less than 10 mg kg−1 (Wilson et al., Citation2010) and 1 µg L−1 (Filella et al., Citation2002), respectively. Under mildly acid, neutral, or alkaline conditions, the main occurring inorganic species are [Sb(OH)6]− and Sb(OH)3 (Filella et al., Citation2002). Similarly to As, Sb can form organic compounds via methylation, with methylstibonic acid (MSA), dimethylstibonic acid (DMSA), and trimethylstiboxide being the most common organic forms (Wilson et al., Citation2010). The toxicity of Sb also greatly depends on its chemical species, varying in the following order: Sb (III) > Sb(V) > methylated species (Filella et al., Citation2002; Wilson et al., Citation2010). Intoxication with Sb can occur through dermal contact, inhalation, and oral ingestion (Saerens et al., Citation2019). In humans, long-term exposure can lead to the development of respiratory problems (e.g., chronic bronchitis, emphysema, pleural adhesions, and pneumoconiosis), immune system alterations, cardiovascular diseases, gastrointestinal problems, reproductive disorders, and lung cancer (Saerens et al., Citation2019).
Both As and Sb are readily mobilizable by geogenic and anthropogenic processes. The main common anthropogenic sources of these two metalloids into the environment are mining operations, metallurgical processes, energy generation from fossil fuels, and ceramic/glass and pigment manufacturing industries. Additionally, agricultural practices are an important source of As, while waste incineration, road traffic, and shooting practices are also significant sources of Sb (Alloway, Citation1995; Kabata-Pendias & Mukherjee, Citation2007). Due to their wide distribution and adverse effects on environment and living organisms, these trace elements are considered priority pollutants by the US EPA (United States Environmental Protection Agency).
2. Geochemical behavior of arsenic and antimony
Distinct geochemical processes govern the mobility of these metalloids in the environment, including precipitation–dissolution, adsorption–desorption, and oxidation–reduction reactions.
Arsenic is readily adsorbed by Fe (oxyhydr)oxides, with low crystalline/amorphous compounds showing the highest adsorption capacities. The maximum adsorption values for As(V) are reached at pH values of 2–4, decreasing progressively as pH increases (Wang & Mulligan, Citation2006; Lee et al., Citation2014). The highest adsorption values for As(III) are attained between pH 7 and 10 (Wang & Mulligan, Citation2006). The adsorption of As(V) on Fe (oxyhydr)oxides mainly takes place via bidentate inner-sphere complexes; this mechanism is also the preferentially involved in the adsorption of As(III), although it may also form outer-sphere surface complexes on low crystalline/amorphous compounds (Waychunas et al., Citation1993; Wang & Mulligan, Citation2008). These compounds, when generated in situ, can also remove both As(V) and As(III) via co-precipitation processes, attaining greater As loads and, therefore, greater As removal efficiencies (Waychunas et al., Citation1993; Kadokura et al., Citation2019). This process plays an important role in the natural attenuation of As in mine scenarios where the weathering of iron sulfides leads to the precipitation of Fe (oxyhydr)oxides (Moldovan et al., Citation2003; Ritchie et al., Citation2013). Additionally, Fe (oxyhydr)oxides can oxidize As(III); this reaction is catalyzed in the presence of light (Wang & Mulligan, Citation2006). Aluminum (oxyhydr)oxides are effective As adsorbents as well, with amorphous compounds showing also much higher adsorption capacities. The adsorption of As(V) is highest within the pH range of 4–6 (Álvarez-Ayuso & Murciego, Citation2021), whereas that of As(III) is reached at the pH range of 6–9.5 (Wang & Mulligan, Citation2006). The adsorption of As(V) by Al (oxyhydr)oxides occurs via the formation of inner-sphere bidentate-binuclear complexes (Gräfe et al., Citation2008; Wang & Mulligan, Citation2008), whereas As(III) is adsorbed by both inner- and outer-sphere complexes on crystalline phases and only by the latter on amorphous compounds (Wang & Mulligan, Citation2008). Manganese (oxyhydr)oxides possess negative surface charges under a wide pH range (above approximately 2.3) (Wang & Mulligan, Citation2006), which hinders As adsorption. Despite this limitation, this process has been found to proceed through the generation of fresh adsorption sites for As(V) resulting from the oxidation of As(III) (Wang & Mulligan, Citation2006). Arsenic(V) is adsorbed on these surfaces through the formation of inner-sphere bidentate-binuclear complexes (Wang & Mulligan, Citation2008).
Antimony is also readily adsorbed by Fe, Al, and Mn (oxyhydr)oxides (Mitsunobu et al., Citation2010; Wilson et al., Citation2010; Biver et al., Citation2011; Ilgen & Trainor, Citation2012). Antimony(III) is adsorbed by these compounds over a wide pH range, whereas the adsorption of Sb(V) is significantly reduced under neutral and alkaline conditions (Wilson et al., Citation2010). The adsorption of both oxidation states occurs via the formation of bidentate inner-sphere complexes on Fe (oxyhydr)oxides (Mitsunobu et al., Citation2010; Wilson et al., Citation2010) as well as bidentate and monodentate inner-sphere complexes on Al (oxyhydr)oxides (Ilgen & Trainor, Citation2012). When generated in situ, Fe (oxyhydr)oxides also remove Sb(V) via co-precipitation, incorporating it in their structure (Mitsunobu et al., Citation2010). This process has been suggested to be an important pathway for attenuating Sb in mining areas (Ritchie et al., Citation2013). Additionally, Fe (oxyhydr)oxides can catalyze the oxidation of Sb(III), with the oxidation rate increasing with the amorphous character of Fe compounds (Wilson et al., Citation2010). As occurs with As(V), the oxidation of Sb(III) is also the process involved in the adsorption of Sb(V) by Mn (oxyhydr)oxides (Wilson et al., Citation2010; Sun et al., Citation2019). This reaction generates exposed reactive sites for Sb(V) adsorption, which occurs by the formation of inner monodentate mononuclear complexes (Sun et al., Citation2019).
Organic matter (OM) also plays an important role in the mobility of these metalloids, mainly through adsorption–desorption and oxidation–reduction reactions. Arsenic, in both oxidation states, can be strongly adsorbed by OM forming ternary complexes in which cationic metals mediate this binding (Ko et al., Citation2004). Nevertheless, As(III) and As(V), particularly the former, can form soluble complexes with OM, thus increasing their mobility (Ko et al., Citation2004). Moreover, under acidic conditions, OM may compete with As for adsorption sites on metal (oxyhydr)oxides (Grafe et al., Citation2002). Organic matter can also catalyze redox reactions of As species (Ko et al., Citation2004). Contrary to As, Sb can be bound directly to OM, particularly in its reduced state (Wilson et al., Citation2010). The adsorption of Sb(III) by OM is greatly decreased above moderately acidic pH values (Wilson et al., Citation2010). In this regard, it has been found that the increase in pH coupled with the increase in OM content promotes Sb mobility in polluted areas (Nakamaru & Martín Peinado, Citation2017). Humic acids present in OM can catalyze the oxidation of Sb(III) (Wilson et al., Citation2010), which can lead to the increased mobilization of this metalloid due to increased Sb(V) mobility.
Clay minerals have a much less significant impact on the mobility of these metalloids as their capacity to adsorb either As (Lin & Puls, Citation2000) or Sb (Xi et al., Citation2011; Biver et al., Citation2011) is much lower than that exhibited by metal (oxyhydr)oxides as well as that exhibited by OM with regard to Sb. In general, clay minerals show higher adsorption capacities for As(V) and Sb(III) than for As(III) and Sb(V), respectively (Lin & Puls, Citation2000; Xi et al., Citation2011). Arsenic or Sb adsorption is performed through variable charges present in the broken bonds or edges of clay minerals (Wilson et al., Citation2010).
3. Characteristics of arsenic- or antimony-bearing mine waste
3.1. Arsenic-bearing mine waste
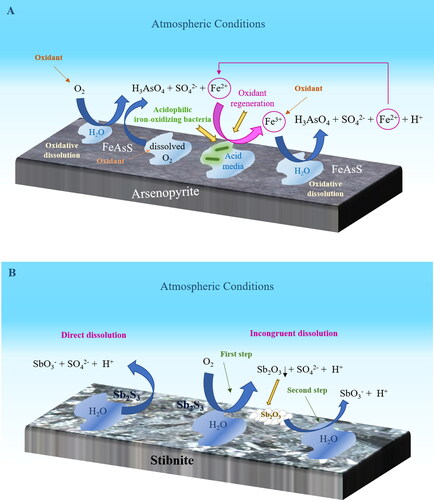
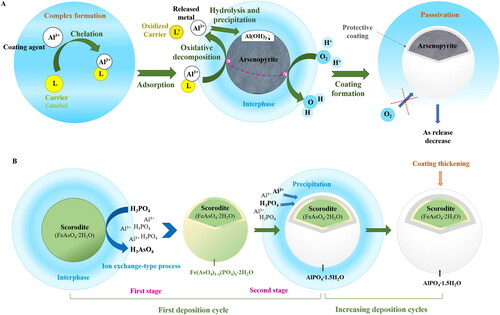
Over 300 minerals can contain or host As. Most are arsenates (roughly 60%) and sulfides or sulfosalts (roughly 20%); the remaining are primarily arsenides, arsenites, oxides, and elemental As (Alloway, Citation1995; Drahota & Filippi, Citation2009). The common primary minerals of As include arsenopyrite (FeAsS), orpiment (As2S3), and realgar (AsS) (Alloway, Citation1995; Kabata-Pendias & Mukherjee, Citation2007). Of these, arsenopyrite is the most common. It is present in many deposits of different origins along with diverse ore minerals source of valuable elements (e.g., Au, Ag, Co, Cu, Pb, Ni, Zn, Sb, Sn, and W) (Alloway, Citation1995). The exploitation of these deposits has generated high amounts of mine waste with important contents of arsenopyrite. This mineral is stable under reducing conditions (Craw et al., Citation2003). Nevertheless, oxidizing conditions dissolve it. The oxidation of arsenopyrite proceeds ()
via oxygen (Mok & Wai, Citation1994):
(1)
(1)
via Fe(III) (Dove & Rimstidt, Citation1985):
(2)
(2)
The oxidation of arsenopyrite occurs more rapidly when facilitated by Fe(III) (Yu et al., Citation2007). Furthermore, the arsenopyrite reactivity is influenced by other factors such as galvanic and biological effects and reaction media. Thus, the occurrence of pyrite (FeS2) together with arsenopyrite increases the solubilization of the latter (Dos Santos et al., Citation2017). Additionally, the oxidation of arsenopyrite can be facilitated by iron-oxidizing bacteria, which are usually present in acid mine drainage (e.g., Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans) (Lottermoser, Citation2010). The biological process occurs more quickly and extensively than the chemical one (Corkhill et al., Citation2008). This fact has been attributed to the oxidation of Fe(II) by such microorganisms and, therefore, to the regeneration of oxidant Fe(III) (Corkhill et al., Citation2008). Regarding reaction media, the oxidation of arsenopyrite was found to occur faster in air-saturated water than in air alone, suggesting that the aqueous phase facilitates the transport of reactants and products to and from the arsenopyrite surface and catalyzes its oxidation (Nesbitt et al., Citation1995). Conversely, when oxidation products develop a layer on arsenopyrite, the diffusion of the oxidant is restricted; consequently, the overall rate of arsenopyrite dissolution is reduced (Asta et al., Citation2010; Flakova et al., Citation2012).
The secondary products resulting from the oxidation of arsenopyrite are diverse, including As oxides, iron arsenates, iron sulfoarsenates and sulfoarsenites, calcium and magnesium arsenates and arsenates of metallic elements such as zinc, lead, and copper (Drahota & Filippi, Citation2009). Among them, the most common are iron arsenates, with scorodite (FeAsO4·2H2O) and amorphous iron arsenates (AFA) being the most frequently observed (Filippi et al., Citation2015), particularly in the crystalline phase. Amorphous iron arsenates are considered the precursors of scorodite. This phase transformation occurs at a pH of approximately 1 (Paktunc, Citation2015). Nevertheless, the kinetics of this reaction are dependent on pH, exponentially slowing as pH increases (Paktunc, Citation2015). Scorodite is much less soluble than AFA, with solubility values within 1–7 orders of magnitude lower (Paktunc & Bruggeman, Citation2010). Generally, other iron arsenates found in areas with Ca or K include arseniosiderite (Ca2Fe3O2(AsO4)3·3H2O) and pharmacosiderite (KFe4(AsO4)3(OH)4·6-7H2O) (Drahota & Filippi, Citation2009), respectively. Arseniosiderite remains stable in Ca-saturated solutions with pH values between 3.5 and 7.5 (Paktunc et al., Citation2015), whereas the formation of pharmacosiderite is favored in the presence of dissolved K, Fe/As molar ratios > 1 and < 2, and neutral-to-alkaline pH conditions (Haffert et al., Citation2010).
Iron sulfoarsenates and sulfoarsenites are mostly found in altered mine waste with the coexistence of arsenopyrite and pyrite due to the acidic conditions and high levels of dissolved sulfate, which promote their formation. The most frequently observed among these are bukovskýite (Fe2(AsO4)(SO4)(OH)·7H2O) (Haffert et al., Citation2010; Jelenová et al., Citation2018) and zýkaite (Fe4(AsO4)3(SO4)(OH)·15H2O) (Haffert et al., Citation2010). Under these conditions, the occurrence of iron hydroxy sulfates such as jarosite (KFe3(SO4)2(OH)6) and schwertmannite (Fe16O16(OH)12(SO4)2) (Jelenová et al., Citation2018) is also typical, with the former being stable up to a pH of approximately 3 and the latter being stable between pH values of 3 and 5 (Cheng et al., Citation2009). Although these are not As minerals, these phases can immobilize significant amounts of this metalloid. Thus, contents up to 3.35 wt.% As2O5 (Filippi et al., Citation2015) and up to 9.20 wt.% As2O5 (Park et al., Citation2016) have been found in mining environments containing these phases. Jarosite removes As(V) either via adsorption on the mineral surface through the formation of bidentate-binuclear complexes (Gräfe et al., Citation2008) or via incorporation into the mineral structure replacing sulfate (Savage et al., Citation2005). Schwertmannite can efficiently retain both As(III) and As(V). Their adsorption occurs via a bidentate binuclear binding mechanism (Paikaray et al., Citation2014). The removal of this metalloid by schwertmannite can also occur via co-precipitation processes, which has been found to be the dominant mechanism in mining areas (Park et al., Citation2016). High As loads destabilize this phase, which differs from the process in which only adsorption is involved in removal (Wang et al., Citation2020). Therefore, schwertmannite is considered to control As mobility only at short time-scales.
Other non-As phases that are ubiquitous in weathered arsenopyrite-bearing mine waste include Fe (oxyhydr)oxides, with hydrous ferric oxides (commonly ferrihydrite (Fe2O3·0.5H2O)), goethite (α-FeOOH), and hematite (α-Fe2O3) being the most abundant (Filippi et al., Citation2015). It is well known that these phases are important sinks for As. Thus, up to approximately 30 (27.4–31.6) wt.% As2O5 have been reported in hydrous ferric oxides occurring in mine waste (Hiller et al., Citation2012; Jelenová et al., Citation2018). Moreover, the presence of high As loads in hydrous ferric oxides has been found to stabilize these phases, limiting their transformation to well-crystallized compounds, thus inhibiting the consequent release of As (Moldovan et al., Citation2003).
Scorodite, the most common weathering product of arsenopyrite, is unstable under reducing conditions, whereas it exhibits relatively low solubilities under oxidizing conditions, with minimum values (approximately 0.25 mg L−1) being reached near a pH of 3. Under such conditions, it dissolves incongruently and promotes Fe (oxyhydr)oxide precipitation, which partially attenuate As release (Paktunc & Bruggeman, Citation2010). The significant dependence of scorodite stability and solubility on redox and pH conditions significantly restricts its effectiveness for immobilizing As. Furthermore, even if the leachable As content in scorodite represents a significantly small fraction of the total As (< 0.01%) (Randall, Citation2012), it is sufficient to classify scorodite as a hazardous material (Lagno et al., Citation2010) due to the significant increase experienced by scorodite solubility at pH values < 2 and > 6 (Paktunc & Bruggeman, Citation2010). Therefore, the inappropriate management of weathered arsenopyrite mine waste can produce a considerable risk of As spreading to the surrounding area with consequent negative impacts on environment and human health.
3.2. Antimony-bearing mine waste
Antimony occurs in more than 100 minerals, mainly as sulfides, oxides, and complex sulfides (mostly with copper, lead, and argent), such as stibnite (Sb2S3), valentinite (β-Sb2O3), tetrahedrite ((Cu,Fe)12Sb4S13), bournonite (PbCuSbS3), and pyrargyrite (Ag3SbS3). Of these, stibnite is the most important Sb mineral and primary commercial source of Sb (Alloway, Citation1995; Kabata-Pendias & Mukherjee, Citation2007). Stibnite is typically associated with sphalerite (ZnS), pyrite, or galena (PbS), and in mercury deposits, albeit less frequently (Alloway, Citation1995). The exploitation of stibnite and other associated elements has generated considerable amounts of mine waste. Stibnite is not thermodynamically stable under atmospheric conditions, excluding highly reducing conditions (Vink, Citation1996). Mine waste containing stibnite easily leaches Sb (Wilson et al., Citation2004). Thus, it has been reported that the dissolution of this mineral released up to 55 mg Sb L−1 (Ashley et al., Citation2003). The dissolution of stibnite occurs quickly in mine waste (within days or weeks). It can occur through two primary mechanisms (Ashley et al., Citation2003) (), which are described below.
Direct dissolution:
(3)
(3)
Incongruent dissolution through which formed intermediate Sb oxides are subsequently partially dissolved:
(4)
(4)
(5)
(5)
The dissolution of this mineral is affected by various abiotic factors such as temperature (25–70 °C) (Çopur et al., Citation1997), pH (1.08–10.63) (Biver & Shotyk, Citation2012a), particle size (0.1061–0.8426 mm) (Çopur et al., Citation1997), organic ligands (Biver & Shotyk, Citation2012b), and light irradiation (λmax = 400–700 nm from 0 to 240 min) (Hu et al., Citation2015), with mild basic conditions (8.85–10.63), raising temperature, decreasing particle size, and light irradiation promoting the Sb release. As with arsenopyrite, the oxidation of stibnite is also influenced by galvanic effects. Thus, the occurrence of pyrite with stibnite enhances the dissolution of the latter (Beauchemin et al., Citation2012; Flakova et al., Citation2012; Yan et al., Citation2020). Pyrite can accelerate the rate of stibnite oxidative dissolution 11.4-fold under sunlight (Yan et al., Citation2020). Considering these characteristics, the weathering degree of these minerals in mine waste can be ordered as follows: stibnite > arsenopyrite > pyrite (Flakova et al., Citation2012). In addition to the abiotic process, it has recently been proven that the dissolution of stibnite can also proceed via bacterially mediation. Microorganisms may dissolve this mineral via pH changes and subsequently oxidize the dissolved Sb(III) to Sb(V), resulting in the formation of secondary phases (Loni et al., Citation2020).
A simple general sequence of stibnite oxidation has been indicated as follows (Roper et al., Citation2012): stibnite (Sb2S3) → kermesite (Sb2S2O) → senarmontite/valentinite (α-Sb2O3)/(β-Sb2O3) → cervantite (α-Sb2O4) → minerals of the roméite group (Ca2Sb2O7) or iron antimonates such as schafarzikite (FeSb2O4) and tripuhyite (FeSbO4). Moreover, it has been reported that the predominance of stibnite weathering products and consequent variable Sb mobility observed in mining areas are dependent on the formation kinetics of secondary Sb phases (Majzlan et al., Citation2016). Thus, as initial phases of stibnite weathering, soluble antimonates and/or sulfates have been reported in different mining areas together with oxides (Filella et al., Citation2009; Majzlan et al., Citation2016).
Common oxides include senarmontite, valentinite, cervantite, and stibiconite (Sb3O6(OH)), with the two latter being mixed-valence oxides (Roper et al., Citation2012). Valentinite is considered a metastable phase, exhibiting higher solubility and dissolution rates than senarmontite, which is its polymorph (Biver & Shotyk, Citation2013). The equilibrium solubility of senarmontite under standard conditions at circumneutral pH has been reported to be approximately 1.3 mg Sb L−1 (Filella et al., Citation2009). Some higher values have been shown at pH 3–2 (2.9–2.6 mg Sb L−1) (Biver & Shotyk, Citation2013). Stibiconite, the oxide found more frequently in mining areas (Roper et al., Citation2012), is approximately one order of magnitude less soluble, with solubility values varying in the range of 153.2–452.0 µg Sb L−1 at pH values ≥ 2 and < 10 (Biver & Shotyk, Citation2013).
Commonly reported sulfates include coquandite (Sb6O8(SO4)·(H2O)), klebelsbergite (Sb4O4(OH)2(SO4)) and peretaite (CaSb4O4(OH)2(SO4)2·2H2O) (Filella et al., Citation2009; Majzlan et al., Citation2016). These compounds require the action of local acidic conditions on stibnite resulting from the oxidation of co-occurring pyrite for their formation (Majzlan et al., Citation2016). Of soluble antimonates, brandholzite (Mg[Sb(OH)6]2·6(H2O)) has been described in several studies on carbonated areas (Filella et al., Citation2009; Majzlan et al., Citation2016) as being formed under humid and near-neutral conditions (Majzlan et al., Citation2016). In this case, the presence of pyrite inhibits its formation due to the prevalent acidic conditions occurring under such circumstances (Majzlan et al., Citation2016). The solubility of this mineral has been reported to be 0.5 g L−1 (Herath et al., Citation2017).
Minerals of the roméite group and iron antimonates are considered to play an important role in restricting the mobility of Sb (Roper et al., Citation2012). Thus, the solubility of Sb from roméite is significantly low, at approximately 0.04 mg L−1, and from iron antimonates, approximately some µg L−1 (Multani et al., Citation2016). Particularly, minerals of the roméite group are stable at high pH values (Courtin-Nomade et al., Citation2012) and can considerably limit the mobility of Sb in alkaline environments with high Ca availability (Roper et al., Citation2012), whereas iron antimonates occur in a wider pH range (Multani et al., Citation2016). Both minerals of the roméite group and iron antimonates, particularly tripuhyite, have been reported in mine environments as important sinks for Sb (Courtin-Nomade et al., Citation2012). In addition to these Sb minerals, other phases also act as important Sb scavengers in mine scenarios, particularly when Fe sulfides coexist with stibnite. These are mainly Fe (oxyhydr)oxides (mostly hydrous ferric oxides and goethite) (Beauchemin et al., Citation2012; Courtin-Nomade et al., Citation2012; Flaková et al., Citation2017); iron hydroxy sulfates such as jarosite (Courtin-Nomade et al., Citation2012) and schwertmannite (Manaka et al., Citation2007) have been reported as well. Antimony adsorption or co-precipitation with hydrous ferric oxides has been reported as the primary attenuation mechanism (Beauchemin et al., Citation2012; Flaková et al., Citation2017).
Although different stibnite oxidation products and other sulfide weathering products can effectively restrict the release of Sb into the environment, the emplacements where mining activities have generated waste with high Sb concentrations can result significantly polluted as the appropriate geochemical conditions to generate insoluble Sb minerals or Sb-adsorbing/precipitating phases do not always occur. Thus, high soluble Sb contents (> 100 mg kg−1) in mine waste derived from the exploitation of Sb mineral ore deposits have been reported.
3.3. Fate and transport of arsenic and antimony from mine waste
Under atmospheric conditions, As and Sb are released from mine waste via the oxidative dissolution of their main primary minerals, which are commonly arsenopyrite and stibnite, respectively. The oxidation of arsenopyrite is favored under acidic conditions, whereas that of stibnite is favored under alkaline conditions (Biver & Shotyk, Citation2012a; Hu et al., Citation2015). Under the typical acidic conditions of the acid mine drainage, the dissolution of arsenopyrite is promoted by acidophilic iron-oxidizing bacteria, promoting the speed and scale at which this process occurs (Corkhill et al., Citation2008). The acid mine drainage conditions (low pH values and high ferric ion concentrations in solution) strongly catalyze the dissolution of stibnite (Biver & Shotyk, Citation2012a). Nevertheless, the stibnite dissolution rate under relatively mild basic conditions exceeds that in acid solution (Biver & Shotyk, Citation2012a). Under such conditions, basic cations (such as Ca2+ and Mg2+) also increase the stibnite dissolution rate (Biver & Shotyk, Citation2012a). The biotic oxidation of stibnite has been only recently reported (Loni et al., Citation2020), and further research is required to effectively elucidate the reactions and their implications. It has been postulated that bacteria may dissolve Sb(III) in stibnite owing to increased pH (Loni et al., Citation2020), which is in agreement with previous findings. Stibnite oxidizes faster than arsenopyrite (Beauchemin et al., Citation2012); therefore, when these minerals co-occur in mine waste, the mobilization of Sb from sulfides is considered to be higher in the early stages of weathering, whereas as weathering progresses higher As mobilization takes place (Flaková et al., Citation2017).
Natural attenuation processes such as the precipitation of secondary As or Sb minerals/phases, phase transformation, and As and/or Sb adsorption/co-precipitation by other sulfide weathering products can reduce the overall release of the soluble forms of these metalloids into the surrounding environment. The occurrence and predominance of these minerals/phases depend on different factors such as the kinetics of their formation, dissolution or transformation, the weathering stage of mine waste, the pH and redox conditions existing in the system, the chemical composition of the medium, determined by the own mineralogy of the exploited deposit, and the climatic conditions. In addition to the transport of dissolved As and Sb species by leaching and/or run-off, the spread of these metalloids into the environment also occurs via mechanical transport of mine waste particles (Alloway, Citation1995). Specifically, this mechanism is regarded as the primary mode of transport in areas with extreme climatic conditions where high-temperature and low-water availability prevail. Under such conditions, the oxidative dissolution of sulfides rarely occurs.
Once released from mine waste, these metalloids can reach soils and water bodies. Extremely high soil As and Sb contents have been observed in areas where mining activities have been performed without the appropriate management of produced wastes. Thus, values > 10000 mg kg−1 of either As (Razo et al., Citation2004) or Sb (Okkenhaug et al., Citation2011) have been reported in soils surrounding several mining areas worldwide. Soils possess mechanisms for the detoxification and attenuation of these elements. Detoxification processes are performed by oxidation of the more toxic reduced forms; these processes can be performed by microorganisms (Herath et al., Citation2017) or by Fe and/or Mn (oxyhydr)oxides and OM (Wilson et al., Citation2010; Sun et al., Citation2019). Particularly, in the case of Sb, its oxidized form is clearly the dominant in soils, what is attributed to the high rate of the oxidation process mediated by Fe and Mn (oxyhydr)oxides (Wilson et al., Citation2010). The immobilization of As and Sb in soils mainly proceeds via adsorption on metal (oxyhydr)oxides as well as on OM in the case of Sb(III) and by precipitation of insoluble compounds in alkaline soils, primarily calcium arsenates and calcium antimonates. Although soils can be important sinks for these elements, the natural attenuation capacity of soils is exceeded at high As or Sb loads, which is associated with the risk of transference to other environmental compartments. The main soil factors governing the attenuation processes are pH, redox potential, texture, amount and kind of OM, content of (oxyhydr)oxides of Fe, Al, and Mn, and microorganisms (Kabata-Pendias & Mukherjee, Citation2007). Of them, pH is considered one of the most decisive, acting onto the surfaces of variable-charge minerals/phases and modifying the speciation of trace elements. Redox conditions determine the stability of (oxyhydr)oxides of Fe and Mn and sulfides and are also especially significant for As and Sb as their oxidation status affects their mobility. In general, heavy-textured soils with important contents of (oxyhydr)oxides of Fe, Al, and Mn, and also OM in the case of Sb, exhibit a higher overall retention capacity for these metalloids. Soil microorganisms have a great impact on all oxidation–reduction and precipitation–dissolution processes occurring in soil (Kabata-Pendias & Mukherjee, Citation2007). All these factors determine the partitioning of these trace elements between the soil solid phase and the soil solution and, consequently, their mobility and availability. Additionally, other conditions such as climatology and water regime control their transference as well. Very high As and Sb concentrations have been also reported in water bodies in areas affected by mining activities (Ashley et al., Citation2003; Razo et al., Citation2004; Filella et al., Citation2009; Hiller et al., Citation2012; Ritchie et al., Citation2013; Cidu et al., Citation2014), with values > 5 mg L−1 in some of them (Razo et al., Citation2004; Cidu et al., Citation2014). The primary pathways for As and Sb attenuation are dilution by unpolluted tributary water and removal by amorphous Fe and Mn oxides present in sediments (Filella et al., Citation2009; Beauchemin et al., Citation2012; Flakova et al., Citation2012; Ritchie et al., Citation2013; Johnston et al., Citation2020). Under oxic conditions, Sb has been observed to be more mobile than As, as Sb is released from solids more efficiently (Hiller et al., Citation2012; Johnston et al., Citation2020); conversely, local reducing conditions increase the aqueous mobility of As and attenuate that of Sb (Johnston et al., Citation2020).
Due to the considerable risk of pollution in areas surrounding mine emplacements and the consequent hazard to ecosystem and human health, various strategies have been proposed for the safe and effective management of mine waste. These approaches primarily function by stabilizing or encapsulating toxic elements within the mine waste.
4. Stabilization and encapsulation strategies of arsenic- or antimony-bearing mine waste
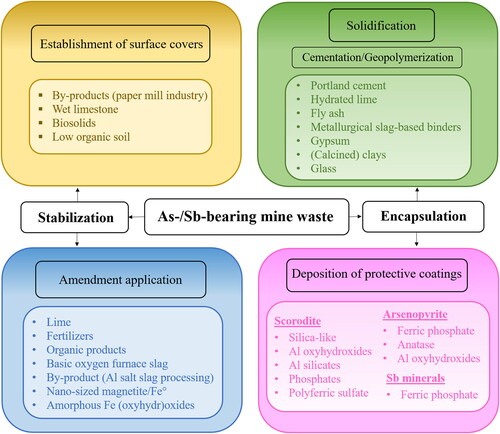
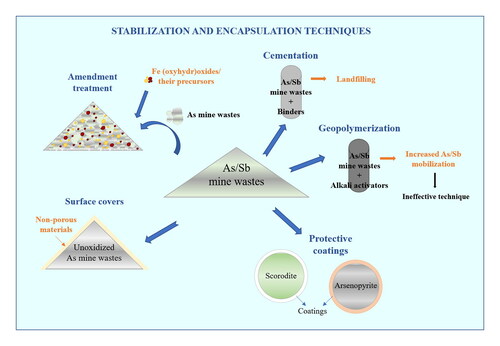
Stabilizing and encapsulating strategies aim to control contaminants to mitigate or terminate their permeation of the surrounding environment. These techniques proceed by either chemical reactions or waste confinement within a matrix or a coating that limits the weathering of wastes. The performances of different strategies have been evaluated in stabilizing or encapsulating mine waste exhibiting high metalloid content (). Mine waste stabilization/encapsulation strategies can be classified as follows: establishment of surface covers, application of amendments, solidification (cementation and geopolymerization), and deposition of protective coatings.
These approaches have been widely evaluated for As-bearing mine waste (Tables S1-S4); however, research attention has been devoted to evaluating the stabilization or encapsulation of Sb-bearing mine waste by means of amendment application, solidification (i.e., cementation or geopolymerization processes), and deposition of protective coatings ().
Table 1. Summary of techniques to treat Sb-bearing mine waste.
4.1. Establishment of surface covers
The establishment of surface covers on wastes seeks mainly for reducing the entrance or availability of the oxidant agent, pH control and, in instances, inhibiting the proliferation of acidophilic bacteria (Lottermoser, Citation2010). Both wet and dry covers have been investigated and applied to treat sulfidic wastes. Wet covers are applied by submerging wastes under water (Lottermoser, Citation2010). Although they are effective, owing to the restriction of oxygen, their application is limited as the disposal of sulfide wastes in lakes and oceans is limited or prohibited in many regions. Accordingly, researchers have begun to focus on dry cover approaches in which wastes are capped with a thick stratum of a solid material with low hydraulic conductivity (Lottermoser, Citation2010). Such a stratum can be composed of a single layer or composite layers. Pyritic wastes are among the most frequently studied, and various materials have been evaluated for use as constituents of dry covers (such as liming or alkaline materials, organic wastes, soils or clay subsoils, waste rocks with low sulfide contents, and oxide-rich wastes) (Lottermoser, Citation2010). This technique is facile and low-cost, particularly when employing by-products as cover constituents. In such cases, only the transport costs from the site of generation to the mine emplacements are involved.
The use of dry covers for treating wastes with high contents of (weathered) arsenopyrite has been also investigated (). The evaluated dry cover materials include alkaline materials, such as wet limestone (Craw et al., Citation2002), lime mud, fly ash, and green liquor dregs (Jia et al., Citation2014, Citation2017); organic wastes (biosolids) (Paktunc, Citation2013); and soil with low organic content (DeSisto et al., Citation2017). The performance of these materials as surface covers has been evaluated after varying periods from their application by mineralogical studies (mainly by X-ray diffraction (XRD)) and/or geochemical studies using different leaching tests. These studies have been performed at field and lab scales, following different procedures. Table S1 presents the results of examined covers on phase transformation and As mobility.
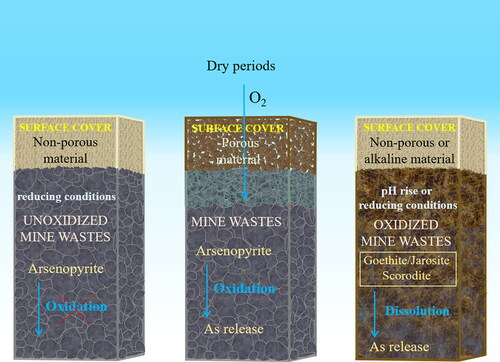
Lab scale studies. Jia et al. (Citation2014) studied the potential of lime mud, fly ash, and green liquor dregs as dry cover materials. Leachates from these alkaline materials were applied to disturbed (oxidized or unoxidized) mine waste. A liquid/solid ratio of 10 L kg−1 and an interaction period of 3 weeks were employed. This experimental approach aimed to simulate a simplified system composed of an alkaline material cover through which rainwater percolates, reacting with different types of mine waste (oxidized or unoxidized). Some studied alkaline materials (lime mud and green liquor dregs) were able to decrease As leaching when applied to unoxidized mine waste under reducing conditions. Notably, when this cover was applied to oxidized mine waste, the As release greatly increased regardless of the redox conditions. This behavior was attributed to the alkaline nature of the cover materials. Alkaline materials may provoke the dissolution of some secondary products present in the studied oxidized mine waste, such as jarosite, or the As desorption from other occurring secondary phases, such as Fe (oxyhydr)oxides (Jia et al., Citation2014). The dissolution of jarosite entails the concomitant release of incorporated or adsorbed As(V) (Savage et al., Citation2005; Gräfe et al., Citation2008).
Craw et al. (Citation2002) also evaluated the performance of wet limestone as surface cover. With this aim, an undisturbed column (9 cm diameter × 8 cm height) of oxidized mine tailings was covered by a layer of limestone (1 cm height) over which an additional layer of water (2 cm height) was placed. Water was replenished periodically to compensate for loss by evaporation and downward seepage. The experiment was conducted over 400 days, after which As levels were measured in tailings pore waters. It was found that the application of wet limestone on oxidized mine tailings provoked As mobilization as a result of the scorodite dissolution caused by the acid neutralizing effect of limestone cover (Craw et al., Citation2002).
DeSisto et al. (Citation2017) investigated the behavior of a cover constituted by low-OM soil. The performance of this approach was evaluated on different weathered mine tailings with the presence of Fe arsenates or Ca arsenates and/or Fe (oxyhydr)oxides and As-containing sulfides. Columns (25 cm thick) of disturbed mine tailings covered by a layer of soil (30 cm thick) were leached with synthetic rainwater following the synthetic precipitation leaching procedure (SPLP). A synthetic rainwater volume equivalent to 2 years of rainfall was percolated through the columns, including two dry periods. It was found that the low-organic content soil cover did not establish reducing conditions; therefore, secondary phases acting as As carriers were not destabilized. Oxygen was observed to penetrate this cover in dry seasons, and some As mobilization was induced owing to the oxidation of arsenopyrite or other As-bearing sulfides (DeSisto et al., Citation2017).
Field scale studies. Paktunc (Citation2013) studied the performance of surface barriers composed of biosolids when applied on oxidized mine waste constituted by pyrite and arsenopyrite enveloped by goethite. This experiment was conducted in a field test plot where mine waste samples were covered with a layer of pulp and paper biosolids (1 m thick). Two years after cover application, the mobility of As was assessed using a modified toxicity characteristic leaching procedure (TCLP). The cover treatment was found to have increased As leaching, primarily in layers near the cover due to the instauration of reducing conditions. Such conditions gave rise to the reductive dissolution of goethite present in wastes and, consequently, to the concomitant release of the retained As (Paktunc, Citation2013). Moreover, covered mine waste exhibited changes in solid As speciation with depth. The proportion of As(III) gradually increased toward the cover from approximately 20 cm depth, as determined by X-ray absorption near-edge structure (XANES) spectroscopy (Paktunc, Citation2013).
Therefore, this strategy was only found to be suitable for treating unoxidized mine waste when a non-porous sealing overlayer is used and reducing conditions that prevent As sulfide oxidation are established. Generally, mine wastes are subject to long periods of weathering that hinder the application of this technique, particularly in former mine locations. Arsenic secondary phases are typically stable under oxidizing conditions, thus being incompatible with treatments aimed to implement reducing environments. Moreover, the geochemical conditions under which different As minerals/phases remain stable are very diverse.
This approach has not been evaluated with regard to mine waste from the exploitation of stibnite deposits. This is expected as stibnite oxidizes under atmospheric conditions within days or weeks, and under strongly reducing conditions, it may be dissolved if alkaline conditions are established (Vink, Citation1996). Dumped mine waste contains partially or totally weathered stibnite. In the first stages of weathering, soluble antimonates and/or sulfates coexist with oxides. Therefore, considering the rapid dissolution of stibnite and further co-occurrence of various minerals with diverse stability, solubility, and dissolution rate conditions, the management of mine waste produced from the exploitation of stibnite deposits is challenging as surface covers are likely ineffective, even if they can prevent the mechanical transport of mine waste particles.
4.2. Amendment application
Amendment application is a stabilization technique based on the incorporation of chemical agents to prevent the off-side migration of soluble and easily extractable forms of toxic elements from a contaminated location. The application of amendments to As- and/or Sb-bearing mine wastes aims to stabilize them via immobilization, mainly through adsorption and/or precipitation processes.
Table S2 summarizes the primary amendment treatments evaluated to stabilize mine waste with high As levels. These treatments have been mostly applied to weathered arsenopyrite mine waste and include lime alone (Jones et al., Citation1997) or together with fertilizer (Noble et al., Citation2016), nano-sized magnetite (Fe3O4) and zero valent iron (Kim et al., Citation2012), basic oxygen blast furnace slag (Kim et al., Citation2018), by-product from aluminum salt slag processing (Álvarez-Ayuso & Murciego, Citation2021), peat and/or Fe-rich sludge (Rakotonimaro et al., Citation2019), and precursor substances of amorphous Fe compounds (Kim et al., Citation2003). Most of these studies were performed at lab scale. In these cases, disturbed mine waste was mixed with fixed amounts of amendments, and the performances of the stabilizing treatments were evaluated using batch or column leaching experiments and extraction procedures.
Lab scale studies. The performance of lime as a stabilizing agent was studied using mine tailings (0 to 0.30 m depth) with aqueous As activity mainly controlled by Fe (oxyhydr)oxides (Jones et al., Citation1997). These mine tailings, with pH values of 3.5–4.1, were treated with a 60% CaCO3/40% Ca(OH)2 mixture at dosages of 3.0–25.6 g kg−1. After liming, the pH of mine tailings increased up to 8.0–9.8 and, parallelly, the soluble As concentrations rose by factors ranging from 10 to 400. The observed pH rises were identified as responsible for As solubilization because high pH values can lead to As desorption from Fe (oxyhydr)oxides (Jones et al., Citation1997).
The use of nano-sized magnetite and zero valent iron, two efficient As-adsorbents (Kanel et al., Citation2005, Citation2006; Liu et al., Citation2015), was investigated in the stabilization of mine tailings presenting close to 1% of their total As in non-specifically adsorbed forms (Kim et al., Citation2012). Nano-sized zero valent iron can remove As in its oxidized and reduced forms within minutes. The removal mechanism of both As(V) and As(III) mainly proceeds through adsorption and co-precipitation by Fe(II) and Fe(III) (oxyhydr)oxides (magnetite/maghemite (γ-Fe2O3) and/or lepidocrocite (γ-FeOOH)), which form during nano-sized zero valent iron oxidation. The adsorption of As(III) and As(V) occurs by the formation of inner-sphere surface complexes (Kanel et al., Citation2005, Citation2006). Nano-sized magnetite can also remove both As(V) and As(III) through adsorption, forming mainly bidentate binuclear complexes with As(V) and tridentate hexanuclear complexes with As(III) (Liu et al., Citation2015). Uncoated and sodium dodecyl sulfate (SDS)-coated nano-sized magnetite and zero valent iron were applied individually to weakly basic mine tailings (pH: 8.2) at an iron/mine tailings rate of 0.34%. Coating was conducted to restrain the aggregation of magnetic materials and their consequent restricted dispersion into mine tailings that could hinder their practical application (Kim et al., Citation2012). All four treatments decreased As leaching from mine tailings by 39.1–69.0% and 52.3–73.9% for uncoated and SDS-coated materials, respectively, with SDS-nano-sized magnetite showing the best restrictive effect. Its higher specific surface and smaller particle size were indicated as factors accounting for such increased As stabilization.
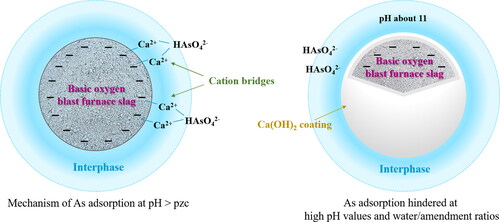
The application of basic oxygen blast furnace slag was investigated for stabilizing mine waste containing approximately 0.1% of leachable As to total As (Kim et al., Citation2018). This material is characterized as rich in iron and calcium oxides (approximately 30 and 40%, respectively), with pH and point of zero charge values of 12.6 and 6.3, respectively; it was incorporated to mine waste at dosages of 3, 5, and 10%. At the highest doses (5 and 10%), significant reductions in the leachable As content of mine waste were achieved in the ranges of 75–92% and 92–95%, respectively. At the lowest basic oxygen blast furnace slag dose, As leaching was significantly influenced by the amount of water present in the system, with the efficiency of this material to immobilize As sharply decreasing as the water level increased. The mechanisms proposed for As immobilization by this amendment include the adsorption of As(V) on its surface via cation (Ca2+) bridges and the precipitation of calcium arsenates (Kim et al., Citation2018). The formation of a Ca(OH)2-coating on the oxygen blast furnace slag surface that hindered As adsorption was indicated as the process responsible for the relatively low efficiency of this material when an excess of water was present in the system (Kim et al., Citation2018). A scheme of these processes is shown in .
The performance of the by-product of aluminum salt slag processing was assessed on weathered arsenopyrite mine waste with the occurrence of AFA and scorodite as main secondary products (Álvarez-Ayuso & Murciego, Citation2021). This alkaline amendment, at its optimal dose (4%), partially neutralized acidity and importantly decreased the leachable As concentration (52-79%). The As adsorption on Al oxides/hydroxides composing this industrial by-product and the conversion of soluble AFA to scorodite were reported as primary processes involved in stabilization.
The utility of peat and/or Fe-rich sludge as As-immobilizing amendments was evaluated for stabilizing mine tailings with the occurrence of arsenopyrite together with löllingite (FeAs2) and iron oxides (hematite and magnetite) as main As-minerals/carrier phases (Rakotonimaro et al., Citation2019). In previous studies, peat has been found to be effective in immobilizing very low As concentrations in tailings, particularly in conjunction with Fe-based materials (Rakotonimaro et al., Citation2019). In these coupled systems, the formation of ternary complexes (As-Fe-OM) has been suggested to be involved in the immobilization of As(V) (De Oliveira et al., Citation2015) and As(III) under alkaline conditions (Hoffmann et al., Citation2013). Therefore, it is hypothesized that peat together with Fe-rich sludge can reduce As leaching in tailings (Rakotonimaro et al., Citation2019). Mine tailings were treated with peat or Fe-rich sludge at dosages of 5 and 10% and with mixtures of both amendments. The increasing peat dosages promoted As leaching and increased the proportion of As(III) in leachates, whereas the application of Fe-rich sludge alone (5%) or in combination with peat considerably reduced As release at levels of 65–80%. The combined application of these materials could yield eventual potential risks because organic products may dissolve Fe (oxyhydr)oxides and promote the reduction of metal(loid)s, even if good performance is achieved early in the treatment process (Lottermoser, Citation2010; Rakotonimaro et al., Citation2019). The dissolution of Fe (oxyhydr)oxides entails the concomitant release of the retained As, which can cause more adverse effects on ecosystems due to its higher toxicity if mobilized in its reduced state (Di et al., Citation2019).
The effectiveness of amorphous Fe compounds to attenuate the As release from mine tailings when generated in situ has been also reported (Kim et al., Citation2003). The application of ferrous or ferric sulfate solutions (20–100 mM) to arsenopyrite-bearing mine tailings was tested at a rate of 10:1 followed by pH adjustment within the range of 3–6 with NaOH or Ca(OH)2. The treatment using ferric sulfate and Ca(OH)2 showed the best performance, achieving As immobilization levels of 70–80%. The adsorption or precipitation of As by freshly formed Fe-precipitates was proposed as the primary mechanism by which As was removed. The precipitation of these phases was much more reduced when using ferrous sulfate solutions (> 70% of supplied iron remained in solution), therefore limiting the ability of this treatment to reduce As extractability.
Field scale studies. The incorporation of lime and fertilizer (N:P:K = 8:4:10) was evaluated at a field scale for the treatment of mine tailings with the occurrence of scorodite as the main As mineral (Noble et al., Citation2016). The amendments were applied to the surfaces of mine tailings at respective doses of 10 t ha−1 and 300 kg ha−1. The application of fertilizer aimed to ameliorate the nutrient status of mine tailings for further revegetation of the neutralized tailings surface. Three years after the treatment application, As levels in on-site surface waters achieved a 100- and 27-fold reduction under dry and wet conditions, respectively. Concomitantly, under the same conditions, the pH of surface waters increased from 3.3 to 4.6 (3.6–5.3) and 6.4 (4.5–8.0), respectively. Such pH increases resulted in the formation of hydrous ferric oxides, hematite, and goethite. These phases are well known scavengers of As. However, As exhibited greater mobility in amended tailings than in subsurface unamended tailings, as derived by single short extractions (2 h) performed with deionized water, dilute salt solutions, and dilute solutions of low-molecular-weight organic acids. Such behavior was attributed to As desorption from phases with variable charge surfaces under alkaline pH conditions. Therefore, when applying neutralizing materials as amendments, special care should be taken regarding pH variations that could lead to As-phases destabilization or As desorption.
Pilot scale studies. The application of ferrous sulfate solutions was also investigated with regard to other kinds of As-containing mine wastes, particularly considering weathered realgar mine tailings. This approach was evaluated at a pilot scale using mine tailings with the occurrence of realgar along with highly soluble pharmacolite (CaHAsO4·2H2O) (Ksp = 10−4.68) as main As-minerals (Wang et al., Citation2019). The As leaching was reduced with the increasing ferrous sulfate additions to mine tailings (0–30%), with a dose of 20% being reported as optimal for reducing As levels in leachates to satisfy the regulatory disposal limits. At this ferrous sulfate dosage, a reduction in As leaching by approximately 99% was achieved with a concomitant pH decrease from 7.6 to 5.3. After treatment, the new formation of the sparingly soluble symplesite (Fe3(AsO4)2·8H2O) (Ksp = 10−33.25) was detected, together with an increased content of amorphous Fe oxides. Concurrently, the realgar and pharmacolite contents were decreased. In accordance with these observations, the transformation of Ca-As and S-As compounds into Fe-As phases, which are less soluble or more stable under oxidizing conditions, was reported as the main process involved in the stabilization of this type of As mine waste (Wang et al., Citation2019).
Some amendment treatments described above have been also evaluated at lab scale to treat mine tailings with low total As content (< 0.15%), exhibiting similar performance as when applied to stabilize mine waste with the occurrence of significant As levels. These are substances containing iron oxides or their precursors such as ochre, zero-valent Fe, red mud, ferrous sulfate, red mud/ferrous sulfate, or calcium carbonate/ferrous sulfate mixtures (Seidel et al., Citation2005; Koo et al., Citation2012). Additionally, other amendments have been investigated as stabilizing agents to treat mine tailings with low total As content (< 0.15%). All these studies have been performed at lab scale. Amendments tested in this respect include phosphate products such as ground bovine bone meal and partially soluble granular superphosphate (Munksgaard & Lottermoser, Citation2013); organic products such as spent mushroom waste, by-product fertilizers, and biochar (Koo et al., Citation2012; Gu et al., Citation2020); and alkaline products such as furnace slag, natural and calcined oyster shells, Portland cement, cement kiln dust, and calcined oyster shells/Portland cement or calcined oyster shells/cement kiln dust combinations (Moon et al., Citation2011; Koo et al., Citation2012). Phosphate products showed a negative impact, increasing the As release by a factor > 2 when amendments were applied at doses of 5%. This increased As mobilization was attributed to phosphate ligand exchange processes (Munksgaard & Lottermoser, Citation2013). Likewise, organic products also generally increased the mobile fractions of As even at relatively low dosages (≥ 1%); the possible boosting of reduction processes and the competition of dissolved organic carbon and phosphate for the As adsorption sites were proposed as the mechanisms responsible for this behavior (Koo et al., Citation2012; Gu et al., Citation2020). Alkaline products that increase the pH of mine waste above neutral did not show positive effects on As immobilization, unless constituents were supplied to precipitate As, such as reactive Ca (Moon et al., Citation2011; Koo et al., Citation2012), and applied at high dosages (25–30%) (Moon et al., Citation2011). Phosphates, organic products, and liming materials have also shown a negative impact when applied as amendments to remediate As-polluted soils (Palansooriya et al., Citation2020).
Considering the previously reported results, mine wastes with the coexistence of As sulfides and their weathering products can be effectively stabilized using amendments that lead to As adsorption or co-precipitation. Among tested materials, Al oxide/hydroxide-containing materials and Fe (oxyhydr)oxides and their precursors have shown a good behavior, greatly reducing the As mobility from mine waste. In any case, the amendment treatments based on Fe (oxyhydr)oxides and their precursors should be restricted to atmosphere-exposed mine wastes. When mine wastes are destined for disposal in landfills, the enduring effectiveness of this treatment is not expected, as the prevalent reducing conditions in landfills can dissolve both amorphous and crystalline Fe (oxyhydr)oxides, concomitantly releasing the retained metalloid.
The assayed amendment methods concerning the treatment of Sb-containing mine waste, the tested materials, and their impact on Sb mobility are indicated in . These stabilization treatments were focused on tailings with the co-occurrence of As and with low total contents of both metalloids (< 0.07%). The tested amendments include phosphate products such as ground bovine bone meal and partially soluble granular superphosphate (Munksgaard & Lottermoser, Citation2013) and organic materials, namely biochar derived from Arundo donax L. straw stems (Gu et al., Citation2020). Both studies were performed at lab scale using disturbed mine tailings.
The performance of bovine bone meal and partially soluble granular superphosphate was evaluated individually at different application doses (1 and 5%; Munksgaard & Lottermoser, Citation2013). These amendments increased the mobilization of As and Sb, particularly when applied at the highest dose (5%). Notably, significant increases followed the application of 5% partially soluble granular superphosphate, reaching 6.8- and 99-fold for As and Sb, respectively. This approach significantly increased the level of soluble phosphate. The occurrence of phosphate ligand exchange processes was proposed as the mechanism responsible for the negative behavior of these amendments (Munksgaard & Lottermoser, Citation2013). It is well known that phosphate can compete effectively with As in both oxidation states for adsorption sites on different geologic materials, including Fe (oxyhydr)oxides (Manning & Goldberg, Citation1996; Jain & Loeppert, Citation2000), whose occurrence is particularly significant in mining areas. Arsenate results affected in more extent (Jain & Loeppert, Citation2000). Arsenate and phosphate are chemical analogues, both exhibiting tetrahedral configuration and having similar acid dissociation constants, but, due to the smaller size and higher charge density of phosphate, phosphate outcompetes arsenate for adsorption sites, even at similar concentrations (Manning & Goldberg, Citation1996). Phosphate can also compete with Sb for adsorption sites on Fe, Al, and Mn (oxyhydr)oxides and clay minerals (Biver et al., Citation2011). In general, it is considered that this competition is less effective for Sb(III) (Wilson et al., Citation2010). Therefore, stabilization treatments based on the application of phosphate-containing materials, although very effective when dealing with Pb polluted places due to the formation of insoluble pyromorphite-like compounds, should be avoided when managing As- and/or Sb-bearing mine waste due to the high risks of further mobilizing these toxic elements. For mine waste revegetation and associated nutrient amelioration, the application of slow‐release phosphate fertilizer at low controlled doses should be considered.
Biochar derived from Arundo donax L. straw stems (pH: 11.3) was applied as a stabilizing treatment at dosages of 1, 3, and 5% (Gu et al., Citation2020). The leachable As and Sb concentrations increased with increasing biochar dosages, up to approximately 3- and 1.5-fold, respectively. Similar behavior has been found when biochar is applied as an amendment to stabilize As and Sb in different soil types (Palansooriya et al., Citation2020). Biochar can increase As and Sb mobility due to its high phosphate and OM content. Phosphate can desorb As and Sb from different geological materials usually present in mine waste (Manning & Goldberg, Citation1996; Jain & Loeppert, Citation2000; Biver et al., Citation2011). Organic matter can form soluble complexes with As, increasing its mobility (Ko et al., Citation2004), and can increase Sb mobility in polluted areas when coupled with increasing pH (Nakamaru & Martín Peinado, Citation2017). In this regard, dissolved OM has been reported to complex Sb(V), with its strongest binding interaction occurring under neutral pH conditions (Fan et al., Citation2019).
Fewer materials have been assessed as amendments for stabilizing Sb-bearing mine waste than for stabilizing Sb-polluted mine soils. Many diverse materials have been proposed and evaluated as amendments to immobilize Sb in soils affected by mining activities, including phosphate materials such fertilizers and hydroxyapatite nanoparticles (Munksgaard & Lottermoser, Citation2010; Arenas-Lago et al., Citation2019); organic products such as wood bark and compost (Munksgaard & Lottermoser, Citation2010; Nakamaru & Martín Peinado, Citation2017); iron (oxyhydr)oxides such as goethite, ferrihydrite, and hematite and maghemite nanoparticles (Álvarez-Ayuso et al., Citation2013; Doherty et al., Citation2017; Arenas-Lago et al., Citation2019); precursors of iron (oxyhydr)oxides such as zero valent iron powder, ferric chloride, and ferric chloride/lime mixtures (Doherty et al., Citation2017); aluminum and manganese oxides (Álvarez-Ayuso et al., Citation2013; Doherty et al., Citation2017); and clays such as kaolinite (Doherty et al., Citation2017). Among these materials, ferrihydrite and iron oxide nanoparticles were reported to be the most effective for stabilizing Sb-polluted mine soils, reaching Sb immobilization levels above 90% at amendment dosages of 5% (Álvarez-Ayuso et al., Citation2013; Doherty et al., Citation2017; Arenas-Lago et al., Citation2019). Despite the good stabilizing performance of these materials, none have been assayed to treat mine waste with high Sb-mineral/phase content. These materials may be suitable amendments for stabilizing Sb-bearing mine waste, representing a feasible approach that warrants further investigation.
The application of amendments to mine waste is difficult when managing wastes that have already been dumped, as this treatment requires the thorough blending of amendments with mine waste (Lottermoser, Citation2010). Therefore, the treatment can be properly applied only when mine waste is dumped. To treat already-dumped wastes, a potential approach may be the use of SDS-coated nano-sized magnetite or similarly coated-Fe (oxyhydr)oxides. This coating process improves the mobility of amendment particles, facilitating their movement through pores of the media and, consequently, the amendment application (Kim et al., Citation2012). The in situ generation of Fe (oxyhydr)oxides by the percolation of iron salt solutions and alkaline agents for pH adjustment may be useful for managing dumped wastes. Ferric sulfate and slaked lime solutions appear to be the most suitable due to the lower pH of Fe(III) precipitation, the lower alkalinity required for precipitation, and relatively low cost of slaked lime. The pH adjustment should be established at a sufficiently high value to assure the practically complete precipitation of Fe(III)—otherwise, it would act as an oxidant of arsenopyrite—but sufficiently low to prevent the increased solubilization of scorodite that occurs at pH values > 6. This approach could allow for the precipitation of Fe (oxyhydr)oxides on the surface of As minerals/phases. The formation of Fe (oxyhydr)oxide rims or coatings on minerals such as arsenopyrite and scorodite has been found to restrict or inhibit their dissolution (Nordstrom & Parks, Citation1987; Asta et al., Citation2010). This approach appears to be more suitable for mine waste dumps that have developed a hardpan layer between the oxidized and reduced zones, as this layer would act as a horizontal barrier to the vertical flow of pore solutions.
4.3. Solidification processes
Both cementation and geopolymerization have been evaluated as solidification techniques to encapsulate As- and/or Sb-bearing mine waste. Summaries of the investigated materials, treatment mechanisms, and their impacts on As and Sb mobility are shown in , respectively.
4.3.1. Cementation
Cementation is defined as the precipitation of a binding material around grains, with the captured material filling the pores of the granulated material. When applied to wastes, waste components are trapped in a stable solid matrix with the aim of transforming potentially hazardous granulated solid wastes into a less hazardous or non-hazardous monolith solid before their final fate, which is usually disposal in a landfill or into mine openings in the case of mine waste. Two general strategies have been employed, the use of low water/binder ratios and high hydraulic binder proportions in relation to solid wastes, or the use of low proportions of hydraulic binders (3–7 wt%) and relatively high global water contents (typically 20–25 wt%) (Coussy et al., Citation2011; Hamberg et al., Citation2015). The difference of the binder proportions lays on the compressive strength achieved by derived materials, when using low binder proportions lower compressive strengths are achieved, but enough to be used as a geotechnical support in hard rock underground mines. This latter approach is referred to as cemented paste backfill (CPB) (Coussy et al., Citation2011; Hamberg et al., Citation2015). In general, cementation is an affordable strategy as cement is a low-cost, readily available material, and relatively easy to handle. Cement costs become much higher when attempting to manage large mines and the subsequent significant amount of derived wastes (Hamberg et al., Citation2015), particularly when not employing the CPB approach. The use of binding agents as an alternative to cement can cheapen this process. Particularly, the use of inexpensive industrial by-products with pozzolanic properties (such as fly ashes or blast furnace slags) is considered the best alternative.
The solidification techniques of arsenopyrite-bearing mine waste by their inclusion within cementing matrices have been evaluated using the following materials as binding agents: Portland cement, blast furnace slag cement, aluminous cement, hydrated lime, and mixtures of Portland cement and fly ashes/blast furnace slags/hydrated lime (Benzaazoua et al., Citation2004; Choi et al., Citation2009; Coussy et al., Citation2011). These materials have been employed in solid mixtures with proportions ranging from 5 to 30%. At these dosages, solidified wastes developed compressive strengths (after 28 d of curing) much higher than that considered suitable to resist typical overburden pressures in landfills (0.35 MPa) (Choi et al., Citation2009). Likewise, the solidification process produced by most of these materials led to a considerable reduction in As leaching compared with unsolidified mine waste, as determined following different leaching tests performed on monolithic (tank leaching test and Soxhlet extraction test) and crushed (weathering cell test, TCLP, SPLP and Korean standard leaching test) samples. These reductions attained levels approximately 80–90% at the lowest binder doses. The highest As immobilization levels (approximately 99%) were reached by Portland cement, hydrated lime, and their mixtures when applied at doses ≥ 10%. In such cases, the primary mechanism involved in As retention is the formation of calcium arsenates, as determined by XRD, scanning electron microscopy (SEM), XANES, and X-ray photoelectron spectroscopy (XPS) (Benzaazoua et al., Citation2004; Coussy et al., Citation2011). Additionally, cemented matrices enriched in hydrated lime present pH conditions and calcium activities appropriate to inhibit arsenopyrite oxidization, as established by electrochemical measurements (Benzaazoua et al., Citation2004). When lower binder proportions were employed (< 5%) or when only fly ashes were used as cementitious material, the developed compressive strengths of the resulting solidified wastes remained below 0.35 MPa (Hamberg et al., Citation2015). Moreover, when highly weathered arsenopyrite wastes with Fe precipitates as the dominant As carrier phases were subjected to solidification at low binder proportions, increased As release was observed. This was attributed to the desorption of As from Fe precipitates and its further release from calcium arsenates or cementitious phases due to the decrease in pH caused after the first stages of leaching (Hamberg et al., Citation2015). Aside from the aforementioned binder mixtures, Portland cement and hydrated lime composites with the incorporation of ferrous sulfate were also assessed to treat scorodite-rich mine waste using binder proportions within the usual range (Randall, Citation2012). The addition of ferrous sulfate was considered to contribute to As retention by newly formed Fe (oxyhydr)oxides. Nevertheless, even if the As release from solidified wastes resulting from this treatment decreased under some leaching conditions (TCLP and deionized water batch leaching tests), diffusion studies and derived effective diffusivities suggested that these cemented wastes were inappropriate for disposal. The obtained leachability index indicated values below that considered suitable for disposal. Moreover, these solidified wastes presented relatively low compressive strengths (< 0.35 MPa).
Research on the cementation of Sb-bearing mine waste has been practically limited to the studies of Salihoglu (Citation2014) and Gao et al. (Citation2020). This encapsulation system has been tested to confine two kinds of Sb-containing mine waste: mine tailings (Gao et al., Citation2020) and slag from the thermal processing of Sb ore (Salihoglu, Citation2014).
Mine tailings evaluated in this respect exhibited considerable total Sb contents (0.18%) co-occurring with high total As content (1.64%) (Gao et al., Citation2020). Metallurgical slag-based binders were subjected to trials using a binder:tailings ratio of 1:4. The constituents of metallurgical slag-based binders (ground granulated blast furnace slag, steel slag powder, and flue gas desulfurization gypsum) were evaluated at different proportions (10–80%, 80–10%, and 10%, respectively). All samples developed compressive strengths (after 28 d of curing) higher than that regarded as appropriate to endure usual overburden pressures in landfills (0.35 MPa) (Choi et al., Citation2009), and even higher than that required for fill materials (1 MPa) (Gao et al., Citation2020). Particularly, the solidified wastes derived employing the highest proportions of ground granulated blast furnace slag (60–80%) in the binder mixture showed the best mechanical performance. Additionally, the solidification processes gave rise to significant reductions in the leachable Sb (67–97%) and As (80–95%) contents, as determined by the Chinese standard leaching test HJ557-2010. Among solidified wastes, those with the highest proportions of steel slag powder in the binder mixture were found to be the most effective for immobilizing As, whereas those with the highest proportions of ground granulated blast furnace slag were the most effective for immobilizing Sb. Physical encapsulation was found to be the primary mechanism involved in mitigating Sb release, whereas precipitation was the prevailing mechanism for mitigating As release. The composition of metallurgical slag-based binders was shown to influence the leaching characteristics of cemented wastes, affecting the dominant immobilization mechanisms of the concerned metalloids. Hence, the As diffusion coefficient values decreased and those of Sb increased as the steel slag powder content of cemented wastes increased, indicating an increasing effect of chemical retardation for As (Gao et al., Citation2020).
The slag from the thermal processing of Sb ore was subjected to cementation processes using different cementitious materials (Salihoglu, Citation2014). The tested binders were Portland cement, fly ash, mixtures of Portland cement and fly ash, and different mixtures containing three of the following materials: Portland cement, fly ash, clay, gypsum, or blast furnace slag. Equal proportions of all materials were employed in the binder mixtures. The cemented solids were generated using a binder:slag ratio of 3:1. Of the different generated solidified wastes, only those derived using Portland cement alone or Portland cement together with fly ash and gypsum exhibited leachable Sb concentrations that were below the regulatory limit for non-hazardous waste landfills as determined by the compliance test for leaching of granular waste materials and sludges EN-12457-4 (Citation2002), representing Sb release decreases approximately 75% (72–78%). Moreover, compressive strengths (after 28 d of curing) above those suitable for landfilling and for fill materials were attained by these samples. The Sb adsorption on calcium-silicate-hydrates (C-S-H) formed during the cementation process was proposed as the mechanism responsible for such Sb attenuation.
Of these strategies for managing cemented wastes, their recycling as fill materials in some civil constructions, even if As or Sb release from them is minimized, would conflict with the environmental aims of many countries. Therefore, their disposal in controlled landfills appears to be the most suitable management strategy. The mine waste/binding agents ratios employed to produce cemented solids should be as high as possible to reduce their generated amounts, provided that they fulfill the criteria for safe storage regarding leaching characteristics and reach the compressive strength requirements.
4.3.2. Geopolymerization
Geopolymerization has been also evaluated as an encapsulation technique for managing As- and/or Sb-bearing mine waste (Salihoglu, Citation2014; Barrie et al., Citation2015; Kiventerä et al., Citation2018). Geopolymerization consists of generating amorphous/semi-crystalline three-dimensional aluminosilicate polymers, usually using alkali activators such as hydroxide or silicate solutions. This technology has been tested for the treatment of diverse wastes containing toxic elements.
Mixtures of As-bearing mine tailings, aggregate (sand) and solid precursors were treated with sodium silicate (NaSil)/NaOH solutions as activators to generate geopolymers (Barrie et al., Citation2015). The employed solid precursors included blast furnace slag and a combination of volcanic glass and calcined halloysite using mine tailings + aggregate:precursor ratios of 75:25. The compressive strength (after 28 d of curing) developed by the derived solidified products achieved relatively high values of approximately 10–30 MPa. These geopolymerization treatments increased As release, as observed by the tank leaching and shaking leaching tests. Additionally, thermal treated mine tailings (900 °C for 6 h) were employed to enhance their reactivity by the formation of CaO and MgO from the present carbonates (Kiventerä et al., Citation2018). The derived geopolymers improved their mechanical performance, increasing their compressive strength by a factor of 2, but the As release remained at similar level, as determined by the compliance test for leaching of granular waste materials and sludges EN-12457-4 (Citation2002). Considering the additional high cost of this thermal treatment and its null impact on the As immobilization, its application generally yields more drawbacks than advantages. In an attempt to reduce As leachability, the geopolymerization process was applied using untreated and thermally treated mine tailings by replacing conventional activators with 5% Ca(OH)2 solutions (Kiventerä et al., Citation2018). The As release from the solidified wastes derived under such circumstances was significantly reduced. In this case, similar reactions to those occurring in cementing processes occurred; the formation of calcium arsenates was identified as the main process responsible for the observed As immobilization (Kiventerä et al., Citation2018).
The slag produced by the thermal processing of Sb ore has been also subjected to geopolymerization (Salihoglu, Citation2014). The geopolymers were produced using NaSil/NaOH or NaOH solutions as activators and Portland cement, fly ash, mixtures of Portland cement and fly ash, and different mixtures containing three of Portland cement, fly ash, clay, gypsum or blast furnace slag as solid precursors. The precursors were used in the geopolymerization process at a precursor:slag ratio of 3:1. All derived geopolymers exhibited higher compressive strengths than solidified wastes arising from the cementation process. Nevertheless, geopolymerization gave rise to increased leachable Sb concentrations compared with that of the untreated slag, as observed by the compliance test for leaching of granular waste materials and sludges EN-12457-4 (Citation2002) (Salihoglu, Citation2014).
Various factors may be involved in the poor performance of geopolymerization processes in reducing the mobility of As and Sb in mine waste: a) the alkaline conditions of these matrices may lead to As and Sb desorption from minerals/phases with variable charge surfaces (generally ubiquitous in mine waste) and the solubilization of some As or Sb minerals/phases, b) the much higher solubility of compounds that oxyanions form with sodium compared with those formed with calcium, and c) the competition of silicate with As and Sb for adsorption sites.
Silicate can compete effectively with As and Sb for adsorption sites on various adsorbent materials (Smith & Edwards, Citation2005; Li et al., Citation2012). Particularly, it is well known that silicate interferes the As adsorption on Fe (oxyhydr)oxides, particularly at pH > 7.5 (Smith & Edwards, Citation2005). Moreover, silicate can also hinder As removal when these Fe phases are precipitated in situ, as it hampers their precipitation and further aggregation. This effect is more significant under high-pH conditions (Liu et al., Citation2007).
Geopolymerization has been found to be unsuitable for managing As- and/or Sb-bearing mine waste. This process cannot effectively immobilize metalloids, and the opposite effect can be observed. This solidification strategy should be definitively ruled out in favor of cementation. The cementation processes of mine waste mitigate As and Sb release from mine wastes and are more affordable due to the lower cost of cement or pozzolanic materials compared with that of NaSil or NaOH, which are the primary activators used in geopolymerization.
4.4. Deposition of protective coatings
Studies on encapsulation methods based on the generation of protective coatings have been focused on the passivation of arsenopyrite and its main weathering product, scorodite (Table S4), whereas only a few efforts have been devoted to encapsulating Sb-bearing mine waste ().
4.4.1. Protective coatings on arsenopyrite
The encapsulation techniques aiming to passivate arsenopyrite have been focused on the development of phosphate (Jones et al., Citation2003; Harris & Lottermoser, Citation2006) and metal (oxyhydr)oxide (Park et al., Citation2018a, Citation2018b, Citation2020) coatings.
Different approaches have been tested at lab scale to generate phosphate coatings. These include biotic processes using acidophilic iron-oxidizing bacteria (Thiobacillus ferrooxidans) suspended in acid growth saline solutions containing KH2PO4 (Jones et al., Citation2003), and abiotic processes using buffered solutions (pH > 5) containing KH2PO4 and an oxidizing agent (H2O2) (Harris & Lottermoser, Citation2006). The biotic approach was performed on polished arsenopyrite laths. This process gave rise to a delicate and permeable overlayer of FePO4 on arsenopyrite, as determined by SEM and XPS, that did not prevent its continuous oxidation and the release of As to the environment. The abiotic coating treatment with KH2PO4-H2O2 was performed via column (5.7 cm diameter × 12 cm height) packed with disturbed mine waste. This treatment did not minimize arsenopyrite oxidation or the consequent As release due to the lack of a continuous phosphate coating on arsenopyrite. In addition to the unsuccessful formation of phosphate layers on arsenopyrite by these methods, the use of coatings based on phosphates is questionable owing to the adverse effects that excessive phosphorous can represent to the environment (Lottermoser, Citation2010).
High phosphorous levels can lead to water eutrophication, promoting an excessive production of algae and cyanobacteria that causes hypoxia/anoxia in poorly mixed bottom waters and in surface waters during the night period under calm and warm conditions (Correll, Citation1998). These circumstances impact the sustainability of aquatic ecosystems, damaging aquatic animals (Correll, Citation1998). To avoid such a process, phosphate coatings should be performed in a leakproof tank or reactor. Otherwise, the establishment of a collector leachate system in the treatment area would be an effective alternative for recovering the excess of phosphate solutions employed during the coating of mine waste, thus minimizing its spread into the environment. Additionally, the disposal of phosphate-coated mine waste together with a phosphate selective-adsorbent is recommended to retain any phosphate release from coatings. Adsorbents with high adsorption capacities and rates, even at low phosphate concentrations, are preferable. Various adsorbents exhibit these characteristics, including single or mixed metal (Fe(III), Al(III), and Zr(IV)) oxides and hydroxides, layered double hydroxides, highly porous metal oxide/polymer composites, and metal-support adsorbents. The chosen adsorbents should also be low-cost and environmentally friendly. Some of these adsorbents also exhibit good adsorbent properties with regard to As(V) and/or As(III).
Unlike phosphate coatings, coatings based on metal (oxyhydr)oxides have exhibited great effectiveness in limiting the oxidation of arsenopyrite and mitigating As release into the environment. These coatings were developed via treatment of mine waste with titanium(IV)-catecholate ([Ti(Cat)3]2−) and aluminum-catecholate ([Al(Cat)]+) complexes. This method, called carrier microencapsulation, uses a redox-sensitive organic compound, namely catechol, to carry and deliver the material responsible for arsenopyrite encapsulation. As the decomposition of metal-organic complexes is electrochemical, these treatments can specifically target minerals that dissolve electrochemically, such as sulfides, avoiding excessive reagent consumption when treating mine wastes (Park et al., Citation2018a, Citation2018b). A scheme of this method according to those proposed by Park et al. (Citation2018a, Citation2018b) is shown in . This process involves the following steps: adsorption of catecholate complexes on the surface of arsenopyrite, oxidative decomposition of catecholate complexes, release of metals, and hydrolysis and precipitation of metals forming the coating of (oxyhydr)oxides (anatase (β-TiO2) in the case of Ti(IV) and boehmite (γ-AlOOH), gibbsite (γ-Al(OH)3), and bayerite (α-Al(OH)3) in the case of Al), as determined by SEM and diffuse reflectance infrared Fourier transform spectroscopy. These protective layers minimized the release of As from arsenopyrite by approximately 4–5 times (Park et al., Citation2018a, Citation2018b). It was found that, as weathering progressed, increasingly acidic conditions provoked a gradual dissolution of the Al oxyhydroxide-coating, thus reducing its protective effect (Park et al., Citation2020). Two strategies were proposed to increase the long-term stability of this method: increasing coating thickness or blending the Al oxyhydroxide-coated arsenopyrite with neutralizing materials to maintain pH conditions within the stability range of Al oxyhydroxides (approximately 4.5–8.5). These approaches should be tested to evaluate the proposed hypothesis. At first, these strategies do not seem feasible. Increasing the coating thickness may only delay the onset of dissolution. Blending Al oxyhydroxide-coated arsenopyrite with neutralizing materials appears to be viable primarily when managing unoxidized mine waste. When used to treat partially oxidized mine waste, strict pH control is required to maintain a pH within the conjoint stability range of Al oxyhydroxides and arsenopyrite weathered products such as scorodite and to avoid As desorption from Fe (oxyhydr)oxides. Unlike Al oxyhydroxides, anatase is stable under very acidic conditions. Moreover, bearing in mind that the treatment based on Ti(IV)-catecholate only achieves a surface coverage of 60%, improving the deposition process of anatase on arsenopyrite to near-full coverage would significantly enhance the protective action of this coating, making it a very attractive system for preventing arsenopyrite oxidation. When attempting to manage unoxidized mine waste, the application of surface covers to prevent arsenopyrite oxidation may be a useful strategy due to its facile application and low associated costs.
4.4.2. Protective coatings on scorodite
The development of protective layers has been investigated at lab scale for passivating scorodite. In this regard, coatings have been designed using silicate gels (Adelman et al., Citation2015), aluminum hydroxyl gels (Leetmaa et al., Citation2016; Guo & Demopoulos, Citation2018), aluminum silicate gels (Ma et al., Citation2019), and aluminum or calcium phosphate (Lagno et al., Citation2010; Rocha et al., Citation2012) or polyferric sulfate (Ke et al., Citation2018) deposition. The effectiveness of these approaches was quite dissimilar.
The silicate gels exhibited the worst performance. A reverse titration method was necessary to produce gels at pH values compatible with scorodite (Adelman et al., Citation2015). Using this method, a Si:As molar ratio of 0.1 was found to be appropriate for producing gels able to physically coat this mineral, as observed by SEM. These coatings exhibited a marginal effect on As attenuation from scorodite under either oxic or anoxic (0 mV) conditions at pH 9. To enhance this behavior, diverse aging processed were assayed (drying at 22 and 44 °C and hydrothermal treatment at 110 and 160 °C). Nearly all processes failed to accomplish attenuation, as As release increased following treatment. The inefficacy of these silica-like coatings was explained as occurring due to an ion-exchange reaction between arsenate and silicate on scorodite surface as determined by XPS, Fourier transform infrared spectroscopy, and Raman spectroscopy. In this regard, the stability constant of the complex that silicate forms with Fe(III) is many orders of magnitude higher than those of complexes that arsenate form with Fe(III), implying that silicate can replace arsenate on scorodite surface (Adelman et al., Citation2015).
The aluminum hydroxyl gels were found to perform much better. These gels were derived from either chloride or sulfate salts and evaluated for scorodite treatment under different Al:As molar ratios and aging conditions (22–70 °C, 1–54 days under closed/open systems) (Leetmaa et al., Citation2016). The aluminum hydroxyl gels were able to create a matrix around scorodite, as observed by SEM, which was considered responsible for mitigating the release of As from scorodite (≥ 20-fold) under oxidizing conditions at pH of approximately 7.0–7.5, particularly when using aluminum sulfate-derived gels. For these, an Al:As molar ratio of 0.1, 1 day ambient aging, and open systems were set as the optimal conditions to encapsulate scorodite. The improved performance of the matrix resulting from the aluminum sulfate-derived gels for controlling As was attributed to the As attenuation mechanisms. In this case, As adsorption and physical protection were involved, whereas aluminum chloride-derived gels mostly performed As adsorption. The aluminum hydroxide matrix formed on scorodite by aluminum sulfate-derived gels presented a SO4-stabilized Keggin Al13 structure. This structure was found to be a prerequisite to crystalline aluminum hydroxide formation upon aging (Leetmaa et al., Citation2016). The mineralization of the aluminum hydroxide matrix is expected to yield additional physical protection for scorodite. Under longer aging periods, a very thin coating composed of gibbsite and bayerite was developed on the surface of scorodite (Guo & Demopoulos, Citation2018). This coating was also able to restrict the release of As from scorodite under reducing conditions (340–210 mV) at pH of approximately 8.0–8.5, reaching similar reduction levels as those indicated previously (Guo & Demopoulos, Citation2018).
Good performance was also reported for aluminum silicate gels, which formed a uniform coating on the surface of scorodite, as observed by SEM, via supersaturation of Al-Si gels using Al:Si and Si:As molar ratios of 0.6 and 0.2–1.5, respectively (Ma et al., Citation2019). After an aging period of 5 h, coatings formed at Si:As molar ratios of 0.2 were reported to be the most effective for decreasing As mobilization from scorodite at pH 4 (approximately 85%), whereas coatings formed at Si:As molar ratios of 1.5 under pH 6 and 8 conditions demonstrated the most effective As mitigation (approximately 60–70%). The coating layer had an amorphous structure, being hypothetically composed of multiphase gels (Si, Al, and Al-Si gels), as inferred by Raman and attenuated total reflectance Fourier-transform infrared spectroscopy. The multiphasic composition of the coating may suppose different stability properties under different pH values, offering a wide pH range under which it may be effective to prevent or decrease As release from scorodite.
Contrary to what happens with arsenopyrite, uniform phosphate coatings can be generated on scorodite surface. These coatings were formed on this mineral via supersaturated metastable solutions of aluminum phosphate prepared at pH 1.7, keeping it constant during the deposition stage (Lagno et al., Citation2010). The coating process was performed using a solid/liquid ratio of 50 g L−1 and increasing deposition cycles (from 1 to 3). The first deposition process is considered to comprise two stages: one in which phosphate is retained by the scorodite surface via ion exchange with arsenate and another in which phosphate is deposited on the scorodite surface via stoichiometric precipitation of aluminum phosphate, generating a crystalline coating of AlPO4·1.5H2O, which thickens with deposition cycles (up to 3.5 µm). These results have been observed using XRD and SEM. A scheme of this encapsulation process is shown in . The coating generated after the third deposition cycle reduced the As release from scorodite by more than one order of magnitude, either under oxic conditions at pH 4, 6, or 8 or under anoxic conditions (100 mV) at pH 7. In spite of the good performance of this treatment, a complete sealing of scorodite was not obtained, being still considered to be hazardous (Lagno et al., Citation2010). Additional attempts have been made to encapsulate scorodite with other phosphates such as hydroxyapatite (Ca5(PO4)3OH) or fluorapatite (Ca5(PO4)3F) (Rocha et al., Citation2012). Scorodite was treated with metastable solutions of P and Ca or P, Ca, and F at controlled pH conditions (7.0–7.6 or 6.0–7.0) under two stages, namely nucleation and growth. Studies by SEM indicated that deposited coatings on scorodite were not continuous, and their effectiveness on restricting As mobilization was greatly dependent on the specific coating and redox conditions. Thus, after subjecting scorodite to these treatments, the As release reductions achieved under oxic or anoxic (150 mV) conditions at pH 9 ranged from approximately 3- to 22-fold.
The feasibility of depositing a protective crystalline coating of polyferric sulfate on the scorodite surface has also been demonstrated. This was accomplished by treating scorodite with ferrous sulfate solutions of different concentrations (0.1, 0.2, 0.3, and 0.4 M) and flowing oxygen to oxidize ferrous ions into ferric ions (Ke et al., Citation2018). The effectiveness of this treatment to restrain the As release was evaluated under oxic (TCLP at pH 4.93, NaOH at pH 9.3, and NaH2PO4-NaOH at pH 8.6) and anoxic (−100–0 mV at pH 7.6 and 8.1) conditions. The polyferric sulfate coatings were formed uniformly on scorodite, as determined by SEM and transmission electron microscopy. Such coatings showed a good performance, particularly when derived from 0.4 M ferrous sulfate solutions, giving rise to As immobilization levels above 95%, except under alkaline solutions containing phosphate (approximately 87%). An ion exchange reaction occurred between arsenate and phosphate that increased the release of the former. In addition to the physical protection provided to scorodite by the polyferric sulfate layer, this coating was suggested to control the As release via adsorption due to the adsorbent properties evidenced by polyferric sulfate.
Although several of the proposed methods exhibit good potential for treating scorodite, some require optimization to improve the level of scorodite coverage or the thickness of the coating. Moreover, generally the pH range under which they have proven their suitability is quite restricted or is dependent on the deposition conditions. Coatings should be able to protect scorodite under the low acidic conditions prevalent in sulfide wastes.
4.4.3. Protective coatings on Sb-bearing mine waste
Research on Sb-bearing mine waste passivation has been practically limited to the study of Harris and Lottermoser (Citation2006), who focused on the development of phosphate coatings. The generation of these coatings were assayed at lab scale on mine waste with the occurrence of tetrahedrite using buffered solutions (pH > 5) containing KH2PO4 and an oxidizing agent (H2O2). The coating treatment with KH2PO4-H2O2 was performed using column (5.7 cm diameter × 12 cm height) packed with disturbed mine waste. After this treatment, tetrahedrite was uncoated or minimally coated, as determined by SEM, and Sb release from wastes was not prevented. The failure to develop phosphate coatings on tetrahedrite was attributed to the lack of iron in the mineral lattice, precluding the formation of the corresponding phosphate.
4.5. Unconventional techniques
Apart from the discussed geochemical approaches, less conventional techniques have been also evaluated for treating As-bearing mine waste. These include the development of passivating coatings comprising alkoxysilanes (Khummalai & Boonamnuayvitaya, Citation2005), the use of epoxy materials for encapsulation processes (Randall, Citation2012), and the application of geosynthetic clay liners as surface covers (Hosney & Rowe, Citation2014; Rowe & Hosney, Citation2013).
The coatings based on alkoxysilanes restrained both the chemical and biological oxidation of arsenopyrite, with methyltrimethoxysilane (MTMOS) being the most effective amongst tested precursors. These coatings were produced at lab scale using sol-gel processes where arsenopyrite was treated with mixtures of the alkoxysilane and water at different H2O/Si molar ratios (2–16). At the optimal H2O/Si molar ratio (2), the thin film created on arsenopyrite using MTMOS as its precursor was able to decrease the rate of the chemical and biological oxidation of this mineral to 5.25 and 12.63%, respectively (Khummalai & Boonamnuayvitaya, Citation2005).
The encapsulation of weathered arsenopyrite-bearing mine waste by epoxy materials was evaluated using Terra-Bond technology, a patented process of Pildysh Technologies, Inc. This process proceeds through the formation of a stable thermoplastic polymeric material that encapsulates wastes after blending them with a molten sulfur binder (Randall, Citation2012). Although this technique proved to minimize As leaching at various pH values, effective diffusivities derived from diffusion studies reported that the mine waste treated in this way were unsuitable for disposal.
The geosynthetic clay liners investigated as surface covers were composed of either natural or polymer-enhanced Na-bentonite comprised between different carrier geotextiles. These were applied at lab/field scale on mine tailings with diverse total As contents (0.45–12.3%) and As porewater concentration (3.5–30 mg L−1) with or without the inclusion of a foundation layer (non-contaminated soil) between them and mine tailings. The geosynthetic clay liners were finally covered by a non-contaminated soil layer. The performance of these systems regarding their As retention capacity was evaluated under laboratory and field conditions during prolonged periods of time (up to 4 years). It was found that the movement of As upwards from mine tailings was most significant in absence of the foundation layer, but As was retained in the applied geosynthetic clay liner. Considering both economic and technical aspects, the recommended systems from top to bottom are as follows (Hosney & Rowe, Citation2014; Rowe & Hosney, Citation2013):
- a layer of clean soil/a layer of geosynthetic clay liner with polymer-enhanced Na-bentonite/a foundation layer
- a layer of clean soil/a layer of geosynthetic clay liner with natural Na-bentonite/a foundation layer.
5. Conclusions and outlook
Under weathering conditions, As and Sb in mine wastes are readily mobilizable. Different stabilization and encapsulation strategies have been investigated to prevent the off-side migration of these toxic metalloids and their adverse impact on surrounding ecosystems. Some of these techniques have shown their feasibility or potential to treat As and/or Sb mine waste. However, their actual effectiveness is greatly dependent on the weathering state, composition and final fate of mine waste.
The effective treatment of As-bearing mine waste can be performed by means of a) non-porous surface covers in the case of unoxidized wastes, b) cementation with Portland cement and/or hydrated lime when landfilling is the final fate of wastes, and c) different amendments, highlighting those composed of Fe (oxyhydr)oxides and their precursors provided that mine waste keep exposed to atmospheric conditions. Concerning the application of amendments, there is a lack of knowledge on their long-term performance. Field scale studies should be performed to evaluate the durability of these treatments and the likely requirement of successive amendment incorporations as weathering progresses to control the release of this toxic metalloid.
Additionally, encapsulation methods based on the development of protective layers show a great potential to encapsulate mine waste containing As sulfides such as arsenopyrite or its main weathering product (scorodite). Up to now, several promising approaches have been proposed, requiring optimization to become actual effective treatment systems. Of the proposed approaches, those aimed to generate an anatase coating exhibit some advantages owing to the wide stability field of this phase. In any case, these treatments should be tested with actual mine waste to facilitate field scale studies. Thus far, these processes have been solely assayed using pure/synthetic phases. Various limitations may arise when evaluating actual mine waste, such as destabilization of other As-bearing minerals/phases present in mine waste under the treatment conditions, an excessive consumption of reactants that may reduce economic viability of a given method, and poorer deposition or performance of coatings than under lab conditions.
In the case of Sb-bearing mine waste, only cementation has been demonstrated to be effective for their treatment. More research is necessary to develop useful stabilization methods and/or protective coatings for these wastes. From the current state of research, the following perspectives on future work were derived: a) application of proven Sb adsorbents as amendments such as Fe, Al, or Mn (oxyhydr)oxides, prioritizing the use of by-products containing them when available, b) application of high-Ca liming materials to promote the formation of the sparingly soluble minerals of the roméite group, and c) forced oxidation of mine waste in presence of available Fe to promote the formation of the highly insoluble Fe antimonates, particularly tripuhyite, which has been found to be an important sink for Sb in mine waste.
Supplemental Material
Download MS Word (35.2 KB)Acknowledgements
The present work was carried out under the framework of the project TERMET (Grant number: RTI2018-095433-B-I00) funded by Ministerio de Ciencia, Innovación y Universidades (MCIU), Spain/Agencia Estatal de Investigación (AEI), Spain/Fondo Europeo de Desarrollo Regional (FEDER), UE.
References
- Adelman, J. G., Elouatik, S., & Demopoulos, G. P. (2015). Investigation of sodium silicate-derived gels as encapsulants for hazardous materials-The case of scorodite. Journal of Hazardous Materials, 292, 108–117. https://doi.org/https://doi.org/10.1016/j.jhazmat.2015.03.008
- Alloway, B. J. (1995). Heavy metals in soils. Blackie Academic & Professional.
- Álvarez-Ayuso, E., & Murciego, A. (2021). Stabilization methods for the treatment of weathered arsenopyrite mine wastes: Arsenic immobilization under selective leaching conditions. Journal of Cleaner Production, 283, 125265. https://doi.org/https://doi.org/10.1016/j.jclepro.2020.125265
- Álvarez-Ayuso, E., Otones, V., Murciego, A., & García-Sánchez, A. (2013). Evaluation of different amendments to stabilize antimony in mining polluted soils. Chemosphere, 90(8), 2233–2239. https://doi.org/https://doi.org/10.1016/j.chemosphere.2012.09.086
- Arenas-Lago, D., Abreu, M. M., Andrade Couce, L., & Vega, F. A. (2019). Is nanoremediation an effective tool to reduce the bioavailable As, Pb and Sb contents in mine soils from Iberian Pyrite Belt? CATENA, 176, 362–371. https://doi.org/https://doi.org/10.1016/j.catena.2019.01.038
- Ashley, P. M., Craw, D., Graham, B. P., & Chappell, D. A. (2003). Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and Southern New Zealand. Journal of Geochemical Exploration, 77(1), 1–14. https://doi.org/https://doi.org/10.1016/S0375-6742(02)00251-0
- Asta, M. P., Cama, J., Ayora, C., Acero, P., & de Giudici, G. (2010). Arsenopyrite dissolution rates in O2-bearing solutions. Chemical Geology, 273(3-4), 272–285. https://doi.org/https://doi.org/10.1016/j.chemgeo.2010.03.002
- Barrie, E., Cappuyns, V., Vassilieva, E., Adriaens, R., Hollanders, S., Garcés, D., Paredes, C., Pontikes, Y., Elsen, J., & Machiels, L. (2015). Potential of inorganic polymers (geopolymers) made of halloysite and volcanic glass for the immobilisation of tailings from gold extraction in Ecuador. Applied Clay Science, 109-110, 95–106. https://doi.org/https://doi.org/10.1016/j.clay.2015.02.025
- Beauchemin, S., Kwong, Y. T. J., Desbarats, A. J., MacKinnon, T., Percival, J. B., Parsons, M. B., & Pandya, K. (2012). Downstream changes in antimony and arsenic speciation in sediments at a mesothermal gold deposit in British Columbia. Canada. Applied Geochemistry, 27(10), 1953–1965. https://doi.org/https://doi.org/10.1016/j.apgeochem.2012.04.003
- Benzaazoua, M., Marion, P., Picquet, I., & Bussière, B. (2004). The use of pastefill as a solidification and stabilization process for the control of acid mine drainage. Minerals Engineering, 17(2), 233–243. https://doi.org/https://doi.org/10.1016/j.mineng.2003.10.027
- Biver, M., & Shotyk, W. (2012a). Stibnite (Sb2S3) oxidative dissolution kinetics from pH 1 to 11. Geochimica et Cosmochimica Acta, 79, 127–139. https://doi.org/https://doi.org/10.1016/j.gca.2011.11.033
- Biver, M., & Shotyk, W. (2012b). Experimental study of the kinetics of ligand-promoted dissolution of stibnite (Sb2S3). Chemical Geology, 294–295, 165–172. https://doi.org/https://doi.org/10.1016/j.chemgeo.2011.11.009
- Biver, M., & Shotyk, W. (2013). Stibiconite (Sb3O6OH), senarmontite (Sb2O3) and valentinite (Sb2O3): Dissolution rates at pH 2-11 and isoelectric points. Geochimica et Cosmochimica Acta, 109, 268–279. https://doi.org/https://doi.org/10.1016/j.gca.2013.01.033
- Biver, M., Krachler, M., & Shotyk, W. (2011). The desorption of antimony(V) from sediments, hydrous oxides, and clay minerals by carbonate, phosphate, sulfate, nitrate, and chloride. Journal of Environmental Quality, 40(4), 1143–1152. https://doi.org/https://doi.org/10.2134/jeq2010.0503
- Cheng, H., Hu, Y., Luo, J., Xu, B., & Zhao, J. (2009). Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. Journal of Hazardous Materials, 165(1-3), 13–26. https://doi.org/https://doi.org/10.1016/j.jhazmat.2008.10.070
- Choi, W.-H., Lee, S.-R., & Park, J.-Y. (2009). Cement based solidification/stabilization of arsenic-contaminated mine tailings. Waste Management (New York, N.Y.), 29(5), 1766–1771. https://doi.org/https://doi.org/10.1016/j.wasman.2008.11.008
- Cidu, R., Biddau, R., Dore, E., Vacca, A., & Marini, L. (2014). Antimony in the soil-water-plant system at the Su Suergiu abandoned mine (Sardinia, Italy): Strategies to mitigate contamination. Science of the Total Environment, 497-498, 319–331. https://doi.org/https://doi.org/10.1016/j.scitotenv.2014.07.117
- Çopur, M., Pekdemir, T., Çelik, C., & Çolak, S. (1997). Determination of the optimum conditions for the dissolution of stibnite in HCl solutions. Industrial & Engineering Chemistry Research, 36(3), 682–687. https://doi.org/https://doi.org/10.1021/ie960258r
- Corkhill, C. L., Wincott, P. L., Lloyd, J. R., & Vaughan, D. J. (2008). The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans. Geochimica et Cosmochimica Acta, 72(23), 5616–5633. https://doi.org/https://doi.org/10.1016/j.gca.2008.09.008
- Correll, D. L. (1998). The role of phosphorus in the eutrophication of receiving waters: A review. Journal of Environmental Quality, 27(2), 261–266. https://doi.org/https://doi.org/10.2134/jeq1998.00472425002700020004x
- Courtin-Nomade, A., Rakotoarisoa, O., Bril, H., Grybos, M., Forestier, L., Foucher, F., & Kunz, M. (2012). Weathering of Sb-rich mining and smelting residues: Insight in solid speciation and soil bacteria toxicity. Geochemistry, 72(S4), 29–39. https://doi.org/https://doi.org/10.1016/j.chemer.2012.02.004
- Coussy, S., Benzaazoua, M., Blanc, D., Moszkowicz, P., & Bussière, B. (2011). Arsenic stability in arsenopyrite-rich cemented paste backfills: A leaching test-based assessment. Journal of Hazardous Materials, 185(2-3), 1467–1476. https://doi.org/https://doi.org/10.1016/j.jhazmat.2010.10.070
- Craw, D., Falconer, D., & Youngson, J. H. (2003). Environmental arsenopyrite stability and dissolution: Theory, experiment, and field observations. Chemical Geology, 199(1-2), 71–82. https://doi.org/https://doi.org/10.1016/S0009-2541(03)00117-7
- Craw, D., Koons, P. O., & Chappell, D. A. (2002). Arsenic distribution during formation and capping of an oxidised sulphidic minesoil, Macraes mine, New Zealand. Journal of Geochemical Exploration, 76(1), 13–29. https://doi.org/https://doi.org/10.1016/S0375-6742(02)00202-9
- De Oliveira, L. K., Melo, C. A., Goveia, D., Lobo, F. A., Armienta Hernández, M. A., Fraceto, L. F., & Rosa, A. H. (2015). Adsorption/desorption of arsenic by tropical peat: Influence of organic matter, iron and aluminium. Environmental Technology, 36(1-4), 149–159. https://doi.org/https://doi.org/10.1080/09593330.2014.939999
- DeSisto, S. L., Jamieson, H. E., & Parsons, M. B. (2017). Arsenic mobility in weathered gold mine tailings under a low-organic soil cover. Environmental Earth Sciences, 76(22), 773. https://doi.org/https://doi.org/10.1007/s12665-017-7041-7
- Di, X., Beesley, L., Zhang, Z., Zhi, S., Jia, Y., & Ding, Y. (2019). Microbial arsenic methylation in soil and uptake and metabolism of methylated arsenic in plants: A review. International Journal of Environmental Research and Public Health, 16(24), 5012. https://doi.org/https://doi.org/10.3390/ijerph16245012
- Doherty, S. J., Tighe, M. K., & Wilson, S. C. (2017). Evaluation of amendments to reduce arsenic and antimony leaching from co-contaminated soils. Chemosphere, 174, 208–217. https://doi.org/https://doi.org/10.1016/j.chemosphere.2017.01.100
- Dos Santos, E. C., Lourenço, M. P., Pettersson, L. G. M., & Duarte, H. A. (2017). Stability, structure, and electronic properties of the pyrite/arsenopyrite solid-solid interface-A DFT study. The Journal of Physical Chemistry C, 121(14), 8042–8051. https://doi.org/https://doi.org/10.1021/acs.jpcc.7b02642
- Dove, P. M., & Rimstidt, J. D. (1985). The solubility and stability of scorodite, FeAsO4·2H2O. American Mineralogist, 70, 838–844.
- Drahota, P., & Filippi, M. (2009). Secondary arsenic minerals in the environment: A review. Environment International, 35(8), 1243–1255. https://doi.org/https://doi.org/10.1016/j.envint.2009.07.004
- EN-12457-4. (2002). Characterization of waste - Leaching-Compliance test for leaching of granular waste materials and sludges - Part 4: One stage batch test at a liquid to solid ratio of 10 l/kg for materials with particle size below 10 mm (without or with size reduction). European Committee for Standardization.
- Fan, Y., Zheng, C., Huo, A., Wang, Q., Shen, Z., Xue, Z., & He, C. (2019). Investigating the binding properties between antimony(V) and dissolved organic matter (DOM) under different pH conditions during the soil sorption process using fluorescence and FTIR spectroscopy. Ecotoxicology and Environmental Safety, 181, 34–42. https://doi.org/https://doi.org/10.1016/j.ecoenv.2019.05.076
- Filella, M., Belzile, N., & Chen, Y.-W. (2002). Antimony in the environment: A review focused on natural waters I. Occurrence. Earth-Science Reviews, 57(1–2), 125–176. https://doi.org/https://doi.org/10.1016/S0012-8252(01)00070-8
- Filella, M., Philippo, S., Belzile, N., Chen, Y., & Quentel, F. (2009). Natural attenuation processes applying to antimony: A study in the abandoned antimony mine in Goesdorf, Luxembourg. Science of the Total Environment, 407(24), 6205–6216. https://doi.org/https://doi.org/10.1016/j.scitotenv.2009.08.027
- Filippi, M., Drahota, P., Machovič, V., Böhmová, V., & Mihaljevič, M. (2015). Arsenic mineralogy and mobility in the arsenic-rich historical mine waste dump. Science of the Total Environment, 536, 713–728. https://doi.org/https://doi.org/10.1016/j.scitotenv.2015.07.113
- Flaková, R., Ženišová, Z., Krčmár, D., Ondrejková, I., & Sracek, O. (2017). Occurrence of antimony and arsenic at mining sites in Slovakia: Implications for their mobility. Carpathian Journal of Earth and Environmental Sciences, 12, 41–48.
- Flakova, R., Zenisova, Z., Sracek, O., Krcmar, D., Ondrejkova, I., Chovan, M., Lalinská, B., & Fendekova, M. (2012). The behavior of arsenic and antimony at Pezinok mining site, southwestern part of the Slovak Republic. Environmental Earth Sciences, 66(4), 1043–1057. https://doi.org/https://doi.org/10.1007/s12665-011-1310-7
- Gao, W., Ni, W., Zhang, Y., Li, Y., Shi, T., & Li, Z. (2020). Investigation into the semi-dynamic leaching characteristics of arsenic and antimony from solidified/stabilized tailings using metallurgical slag-based binders. Journal of Hazardous Materials, 381, 120992. https://doi.org/https://doi.org/10.1016/j.jhazmat.2019.120992
- Gräfe, M., Beattie, D. A., Smith, E., Skinner, W. M., & Singh, B. (2008). Copper and arsenate co-sorption at the mineral-water interfaces of goethite and jarosite. Journal of Colloid and Interface Science, 322(2), 399–413. https://doi.org/https://doi.org/10.1016/j.jcis.2008.02.044
- Grafe, M., Eick, M. J., Grossl, P. R., & Saunders, A. M. (2002). Adsorption of arsenate and arsenite on ferrihydrite in the presence and absence of dissolved organic carbon. Journal of Environmental Quality, 31(4), 1115–1123. https://doi.org/https://doi.org/10.2134/jeq2002.1115
- Gu, J., Yao, J., Jordan, G., Roha, B., Min, N., Li, H., & Lu, C. (2020). Arundo donax L. stem-derived biochar increases As and Sb toxicities from nonferrous metal mine tailings. Environmental Science and Pollution Research International, 27(3), 2433–2443. https://doi.org/https://doi.org/10.1007/s11356-018-2780-x
- Guo, F., & Demopoulos, G. P. (2018). Development of an encapsulation process to extend the stability of scorodite under wider pH and redox potential range conditions. In B. Davis (Eds.), Extraction 2018 (pp. 1411–1420). The Minerals, Metals & Materials Series. Springer.
- Haffert, L., Craw, D., & Pope, J. (2010). Climatic and compositional controls on secondary arsenic mineral formation in high-arsenic mine wastes, South Island, New Zealand. New Zealand Journal of Geology and Geophysics, 53(2-3), 91–101. https://doi.org/https://doi.org/10.1080/00288306.2010.498403
- Hamberg, R., Maurice, C., & Alakangas, L. (2015). The use of low binder proportions in cemented paste backfill - Effects on As-leaching. Minerals Engineering, 78, 74–82. https://doi.org/https://doi.org/10.1016/j.mineng.2015.04.017
- Harris, D. L., & Lottermoser, B. G. (2006). Phosphate stabilization of polyminerallic mine wastes. Mineralogical Magazine, 70(1), 1–13. https://doi.org/https://doi.org/10.1180/0026461067010309
- Herath, I., Vithanage, M., & Bundschuh, J. (2017). Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environmental Pollution (Barking, Essex : 1987), 223, 545–559. https://doi.org/https://doi.org/10.1016/j.envpol.2017.01.057
- Hiller, E., Lalinská, B., Chovan, M., Jurkovič, Ľ., Klimko, T., Jankulár, M., Hovorič, R., Šottník, P., Fľaková, R., Ženišová, Z., & Ondrejková, I. (2012). Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Applied Geochemistry, 27(3), 598–614. https://doi.org/https://doi.org/10.1016/j.apgeochem.2011.12.005
- Hoffmann, M., Mikutta, C., & Kretzschmar, R. (2013). Arsenite binding to natural organic matter: Spectroscopic evidence for ligand exchange and ternary complex formation. Environmental Science & Technology, 47(21), 12165–12173. https://doi.org/https://doi.org/10.1021/es4023317
- Hosney, M. S., & Rowe, R. K. (2014). Performance of three GCLs used for covering gold mine tailings for 4 years under field and laboratory exposure conditions. Geosynthetics International, 21(3), 197–212. https://doi.org/https://doi.org/10.1680/gein.14.00009
- Hu, X., He, M., & Kong, L. (2015). Photopromoted oxidative dissolution of stibnite. Applied Geochemistry, 61, 53–61. https://doi.org/https://doi.org/10.1016/j.apgeochem.2015.05.014
- Ilgen, A. G., & Trainor, T. P. (2012). Sb(III) and Sb(V) sorption onto al-rich phases: Hydrous al oxide and the clay minerals kaolinite KGa-1b and oxidized and reduced nontronite NAu-1. Environmental Science & Technology, 46(2), 843–851. https://doi.org/https://doi.org/10.1021/es203027v
- Jain, A., & Loeppert, R. H. (2000). Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. Journal of Environmental Quality, 29(5), 1422–1430. https://doi.org/https://doi.org/10.2134/jeq2000.00472425002900050008x
- Jelenová, H., Majzlan, J., Amoako, F. Y., & Drahota, P. (2018). Geochemical and mineralogical characterization of the arsenic-, iron-, and sulfur-rich mining waste dumps near Kaňk, Czech Republic. Applied Geochemistry, 97, 247–255. https://doi.org/https://doi.org/10.1016/j.apgeochem.2018.08.029
- Jia, Y., Maurice, C., & Öhlander, B. (2014). Effect of the alkaline industrial residues fly ash, green liquor dregs, and lime mud on mine tailings oxidation when used as covering material. Environmental Earth Sciences, 72(2), 319–334. https://doi.org/https://doi.org/10.1007/s12665-013-2953-3
- Jia, Y., Stahre, N., Mäkitalo, M., Maurice, C., & Öhlander, B. (2017). Elemental mobility in sulfidic mine tailings reclaimed with paper mill by-products as sealing materials. Environmental Science and Pollution Research International, 24(25), 20372–20389. https://doi.org/https://doi.org/10.1007/s11356-017-9650-9
- Johnston, S. G., Bennett, W. W., Doriean, N., Hockmann, K., Karimian, N., & Burton, E. D. (2020). Antimony and arsenic speciation, redox-cycling and contrasting mobility in a mining-impacted river system. Science of the Total Environment, 710, 136354. https://doi.org/https://doi.org/10.1016/j.scitotenv.2019.136354
- Jones, C. A., Inskeep, W. P., & Neuman, D. R. (1997). Arsenic transport in contaminated mine tailings following liming. Journal of Environmental Quality, 26(2), 433–439. https://doi.org/https://doi.org/10.2134/jeq1997.00472425002600020014x
- Jones, R. A., Koval, S. F., & Nesbitt, H. W. (2003). Surface alteration of arsenopyrite (FeAsS) by Thiobacillus ferrooxidans. Geochimica et Cosmochimica Acta, 67(5), 955–965. https://doi.org/https://doi.org/10.1016/S0016-7037(02)00996-1
- Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Springer-Verlag.
- Kadokura, M., Suzuki, K., Kato, T., Grabda, M., Tokoro, C. (2019). Mechanism investigation and surface complexation modeling of arsenite sorption on ferrihydrite in adsorption/co-precipitation processes. Proceedings of the 10th European Metallurgical Conference (EMC) 2019, 727–738.
- Kanel, S. R., Greneche, J.-M., & Choi, H. (2006). Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environmental Science & Technology, 40(6), 2045–2050. https://doi.org/https://doi.org/10.1021/es0520924
- Kanel, S. R., Manning, B., Charlet, L., & Choi, H. (2005). Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environmental Science & Technology, 39(5), 1291–1298. https://doi.org/https://doi.org/10.1021/es048991u
- Ke, P., Song, K., & Liu, Z. (2018). Encapsulation of scorodite using crystalline polyferric sulfate precipitated from the Fe(II)-SO42−-O2-H2O system. Hydrometallurgy, 180, 78–87. https://doi.org/https://doi.org/10.1016/j.hydromet.2018.07.011
- Khummalai, N., & Boonamnuayvitaya, V. (2005). Suppression of arsenopyrite surface oxidation by sol-gel coatings. Journal of Bioscience and Bioengineering, 99(3), 277–284. https://doi.org/https://doi.org/10.1263/jbb.99.277
- Kim, J.-Y., Davis, A. P., & Kim, K.-W. (2003). Stabilization of available arsenic in highly contaminated mine tailings using iron. Environmental Science & Technology, 37(1), 189–195. https://doi.org/https://doi.org/10.1021/es020799+
- Kim, K.-R., Lee, B.-T., & Kim, K.-W. (2012). Arsenic stabilization in mine tailings using nano-sized magnetite and zero valent iron with the enhancement of mobility by surface coating. Journal of Geochemical Exploration, 113, 124–129. https://doi.org/https://doi.org/10.1016/j.gexplo.2011.07.002
- Kim, S. H., Jeong, S., Chung, H., & Nam, K. (2018). Stabilization mechanism of arsenic in mine waste using basic oxygen furnace slag: The role of water contents on stabilization efficiency. Chemosphere, 208, 916–921. https://doi.org/https://doi.org/10.1016/j.chemosphere.2018.05.173
- Kiventerä, J., Sreenivasan, H., Cheeseman, C., Kinnunen, P., & Illikainen, M. (2018). Immobilization of sulfates and heavy metals in gold mine tailings by sodium silicate and hydrated lime. Journal of Environmental Chemical Engineering, 6(5), 6530–6536. https://doi.org/https://doi.org/10.1016/j.jece.2018.10.012
- Ko, I., Kim, J.-Y., & Kim, K.-W. (2004). Arsenic speciation and sorption kinetics in the As-hematite-humic acid system. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 234(1-3), 43–50. https://doi.org/https://doi.org/10.1016/j.colsurfa.2003.12.001
- Koo, N., Lee, S.-H., & Kim, J.-G. (2012). Arsenic mobility in the amended mine tailings and its impact on soil enzyme activity. Environmental Geochemistry and Health, 34(3), 337–348. https://doi.org/https://doi.org/10.1007/s10653-011-9419-x
- Lagno, F., Rocha, S. D. F., Chryssoulis, S., & Demopoulos, G. P. (2010). Scorodite encapsulation by controlled deposition of aluminum phosphate coatings. Journal of Hazardous Materials, 181(1-3), 526–534. https://doi.org/https://doi.org/10.1016/j.jhazmat.2010.05.046
- Lee, W. C., Kim, S.-O., Ranville, J., Yun, S.-T., & Choi, S. H. (2014). Sequestration of arsenate from aqueous solution using 2-line ferrihydrite: Equilibria, kinetics, and X-ray absorption spectroscopic analysis. Environmental Earth Sciences, 71(8), 3307–3318. https://doi.org/https://doi.org/10.1007/s12665-013-2717-0
- Leetmaa, K., Guo, F., Becze, L., Gomez, M. A., & Demopoulos, G. P. (2016). Stabilization of iron arsenate solids by encapsulation with aluminum hydroxyl gels. Journal of Chemical Technology & Biotechnology, 91(2), 408–415. https://doi.org/https://doi.org/10.1002/jctb.4590
- Li, X., Dou, X., & Li, J. (2012). Antimony(V) removal from water by iron-zirconium bimetal oxide: Performance and mechanism. Journal of Environmental Sciences (China), 24(7), 1197–1203. https://doi.org/https://doi.org/10.1016/S1001-0742(11)60932-7
- Lin, Z., & Puls, R. W. (2000). Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environmental Geology, 39(7), 753–759. https://doi.org/https://doi.org/10.1007/s002540050490
- Liu, C.-H., Chuang, Y.-H., Chen, T.-Y., Tian, Y., Li, H., Wang, M.-K., & Zhang, W. (2015). Mechanism of arsenic adsorption on magnetite nanoparticles from water: Thermodynamic and spectroscopic studies. Environmental Science & Technology, 49(13), 7726–7734. https://doi.org/https://doi.org/10.1021/acs.est.5b00381
- Liu, R., Qu, J., Xia, S., Zhang, G., & Li, G. (2007). Silicate hindering in situ formed ferric hydroxide precipitation: Inhibiting arsenic removal from water. Environmental Engineering Science, 24(5), 707–715. https://doi.org/https://doi.org/10.1089/ees.2006.0074
- Loni, P. C., Wu, M., Wang, W., Wang, H., Ma, L., Liu, C., Song, Y., & H Tuovinen, O. (2020). Mechanism of microbial dissolution and oxidation of antimony in stibnite under ambient conditions. Journal of Hazardous Materials, 385, 121561. https://doi.org/https://doi.org/10.1016/j.jhazmat.2019.121561
- Lottermoser, B. G. (2010). Mine wastes-Characterization, treatment and environmental impacts (3rd ed.). Springer-Verlag.
- Ma, X., Li, S., Yuan, Z., Yao, S., Jia, Y., & Wang, S. (2019). Stabilization of scorodite by aluminum silicate microencapsulation. Journal of Environmental Engineering, 145(4), 04019010. https://doi.org/https://doi.org/10.1061/(ASCE)EE.1943-7870.0001511
- Majzlan, J., Števko, M., & Lánczos, T. (2016). Soluble secondary minerals of antimony in Pezinok and Kremnica (Slovakia) and the question of mobility or immobility of antimony in mine waters. Environmental Chemistry, 13(6), 927–935. https://doi.org/https://doi.org/10.1071/EN16013
- Manaka, M., Yanase, N., Sato, T., & Fukushi, K. (2007). Natural attenuation of antimony in mine drainage water. Geochemical Journal, 41(1), 17–27. https://doi.org/https://doi.org/10.2343/geochemj.41.17
- Mandal, B. K., & Suzuki, K. T. (2002). Arsenic round the world: A review. Talanta, 58(1), 201–235.
- Manning, B. A., & Goldberg, S. (1996). Modeling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals. Soil Science Society of America Journal, 60(1), 121–131. https://doi.org/https://doi.org/10.2136/sssaj1996.03615995006000010020x
- Mitsunobu, S., Takahashi, Y., Terada, Y., & Sakata, M. (2010). Antimony(V) incorporation into synthetic ferrihydrite, goethite, and natural iron oxyhydroxides. Environmental Science & Technology, 44(10), 3712–3718. https://doi.org/https://doi.org/10.1021/es903901e
- Mok, W. M., & Wai, C. M. (1994). Mobilization of arsenic in contaminated river waters. In J. O. Nriagu (Ed.), Arsenic in the environment-Part I: Cycling and characterization (pp. 99–108). John Wiley Interscience.
- Moldovan, B. J., Jiang, D. T., & Hendry, M. J. (2003). Mineralogical characterization of arsenic in uranium mine tailings precipitated from iron-rich hydrometallurgical solutions. Environmental Science & Technology, 37(5), 873–879. https://doi.org/https://doi.org/10.1021/es025947a
- Moon, D. H., Kim, K.-W., Yoon, I.-H., Grubb, D. G., Shin, D.-Y., Cheong, K. H., Choi, H.-I., Ok, Y. S., & Park, J.-H. (2011). Stabilization of arsenic-contaminated mine tailings using natural and calcined oyster shells. Environmental Earth Sciences, 64(3), 597–605. https://doi.org/https://doi.org/10.1007/s12665-010-0890-y
- Multani, R. S., Feldmann, T., & Demopoulos, G. P. (2016). Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy, 164, 141–153. https://doi.org/https://doi.org/10.1016/j.hydromet.2016.06.014
- Munksgaard, N. C., & Lottermoser, B. G. (2010). Effects of wood bark and fertilizer amendment on trace element mobility in mine soils, broken hill, Australia: Implications for mined land reclamation. Journal of Environmental Quality, 39(6), 2054–2062. https://doi.org/https://doi.org/10.2134/jeq2010.0201
- Munksgaard, N. C., & Lottermoser, B. G. (2013). Phosphate amendment of metalliferous tailings, Cannington Ag-Pb-Zn mine, Australia: Implications for the capping of tailings storage facilities. Environmental Earth Sciences, 68(1), 33–44. https://doi.org/https://doi.org/10.1007/s12665-012-1711-2
- Nakamaru, Y. M., & Martín Peinado, F. J. (2017). Effect of soil organic matter on antimony bioavailability after the remediation process. Environmental Pollution (Barking, Essex : 1987), 228, 425–432. https://doi.org/https://doi.org/10.1016/j.envpol.2017.05.042
- Nesbitt, H. W., Muir, I. J., & Prarr, A. R. (1995). Oxidation of arsenopyrite by air and air-saturated, distilled water, and implications for mechanism of oxidation. Geochimica et Cosmochimica Acta, 59(9), 1773–1786. https://doi.org/https://doi.org/10.1016/0016-7037(95)00081-A
- Noble, T. L., Lottermoser, B. G., & Townsend, A. T. (2016). Mobility of arsenic and environmentally significant elements in mine tailings following liming. Australian Journal of Earth Sciences, 63(6), 781–793. https://doi.org/https://doi.org/10.1080/08120099.2016.1249955
- Nordstrom, D. K., & Parks, G. A. (1987). Solubility and stability of scorodite, FeAsO4·2H2O: Discussion. American Mineralogist, 72, 849–851.
- Okkenhaug, G., Zhu, Y.-G., Luo, L., Lei, M., Li, X., & Mulder, J. (2011). Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environmental Pollution (Barking, Essex : 1987), 159(10), 2427–2434. https://doi.org/https://doi.org/10.1016/j.envpol.2011.06.028
- Paikaray, S., Essilfie-Dughan, J., Göttlicher, J., Pollok, K., & Peiffer, S. (2014). Redox stability of As(III) on schwertmannite surfaces. Journal of Hazardous Materials, 265, 208–216. https://doi.org/https://doi.org/10.1016/j.jhazmat.2013.11.068
- Paktunc, D. (2013). Mobilization of arsenic from mine tailings through reductive dissolution of goethite influenced by organic cover. Applied Geochemistry, 36, 49–56. https://doi.org/https://doi.org/10.1016/j.apgeochem.2013.05.012
- Paktunc, D. (2015). Phase transformations in the system Fe-AsO4-SO4 and the structure of amorphous ferric arsenate: Implications for arsenic stabilization in mine drainage and industrial effluents. The Canadian Mineralogist, 53(5), 921–936. https://doi.org/https://doi.org/10.3749/canmin.1500040
- Paktunc, D., & Bruggeman, K. (2010). Solubility of nanocrystalline scorodite and amorphous ferric arsenate: Implications for stabilization of arsenic in mine wastes. Applied Geochemistry, 25(5), 674–683. https://doi.org/https://doi.org/10.1016/j.apgeochem.2010.01.021
- Paktunc, D., Majzlan, J., Huang, A., Thibault, Y., Johnson, M. B., & White, M. A. (2015). Synthesis, characterization, and thermodynamics of arsenates forming in the Ca-Fe(III)-As(V)-NO3 system: Implications for the stability of Ca-Fe arsenates. American Mineralogist, 100(8-9), 1803–1820. https://doi.org/https://doi.org/10.2138/am-2015-5199
- Palansooriya, K. N., Shaheen, S. M., Chen, S. S., Tsang, D. C. W., Hashimoto, Y., Hou, D., Bolan, N. S., Rinklebe, J., & Ok, Y. S. (2020). Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environment International, 134, 105046. https://doi.org/https://doi.org/10.1016/j.envint.2019.105046
- Park, I., Tabelin, C. B., Magaribuchi, K., Seno, K., Ito, M., & Hiroyoshi, N. (2018a). Suppression of the release of arsenic from arsenopyrite by carrier-microencapsulation using Ti-catechol complex. Journal of Hazardous Materials, 344, 322–332. https://doi.org/https://doi.org/10.1016/j.jhazmat.2017.10.025
- Park, I., Tabelin, C. B., Seno, K., Jeon, S., Inano, H., Ito, M., & Hiroyoshi, N. (2020). Carrier-microencapsulation of arsenopyrite using Al-catecholate complex: Nature of oxidation products, effects on anodic and cathodic reactions, and coating stability under simulated weathering conditions. Heliyon, 6(1), e03189. https://doi.org/https://doi.org/10.1016/j.heliyon.2020.e03189
- Park, I., Tabelin, C. B., Seno, K., Jeon, S., Ito, M., & Hiroyoshi, N. (2018b). Simultaneous suppression of acid mine drainage formation and arsenic release by carrier-microencapsulation using aluminum-catecholate complexes. Chemosphere, 205, 414–425. https://doi.org/https://doi.org/10.1016/j.chemosphere.2018.04.088
- Park, J. H., Han, Y.-S., & Ahn, J. S. (2016). Comparison of arsenic co-precipitation and adsorption by iron minerals and the mechanism of arsenic natural attenuation in a mine stream. Water Research, 106, 295–303. https://doi.org/https://doi.org/10.1016/j.watres.2016.10.006
- Rakotonimaro, T. V., Guittonny, M., Neculita, C. M., Trépanier, F., & Pépin, G. (2019). Evaluation of arsenic leaching potential in gold mine tailings amended with peat and mine drainage treatment sludge. Journal of Environmental Quality, 48(3), 735–774. https://doi.org/https://doi.org/10.2134/jeq2018.11.0392
- Randall, P. M. (2012). Arsenic encapsulation using Portland cement with ferrous sulfate/lime and Terra-Bond™ technologies - Microcharacterization and leaching studies . The Science of the Total Environment, 420, 300–312. https://doi.org/https://doi.org/10.1016/j.scitotenv.2011.12.066
- Razo, I., Carrizales, L., Castro, J., Díaz-Barriga, F., & Monroy, M. (2004). Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water, Air, and Soil Pollution, 152(1-4), 129–152. https://doi.org/https://doi.org/10.1023/B:WATE.0000015350.14520.c1
- Ritchie, V. J., Ilgen, A. G., Mueller, S. H., Trainor, T. P., & Goldfarb, R. J. (2013). Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chemical Geology, 335, 172–188. https://doi.org/https://doi.org/10.1016/j.chemgeo.2012.10.016
- Rocha, S. D. F., Katsarou, L., Demopoulos, G. P., et al. (2012). Reduction of arsenic releasing in aqueous environment by scorodite encapsulation. In C. Q. Jia (Eds.), Sustainable issues in mineral and metal extraction (WALSIM II) (pp. 215–224). MetSoc of CIM.
- Roper, A. J., Williams, P. A., & Filella, M. (2012). Secondary antimony minerals: Phases that control the dispersion of antimony in the supergene zone. Geochemistry, 72(S4), 9–14. https://doi.org/https://doi.org/10.1016/j.chemer.2012.01.005
- Rowe, R. K., & Hosney, M. S. (2013). Laboratory investigation of GCL performance for covering arsenic contaminated mine wastes. Geotextiles and Geomembranes, 39, 63–77. https://doi.org/https://doi.org/10.1016/j.geotexmem.2013.06.003
- Saerens, A., Ghosh, M., Verdonck, J., & Godderis, L. (2019). Risk of cancer for workers exposed to antimony compounds: A systematic review. International Journal of Environmental Research and Public Health, 16(22), 4474. https://doi.org/https://doi.org/10.3390/ijerph16224474
- Salihoglu, G. (2014). Immobilization of antimony waste slag by applying geopolymerization and stabilization/solidification technologies. Journal of the Air & Waste Management Association (1995), 64(11), 1288–1298. https://doi.org/https://doi.org/10.1080/10962247.2014.943352
- Savage, K. S., Bird, D. K., & O'Day, P. A. (2005). Arsenic speciation in synthetic jarosite. Chemical Geology, 215(1-4), 473–498. https://doi.org/https://doi.org/10.1016/j.chemgeo.2004.06.046
- Seidel, H., Görsch, K., Amstätter, K., & Mattusch, J. (2005). Immobilization of arsenic in a tailings material by ferrous iron treatment. Water Research, 39(17), 4073–4082. https://doi.org/https://doi.org/10.1016/j.watres.2005.08.001
- Smith, S. D., & Edwards, M. (2005). The influence of silica and calcium on arsenate sorption to oxide surfaces. Journal of Water Supply: Research and Technology-Aqua, 54(4), 201–211. https://doi.org/https://doi.org/10.2166/aqua.2005.0019
- Sun, Q., Cui, P.-X., Liu, C., Peng, S.-M., Alves, M. E., Zhou, D.-M., Shi, Z.-Q., & Wang, Y.-J. (2019). Antimony oxidation and sorption behavior on birnessites with different properties (Δ-MnO2 and triclinic birnessite). Environmental Pollution (Barking, Essex: 1987), 246, 990–998. https://doi.org/https://doi.org/10.1016/j.envpol.2018.12.027
- Vink, B. W. (1996). Stability relations of antimony and arsenic compounds in the light of revised and extended Eh-pH diagrams. Chemical Geology, 130(1-2), 21–30. https://doi.org/https://doi.org/10.1016/0009-2541(95)00183-2
- Wang, S., & Mulligan, C. N. (2006). Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. Journal of Hazardous Materials, 138(3), 459–470. https://doi.org/https://doi.org/10.1016/j.jhazmat.2006.09.048
- Wang, S., & Mulligan, C. N. (2008). Speciation and surface structure of inorganic arsenic in solid phases: A review. Environment International, 34(6), 867–879. https://doi.org/https://doi.org/10.1016/j.envint.2007.11.005
- Wang, X., Zhang, H., Wang, L., Chen, J., Xu, S., Hou, H., Shi, Y., Zhang, J., Ma, M., Tsang, D. C. W., & Crittenden, J. C. (2019). Transformation of arsenic during realgar tailings stabilization using ferrous sulfate in a pilot-scale treatment. Science of the Total Environment, 668, 32–39. https://doi.org/https://doi.org/10.1016/j.scitotenv.2019.02.289
- Wang, Y., Gao, M., Huang, W., Wang, T., & Liu, Y. (2020). Effects of extreme pH conditions on the stability of As(V)-bearing schwertmannite. Chemosphere, 251, 126427. https://doi.org/https://doi.org/10.1016/j.chemosphere.2020.126427
- Waychunas, G. A., Rea, B. A., Fuller, C. C., & Davis, J. A. (1993). Surface chemistry of ferrihydrite: Part 1. EXAFS studies of the geometry of coprecipitated and adsorbed arsenate. Geochimica et. Cosmochimica Acta, 57(10), 2251–2269. https://doi.org/https://doi.org/10.1016/0016-7037(93)90567-G
- Wilson, N. J., Craw, D., & Hunter, K. (2004). Antimony distribution and environmental mobility at an historic antimony smelter site, New Zealand. Environmental Pollution, 129(2), 257–266. https://doi.org/https://doi.org/10.1016/j.envpol.2003.10.014
- Wilson, S. C., Lockwood, P. V., Ashley, P. M., & Tighe, M. (2010). The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environmental Pollution, 158(5), 1169–1181. https://doi.org/https://doi.org/10.1016/j.envpol.2009.10.045
- Xi, J., He, M., & Lin, C. (2011). Adsorption of antimony(III) and antimony(V) on bentonite: Kinetics, thermodynamics and anion competition. Microchemical Journal, 97(1), 85–91. https://doi.org/https://doi.org/10.1016/j.microc.2010.05.017
- Yan, L., Chan, T., & Jing, C. (2020). Mechanistic study for stibnite oxidative dissolution and sequestration on pyrite. Environmental Pollution, 262, 114309. https://doi.org/https://doi.org/10.1016/j.envpol.2020.114309
- Yu, Y., Zhu, Y., Gao, Z., Gammons, C. H., & Li, D. (2007). Rates of arsenopyrite oxidation by oxygen and Fe(III) at pH 1.8-12.6 and 15-45 °C. Environmental Science & Technology, 41(18), 6460–6464. https://doi.org/https://doi.org/10.1021/es070788m