Abstract
The exposure of plants to pharmaceuticals via treated wastewater irrigation and biosolid application presents an important route of chronic exposure of crops to a wide variety of bioactive pollutants. This paper presents a novel approach which aims to improve our understanding of the interactions of bioactive pollutants with plants through the concept of plant pharmacology and two main sub-divisions: (i) plant pharmacokinetics which describes the fate of exogenous xenobiotics in the plant based on the processes of absorption, distribution, metabolism and accumulation (ADMA), processes that are analogous to pharmacokinetics in animals; and (ii) plant pharmacodynamics that proposes that exogenous xenobiotics interact with plant enzymes and biochemical pathways, establishing a relationship with pharmacological concepts and emphasizing the importance of exposure-response interactions. The concept of plant pharmacology and its two subdivisions provide a foundation for the development of in-depth knowledge regarding the fate of xenobiotics in plants and establishing plant pharmacokinetic-pharmacodynamic models that include both the ADMA processes and time-dependent response of the plant to these compounds. This concept provides a new perspective on pharmacovigilance, focusing on plant-xenobiotic compound interactions, and a conceptual framework for understanding the fate and interactions of these bioactive molecules in agricultural systems, to enable more accurate risks assessments of environmental and human health.
Graphical abstract

HANDLING EDITORS:
Introduction
Global water stress, a consequence of population growth, urbanization, higher living standards, and climate change, has increased the reliance of agriculture on treated wastewater as a vital source for irrigation worldwide (Hettiarachchi & Ardakanian, Citation2018; Yi et al., Citation2011). However, there are unknown potential environmental and human health consequences with the practice that must be considered (Fu et al., Citation2019; Vergine et al., Citation2017). In accordance with government regulations, treated effluent is discharged into the environment or reused for agricultural purposes (Hettiarachchi & Ardakanian, Citation2018). However, the ability of conventional treatment plants to remove pharmaceuticals is limited, resulting in their omnipresence in treated effluents (Evgenidou et al., Citation2015; Gracia-Lor et al., Citation2012; Kasprzyk-Hordern et al., Citation2009). Hence, the use of treated wastewater for irrigation exposes agricultural soil and crops to biologically active pharmaceuticals (Malchi et al., Citation2014; Paz et al., Citation2016). The accumulation of pharmaceuticals in edible crops introduces these compounds into the food chain and results in involuntary human exposure and subsequent unknown consequences (Paltiel et al., Citation2016; Schapira et al., Citation2020).
Similar to past research on pesticides, the current literature examining pharmaceuticals in the agricultural ecosystem indicates that their fate is likely to be governed by the physicochemical properties of each compound (Coleman et al., Citation1997; Van Eerd et al., Citation2006; Sandermann, Citation1992; Siminszky, Citation2006). However, the pattern of crop exposure to pharmaceuticals differs from that to pesticides. Pesticides are applied as a known formulation at measured concentrations and specific timing. In contrast, the exposure of plants to pharmaceuticals is not controlled by the farmer and occurs at chronic levels as a mixture of largely unknown ingredients with temporal variation (Kasprzyk-Hordern et al., Citation2009; Ort et al., Citation2014; Sui et al., Citation2011).
Current scientific knowledge has many gaps hampering our understanding of the interactions and processes of xenobiotic pollutants (e.g., drugs) in plants. An illustration of gaps is the consistent exclusion of the metabolic parameter in modeling the fate of organic compounds in plants (Fantke et al., Citation2013; Rein et al., Citation2011; Trapp, Citation2004); or conflicting results related to the implication of compound properties on their uptake and distribution within the plant (Dettenmaier et al., Citation2009; Limmer & Burken, Citation2014; Schriever & Lamshoeft, Citation2020). Beyond mathematical modeling, there is a need to develop a clear conceptual model that can reduce uncertainty and misinterpretation of data.
Plants share with mammals key mechanisms that govern xenobiotic distribution and detoxification. As these processes have been extensively modeled in humans and animals, this paper seeks to build an interdisciplinary understanding, connecting the processes and fate of xenobiotics in plants and animals. The paper offers a unified concept for understanding the interactions between plants and xenobiotic compounds, and proposes the introduction and redefinition of appropriate parameters and metrics for standardization of results in the research of xenobiotic compounds in plants. This standardization is based on useful pharmacological parameters in animals that enable a common language for reporting the results of similar studies in plants. The concept of plant pharmacology establishes a new paradigm for understanding plant–pharmaceutical interactions, one that is required for substantiated risk assessments for environmental and human health, and more accurate computational modeling of xenobiotics in the environment.
Plant pharmacology
The dichotomy in biology between plants and animals, botany and zoology, can be traced back to the age of Aristotle and Theophrastus (4th century BC) who correlated morphology, anatomy and functionality of the plant and animal kingdoms (Thanos, Citation1994). Charles Darwin renewed this discussion through comparison of the similar evolutionary processes in domesticated animals and cultivated plants, and in the functional similarity between these two kingdoms. In his book, he developed a novel comparison of plant roots to the central nervous system in animals, as both systems have the ability to detect, evaluate, and respond to the environment (Darwin, Citation1880). The initial concepts developed by Darwin are manifested in subsequent scientific research related to electrical, hydraulic, and chemical signaling in plants, which indicates that plants have many biochemical processes and functions corresponding to those of animals. At the cellular and organ level, structures and functions are not merely analogous, but even homologous (Baluška & Mancuso, Citation2009; Enomoto & Goto, Citation2008; Morth et al., Citation2011; Nagata et al., Citation2004).
Plant pharmacology offers a comparative understanding of the interaction of xenobiotic compounds with plants and animals. For the premise of the following discussion, pharmacology is defined as the study of the interactions between exogenous chemicals and living systems and the manner in which the functions of living systems are affected by exogenous chemical agents (Rang et al., Citation2019). Xenobiotics are substances that are foreign to the biological system and include natural or synthetic chemicals, medicinal drugs, agricultural and industrial chemicals, environmental contaminants and other exogenous substances (Howland, Citation2015). The term drug or pharmaceutical refers to a xenobiotic chemical substance to which organisms are exposed and that can potentially cause a biochemical or physiological effect at the cell, tissue, organ, or organism level (Buxton & Benet, Citation2013). Pharmaceutical interactions are distinguished into: “what the biological system does to the drug,” i.e., pharmacokinetics, and “what the drug does to the biological systems,” i.e., pharmacodynamics (Goodman et al., Citation2000). Pharmacokinetics is the study of the effects of biological systems to drugs in terms of drug absorption, distribution, metabolism and elimination (ADME). Pharmacodynamics studies the effects of a drug to the biological system and its mechanism of action, elucidating the relationship between drug concentration at the site of action and its biochemical or physiological effects. In classic pharmacology, pharmacokinetics and pharmacodynamics are two sub-divisions of a conceptual model that enables quantitative modeling and prediction of drug effects on a living system. In understanding the different processes that translate into dose-response relationships, it is evident that a compound's kinetics and dynamics are interrelated processes (Shargel et al., Citation2012).
The study of xenobiotics in the agricultural environment requires a similar approach, thus we propose the terms plant pharmacokinetics and plant pharmacodynamics, and their interrelation, as essential concepts in the study of the processes involved and the physical or biological effects of xenobiotic compounds on plants. For example, the rate and extent of absorption of a compound from the rhizosphere into the plant roots, as well as its distribution to different plant organs, will determine whether the compound will reach relevant concentrations at a particular site of action and have an effect on the plant.
Plant pharmacokinetics
All pharmacokinetic processes, in animals and plants, depend on the transfer of compounds across membranes by passive diffusion and/or membrane transporters or receptors. Passive diffusion is described by Fick’s law of diffusion (Taiz et al., Citation2014). Transporter or receptor mediated transport involves specific binding to a membrane protein that enables the compound to cross a membrane. This process may or may not require energy and can drive compound accumulation in compartments on either side of the membrane (Buxton & Benet, Citation2013). The ability of a compound to passively diffuse across membranes depends on the physicochemical properties of the molecule as described by Lipinski’s “rule of five” (Lipinski, Citation2004) which predicts membrane crossing if the molecule: (i) is neutrally charged, (ii) has a molecular mass <500 Da, (iii) has less than 5 hydrogen bond donors, (iv) has less than 10 hydrogen bond acceptors, and (v) lipophilicity (log D) between 1 and 5. The kinetics of molecules whose transfer across membranes is mediated by active transport does not follow these rules.
Using the plant pharmacokinetics approach, a pharmacokinetic sequence analogous to ADME is proposed. It is composed of absorption, distribution, metabolism and accumulation (instead of elimination), termed ADMA (). Understanding the ADMA processes provides a conceptual understanding of the fate of xenobiotics in the plant system. These processes of plant and xenobiotic interactions enable the prediction and modeling its fate and effects in the plant system.
Figure 1. Comparison of pharmacokinetic processes in the human body and in the plant. In the human body, the fate of a pharmaceutical is defined along the processes of absorption, distribution, metabolism, and excretion (ADME); in the plant, these processes are absorption, distribution, metabolism and accumulation (ADMA).
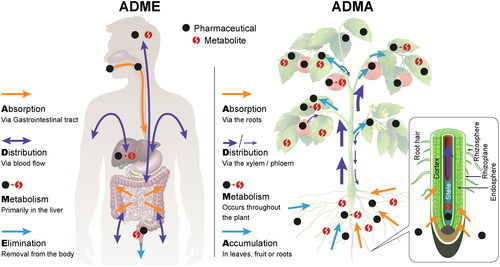
Absorption in human pharmacology describes the movement of a drug from its site of administration into the blood stream (Buxton & Benet, Citation2013). For orally administered compounds, the site of absorption is the gastrointestinal tract, composed of the mouth, pharynx, esophagus, stomach, small intestine and large intestine. The corresponding system in plants is the rhizosphere continuum; i.e. the rhizosphere (soil and water adjacent to the root), the rhizoplane (root surface area, epidermis and mucigel), and the endosphere (root cortex and endodermis) (Bakker et al., Citation2013; de la Fuente Cantó et al., Citation2020; York et al., Citation2016). In plant pharmacology, absorption is the compound's movement from the rhizosphere to the plant's root plasmodesmata, i.e. the cytoplasmic channels between cells providing intracellular continuity along the symplast. The plasmodesmata are analogous to gap junctions between animal cells, serving an essential role in intercellular communication (Bloemendal & Kück, Citation2013). Once in the symplast, compounds are able to interact with various cellular organelles, proteins and enzymes within the cytoplasm matrix (Taiz et al., Citation2014). Compounds may also be absorbed via direct exposure of foliage, however this is considered a less relevant route for wastewater-derived xenobiotics which are introduced via irrigation and is thus not included in further discussion in this paper.
The rhizosphere continuum can be understood as an inside out gastrointestinal tract, with multiple analogies existing between these two systems (Alaoui-Sossé et al., Citation2004; Barberon & Geldner, Citation2014). Both systems are responsible for nutrient acquisition and functions related to immunity and defense. Both systems are analogous in terms of their functions and roles of their immense and diverse symbiotic microbiome. Furthermore, the formation and function of the microvilli in the small intestine are analogous to plant root hairs, as both structure increase surface area for nutrient absorption. These two systems function in different environments in terms of pH, oxygen levels and nutrient concentration and gradients (Mendes & Raaijmakers, Citation2015; Ramírez-Puebla et al., Citation2013). The absorption pathways, although described with different terminologies, are also analogous to each other (Buxton & Benet, Citation2013).
Following oral administration of a drug to humans, the compound dissolves in the aqueous digestive fluids of the gastrointestinal tract and passes through the gastrointestinal membrane into the blood (Dahlgren & Lennernäs, Citation2019; Hirtz, Citation1985). The rate and extent of absorption are determined by both the physicochemical properties of the drug and the characteristics of the site of administration. These include dissolution, solubility, stability, gastrointestinal motility, and rate of diffusion across membranes (Dahlgren & Lennernäs, Citation2019). The major pharmacokinetic parameters that describe absorption are bioavailability (the fraction of the dose that reaches the blood stream), maximal concentration, and time to maximal concentration.
Root uptake is often used as a term to describe absorption in plants, particularly when referring to nutrients, organic compounds and water, indicating that the compound has entered the root cell cytoplasm. Paralleling human absorption of drugs, the ability of a compound to enter the plant's root system, depends on absorption of the compound from the rhizosphere. Absorption of organic compounds into the root is considered passive, however several studies and reviews suggest the potential for active uptake in plants (Eggen et al., Citation2011; Miller et al., Citation2016; Reinhold et al., Citation2010). However, experimental data has yet to validate such mechanisms.
The bioavailability of a compound, i.e., its ability to enter the plant system, is determined by its physicochemical properties, which determine the partitioning of the compound between solid (minerals and organic matter) and liquid phases of the soil, as well as its rate of degradation (Fu et al., Citation2019; Grossberger et al., Citation2014). Compounds which are highly hydrophobic will be adsorbed into the solid soil fraction and root exposure is dependent on the available fraction in the soil solution. Pharmaceutical compounds that are bioavailable in the soil solution undergo diffusive transport past the root epidermis into the root’s endosphere, i.e. through the apoplastic spaces in the root cortex (de la Fuente Cantó et al., Citation2020; York et al., Citation2016), see . Once in the endosphere, compounds need to permeate cell membranes into the cell cytoplasm and the plasmodesmata continuum from where they can enter the xylem and be distributed to the stem, leaves and fruit. The rate and extent of absorption from the rhizosphere continuum into the plant symplast are determined by the drug's physicochemical properties (e.g., Log D, pKa), soil properties (e.g., organic matter content, pH and clay content), plant characteristics (e.g., root lipid content and age), environmental conditions (e.g., temperature and humidity), concentration and time of exposure.
In complex animals, a drug can enter the blood stream from the gastrointestinal tract by a transcellular pathway which requires compound penetration through a cell membrane and this is the main mechanism of transport (Homayun et al., Citation2019). Hydrophilic compounds that are not able to permeate the lipid membrane may be absorbed via a paracellular pathway requiring movement between cells (Laksitorini et al., Citation2014). Root transport is often defined in terms of apoplastic or symplastic pathways in the cortex leading to the vascular tissue. The apoplastic pathway (paracellular) runs along cell walls and extracellular spaces of the root, while the symplastic pathway (transcellular) is via the plasmodesmata continuum. The terms symplastic and apoplastic pathways are, respectively, similar to the transcellular and paracellular pathways in pharmacological terminology.
Relying on the apparent analogies in drug absorption between complex animals and plants, multidisciplinary research can help predict the fate of organic compounds in plants. The Biopharmaceutics Classification System (Shargel & Yu, Citation2015; Yu et al., Citation2002) has been developed to differentiate drugs, on the basis of their aqueous solubility and intestinal permeability, into four classes. Class I pharmaceuticals have high permeability and high solubility; these compounds (e.g., alprazolam, fluoxetine and sildenafil) are well-absorbed. Class II pharmaceuticals have high permeability and low solubility; these compounds (e.g., carbamazepine, ibuprofen and lamotrigine) have limited bioavailability due to their low solubility. Class III pharmaceuticals (e.g., atenolol, gabapentin and pravastatin) exhibit low permeability and high solubility; this means that the drug is available, but absorption is limited due to limited membrane permeation. Class IV pharmaceuticals have limited permeability and low solubility; these drugs (e.g., acetaminophen, sulfadiazine and trimethoprim) have low bioavailability. In animal pharmacology this classification system can assist in selecting compounds for further development based on their absorption properties and cutoff values. Adopting this pharmacological classification system in plant pharmacology, compounds that are classified as Class I and II have high permeability and are more likely to undergo transcellular transport. Therefore, if they are detected in treated wastewater and subsequently in the soil solution, then they are likely to undergo absorption by the plant roots.
Distribution is the bidirectional transfer of the drug between the circulation compartment (plasma) and tissues. In the human body, the distribution of a drug depends on physiological factors such as organ-blood flow rate, and physiochemical factors such as the drug's molecular size, polarity, binding affinity to tissue and plasma proteins, lipid solubility and pH-based partitioning (Buxton & Benet, Citation2013). Distribution into specific organs is regulated by biological barriers (i.e., membranes) that prevent or regulate the transport of xenobiotics into different organs and only allow small molecules with specific characteristics (Lipinski’s rule of 5) to cross the membrane. Examples of such biological barriers in the human body are the blood–brain barrier (BBB) and the placenta. The BBB is an anatomical and functional barrier that regulates the movement of substances from the blood stream into the brain. Compounds able to passively cross the BBB includes carbamazepine, mianserin, and heroin (Alavijeh et al., Citation2005; Banks, Citation2016). Few central nervous system drugs are known to use transporters to cross the BBB, examples of such drugs include gabapentin, verapamil, and levofloxacin (Banks, Citation2016; Mikitsh & Chacko, Citation2014). This diffusional barrier is mostly formed by tight junctions between endothelial cells that line the walls of the brain capillaries and prevent paracellular diffusion. The functional barrier consists of multiple transporters and drug-metabolizing enzymes (Eyal et al., Citation2009).
Distribution is described via the theoretical parameter of "volume of distribution," which refers to the ratio between the administered dose and the drug’s concentration in the plasma; this parameter reflects the difficulties involved in measuring drug concentrations in body tissues compared to the plasma. A larger volume of distribution would suggest drug accumulation within extravascular compartments, although it cannot indicate the tissue in which the drug is accumulating. When measurements of drug levels within body tissues are available, the extent of their distribution can be described by tissue-to-plasma ratio. The commonly used brain-to-plasma ratio reflects the extent of drug distribution from the circulation into the brain across the BBB. The tissue–plasma concentrations of drugs are affected by the drug binding to plasma proteins and to tissue components, and their ability to cross the plasma–tissue barrier (Buxton & Benet, Citation2013; Eyal et al., Citation2009; Howland, Citation2015).
In plants, the Casparian strip can be considered analogous to the BBB. The Casparian strip is formed by lignin polymerization in the primary cell wall of the endodermis that separates the apoplast of the root cortex from the vascular system of the xylem and phloem (Robbins et al., Citation2014; Steudle, Citation1998). Comparable to the BBB, the Casparian strip regulates and/or restricts the passage of solutes into the plant's vascular system and necessitates a compound to pass a cell membrane in order to enter the plant's transport system. Additional similarities between the two biological barriers are the formation of a protein-rich membrane domain and a high concentration of influx and efflux carriers at endodermal plasma membranes, giving it the name of Casparian strip domain (Robbins et al., Citation2014).
There have been ample reports on diffusion across these two biological barriers and the range of physicochemical properties enabling a xenobiotic molecule to cross them. Studies indicate similarity in optimal values of molecular descriptor for the BBB and Casparian strip, and that passage across the Casparian strip can be predicted according to Lipinski’s “rule of 5” (Briggs et al., Citation1982; Clark, Citation2003; Kelder et al., Citation1999; Limmer & Burken, Citation2014; Lipinski, Citation2004). Accordingly, compounds able to cross the BBB, are likely to be able to cross into a plant's vascular system.
Studies have consistently shown that compounds that are distributed at high rates from roots to the leaves in plants are those that can also cross the BBB in animals (Ben Mordechay et al., Citation2018; Malchi et al., Citation2014; Wu et al., Citation2013). Compounds able to cross the BBB and whose properties are not altered by the cellular environment are thus also more likely to be distributed to the shoot. The ability to screen for compounds capable of penetrating the BBB can be done through digital platforms such as SwissADME which provides physicochemical descriptors and ADME parameters (Daina et al., Citation2017). Studies on exposure of plants to pharmaceuticals and other xenobiotics can use such tools to screen for and predict the absorption and distribution patterns in plants.
Metabolism in the human body is a biochemical modification of xenobiotic organic compounds to a more water-soluble and generally less pharmacologically active form, enabling their elimination through excretion. The main site of metabolism is the liver. However, metabolic enzymes are present in practically every organ, including the lungs, kidney, gastrointestinal tract and brain (Krishna & Klotz, Citation1994; Patrick, Citation2017). Pharmaceuticals may undergo a two-phase metabolic process of modification/bioactivation and conjugation, followed by their excretion.
Xenobiotic metabolism in plants has been well documented for some agrochemicals (Coleman et al., Citation1997; Sandermann, Citation1992; Siminszky, Citation2006; Van Eerd et al., Citation2006). The Green Liver Model is most commonly referred to when explaining plant metabolism of xenobiotics, reflecting a plant process analogous to that in mammals (Sandermann, Citation1992). According to this model, plant metabolism of xenobiotic organic compounds is divided into three phases. In phase I, the parent compound is transformed through hydroxylation, N-demethylation, reduction or oxidation into an activated form, carrying groups such as hydroxyls, epoxides or carboxylic acid (Chang & Vanden Born, Citation1971; Van Eerd et al., Citation2006; Ford & Casida, Citation2008; Roberts et al., Citation1999). Many of the phase I reactions are mediated by oxidative enzymes, such as cytochrome P450s, peroxidases, and polyphenol oxidases. The cytochrome P450 family of enzymes is known to be important in phase I metabolism in animals, and has been found to be central in plants as well (Anzenbacher & Anzenbacherová, Citation2001; Ohkawa & Inui, Citation2015; Zanger & Schwab, Citation2013). Cytochrome P450 enzymes play vital roles in promoting plant growth and development and protecting plants from stresses via multiple biosynthetic and detoxification pathways (Xu et al., Citation2015). Similarly, in mammals, cytochrome P450 enzymes are vital in the production and metabolism of many molecules (including xenobiotics) and play an important role in various physiological functions (Seliskar & Rozman, Citation2007). Phase II reactions in plants, as in humans, involve conjugation of the xenobiotic compound or its moiety with a sugar, glutathione, amino acid or other small groups to increase its hydrophilicity. The conjugation (i.e., anabolic process) usually results in lower biological activity and higher water solubility in comparison to the parent compound. Both phase I and II reactions in plants are analogous (but not identical) to those in animals (Aliferis & Chrysayi-Tokousbalides, Citation2011; Cole, Citation1994; Mlynek et al., Citation2020).
Phase III metabolism differs between animals and plants. In the latter, phase III is the transport of the xenobiotic conjugates from the cytoplasm to the vacuole or apoplast, where it cannot interfere with other biochemical processes (Coleman et al., Citation1997; Siminszky, Citation2006; Van Eerd et al., Citation2006). Specific transporters have been identified which actively transfer the conjugated compounds across membranes (Wink, Citation2010). In mammals, ATP-binding cassette (ABC) transporters are known as active transporters of pharmaceuticals (Eyal et al., Citation2009). Hepatic ABC transporters transfer drugs and their metabolites from the hepatocytes into the bile, enabling their biliary excretion (Buxton & Benet, Citation2013). In plants, ABC transporters are involved in vacuole transport as well as other diverse processes related to organ growth, plant nutrition, plant development, response to abiotic stress and plant interactions with the environment (Coleman et al., Citation1997; Kang et al., Citation2011).
Understanding plant xenobiotic metabolism and the ability of the plant to deactivate exogenous compounds is critical for understanding both plant response and human exposure. The ability of the plant to deactivate the compound to inactive metabolites would prevent any potential biological effect of the xenobiotic. The investigation of the metabolism of xenobiotics by plants may be performed by targeted or untargeted analysis. The targeted analysis of metabolites, which is based on metabolite pathways in mammalian systems, has been the most common method to investigate plant metabolism. The use of an untargeted approach for detection of pharmaceutical metabolites in plants is being more gradually utilized for the quantification of unknown metabolites (Mejías et al., Citation2021; Mlynek et al., Citation2020; Villette et al., Citation2019). Beyond the identification of metabolic pathways and potential active metabolites, additional factors need to be understood, such as the location of compound metabolism in the plant, the metabolic rates of the parent compound and metabolites, the saturation or capacity-limitations of metabolism, and the biotic or abiotic factors affecting plant metabolism.
Elimination is the excretion of compounds and their metabolites from the human body via the kidneys (urine) or bile (feces). Xenobiotics may also be excreted into body fluids such as sweat, tears, and breast milk. Elimination through these minor routes does not significantly affect the drug’s concentrations in the plasma and, with the exception of breast milk, does not have clinical implications. The major pharmacokinetic parameter describing the rate of elimination (by metabolism, excretion, or both) is clearance: the volume of plasma which is completely cleared of the drug within a certain unit of time. Both volume of distribution and clearance determine the time required for the drug to be eliminated from plasma, and most drugs are eliminated within a range of hours to days. However, some drugs or their metabolites accumulate in the cells or cellular compartments. Examples are the cardiovascular drug amiodarone, which accumulates in the vacuoles for weeks or even months, and bisphosphonates, which are retained in human bones for years (Lin, Citation1996; Morissette et al., Citation2009).
Accumulation in plants is proposed to be analogous to elimination in animals. Plants lack major excretion pathways for xenobiotics. In higher plants, leaf abscission can be considered a form of excretion, but this is limited to specific crops, mostly deciduous fruit trees. Fruit may also be considered part of the excretion pathway, where fruit abscission constitutes an excretion pathway of unwanted compounds. However, this pathway is considered minimal, with studies demonstrating that the accumulation of these compounds in cucumber and tomato fruit are less than 1% of the accumulation in the leaf (Goldstein et al., Citation2014). Root exudation may also be another possible mechanism of excretion. This is supported by literature which demonstrates that for most agricultural crops, exposure to pharmaceuticals results in accumulation of the parent compound and/or its metabolites, while the root exudate may rather facilitate microbial degradation (Zhang et al., Citation2016). These routes are all considered minor or plant-specific, analogous to minor excretion pathways in animals such as tears or sweat. Since there is no prevailing excretion route in plants, the elimination process is replaced by accumulation of the compound. Compounds will accumulate in roots or shoots, depending on the exposure concentration, processes of absorption and distribution, and the relative rate of metabolism compared to rate of accumulation in the specific organ.
At the cellular level, xenobiotics may undergo storage of soluble metabolites in plant cell vacuoles or their deposition as bound residues into biopolymers found in the cell walls of plant cells (pectin, lignin, polysaccharides, and proteins) (Cummins et al., Citation2011; Roberts et al., Citation1999). While it has been proposed that pesticides undergo incorporation into different cell-wall components, may undergo biological reactivation or further transformation of the bound xenobiotic, there is a lack of published data regarding this processes (Hock & Elstner, Citation2004; Sandermann, Citation2004). The accumulation of active xenobiotic compounds results in greater cellular concentrations and the potential for pharmacodynamic interactions between the compound and the plant.
Pharmacodynamics
In humans, a pharmacological effect usually starts with binding of the drug to a cellular receptor to modulate the cell's biochemical or electrical signals (Jusko, Citation2016). Target modulation by the drug leads to effects on tissues and organ systems (Raj & Raveendran, Citation2019). For example, the drug ibuprofen inhibits the activity of cyclooxygenases (i.e., drug's mechanism of action), which results in analgesic and anti-inflammatory responses (i.e., drug effect) (Bushra & Aslam, Citation2010). Plant pharmacodynamics provides a similar concept for evaluating the response of plants to bioactive compounds, from a molecular to system level. Plant pharmacodynamics aims to reproduce the basic concepts of pharmacodynamics in complex animals, in order to understand a drug's mechanism of action and effect, drug–drug interactions, and the time course and intensity of those interactions.
Ligand—receptor interactions
Drugs generally do not create a new effect, but rather, activate or inhibit a specific receptor-related activity. Receptors are sites to which specific ligands bind, thereby altering the cell's biochemical activity. An agonist binds to a receptor and activates a sequence of events that leads to a response. An antagonist binds to a receptor to inhibit the action, without initiating any effect itself. Drugs conferring a short duration of receptor activation generally interact via weaker bonds (ionic, hydrogen or van der Waals), whereas long-duration or irreversible drug–receptor interactions may form stronger bonds, such as covalent bonds. A drug’s ability to affect a specific receptor is related to its affinity and efficacy, which are parameters determined by its chemical structure. Affinity is defined as the probability of a drug occupying its target receptor upon interaction. Efficacy is defined as the extent to which a drug activates a receptor, resulting in a cellular response also known as drug effect (Buxton & Benet, Citation2013; Page & Maddison, Citation2008; Raj & Raveendran, Citation2019).
There are numerous examples of analogies and homologies of receptors between animals and plants, and examples of such are transmembrane ion-channel receptors, transmembrane G-protein-coupled receptors and transmembrane receptors within cytosolic domains. Transmembrane ion-channel receptors such as voltage-gated ion channels regulate the ionic balance of the cell and cellular processes. Plant ion channel families exhibit homologies to animal proteins, and include hyperpolarization-and depolarization-activated Shaker-type potassium channels, chloride transporters/channels, cyclic nucleotide–gated channels, and ionotropic glutamate receptor homologs (Ward et al., Citation2009). Transmembrane G-protein-coupled receptors can activate a signal-transduction pathway that alters cellular processes through the activation of a second messenger system. Heterotrimeric G protein signaling regulates a wide range of growth and developmental processes in both animals and plants, but the two kingdoms are believed to have differences in protein structure, subunit composition and different G-protein-associated receptors (Stateczny et al., Citation2016; Trusov & Botella, Citation2016);
Transmembrane receptors within cytosolic domains cause enzyme activation or modification of the influx/efflux of endogenous compounds. Transmembrane proteins with domains on both sides of the membrane are poised structurally to transmit information from one side of the membrane to the other. Plant proteins have been identified that resemble the receptor protein kinases of animal cells, known as receptor-like protein kinases (Braun & Walker, Citation1996; He et al., Citation2018). In addition to receptor activity, xenobiotics compounds may modify cell membrane structure and function (Page & Maddison, Citation2008; Wink, Citation2010).
Dose—reponses
The Renaissance physician Paracelsus (1493–1541) is credited with the dictum “What is there that is not poison? All things are poison, and nothing is without poison. Solely the dose determines that a thing is not a poison.” In other words, the dose makes the poison (Grandjean, Citation2016). In humans, the relationship between concentration/exposure and effect is described by a dose-response curve, which presents the percentage of the maximum response against the drug plasma concentration. As the drug concentration increases, the pharmacological response is enhanced. Dose-response curves exhibit a sigmoidal shape with three basic properties: threshold (lowest dose that has an effect), slope (change in effect with concentration), and maximal effect (obtained when all receptor sites are occupied) (Buxton & Benet, Citation2013; Raj & Raveendran, Citation2019). The intensity of the pharmacological effect for a drug administered at equivalent concentrations varies in different individuals or populations, and it may even vary in a single individual at exposure to identical concentrations. The response to a drug can be influenced by various factors related to the patient's characteristics (e.g., age, body weight, nutritional status), underlying disease, and genetic variation (Buxton & Benet, Citation2013; Raj & Raveendran, Citation2019). The drug's mechanism of action and resulting effects are based on ligand–receptor interactions. Drugs can exert similar effects through different mechanisms. For example, two antiseizure medications, carbamazepine and gabapentin, reduce neuronal excitability to help control seizures. This is done via blocking of voltage-gated sodium and calcium channels, respectively (Málaga et al., Citation2019; Sirven et al., Citation2012).
Drug-drug interactions
Exposure to, or administration, of multiple drugs may result in pharmacokinetic or pharmacodynamic drug–drug interactions. Pharmacokinetic mechanisms of drug–drug interactions include alterations in the ADME process. Absorption is changed due to pH-altering drugs or drugs that act as chelators or adsorbents. Distribution is modified due to displacement from binding sites or modulation of transporter functions at blood–tissue barriers. Metabolism is altered due to activation or inhibition of specific enzymes, which is well documented for antiepileptic drugs (Ashraf & Lionel, Citation2012; Perucca, Citation2006). Pharmacodynamic interactions involve additive, antagonistic or synergistic effects at the receptor level. Additive interactions are when the effect of two drugs is the sum of the effect of the two chemicals taken individually. Antagonistic interactions are when one drug reduces or eliminates the effect of the other drug. Synergistic interactions are when the combined effect of two drugs is greater than the sum of the effects of each drug given alone (Jonker et al., Citation2005; Zheng, Citation2020). Nonspecific pharmacodynamic drug interactions can occur when two drugs react with different receptors of the sample complex or as a result of alterations in electrolyte balance and electrochemical potential (Buxton & Benet, Citation2013; Corrie & Hardman, Citation2014). Due to a lack of knowledge and data, pharmacodynamic drug–drug interactions are not well understood and are more difficult to predict than pharmacokinetic interactions.
Plant pharmacodynamics
Concepts establishing the foundation of plant pharmacodynamics can be traced back to “The Action of Drugs on Plants” (Bose, Citation1915). Sir Jagadish Chandra Bose demonstrated that plants have a concentration-dependent response to chemicals, whereas a poisonous reagent at high concentration will cause depression of electrical signals and even death, whereas at low concentrations, the same compound will stimulate electrical signals. Bose demonstrated the occurrence of a dose-response in plants, similar to that in animals, and that different plants respond differently to the same dose of a specific compound, indicating a plant-specific response. In dose-response studies of herbicides, results indicated that a lethal compound given at low concentrations can have the opposite effect: rather than death, plant growth rate is stimulated (Cedergreen, Citation2008), supporting Bose's assertions (Bose, Citation1915).
The application of dose-response studies to herbicides has enabled understanding of their efficacy and mechanisms of action, and has provided insight on plant-level responses (Kudsk & Moss, Citation2017; Michel et al., Citation2004). The dose-response curve also provides critical data for understanding variations attributed to time, plant species, or environmental parameters (Belz et al., Citation2005). Beyond herbicides, there are examples of compounds, such as elicitor, which at low doses are used to induce or enhance stress mechanisms in plants to stimulate secondary metabolite production, but at elevated concentration are fatal (Kahromi & Khara, Citation2021; Narayani & Srivastava, Citation2017). The "exposure-response" of plants to various exogenous xenobiotics may result in similar, stimulatory or inhibitory effects.
Pharmacodynamics of herbicides
Herbicides, whose mechanisms of action include inhibiting photosynthesis, mimicking plant growth regulators, and blocking amino acid synthesis, among others, are dependent on the availability of adequate concentrations at the site of action. For example, triazine herbicides binds to the quinone-binding protein D1 of the plant photosystem II complex, thereby blocking photosynthetic electron transport by displacing plastoquinone from a specific binding site on the D1 protein (Duke, Citation1990). The desired action of triazine is dependent on ADMA processes, which result in accumulation of the required concentration at the site of action, the chloroplasts. Exposure to low concentrations of triazine has been shown to have non-photosystem II complex interactions which impact root development and molecular signaling networks, as well as hormone response that involves processes regulated by energy, stress, abscisic acid and cytokinin (Alberto et al., Citation2018). The exposure of plants to low concentrations of non-target herbicides affects plant growth and timing, influencing the plant-insect relationships and ecosystem interactions (Bohnenblust et al., Citation2016; Russo et al., Citation2020).
The mechanism of action is also dependent on the plant species and its ability to overcome the effect of the compound through tolerance. Plants that are tolerant to specific herbicides may have specific enzymes that metabolize the compound to a nontoxic form and incorporate it into the plant structure (Van Eerd et al., Citation2006). Sensitive plants accumulate the toxic moiety, which then acts on a variety of plant functions to the detriment of growth and function (Roberts et al., Citation1999). In animals, chronic exposure to drugs can result in tolerance, i.e., a drug-induced reduction in the effect upon administration of the compound. Tolerance may be pharmacokinetic, e.g. increased metabolic rates, or pharmacodynamic, e.g. related to functional tolerance such as downregulation of receptor numbers or decrease in receptor affinity, all of which can result in a reduced drug response (Goodman et al., Citation2000; Shargel & Yu, Citation2015). Similarly, in plants, xenobiotic compounds may produce pharmacokinetic or pharmacodynamic tolerance, resulting in the plant overcoming the potential effect of the exogenous compound.
Pharmacodynamics of pharmaceuticals
Recent studies suggest that pharmaceuticals, similar to low doses of herbicides, may have a negative impact on plant development and agricultural production (Bigott et al., Citation2021; Carter et al., Citation2015; Christou et al., Citation2016; Gorovits et al., Citation2020, Citation2021). For example, plant exposure to carbamazepine has indicated an effect on plant development, with changes in nutrient and plant hormone concentrations, interference of cell signaling and plant defense and changes in amino acid concentrations and additional osmo-protectants accumulation (Carter et al., Citation2015; Gorovits et al., Citation2020, Citation2021). Several studies have also demonstrated that plant exposure to pharmaceuticals may result in changes in the expression of stress related genes (Bigott et al., Citation2021; Christou et al., Citation2016).
Metabolism is the plant’s attempt to remove the active compound from its system or to prevent its interaction with a specific receptor. Plant pharmacodynamics emphasizes that enzymatic activity may be related to more than just metabolism. Deciphering the plant response to a pharmaceutical compound requires an understanding beyond the metabolic and stress response of the plant and elucidating the potential receptor–ligand interactions. Pharmacodynamic interactions may result in changes at the levels of gene expression, regulation, and signal transduction. If the plant is under stress, defense responses through a network of specific genes may be triggered, which influence plant metabolism and affect the availability of precursors for epigenetic processes, resulting in alterations of expression of specific genes (Markus et al., Citation2018).
Despite the growing number of studies published concerning pharmaceuticals in the environment in the last two decades, the interactions of such exogenous compounds with plants are not entirely understood. While interactions between pharmaceutical compounds and plants are measurable through biomarkers of plant stress, the mechanisms of action, toxicity level, molecular reactions, and effect are largely unknown. Plant pharmacodynamics depend on the ADMA processes which govern the accumulation of active compounds following the plant's physiological or biological response. Using the ADMA processes, we can infer that a neutral and slightly hydrophobic compound such as carbamazepine will accumulate in the leaves, whereas a hydrophobic compound such as triclosan will accumulate in the roots (Malchi et al., Citation2014; Mathews et al., Citation2014; Wu et al., Citation2013). Plant pharmacodynamics can enable a compound-specific and multicompound specific understanding of exposure-response in relation to the sites of accumulation and action. Establishment of exposure-response curves for compounds could enable understanding of plant pharmacodynamic effects and how these may relate to exposure concentration, chronic exposure, compound mixtures (i.e., co-exposure with other compounds), environmental factors, plant species, and agronomic practices.
Conceptual pharmacokinetic/pharmacodynamic model
The pharmacological perspective aims to establish an understanding of, and the ability to predict, the behavior of xenobiotics in plants, as well as to understand outcomes related to plant health (plant pharmacology) and human health risks (human pharmacology), as a needed step toward pharmacovigilance legislation to examine the scale of pollution with bioactive pharmaceuticals in waters, soils and crops. Today's models assessing plant exposure to xenobiotic compounds are mostly limited to pharmacokinetics, extrapolating the fate of the compound based on its ability to enter the plant system and undergo translocation, with the aim of determining accumulation for the sake of human or environmental exposure. These models are largely based on the physicochemical properties of the compounds, particularly their octanol–water partitioning (Schriever & Lamshoeft, Citation2020; Trapp, Citation2004). Models of xenobiotic compound interactions with plants should expand to include all ADMA processes and a pharmacodynamic component, linking the drug concentration to an efficacy or response metric. The development of a conceptual plant pharmacokinetic/pharmacodynamic (PK/PD) model provides a mechanistic approach and paves the way for a future computational model that considers both ADMA processes and time-dependent exposure/response of the plant to xenobiotics (Jambhekar & Breen, Citation2012; Shargel & Yu, Citation2015).
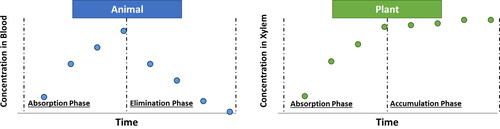
In animals, drug concentration in the blood rises in a time-dependent manner following absorption, while the compound is simultaneously distributed in the body, metabolized and excreted (). Parameters for each process, such as absorption and excretion rate constants, are important for understanding the processes underlying the compound's therapeutic effects or toxicity (Jambhekar & Breen, Citation2012; Shargel & Yu, Citation2015). In plants, analogous parameters can be developed based on time-dependent concentrations. For example, measuring the time-dependent concentration in the xylem sap () will enable establishing parameters based on the compound's distribution, such as the transpiration stream concentration factor (TSCF). The accumulation phase depends on exposure concentrations, plant physiological parameters, transpiration rates, root metabolism and compound physicochemical properties which can be translated into ADMA parameters.
Figure 2. Schematic illustration of time-dependent change in concentrations of a xenobiotic compound in animals (left) and plants (right).
The pharmacokinetic part of the conceptual model can be used to simplify processes and to overcome the inability to quantify rate processes in the plant. A single-compartment model defines the initial and terminal points. In human pharmacology, the one-compartment model assumes that the body is a single, uniform compartment which the drugs enter and leave. It assumes instant administration, rapid distribution concurrent with compound excretion. In plants, we can similarly assume that with chronic exposure, compounds undergo absorption at equilibrium and accumulate according to a relative constant (). This simplified approach can be useful in phytoremediation or environmental studies examining pathways of environmental exposure. Multicompartmental models can more precisely simulate the pharmacokinetics of the drug for different organs while simplifying the ADME/ADMA processes, such as assuming homogeneity of a drug's concentration in a plant organ. A three-compartment model of plant pharmacokinetics () is useful for health risk assessments and in understanding compound accumulation in edible organs. This model simplifies the processes to define fundamental questions: (1) is the compound absorbed through the root or leaves; (2) is the compound distributed in the plant; (3) in which compartments can the compound be metabolized; and (4) in which compartments do the compound accumulate. This conceptual modeling approach can provide a simple pharmacokinetic profile of a compound in a given crop and can greatly assist in prioritizing and standardizing this important field of research.
Figure 3. Conceptual plant pharmacokinetic/pharmacodynamic (PK/PD) models. (A) Single-compartment model. (B) Three-compartment model. (C) Multicompartment model. TWW, treated wastewater.
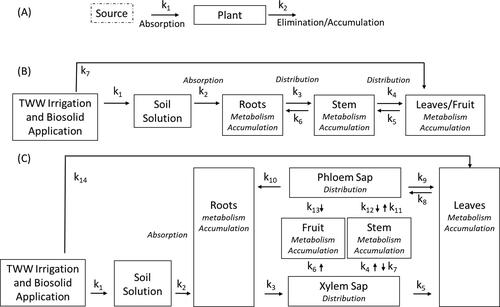
A more complex multicompartment model () is relevant for a PK/PD model examining the detailed interactions between the compound and the plant, such as transport of the compound, its variation with time and plant age, metabolites in the phloem and xylem, or various interactions of the compound with the plant’s biochemical systems. The PK/PD model seeks to understand the interaction of the compound with the plant beyond metabolism, and to understand what sorts of ligand–receptor interactions are occurring. Pharmacokinetics modeling and profiling of compounds could be established using hydroponic studies to evaluate the absorption, distribution, metabolism and accumulation of a compound in a controlled environment. The modeling of xenobiotic compounds in the plant should be based on exposure concentration, tissue concentration of the parent compound and metabolites, and concentration in the xylem sap and phloem, all of which can produce the required parameters for a plant pharmacokinetic profile. A well-characterized PK/PD model would then relate the plant pharmacokinetic profile to the drug concentration at the site of action or plant organ, and the intensity and time course of the plant's response. The model would describe how exposure to xenobiotics over time can be related to desired and undesirable drug effects. Time-dependent exposure should consider changes with plant development considering the exposure relative to the biomass, transpiration rate and accumulation as a function of plant growth.
Parameters for evaluation and modeling
In establishing the conceptual model, there is a necessity to establish experimental parameters for comparison across studies which vary in xenobiotic concentration, time of exposure, compound, and environmental conditions. The ADMA model calls for establishing pharmacokinetic profiles of xenobiotic compounds that describe the associated processes in plants. Experiments would be able to test the effect of various treatments and environmental factors on the parameters and develop clear knowledge of the processes. The following parameters should be understood and determined for experimental comparison within the multicompartment PK/PD model ().
Absorption parameter
The root concentration factor is a commonly used parameter to measure the entry of a compound in the root (Eggen et al., Citation2011; Li et al., Citation2019; Wu et al., Citation2013). The root concentration factor is the concentration in the root divided by the concentration in the soil solution or nutrient solution for hydroponic studies. The value provides information for the amount of a compound at a specific time, however, does not consider the total amount of the compound that enters the plant and is not a measurement of absorption. Plant pharmacology proposes the establishment of an absorption parameter that would determine how much of the compound has entered the roots and quantifies rates of absorption.
Distribution parameter
The distribution of a compound is often described using the TSCF, a ratio between the xylem sap concentration and the concentration in the soil/nutrient solution (Briggs et al., Citation1982; Dettenmaier et al., Citation2009; Hsu et al., Citation1990; Schriever & Lamshoeft, Citation2020). There are several methods of xylem sap measurements: (1) root pressure exudation, (2) pressure chamber, (3) vacuum pump (Alexou & Peuke, Citation2013). There is a lack of comparative data between these methods and a need for standards and quality control of xylem sap measurements. Experiments need to take into consideration the rate of absorption, time of exposure, concentration dependence, circadian rhythm, pharmacodynamic response, effect of mixtures and plant health (Alberto et al., Citation2018; Belbin et al., Citation2019). Erroneous results and conclusions could be the result of any of these factors, and have caused misinterpretation and confusion in the field. Furthermore, the calculation of a TSCF or translocation factor based on compound concentration in plant leaves divided by concentration in plant roots does not properly reflect distribution of a compound as it fails to take into consideration metabolic processes. Interpretation of distribution based on the translocation factor and organ concentrations is incorrect and does not represent the distribution process, so the TSCF should be reported only as a function of direct measurements of the xylem sap. For phloem distribution, there is a general lack of data due to difficulty in measurements. Phloem distribution needs to be quantified through innovative sampling methods. Plant pharmacology proposes the establishment of standardized measurements for xylem and phloem distribution.
Metabolic parameter
Current investigations have largely focused on two methods for studying plant metabolism of pharmaceuticals, i.e. evaluation of enzymatic activity and identification/quantification of metabolites (Carter et al., Citation2018; Macherius et al., Citation2014; Malchi et al., Citation2014; Riemenschneider et al., Citation2017). Metabolic parameters need to quantify the rate and location of metabolism, and consider the absorption and distribution processes, as to determine saturation and concentration dependent metabolism of compounds. Plant pharmacology proposes the establishment of metabolic parameters that improve our knowledge of how abiotic or biotic factors affect metabolic processes.
Accumulation parameter
Quantification of accumulation of compounds and active metabolites is important for understanding plant pharmacodynamics, concentration response and human health aspects. An accumulation parameter of organ-specific concentration factors is widely used and provides important data relating exposure concentration to bioaccumulation. Plant pharmacology proposes the establishment of standardization of results of plant xenobiotic accumulation that enables comparison between methods, plants and studies, and correlates to exposure concentrations, time period of exposure, biotic and abiotic factors, and is relevant to the actual exposure concentrations of the xenobiotic in agricultural ecosystems.
Pharmacodynamic parameters
The evaluation of pharmacodynamic effect on plants needs to take into consideration the effect of compounds on plant growth and development. In order to evaluate the effects on the plant, biomarkers are required for evaluation of different functions. In human pharmacology, biomarkers can range from measurements of blood pressure or temperature to performing complex laboratory blood/organ tests (Shargel & Yu, Citation2015; Strimbu & Tavel, Citation2010). The determination of plant pharmacodynamic biomarkers aims to characterize the exposure or time dependent response of a plant upon exposure to xenobiotic compounds. Biomarker categories include predictive biomarkers which measure any general response/lack of response, or mechanism-of-action biomarkers that allow insights into the pharmacodynamic effects of a drug as well as insight into cellular or sub-cellular responses (Carter et al., Citation2015; Garralda et al., Citation2017; Gorovits et al., Citation2020, Citation2021). Plant pharmacology proposes the establishment of plant pharmacodynamic biomarkers that expand beyond the traditional morphological variations and biochemical changes in plants. Plant pharmacodynamic biomarkers should be related to plant physiology and molecular biology, and include genomic, transcriptomic and proteomic approaches that measure signaling molecules, amino acids, plant hormones, secondary metabolites, plant nutrients, enzyme activity and reactive oxygen species (Mansilla et al., Citation2021).
Concluding remarks
The concept proposed in this article is anticipated to improve understanding of the fate and risks related to xenobiotics in the agricultural environment, in agricultural products, and subsequently, in livestock and humans. The concept of plant pharmacology provides a novel perspective for assessing plant pharmacokinetic processes and potential interactions between plants and xenobiotic compounds, establishing relationships to the science of human pharmacology. The association between these two fields allows sharing of experimental and analytical methods, developing new methods and techniques, improving our understanding of biological processes and enabling insight into the interaction of xenobiotic compounds with biological systems. Furthermore, our extensive knowledge of pharmacodynamics and pharmacokinetics in complex animals should also be useful in environmental and plant sciences. Both fields would prosper from joint research and scientific interaction.
In addition to pharmacology, other fields of plant science could benefit from insight and understanding of the interactions of xenobiotic compounds and plants. Of specific relevance is pharmacognosy, i.e. the study of plants as producers of phytochemicals (primary and secondary metabolites). Secondary metabolites encompass a wide range of compounds (>200,000) and have been used for medicinal purposes throughout human history, while they continue to be the structural basis for many of the drugs we use today (Wink, Citation2010; Van Wyk & Wink, Citation2017). Furthermore, elicitors, i.e. exogenous compounds which induce or stimulate a defense response in plants for enhancement of secondary metabolite production, alter plant cellular activities at biochemical and molecular level and could provide even greater insight of the in-planta kinetic and dynamic processes. Understanding the biochemical processes, distribution, metabolism, accumulation and function of secondary metabolites and elicitors should be related to the study of plant pharmacology and provide mutual benefit, expanding our insight.
Through a multidisciplinary understanding of xenobiotic compounds and living systems, plant pharmacology provides a foundation for a detailed quantifiable model and appropriate parameters that can accurately determine the fate and effect of exogenous xenobiotic compounds in the plant system. Future research needs to validate plant parameters that can measure the pharmacokinetic processes and investigate the pharmacodynamic effect of xenobiotic compounds with emphasis on exposure-response relationships. Insights from pharmacology and pharmacognosy, together with current knowledge of pesticides, pharmaceuticals and other xenobiotics in plants, can provide mutual benefit to many fields of plant and environmental research, and generate new knowledge of the mechanisms and processes occurring during the interactions between exogenous xenobiotic compounds and specific biological systems.
Disclosure statement
There were no potential competing financial interests.
Additional information
Funding
References
- Alaoui-Sossé, B., Genet, P., Vinit-Dunand, F., Toussaint, M.-L., Epron, D., & Badot, P.-M. (2004). Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrateaccumulation and changes in ion contents. Plant Science, 166(5), 1213–1218. https://doi.org/https://doi.org/10.1016/j.plantsci.2003.12.032
- Alavijeh, M. S., Chishty, M., Qaiser, M. Z., & Palmer, A. M. (2005). Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx, 2(4), 554–571. https://doi.org/https://doi.org/10.1602/neurorx.2.4.554
- Alberto, D., Couée, I., Pateyron, S., Sulmon, C., & Gouesbet, G. (2018). Low doses of triazine xenobiotics mobilize ABA and cytokinin regulations in a stress- and low-energy-dependent manner. Plant Science, 274, 8–22. https://doi.org/https://doi.org/10.1016/j.plantsci.2018.04.025
- Alexou, M., & Peuke, A. D. (2013). Methods for xylem sap collection. Methods in Molecular Biology (Clifton, N.J.), 953, 195–207. https://doi.org/https://doi.org/10.1007/978-1-62703-152-3_13
- Aliferis, K. A., & Chrysayi-Tokousbalides, M. (2011). Metabolomics in pesticide research and development: Review and future perspectives. Metabolomics, 7(1), 35–53. https://doi.org/https://doi.org/10.1007/s11306-010-0231-x
- Anzenbacher, P., & Anzenbacherová, E. (2001). Review: Cellular and molecular life sciences cytochromes P450 and metabolism of xenobiotics. Cellular and Molecular Life Sciences, 58(5–6), 737–747. https://doi.org/https://doi.org/10.1007/pl00000897
- Ashraf, M., & Lionel, R. (2012). Handbook of Drug Interactions. Humana Press.
- Bakker, P. A. H. M., Berendsen, R. L., Doornbos, R. F., Wintermans, P. C. A., & Pieterse, C. M. J. (2013). The rhizosphere revisited: Root microbiomics. Frontiers in Plant Science, 4, 165. https://doi.org/https://doi.org/10.3389/fpls.2013.00165
- Baluška, F., & Mancuso, S. (2009). Plant neurobiology: From sensory biology, via plant communication, to social plant behavior. Cognitive Processing, 10(S1), 3–7. https://doi.org/https://doi.org/10.1007/s10339-008-0239-6
- Banks, W. A. (2016). From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nature Reviews Drug Discovery, 15(4), 275–292. https://doi.org/https://doi.org/10.1038/nrd.2015.21
- Barberon, M., & Geldner, N. (2014). Radial transport of nutrients: The plant root as a polarized epithelium. Plant Physiology, 166(2), 528–537. https://doi.org/https://doi.org/10.1104/pp.114.246124
- Belbin, F. E., Hall, G. J., Jackson, A. B., Schanschieff, F. E., Archibald, G., Formstone, C., & Dodd, A. N. (2019). Plant circadian rhythms regulate the effectiveness of a glyphosate-based herbicide. Nature Communications, 10(1), 3704. https://doi.org/https://doi.org/10.1038/s41467-019-11709-5
- Belz, R. G., Hurle, K., & Duke, S. O. (2005). Dose-response—A challenge for allelopathy? Nonlinearity in Biology, Toxicology, Medicine, 3(2), 173–211. https://doi.org/https://doi.org/10.2201/nonlin.003.02.002
- Ben Mordechay, E., Tarchitzky, J., Chen, Y., Shenker, M., & Chefetz, B. (2018). Composted biosolids and treated wastewater as sources of pharmaceuticals and personal care products for plant uptake: A case study with carbamazepine. Environmental Pollution (Barking, Essex : 1987), 232, 164–172. https://doi.org/https://doi.org/10.1016/j.envpol.2017.09.029
- Bigott, Y., Chowdhury, S. P., Pérez, S., Montemurro, N., Manasfi, R., & Schröder, P. (2021). Effect of the pharmaceuticals diclofenac and lamotrigine on stress responses and stress gene expression in lettuce (Lactuca sativa) at environmentally relevant concentrations. Journal of Hazardous Materials, 403, 123881. https://doi.org/https://doi.org/10.1016/j.jhazmat.2020.123881
- Bloemendal, S., & Kück, U. (2013). Cell-to-cell communication in plants, animals, and fungi: A comparative review. Die Naturwissenschaften, 100(1), 3–19. https://doi.org/https://doi.org/10.1007/s00114-012-0988-z
- Bohnenblust, E. W., Vaudo, A. D., Egan, J. F., Mortensen, D. A., & Tooker, J. F. (2016). Effects of the herbicide dicamba on nontarget plants and pollinator visitation. Environmental Toxicology and Chemistry, 35(1), 144–151. https://doi.org/https://doi.org/10.1002/etc.3169
- Bose, J. C. (1915). The action of drugs on plants. Proceedings of the Royal Society of Medicine, 8(Gen Rep), 1–40. https://doi.org/https://doi.org/10.1177/003591571500800501
- Braun, D. M., & Walker, J. C. (1996). Plant transmembrane receptors: New pieces in the signaling puzzle. Trends in Biochemical Sciences, 21(2), 70–73. https://doi.org/https://doi.org/10.1016/S0968-0004(96)80185-X
- Briggs, G. G., Bromilow, R. H., & Evans, A. A. (1982). Relationships between lipophilicity and root uptake and translocation of non‐ionised chemicals by barley. Pesticide Science, 13(5), 495–504. https://doi.org/https://doi.org/10.1002/ps.2780130506
- Bushra, R., & Aslam, N. (2010). An overview of clinical pharmacology of ibuprofen. Oman Medical Journal, 25(3), 155–161. https://doi.org/https://doi.org/10.5001/omj.2010.49
- Buxton, I. O., & Benet, L. Z. (2013). Pharmacokinetics: The dynamics of drug absorption, distribution, metabolism, and elimination. Goodman & Gilman’s: The pharmacological basis of therapeutics. 13th ed. Mcgraw-Hilll.
- Carter, L. J., Williams, M., Böttcher, C., & Kookana, R. S. (2015). Uptake of pharmaceuticals influences plant development and affects nutrient and hormone homeostases. Environmental Science & Technology, 49(20), 12509–12518. https://doi.org/https://doi.org/10.1021/acs.est.5b03468
- Carter, L. J., Williams, M., Martin, S., Kamaludeen, S. P. B., & Kookana, R. S. (2018). Sorption, plant uptake and metabolism of benzodiazepines. The Science of the Total Environment, 628–629, 18–25. https://doi.org/https://doi.org/10.1016/j.scitotenv.2018.01.337
- Cedergreen, N. (2008). Herbicides can stimulate plant growth. Weed Research, 48(5), 429–438. https://doi.org/https://doi.org/10.1111/j.1365-3180.2008.00646.x
- Chang, F. Y., & Vanden Born, W. H. (1971). Dicamba uptake, translocation, metabolism, and selectivity. Weed Science, 19(1), 113–117. https://doi.org/https://doi.org/10.1017/S0043174500048414
- Christou, A., Antoniou, C., Christodoulou, C., Hapeshi, E., Stavrou, I., Michael, C., Fatta-Kassinos, D., & Fotopoulos, V. (2016). Stress-related phenomena and detoxification mechanisms induced by common pharmaceuticals in alfalfa (Medicago sativa L.) plants. The Science of the Total Environment, 557–558, 652–664. https://doi.org/https://doi.org/10.1016/j.scitotenv.2016.03.054
- Clark, D. E. (2003). In silico prediction of blood–brain barrier permeation. Drug Discovery Today, 8(20), 927–933. https://doi.org/https://doi.org/10.1016/S1359-6446(03)02827-7
- Cole, D. J. (1994). Detoxification and activation of agrochemicals in plants. Pesticide Science, 42(3), 209–222. https://doi.org/https://doi.org/10.1002/ps.2780420309
- Coleman, J., Blake-Kalff, M., & Davies, E. (1997). Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends in Plant Science, 2(4), 144–151. https://doi.org/https://doi.org/10.1016/S1360-1385(97)01019-4
- Corrie, K., & Hardman, J. G. (2014). Mechanisms of drug interactions: Pharmacodynamics and pharmacokinetics. Anaesthesia & Intensive Care Medicine, 15(7), 305–308. https://doi.org/https://doi.org/10.1016/j.mpaic.2014.04.005
- Cummins, I., Dixon, D. P., Freitag-Pohl, S., Skipsey, M., & Edwards, R. (2011). Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metabolism Reviews, 43(2), 266–280. https://doi.org/https://doi.org/10.3109/03602532.2011.552910
- Dahlgren, D., & Lennernäs, H. (2019). Intestinal permeability and drug absorption: Predictive experimental, computational and in vivo approaches. Pharmaceutics, 11(8), 411. https://doi.org/https://doi.org/10.3390/pharmaceutics11080411
- Daina, A., Michielin, O., & Zoete, V. (2017). SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports, 7(1), 1–13. https://doi.org/https://doi.org/10.1038/srep42717
- Darwin, C. (1880). The Power of Movement in Plants. Cambridge University Press.
- de la Fuente Cantó, C., Simonin, M., King, E., Moulin, L., Bennett, M. J., Castrillo, G., & Laplaze, L. (2020). An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. The Plant Journal, 103(3), 951–964. https://doi.org/https://doi.org/10.1111/tpj.14781
- Dettenmaier, E. M., Doucette, W. J., & Bugbee, B. (2009). Chemical hydrophobicity and uptake by plant roots. Environmental Science & Technology, 43(2), 324–329. https://doi.org/https://doi.org/10.1021/es801751x
- Duke, S. O. (1990). Overview of herbicide mechanisms of action. Environmental Health Perspectives, 87, 263–271. https://doi.org/https://doi.org/10.1289/ehp.9087263
- Eggen, T., Asp, T. N., Grave, K., & Hormazabal, V. (2011). Uptake and translocation of metformin, ciprofloxacin and narasin in forage- and crop plants. Chemosphere, 85(1), 26–33. https://doi.org/https://doi.org/10.1016/j.chemosphere.2011.06.041
- Enomoto, Y., & Goto, F. (2008). Long-distance signaling of iron deficiency in plants (K.G. Kozminski, editor). Plant Signaling & Behavior, 3(6), 396–397. https://doi.org/https://doi.org/10.4161/psb.3.6.5419
- Evgenidou, E. N., Konstantinou, I. K., & Lambropoulou, D. A. (2015). Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. The Science of the Total Environment, 505, 905–926. https://doi.org/https://doi.org/10.1016/j.scitotenv.2014.10.021
- Eyal, S., Hsiao, P., & Unadkat, J. D. (2009). Drug interactions at the blood-brain barrier: Fact or fantasy? Pharmacology & Therapeutics, 123(1), 80–104. https://doi.org/https://doi.org/10.1016/j.pharmthera.2009.03.017
- Fantke, P., Wieland, P., Wannaz, C., Friedrich, R., & Jolliet, O. (2013). Dynamics of pesticide uptake into plants: From system functioning to parsimonious modeling. Environmental Modelling & Software, 40, 316–324. https://doi.org/https://doi.org/10.1016/j.envsoft.2012.09.016
- Ford, K. A., & Casida, J. E. (2008). Comparative metabolism and pharmacokinetics of seven neonicotinoid insecticides in spinach. Journal of Agricultural and Food Chemistry, 56(21), 10168–10175. https://doi.org/https://doi.org/10.1021/jf8020909
- Fu, Q., Malchi, T., Carter, L. J., Li, H., Gan, J., & Chefetz, B. (2019). Pharmaceutical and personal care products: From wastewater treatment into agro-food systems. Environmental Science & Technology, 53(24), 14083–14090. https://doi.org/https://doi.org/10.1021/acs.est.9b06206
- Garralda, E., Dienstmann, R., & Tabernero, J. (2017). Pharmacokinetic/pharmacodynamic modeling for drug development in oncology. American Society of Clinical Oncology Educational Book, 37, 210–215. https://doi.org/https://doi.org/10.1200/EDBK_180460
- Goldstein, M., Shenker, M., & Chefetz, B. (2014). Insights into the uptake processes of wastewater-borne pharmaceuticals by vegetables. Environmental Science & Technology, 48(10), 5593–5600. https://doi.org/https://doi.org/10.1021/es5008615
- Goodman, A., Tw, R., Nies, A., & Taylor, P. (2000). Goodman & Gilman’s: The pharmacological basis of therapeutics. 10th ed. Mcgraw-Hilll.
- Gorovits, R., Shteinberg, M., Mishra, R., Ari, J. B., Malchi, T., Chefetz, B., Anfoka, G., & Czosnek, H. (2021). Interplay of stress responses to carbamazepine treatment, whitefly infestation and virus infection in tomato plants. Plant Stress, 1, 100009. https://doi.org/https://doi.org/10.1016/j.stress.2021.100009
- Gorovits, R., Sobol, I., Akama, K., Chefetz, B., & Czosnek, H. (2020). Pharmaceuticals in treated wastewater induce a stress response in tomato plants. Scientific Reports, 10(1), 1–13. https://doi.org/https://doi.org/10.1038/s41598-020-58776-z
- Gracia-Lor, E., Sancho, J. V., Serrano, R., & Hernández, F. (2012). Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere, 87(5), 453–462. https://doi.org/https://doi.org/10.1016/j.chemosphere.2011.12.025
- Grandjean, P. (2016). Paracelsus revisited: The dose concept in a complex world. Basic & Clinical Pharmacology & Toxicology, 119(2), 126–132. https://doi.org/https://doi.org/10.1111/bcpt.12622
- Grossberger, A., Hadar, Y., Borch, T., & Chefetz, B. (2014). Biodegradability of pharmaceutical compounds in agricultural soils irrigated with treated wastewater. Environmental Pollution (Barking, Essex : 1987), 185, 168–177. https://doi.org/https://doi.org/10.1016/j.envpol.2013.10.038
- He, Y., Zhou, J., Shan, L., & Meng, X. (2018). Plant cell surface receptor-mediated signaling – a common theme amid diversity. Journal of Cell Science, 131(2), jcs209353. https://doi.org/https://doi.org/10.1242/jcs.209353
- Hettiarachchi, H., & Ardakanian, R. (2018). Safe Use of Wastewater in Agriculture from Concept to Implementation. 1st ed. Springer International Publishing.
- Hirtz, J. (1985). The gastrointestinal absorption of drugs in man: A review of current concepts and methods of investigation. British Journal of Clinical Pharmacology, 19(S2), 77S–83S. https://doi.org/https://doi.org/10.1111/j.1365-2125.1985.tb02746.x
- Hock, B., & Elstner, E. (2004). Plant Toxicology. 4th ed. Taylor & Francis Inc.
- Homayun, B., Lin, X., & Choi, H.-J. (2019). Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics, 11(3), 129. https://doi.org/https://doi.org/10.3390/pharmaceutics11030129
- Howland, M. A. (2015). Pharmacokinetic and toxicokinetic principles. In R. S. Hoffman, M. Howland, N. A. Lewin, L. S. Nelson, & L. R. Goldfrank (Eds.), Goldfrank’s toxicologic emergencies (10th ed., pp. 1–22). McGraw-Hill.
- Hsu, F. C., Marxmiller, R. L., & Yang, A. Y. S. (1990). Study of root uptake and xylem translocation of cinmethylin and related compounds in detopped soybean roots using a pressure chamber technique. Plant Physiology, 93(4), 1573–1578. https://doi.org/https://doi.org/10.1104/pp.93.4.1573
- Jambhekar, S. S., & Breen, P. J. (2012). Basic pharmacokinetics. Pharmaceutical Press.
- Jonker, D. M., Visser, S. A. G., van der Graaf, P. H., Voskuyl, R. A., & Danhof, M. (2005). Towards a mechanism-based analysis of pharmacodynamic drug-drug interactions in vivo. Pharmacology & Therapeutics, 106(1), 1–18. https://doi.org/https://doi.org/10.1016/j.pharmthera.2004.10.014
- Jusko, W. J. (2016). Foundations of pharmacodynamic systems analysis (AAPS advances in the pharmaceutical sciences series, pp. 161–175). Springer Verlag.
- Kahromi, S., & Khara, J. (2021). Chitosan stimulates secondary metabolite production and nutrient uptake in medicinal plant Dracocephalum kotschyi. Journal of the Science of Food and Agriculture, 101(9), 3898–3907. https://doi.org/https://doi.org/10.1002/jsfa.11030
- Kang, J., Park, J., Choi, H., Burla, B., Kretzschmar, T., Lee, Y., & Martinoia, E. (2011). Plant ABC transporters. The Arabidopsis Book, 9, e0153. https://doi.org/https://doi.org/10.1199/tab.0153
- Kasprzyk-Hordern, B., Dinsdale, R. M., & Guwy, A. J. (2009). The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Research, 43(2), 363–380. https://doi.org/https://doi.org/10.1016/j.watres.2008.10.047
- Kelder, J., Grootenhuis, P. D. J., Bayada, D. M., Delbressine, L. P. C., & Ploemen, J. (1999). Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharmaceutical Research, 16(10), 1514–1519. https://doi.org/https://doi.org/10.1023/A:1015040217741
- Krishna, D. R., & Klotz, U. (1994). Extrahepatic metabolism of drugs in humans. Clinical Pharmacokinetics, 26(2), 144–160. https://doi.org/https://doi.org/10.2165/00003088-199426020-00007
- Kudsk, P., & Moss, S. (2017). Herbicide dose: What is a low dose? (ACS symposium series, pp. 15–24). American Chemical Society.
- Laksitorini, M., Prasasty, V. D., Kiptoo, P. K., & Siahaan, T. J. (2014). Pathways and progress in improving drug delivery through the intestinal mucosa and blood-brain barriers. Therapeutic Delivery, 5(10), 1143–1163. https://doi.org/https://doi.org/10.4155/tde.14.67
- Li, Y., Chiou, C. T., Li, H., & Schnoor, J. L. (2019). Improved prediction of the bioconcentration factors of organic contaminants from soils into plant/crop roots by related physicochemical parameters. Environment International, 126, 46–53. https://doi.org/https://doi.org/10.1016/j.envint.2019.02.020
- Limmer, M. A., & Burken, J. G. (2014). Plant translocation of organic compounds: Molecular and physicochemical predictors. Environmental Science & Technology Letters, 1(2), 156–161. https://doi.org/https://doi.org/10.1021/ez400214q
- Lin, J. H. (1996). Bisphosphonates: A review of their pharmacokinetic properties. Bone, 18(2), 75–85. https://doi.org/https://doi.org/10.1016/8756-3282(95)00445-9
- Lipinski, C. A. (2004). Lead- and drug-like compounds: The rule-of-five revolution. Drug Discovery Today Technologies, 1(4), 337–341. https://doi.org/https://doi.org/10.1016/j.ddtec.2004.11.007
- Macherius, A., Seiwert, B., Schröder, P., Huber, C., Lorenz, W., & Reemtsma, T. (2014). Identification of plant metabolites of environmental contaminants by UPLC-QToF-MS: The in vitro metabolism of triclosan in horseradish. Journal of Agricultural and Food Chemistry, 62(5), 1001–1009. https://doi.org/https://doi.org/10.1021/jf404784q
- Málaga, I., Sánchez-Carpintero, R., Roldán, S., Ramos-Lizana, J., & García-Peñas, J. J. (2019). New anti-epileptic drugs in paediatrics. Anales de Pediatría (English Edition), 91(6), 415.e1–415.e10. https://doi.org/https://doi.org/10.1016/j.anpede.2019.09.005
- Malchi, T., Maor, Y., Tadmor, G., Shenker, M., & Chefetz, B. (2014). Irrigation of root vegetables with treated wastewater: Evaluating uptake of pharmaceuticals and the associated human health risks. Environmental Science & Technology, 48(16), 9325–9333. https://doi.org/https://doi.org/10.1021/es5017894
- Mansilla, S., Portugal, J., Bayona, J. M., Matamoros, V., Leiva, A. M., Vidal, G., & Piña, B. (2021). Compounds of emerging concern as new plant stressors linked to water reuse and biosolid application in agriculture. Journal of Environmental Chemical Engineering., 9(3), 105198. https://doi.org/https://doi.org/10.1016/j.jece.2021.105198
- Markus, C., Pecinka, A., Karan, R., Barney, J. N., & Merotto, A. (2018). Epigenetic regulation - contribution to herbicide resistance in weeds? Pest Management Science, 74(2), 275–281. https://doi.org/https://doi.org/10.1002/ps.4727
- Mathews, S., Henderson, S., & Reinhold, D. (2014). Uptake and accumulation of antimicrobials, triclocarban and triclosan, by food crops in a hydroponic system. Environmental Science and Pollution Research International, 21(9), 6025–6033. https://doi.org/https://doi.org/10.1007/s11356-013-2474-3
- Mejías, C., Martín, J., Santos, J. L., Aparicio, I., & Alonso, E. (2021). Occurrence of pharmaceuticals and their metabolites in sewage sludge and soil: A review on their distribution and environmental risk assessment. Trends in Environmental Analytical Chemistry, 30, e00125. https://doi.org/https://doi.org/10.1016/j.teac.2021.e00125
- Mendes, R., & Raaijmakers, J. M. (2015). Cross-kingdom similarities in microbiome functions. The ISME Journal, 9(9), 1905–1907. https://doi.org/https://doi.org/10.1038/ismej.2015.7
- Michel, A., Johnson, R. D., Duke, S. O., & Scheffler, B. E. (2004). Dose-response relationships between herbicides with different modes of action and growth of Lemna paucicostata: An improved ecotoxicological method. Environmental Toxicology and Chemistry, 23(4), 1074–1079. https://doi.org/https://doi.org/10.1897/03-256
- Mikitsh, J. L., & Chacko, A. M. (2014). Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspectives in Medicinal Chemistry, 6, 11–24. https://doi.org/https://doi.org/10.4137/PMc.s13384
- Miller, E. L., Nason, S. L., Karthikeyan, K. G., & Pedersen, J. A. (2016). Root uptake of pharmaceuticals and personal care product ingredients. Environmental Science & Technology, 50(2), 525–541. https://doi.org/https://doi.org/10.1021/acs.est.5b01546
- Mlynek, F., Himmelsbach, M., Buchberger, W., & Klampfl, C. W. (2020). Analytical approaches for the determination and identification of drug metabolites in plants after uptake (pp. 493–523). Springer.
- Morissette, G., Ammoury, A., Rusu, D., Marguery, M. C., Lodge, R., Poubelle, P. E., & Marceau, F. (2009). Intracellular sequestration of amiodarone: Role of vacuolar ATPase and macroautophagic transition of the resulting vacuolar cytopathology. British Journal of Pharmacology, 157(8), 1531–1540. https://doi.org/https://doi.org/10.1111/j.1476-5381.2009.00320.x
- Morth, J. P., Pedersen, B. P., Buch-Pedersen, M. J., Andersen, J. P., Vilsen, B., Palmgren, M. G., & Nissen, P. (2011). A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nature Reviews Molecular Cell Biology, 12(1), 60–70. https://doi.org/https://doi.org/10.1038/nrm3031
- Nagata, T., Iizumi, S., Satoh, K., Ooka, H., Kawai, J., Carninci, P., Hayashizaki, Y., Otomo, Y., Murakami, K., Matsubara, K., & Kikuchi, S. (2004). Comparative analysis of plant and animal calcium signal transduction element using plant full-length cDNA data. Molecular Biology and Evolution, 21(10), 1855–1870. https://doi.org/https://doi.org/10.1093/molbev/msh197
- Narayani, M., & Srivastava, S. (2017). Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochemistry Reviews, 16(6), 1227–1252. https://doi.org/https://doi.org/10.1007/s11101-017-9534-0
- Ohkawa, H., & Inui, H. (2015). Metabolism of agrochemicals and related environmental chemicals based on cytochrome P450s in mammals and plants. Pest Management Science, 71(6), 824–828. https://doi.org/https://doi.org/10.1002/ps.3871
- Ort, C., Nuijs, A. L. N., Berset, J.‐D., Bijlsma, L., Castiglioni, S., Covaci, A., Voogt, P., Emke, E., Fatta‐Kassinos, D., Griffiths, P., Hernández, F., González‐Mariño, I., Grabic, R., Kasprzyk‐Hordern, B., Mastroianni, N., Meierjohann, A., Nefau, T., Östman, M., Pico, Y., … Thomas, K. V. (2014). Spatial differences and temporal changes in illicit drug use in Europe quantified by wastewater analysis. Addiction, 109(8), 1338–1352. https://doi.org/https://doi.org/10.1111/add.12570
- Page, S. W., & Maddison, J. E. (2008). Principles of clinical pharmacology. Small animal clinical pharmacology (pp. 1–26). Elsevier.
- Paltiel, O., Fedorova, G., Tadmor, G., Kleinstern, G., Maor, Y., & Chefetz, B. (2016). Human exposure to wastewater-derived pharmaceuticals in fresh produce: A randomized controlled trial focusing on carbamazepine. Environmental Science & Technology, 50(8), 4476–4482. https://doi.org/https://doi.org/10.1021/acs.est.5b06256
- Patrick, G. L. (2017). An introduction to medicinal chemistry. 6th ed. Oxford University Press.
- Paz, A., Tadmor, G., Malchi, T., Blotevogel, J., Borch, T., Polubesova, T., & Chefetz, B. (2016). Fate of carbamazepine, its metabolites, and lamotrigine in soils irrigated with reclaimed wastewater: Sorption, leaching and plant uptake. Chemosphere, 160, 22–29. https://doi.org/https://doi.org/10.1016/j.chemosphere.2016.06.048
- Perucca, E. (2006). Clinically relevant drug interactions with antiepileptic drugs. British Journal of Clinical Pharmacology, 61(3), 246–255. https://doi.org/https://doi.org/10.1111/j.1365-2125.2005.02529.x
- Raj, G. M., & Raveendran, R. (2019). Introduction to basics of pharmacology and toxicology: Volume 1: General and molecular pharmacology: Principles of drug action. Springer.
- Ramírez-Puebla, S. T., Servín-Garcidueñas, L. E., Jiménez-Marín, B., Bolaños, L. M., Rosenblueth, M., Martínez, J., Rogel, M. A., Ormeño-Orrillo, E., & Martínez-Romero, E. (2013). Gut and root microbiota commonalities. Applied and Environmental Microbiology, 79(1), 2–9. https://doi.org/https://doi.org/10.1128/AEM.02553-12
- Rang, H. P., Ritter, J. M., Humphrey, G., Loke, Y. K., & MacEwan, D. (2019). Rang & Dale’s pharmacology. 9th ed. Churchill Livingstone.
- Rein, A., Legind, C. N. N., & Trapp, S. (2011). New concepts for dynamic plant uptake models. SAR and QSAR in Environmental Research, 22(1-–), 191–215. https://doi.org/https://doi.org/10.1080/1062936X.2010.548829
- Reinhold, D., Vishwanathan, S., Park, J. J., Oh, D., & Saunders, F. M. (2010). Assessment of plant-driven removal of emerging organic pollutants by duckweed. Chemosphere, 80(7), 687–692. https://doi.org/https://doi.org/10.1016/j.chemosphere.2010.05.045
- Riemenschneider, C., Seiwert, B., Moeder, M., Schwarz, D., & Reemtsma, T. (2017). Extensive transformation of the pharmaceutical carbamazepine following uptake into intact tomato plants. Environmental Science & Technology, 51(11), 6100–6109. https://doi.org/https://doi.org/10.1021/acs.est.6b06485
- Robbins, N. E., Trontin, C., Duan, L., & Dinneny, J. R. (2014). Beyond the barrier: Communication in the root through the endodermis. Plant Physiology, 166(2), 551–559. https://doi.org/https://doi.org/10.1104/pp.114.244871
- Roberts, T. R., Hutson, D. H., Lee, P. W., Nicholls, P. H., & Plimmer, J. R. (1999). Metabolic pathways of agrochemicals (T. R. Roberts, D. H. Hutson, P. W. Lee, P. H. Nicholls, & J. R. Plimmer, Eds.). Royal Society of Chemistry.
- Russo, L., Buckley, Y. M., Hamilton, H., Kavanagh, M., & Stout, J. C. (2020). Low concentrations of fertilizer and herbicide alter plant growth and interactions with flower-visiting insects. Agriculture Ecosystems and Environment., 304, 107141. https://doi.org/https://doi.org/10.1016/j.agee.2020.107141
- Sandermann, H. (1992). Plant metabolism of xenobiotics. Trends in Biochemical Sciences, 17(2), 82–84. https://doi.org/https://doi.org/10.1016/0968-0004(92)90507-6
- Sandermann, H. (2004). Bound and unextractable pesticidal plant residues: Chemical characterization and consumer exposure. Pest Management Science, 60(7), 613–623. https://doi.org/https://doi.org/10.1002/ps.888
- Schapira, M., Manor, O., Golan, N., Kalo, D., Mordehay, V., Kirshenbaum, N., Goldsmith, R., Chefetz, B., & Paltiel, O. (2020). Involuntary human exposure to carbamazepine: A cross-sectional study of correlates across the lifespan and dietary spectrum. Environment International, 143, 105951. https://doi.org/https://doi.org/10.1016/j.envint.2020.105951
- Schriever, C., & Lamshoeft, M. (2020). Lipophilicity matters – A new look at experimental plant uptake data from literature. Science of the Total Environment, 713, 136667. https://doi.org/https://doi.org/10.1016/j.scitotenv.2020.136667
- Seliskar, M., & Rozman, D. (2007). Mammalian cytochromes P450-importance of tissue specificity. Biochimica et Biophysica Acta, 1770(3), 458–466. https://doi.org/https://doi.org/10.1016/j.bbagen.2006.09.016
- Shargel, L., Wu-Pong, S., & Yu, A. B. C. (2012). Chapter 19. Relationship between pharmacokinetics and pharmacodynamics. In Applied biopharmaceutics & pharmacokinetics, 6e. The McGraw-Hill Companies.
- Shargel, L., & Yu, A. B. C. (2015). Applied biopharmaceutics & pharmacokinetics. 7th ed. Appleton & Lange Reviews/McGraw-Hill, Medical Pub.
- Siminszky, B. (2006). Plant cytochrome P450-mediated herbicide metabolism. Phytochemistry Reviews, 5(2–3), 445–458. https://doi.org/https://doi.org/10.1007/s11101-006-9011-7
- Sirven, J. I., Noe, K., Hoerth, M., & Drazkowski, J. (2012). Antiepileptic drugs 2012: Recent advances and trends. Mayo Clinic Proceedings, 87(9), 879–889. https://doi.org/https://doi.org/10.1016/j.mayocp.2012.05.019
- Stateczny, D., Oppenheimer, J., & Bommert, P. (2016). G protein signaling in plants: Minus times minus equals plus. Current Opinion in Plant Biology, 34, 127–135. https://doi.org/https://doi.org/10.1016/j.pbi.2016.11.001
- Steudle, E. (1998). Review article. How does water get through roots? Journal of Experimental Botany, 49(322), 775–788. https://doi.org/https://doi.org/10.1093/jexbot/49.322.775
- Strimbu, K., & Tavel, J. A. (2010). What are biomarkers? Current Opinion in HIV and AIDS, 5(6), 463–466. https://doi.org/https://doi.org/10.1097/COH.0b013e32833ed177
- Sui, Q., Huang, J., Deng, S., Chen, W., & Yu, G. (2011). Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes. Environmental Science & Technology, 45(8), 3341–3348. https://doi.org/https://doi.org/10.1021/es200248d
- Taiz, L., Zeiger, E., Møller, I. M., & Murphy, A. (2014). Plant physiology and development. 6th ed. SINAUER Associates Inc.
- Thanos, C. A. (1994). Aristotle and Theophrastus on plant-animal interactions. In Plant-animal interactions in Mediterranean-type ecosystems (pp. 3–11). Springer.
- Trapp, S. (2004). Plant uptake and transport models for neutral and ionic chemicals. Environmental Science and Pollution Research International, 11(1), 33–39. https://doi.org/https://doi.org/10.1065/espr2003.08.169
- Trusov, Y., & Botella, J. R. (2016). Plant G-proteins come of age: Breaking the bond with animal models. Frontiers in Chemistry, 4, 24. https://doi.org/https://doi.org/10.3389/fchem.2016.00024
- Van Eerd, L. L., Hoagland, R. E., Zablotowicz, R. M., & Hall, J. C. (2006). Pesticide metabolism in plants and microorganisms. Weed Science, 51(4), 472–495. https://doi.org/https://doi.org/10.1614/0043-1745(2003)051[0472:PMIPAM2.0.CO;2]
- Van Wyk, B.-E., & Wink, M. (2017). Medicinal plants of the world: An illustrated scientific guide to important medicinal plants and their uses (2nd ed.). CABI.
- Vergine, P., Salerno, C., Libutti, A., Beneduce, L., Gatta, G., Berardi, G., & Pollice, A. (2017). Closing the water cycle in the agro-industrial sector by reusing treated wastewater for irrigation. Journal of Cleaner Production, 164, 587–596. https://doi.org/https://doi.org/10.1016/j.jclepro.2017.06.239
- Villette, C., Maurer, L., Wanko, A., & Heintz, D. (2019). Xenobiotics metabolization in Salix alba leaves uncovered by mass spectrometry imaging. Metabolomics, 15(9), 122. https://doi.org/https://doi.org/10.1007/s11306-019-1572-8
- Ward, J. M., Mäser, P., & Schroeder, J. I. (2009). Plant ion channels: Gene families, physiology, and functional genomics analyses. Annual Review of Physiology, 71(1), 59–82. https://doi.org/https://doi.org/10.1146/annurev.physiol.010908.163204
- Wink, M. (2010). Introduction: Biochemistry, physiology and ecological functions of secondary metabolites. In: M. Wink (Ed.), Biochemistry of plant secondary metabolism (pp. 1–19). Wiley-Blackwell.
- Wu, X., Ernst, F., Conkle, J. L., & Gan, J. (2013). Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environment International, 60, 15–22. https://doi.org/https://doi.org/10.1016/j.envint.2013.07.015
- Xu, J., Wang, X., & Guo, W. (2015). The cytochrome P450 superfamily: Key players in plant development and defense. Journal of Integrative Agriculture, 14(9), 1673–1686. https://doi.org/https://doi.org/10.1016/S2095-3119(14)60980-1
- Yi, L., Jiao, W., Chen, X., & Chen, W. (2011). An overview of reclaimed water reuse in China. Journal of Environmental Sciences, 23(10), 1585–1593. https://doi.org/https://doi.org/10.1016/S1001-0742(10)60627-4
- York, L. M., Carminati, A., Mooney, S. J., Ritz, K., & Bennett, M. J. (2016). The holistic rhizosphere: Integrating zones, processes, and semantics in the soil influenced by roots. Journal of Experimental Botany, 67(12), 3629–3643. https://doi.org/https://doi.org/10.1093/jxb/erw108
- Yu, L. X., Amidon, G. L., Polli, J. E., Zhao, H., Mehta, M. U., Conner, D. P., Shah, V. P., Lesko, L. J., Chen, M. ‐L., Lee, V. H. L., & Hussain, A. S. (2002). Biopharmaceutics classification system: The scientific basis for biowaiver extensions. Pharmaceutical Research, 19(7), 921–925. https://doi.org/https://doi.org/10.1023/A:1016473601633
- Zanger, U. M., & Schwab, M. (2013). Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacology & Therapeutics, 138(1), 103–141. https://doi.org/https://doi.org/10.1016/j.pharmthera.2012.12.007
- Zhang, Y., Lv, T., Carvalho, P. N., Arias, C. A., Chen, Z., & Brix, H. (2016). Removal of the pharmaceuticals ibuprofen and iohexol by four wetland plant species in hydroponic culture: Plant uptake and microbial degradation. Environmental Science and Pollution Research International, 23(3), 2890–2898. https://doi.org/https://doi.org/10.1007/s11356-015-5552-x
- Zheng, M. (2020). Pharmacodynamic drug–drug interactions. In Drug discovery and evaluation: Methods in clinical pharmacology (pp. 603–612). Springer International Publishing.