Abstract
Hydrothermal carbonization (HTC) is the method of choice to convert wet waste biomass to hydrochars. Their porous structure can serve as a microenvironment to plant-growth-promoting rhizobacteria (PGPR), supporting their growth, survival, and activities. As published work lacks the systematic compilation of pore characteristics of hydrochars related to bacterial colonization, we collect available data and elaborate on their dependence on the carbonization process conditions, feedstocks, and methodology of pore system characterization. Our analysis indicates a high abundance of pores sized between 1 and 20 μm relevant for the protection of PGPR from predators, and of nutrients and labile C in hydrochars supporting bacterial growth. In addition to the selection of optimized process parameters and feedstocks (240–260 °C, low feedstock pH, non-lignocellulosic biomass), adding mineral amendments prior to HTC offers opportunities for engineering hydrochars with an even larger share of pore space suited for bacterial colonization. Using the comprehensive literature on biochars, we demonstrate that the interior pore space in chars determines the potential to serve as an inoculum carrier to PGPR, thereby enhancing nutrient acquisition and protecting plants from diseases and abiotic stresses. The pore characteristics of hydrochars are comparable to biochars, and hydrochars are generally superior in providing a labile C reservoir that PGPR can readily access. We argue that HTC provides a cost-effective conversion route to produce PGPR vectors/carriers from wet (waste) biomass serving various environmental management objectives (waste recycling, soil fertility, soil remediation technologies) and circular bioeconomy (sustainable agriculture, substituting non-renewable carrier materials and fertilizers).
We review the role of pore characteristics of hydrochars for bacterial colonization
We identify opportunities for engineering hydrochars to provide favorable habitat conditions to PGPR
240–260 °C, low pH, non-lignocellulosic feedstocks, and adding mineral amendments increase the habitable pore space
Hydrochars offer suitable pore characteristics and high labile C amounts and are promising PGPR carriers/vectors
Highlights
Graphical abstract

HANDLING EDITORS:
1. Introduction
The globally rising food demand, driven by population growth and dietary changes, triggers the world’s growth rate for agricultural fertilizer (FAO, Citation2015). The dependence on agrochemicals and the necessity to improve agronomic productivity has motivated experts to find more efficient practices to enhance nutrient availability in soils, including the inoculation with plant-growth-promoting rhizobacteria (PGPR) (Bashan et al., Citation2014; Postma et al., Citation2008; Schnee et al., Citation2016; Tripti et al., Citation2017). Their ability to support plant growth involves processes including N fixation, P solubilization, Fe sequestration, and suppression of stress ethylene production by roots, creating anti-fungal compounds and antibiotics, and eliminating plant pathogens (Hale et al., Citation2014). PGPR thereby protect plants from diseases and abiotic stresses through several mechanisms (Ambrosini et al., Citation2016).
A major drawback, however, relates to the low survival rate and time of PGPR after soil inoculation that impedes the development of a sufficiently large population, making their advantageous utilization in agriculture often unsatisfactory (Acea et al., Citation1988; Bashan et al., Citation1995, Citation2014). Due to their weakness to resist predation, PGPR require a protective microenvironment to grow, feed, and reproduce (Bashan et al., Citation2014).
Various studies explored the concept of using materials as carriers/vectors to increase the survival and activity of PGPR (Egamberdieva et al., Citation2017; Foster et al., Citation2020; Hale et al., Citation2014, Citation2015; Postma et al., Citation2010, Citation2013; Sun et al., Citation2016). To this end, the beneficial microorganisms colonize the pore system of materials that serve as a protective habitat and source of nutrients. Ideally, secondary resources are used as feedstocks to generate carrier materials. However, existing work has focused on using non-renewable resources, including peat and vermiculite (Albareda et al., Citation2008; Sangeetha & Stella, Citation2012). Unfortunately, notwithstanding their effectiveness of PGPR inoculation, peat and vermiculite are unavailable in most regions of the planet and not capable of effectively reducing salinity and water stress (Egamberdieva et al., Citation2017; Hale et al., Citation2014, Citation2015; Vanek & Thies, Citation2016).
A growing body of literature examines the potential of hydrochars from hydrothermal carbonization (HTC) (Egamberdieva et al., Citation2017) and biochars from pyrolysis as bacterial vectors (Hale et al., Citation2014, Citation2015; Postma et al., Citation2013; Sun et al., Citation2016). Pyrolysis produces carbonaceous material at elevated temperatures (typically 400–600 °C) in an inert atmosphere. HTC takes place in aqueous solution under sub-critical conditions, i.e., typically at 160–300 °C and autogenous pressure, producing solid materials similar to peat or lignite (i.e., hydrochar), process water containing a significant fraction of nutrients (N, P, K) and other hydrocarbons, and a small gas fraction (basically CO2, CH4) (Fu et al., Citation2019; Singh Kambo & Dutta, Citation2015). The highly porous structure of hydrochars and biochars holds promise to serve as a habitat for PGPR that supports microbial colonization and growth through space and nutrient provision in their large pore-volumes and surface area (SSA) while reducing predation by nematodes and protozoa (Hale et al., Citation2014; Quilliam et al., Citation2013).
Recent studies on their performance as microbial carriers recommended biochars as suitable to shelter PGPR and associated improved nutrient acquisition and plant growth to prolonged survival in the internal pore system of biochars (Egamberdieva et al., Citation2017; He et al., Citation2014; Postma et al., Citation2013). In addition, a growing body of literature on pore characteristics of biochars is available and positive effects on microorganisms are well-documented (Gul et al., Citation2015; Lehmann et al., Citation2011; Luo et al., Citation2013; Zhou et al., Citation2017).
Hydrochars, however, received less attention and information on pore characteristics, and their interaction with microorganisms is limited. As the standard technique, i.e., N2-adsorption measurement to analyze pore properties (Eibisch et al., Citation2015; Garlapalli et al., Citation2016; Reza et al., Citation2015) is solely proficient in detecting smaller micropores (in the order of nanometers), the existence of macropores (in the order of micrometers) appropriate for bacterial colonization often remains undiscovered. The few investigations that measured pore characteristics of hydrochars by methods that can capture a broader level unveiled plenty of pores in appropriate size (Gascó et al., Citation2018; Hyväluoma et al., Citation2017; Zhang et al., Citation2014). Thus, hydrochars could also provide adequate interior pore space for colonization by PGPR. Additionally, concentrations of soluble carbon (DOC), and molar O/C and H/C ratios in hydrochars exceed those of biochars, indicating a labile C fraction in hydrochars that PGPR can readily access (Bai et al., Citation2013; Bargmann et al., Citation2014; Crombie et al., Citation2013; Fuertes et al., Citation2010). Experimental evidence suggests that the highly positive effects of hydrochars on microbial growth may be even superior to that of biochars (Bamminger et al., Citation2014; Bargmann et al., Citation2014). Owing to the decisive advantage of HTC to carbonize moist feedstocks more energy-efficient than through pyrolysis, abundant wet waste biomass such as animal manure, sewage sludge, food waste, or biogas slurry (Libra et al., Citation2011; Singh Kambo & Dutta, Citation2015) could be reutilized as a substrate for the production of porous carrier materials. Consequently, hydrochars may be an economical and sustainable alternative for the fertilizer industry to maintain soil fertility and recuperate degraded soils.
Recent research reviewed biochars as a carrier for PGPR (Ajeng et al., Citation2020; Egamberdieva et al., Citation2018). However, the novelty of this review is the compilation of available data of pore characteristics, including the SSA, total pore-volume, average pore diameter, and pore size distributions, focussing on hydrochars. While existing literature lacks addressing the mechanisms that affect the pore characteristics of hydrochars and their role in bacterial colonization, this work extends the knowledge about hydrochars as bacterial vectors by exploring the effects of feedstocks, HTC process parameters, and mineral amendments on pore characteristics. We thereby identify opportunities for engineering hydrochars to provide ideal habitat conditions for increased survival of PGPR. We further elaborate on the role of pore characteristics for bacterial colonization and compile results on the microbial performance and effects of inoculated chars on amended crops. We refer to biochars for comparison and where information for hydrochars is scarce.
2. Pore characteristics of hydrochars
As the information on pore characteristics and their modification by feedstocks and process conditions of HTC is a prerequisite for targeted engineering of the pore system to obtain suitable pore size for bacterial colonization (Lehmann et al., Citation2011; Schnee et al., Citation2016), this section provides a compilation of published works on pore characteristics. Section 2.1. gives an overview of the most common techniques applied for pore analysis, section 2.2. and section 2.3. evaluates effects of feedstocks and HTC process parameters on SSA, total pore-volume, and average pore diameter, and section 2.4. showcases how mineral amendments can be utilized for engineering the optimal pore size distribution of hydrochars. Finally, section 2.5 provides a compilation of available data on pore properties of hydrochars as compared to biochars. In line with most studies, we refer to micro-, meso, and macropores as pores having average diameters <2 nm, 2–50 nm, and >50 nm, respectively. Porosity designates the ratio of the void volume and the total volume (in percentage), total pore-volume the void volume per mass (in cm3 g−1), and pore size distribution the porosity in a specific pore range. As justified in section 2.4., activated hydrochars are not included in our compilation. Because the effects of feedstocks and process parameters on pore characteristics of biochars have been reviewed elsewhere (Lehmann et al., Citation2011; Li et al., Citation2017; Tomczyk et al., Citation2020), sections 2.2. and 2.3. concentrate on hydrochars.
2.1. Methods to characterize pores
Several methods can analyze the pore characteristics SSA, pore-volume, pore diameter, and total porosity; each is qualified for analyzing a specific range of pore sizes (). The methods that have been employed to chars include N2-adsorption measurements (working range ∼2–25 nm) (Li et al., Citation2016; Saqib et al., Citation2015; Sevilla & Fuertes, Citation2009), 3D-imaging through X-ray computer microtomography (∼1 μm–1 mm) (Hyväluoma et al., Citation2017; Jeffery et al., Citation2015), scanning electron microscopy (SEM) (∼0.5 nm–1 mm) (Hardie et al., Citation2014; Nizamuddin et al., Citation2015), pycnometry (∼0.2 nm–200 μm) (Abel et al., 2013; Brewer et al., Citation2014), and Hg-porosimetry (∼20 nm–300 μm) (Baltrėnas et al., Citation2015; Cárdenas-Aguiar et al., Citation2019; Méndez et al., Citation2019; Yue et al., Citation2017). Most hydrochar-related studies determined the pore structure through isothermal N2-adsorption measurements due to its low cost and practicability. This method, however, cannot detect larger pore sizes at the micrometer scale, which are relevant for microbial colonization (Antal & Grønli, Citation2003; Lehmann et al., Citation2011). Small-angle X-ray scattering combined with 3D imaging is a non-destructive technique based on X-ray beams that can determine larger macropores (Bird et al., Citation2008; Hyväluoma et al., Citation2017; Jones et al., Citation2015). However, hydrochars were so far not analyzed by 3D imaging. Scanning electron microscopy (SEM) to characterize hydrochars gives a rough overview of the pore distribution and investigates the influence of the process setting on the hydrochar’s microscopic structure (Hardie et al., Citation2014; Martínez et al., Citation2006). Challenges remain in applying SEM and 3D imaging, including selecting representative samples and viewing orientations, defining edges between solid and pore, and developing image analysis protocols to quantify porosity (Bird et al., Citation2008). Pycnometry determines the pore-volume by analyzing the density of the sample but does not provide data about the pore size distribution (Brewer et al., Citation2014). Mercury (Hg) porosimetry captures a broad range of pore sizes and provides detailed information on total pore-volume, SSA, average pore diameter, and porosity from a single measurement. Pore measurements through Hg-porosimetry are based on the fact that Hg behaves like a non-wetting liquid towards most solids. Owing to this property, Hg only penetrates the open pores in response to increasing pressure. By measuring the amount of Hg that has penetrated the samplés pores and the equilibrium pressure at which the intrusion occurs, the experimental data are obtained, from which the pore-volume distribution can be calculated as a function of the pore radius (Giesche, Citation2006). While there are limitations concerning the safety and handling of Hg and the destructive nature of the method, the method has proven helpful in determining pore characteristics of chars.
Figure 1. Pore size ranges detected from different methods. The solid red line indicates the method that can measure pore characteristics (SSA, pore-volume, average pore diameter) and the pore size distribution (porosity in percentage in a certain pore size range), dotted black lines indicate methods that give cumulative pore-volumes, and dashed black lines indicate methods that measure pore characteristics without giving information on the pore size distribution. The green area shows the favorable habitat size for bacteria. The graph is modified from Brewer et al. (Citation2014).
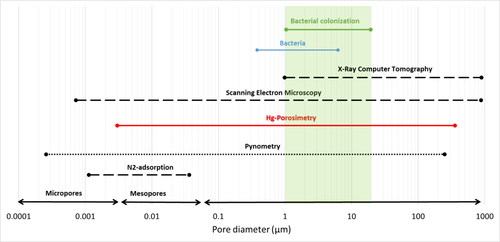
Because no single technique can capture the entire extent of pore sizes, it is challenging to conduct a comparative literature analysis of pore characteristics. Nevertheless, based on the compilations of the reviewed literature data, we attempt to derive some general relationships in the following sections.
2.2. Influence of feedstock
The feedstock is essential for the abundance and composition of macropores (Brewer et al., Citation2014; Hyväluoma et al., Citation2018). When feedstocks undergo thermal conversion, the residual C structure partly preserves the porosity during the release of volatile organic matter (Antal & Grønli, Citation2003). In plant-derived feedstocks, the macroporosity generally arises from the remaining cellular structure (Wildman & Derbyshire, Citation1991). The initial thermo-chemical conversion step is drying the surface and pore-associated water; temperatures >120 °C start releasing the chemically bonded moisture. In lignocellulosic biomass, the cellulose starts to decompose at temperatures between 220 °C and 350 °C, the hemicellulose at 200 °C to 260 °C, and the lignin between 280 °C and 500 °C. Hence, the physical structure of chars relies on the share of these components (Lehmann, Citation2015).
Laine et al. (Citation1991) studied the relationship between SSA and the share of micro- and macropores. They found micropores having a SSA of 750–1360 m2 g−1 and a pore-volume of 0.2–0.5 cm3 g−1, whereas macropores exhibit a SSA of about 50–150 m2 g−1 while creating a larger pore-volume of 0.6–1 cm3 g−1. Consequently, a large SSA is related to a large share of micropores, whereas a sizeable total pore volume connects with a well-developed macropore system.
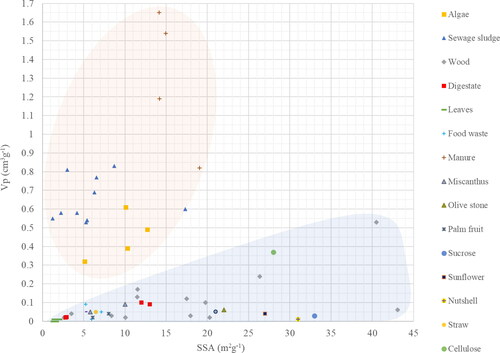
Our compilation of literature data () shows that a large SSA but small total pore-volume characterizes hydrochars from lignocellulosic feedstocks (bamboo, pinewood, wood chips, miscanthus), indicating a low share of macropores. Conversely, hydrochars produced from materials meager in lignocelluloses such as manure, sewage sludge, and algae display high total pore-volumes but small to moderate SSA, indicating a large number of macropores. Moreover, the relatively large average pore diameter of hydrochars derived from sewage sludge (0.14–1.8 µm) and manure (1.7–4.6 µm) explains the existing macroporosity of hydrochars from non-lignocellulosic feedstocks (Table 1S in the Supplementary Information [SI]). However, Hg porosimetry analyzed hydrochars from manure and sewage sludge, which can also detect wider pores. Contrarily, N2-adsorption cannot capture wider pores and analyzed chars derived from lignocellulosic biomass in our compilation ().
Figure 2. Total pore-volume of hydrochars from different feedstocks plotted against the SSA. The red area covers data obtained from Hg-porosimetry and the blue area indicates data generated from N2-adsorption. The figure is based on data shown in Table 1S in the SI.
The relationship between the decomposition of macromolecular compounds and the formation of pores is poorly understood. However, porosity positively correlates to H/C and O/C ratios; higher H/C and O/C ratios indicate higher porosity (Zhu et al., Citation2015). As lignin in lignocellulosic biomass is the most stable compound during HTC (Borrero-López et al., Citation2018; Wang et al., Citation2018), the residual C of the lignin after HTC leads to lower O/C and H/C ratios (see also section 3.1.3), creating a less developed porous structure. Conversely, hydrochars from non-lignocellulosic biomass such as sewage sludge are generally characterized by less stable C compounds (Liu et al., Citation2021; Wilk et al., Citation2019), resulting in larger O/C and H/C ratios after HTC, enhanced transfer of C to the liquid phase, and increased porosity.
We conclude that hydrochars from municipal and husbandry waste show a well-developed macropore structure compared to lignocellulosic biomass from e.g., forest residues (). However, comparative studies using the same methodology to determine the pore structure including the macropores are required.
2.3. Influence of HTC process parameters
Apart from the feedstock, the process parameters temperature, residence time, and pH considerably affect pore characteristics (Lehmann, Citation2015; Parshetti et al., Citation2013; Reza et al., Citation2015). We disregard the influence of pressure since, depending on the applied temperature, it is autogenous for HTC. Furthermore, we dismiss catalysts because information of pore characteristics based on the application of catalysts is hardly available.
2.3.1. Reaction temperature
The most fundamental parameter that controls the physicochemical properties of chars is the reaction temperature (Biederman & Harpole, Citation2013; Funke et al., Citation2013; Lehmann, Citation2015). It primarily regulates the decomposition of cellulose, hemicellulose, and lignin during thermochemical conversion. Therefore, rising reaction temperature removes organic compounds, increasing the SSA and porosity (Singh Kambo & Dutta, Citation2015).
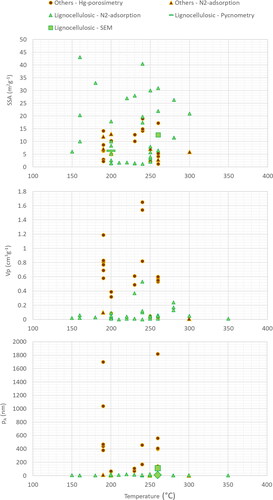
compiles literature data by relating the pore characteristics of hydrochars to the process temperature; we categorize the feedstocks into two groups, i.e., rich and poor in lignocellulose contents. A geometric relation can be expected between SSA and porosity, which, however, can be significantly modified by the specific pore characteristics and heterogeneity of a material. SSA and total pore-volume vary remarkably with HTC process conditions and feedstocks (). However, depending on the measurement technique, we also observe a tendency to increase SSA, total pore-volume, and average pore diameter as the temperature increases up to 240–260 °C, followed by a reversed trend at higher temperatures. Subsequently, SSA, total pore volume, and average diameter decline due to the de-volatilization/condensation of volatile organic compounds resulting in pore blockage (Kloss et al., Citation2012; Singh Kambo & Dutta, Citation2015).
Figure 3. SSA, total pore-volume (Vp), and average pore diameter pA of hydrochars in relation to HTC temperature (in degree celcius). The figure is based on data shown in Table 1S in the SI
2.3.2. Residence time
Gao et al. (Citation2013) studied the effect of residence time on structural properties of hydrochars obtained from water hyacinth. Using SEM, they found increasing porosity along the prolonged HTC process’s residence time from 30 minutes to 24 hours. Extended residence time transforms cellulose and hemicellulose, leading to higher porosity in hydrochars from lignocellulosic biomass. Complete chemical reactions during biomass degradation require sufficient time. Consequently, a short residence time may leave behind an unfinished decomposition product. However, from a chemical engineering perspective, reducing the residence time is desired concerning productivity.
Our data compilation (Figure 1S in the SI) shows that the average pore diameter tends to increase with residence time, indicating the formation of macropores, while we observe no noticeable effect on total pore volume and SSA. Whereas effects of residence time may have been obscured to some extent by different methodologies for assessing the pore and surface characteristics, and possibly also by the variation of feedstock, Lehmann (Citation2015) considered its influence as minor.
2.3.3. pH
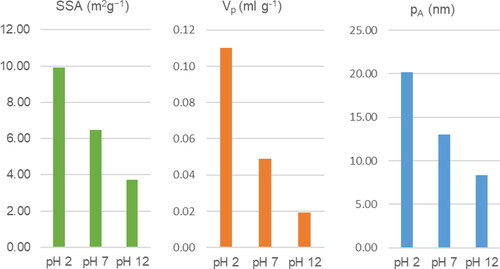
Proton activity (pH) can have relevant impacts on the pore characteristics of hydrochars. Reza et al. (Citation2015) showed that the average pore diameter, pore-volume, and SSA increased substantially in acidic feedwater (). The average pore diameter of hydrochars produced from wheat straw was around 2.4 times larger in acidic feedwater (pH 2) than hydrochars obtained from the same feedstocks under alkaline conditions (pH 12). The SSA generated in acidic feedwater was more than 2.7 times larger. The authors link the effects of pH on the SSA to decomposition reactions of hemicelluloses in the feedstock during HTC. Although the temperature primarily degrades the biomass components, an acidic milieu induces fracturing of hydrogen bonds, leading to relatively greater degradation of hemicellulose, while cellulose and lignin are less affected. Hence, the increase of pore volume, average pore diameter, and the SSA are closely associated with enhanced mass loss of hemicellulose at lower pH. Whereas Fu et al. (Citation2019) found insignificant effects of pH influencing surface area, the work of Wang et al. (Citation2018) supports the effects of low pH on increasing the SSA and porosity.
Figure 4. Effects of feedstock pH on SSA, total pore-volume (Vp), and average pore diameter (pA). Data is obtained from Reza et al. (Citation2015)
Note that during HTC, the pH is typically decreased due to the release of furfural (from lignocellulosic biomass) and organic acids, whereas a lower pH at the beginning forces conversion rates. Furfural arises as an intermediate product from dehydration of hexoses and pentoses (mainly from hemicellulose) that further undergoes hydrolysis, resulting in the breakdown into acids, aldehydes, and phenols, and finally inorganic elements (Gai et al., Citation2016).
2.4. Engineering pore characteristics of hydrochars for improved shelter provision
Physical and chemical activation can increase the SSA of chars up to 1000 m2 g−1, and to generate high porosity due to the alteration and opening of the internal porous structure (Başakçılardan Kabakcı & Baran, Citation2019; Gratuito et al., Citation2008; Hao et al., Citation2013; Jain et al., Citation2016).
Physical activation exposes hydrochars or biochars to elevated temperatures (>900 °C) and the presence of CO2, steam, air, or a combination of these, leading to mass loss (gasification of char); note that pyrolysis takes place in the absence of O2 and is not comparable to physical activation. Consequently, SSA and pore-volume expand (Singh Kambo & Dutta, Citation2015; Zhang et al., Citation2004), but pore size increases mainly within the micropore domain (Alaya et al., Citation2000). Therefore, physical activation is less suited for enhancing the pore space appropriate for bacterial colonization. Moreover, the physical activation of hydrochars is conducted after the production of hydrochars in a separate reactor. Thus, besides the significant energy input, also additional equipment and process steps are necessary.
During chemical activation, potassium hydroxide (KOH) acts as an activating agent that separates the lamellae of crystallites (microscopic crystal) through redox reactions involving the K and the C compounds at char surfaces (Oginni et al., Citation2019). Subsequently, water removes K, resulting in the formation of interlayer space with increased porosity and SSA (Jain et al., Citation2016; Yu et al., Citation2020). If the quantity of KOH increases, the total pore-volume expands, and the pore size distribution shifts from micropores to meso- and macropores. If hydrochars undergo chemical activation at high temperatures (∼280 °C), the pore size distribution becomes considerably narrower than hydrochars produced at temperatures between 180 and 240 °C (Titirici et al., Citation2012). However, increasing porosity and the SSA through chemical activation requires surcharging zinc salts or phosphoric acid, creating additional costs and disposal problems (Zhang et al., Citation2004). Additionally, the production of KOH involves significant amounts of chemicals and energy. Hence, additional equipment and wastewater treatment are necessary (Montes & Hill, Citation2018). Hydrogen peroxide (H2O2) or nitric acid (HNO3) can also perform chemical activation (Ajeng et al., Citation2020). However, H2O2 is harmful to most bacteria (Nathan & Cunningham-Bussel, Citation2013), and HNO3 produces oxygen-containing functional groups that can block the pore entrances from hydrophobic compounds and reduce the share of sites for microbial adsorption due to hydration (Ajeng et al., Citation2020).
Because of these technical, economic, and sustainability drawbacks, activation technologies will not be considered further in this review. However, they may offer exciting opportunities in other applications, such utilizing hydrochars as adsorbents for gas cleaning (Fang et al., Citation2018).
As an alternative, supplementing amendments from secondary (recycled) resources can engineer pore characteristics of hydrochars. This offers benefits regarding experimental equipment and simplicity of the processes since the additives can be added already prior to HTC. shows the pore size distribution of hydrochars from swine manure with and without selected mineral amendments and seagrass added before carbonization, based on data obtained from Pienisch (Citation2018). In unamended hydrochars, we observe a high abundance of macropores in the range between 1 and 100 µm, while the proportion of pores with diameters <0.1 µm is small (<5%). All additives—calcium sulfate, calcium carbonate, rock phosphate, wood ash, and seagrass—cause a shift towards the lower micrometer scale (1–20 µm), probably because of blockage of the largest macropores (>50 µm). Concomitantly, the share of micro- and mesopores <0.1 µm decreases.
Figure 5. Pore size distribution of hydrochars from swine manure with 3% amendments (with respect to total carbonization material) of calcium carbonate (CaCO3), seagrass, calcium sulfate (CaSO4), rock phosphate and wood ash compared to no amendment. They grey area indicates the pore size range suitable for bacterial colonization. The vertical axis represents the fraction of the respective pore size range indicated on the horizontal axis. The figure is based on Pienisch (Citation2018) with data shown in Table 3S in the SI.
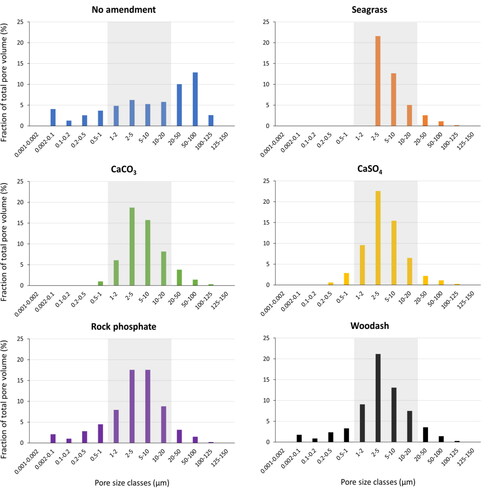
The amendments increased the fraction of pores in the range of 1–20 from 38% (unamended) to 79%–89 %. Moreover, additives increase total porosity (+1%–24%, except for calcium carbonate), total pore-volume (+13%–45%), and average pore diameter (+8%–1853%) but decrease the SSA (+16%–93%). The numbers represent changes caused by amendments relative to the unamended controls. The latter finding is related to the loss of meso- and micropores. HTC cannot thermochemically convert the inert additives; hence, they are deemed to clog the macropores in this order of magnitude depending on their grain size. These results show that additives can engineer hydrochar’s pore characteristics to improve their suitability as a microbial habitat.
2.5. Comparison of pore characteristics between hydrochars and biochars
The number of scientific publications investigating pore characteristics of hydrochars is limited, calling for further research. Because of scarce data on hydrochars and the more advanced understanding of the role of pore characteristics of biochars in microbial inoculation, we compare pore characteristics of hydrochars to biochars in this subsection. Apart from differential physicochemical properties, this comparison can offer valuable insights into the suitability of hydrochars as we can draw from a larger body of literature and related empirical evidence of successful implementation of the carrier/vector approach.
Literature provides partly contrasting results on pore-related differences among both char types. Most studies advocate that biochars incorporate higher porosity and SSA than hydrochars (Sevilla et al., Citation2011; Singh Kambo & Dutta, Citation2015; Titirici et al., Citation2012), while some others show larger total pore-volume of hydrochars (Gascó et al., Citation2018). In general, biochars incorporate a large SSA that generally increases with pyrolysis temperature (Li et al., Citation2017). This is because the exposure to high temperatures induces a regular and highly ordered spacing between the planes of biochar C structure. As the molecules are more ordered, the interplanar distances decline, resulting in a larger SSA per unit pore-volume (Lehmann, Citation2015). As stated in section 2.2, a large SSA and small total pore-volumes indicate abundant micropores, and vice versa, a small SSA and large total pore-volume the presence of macropores. Therefore, as pyrolysis temperature increases, the SSA and the share of micropores increase accordingly (Antal & Grønli, Citation2003). However, under very high reaction temperatures during pyrolysis (750–900 °C), the formation of tar can lead to pore blockage, hence, decreasing the SSA while pore walls of adjacent pores are eliminated, which can also cause increased macroporosity (Brown et al., Citation2006; Chun et al., Citation2004; Jin et al., Citation2016). Using 3D imaging, Hyväluoma et al. (Citation2017) analyzed the pore size distribution of biochars and hydrochars from lignocellulosic biomass generated at different temperatures. Pore sizes between 10 and 20 µm were predominant, and both char types displayed a unimodal pore size distribution with porosity peaks between 5 and 20 μm. The difference between biochars produced at varying temperatures was negligible, suggesting that increased pyrolysis temperature (an increase from 375 to 475 °C) did not affect the number of macropores, but perhaps the number of micropores that 3D imaging could not identify. Differences in the porosity of hydrochars and biochars are thus likely related to microporosity rather than macroporosity.
Our compilation of literature data comparing hydrochars and biochars (Figure 2S in the SI) shows that some biochars indeed have a substantially larger SSA; however, the total pore-volume covers a similar range. At any given SSA, both types of char show a large variability of the total pore-volume, indicating related variation of the share of macropores. As can be depicted from the graph, the total pore-volume of hydrochars, depending on the technique applied to assess the pore structure, resembles that of biochars.
Several studies have reported a higher porosity in biochars characterized by N2- adsorption, which cannot detect larger macropores. However, the measured quantity of meso- and micropores is not relevant for microbial colinization and gives insufficient information on the pore characteristics of chars. Especially biochars analyzed by Hg porosimetry, 3D imaging, and SEM reveal a significant portion of macropores in the micrometer range up to 24 μm in diameter (Jeffery et al., Citation2015), and a high total porosity that can reach nearly 85% (Gray et al., Citation2014) (see Table S2 in the SI). Lu and Zong (Citation2018) found 84% of the total pore-volume allocated to pore diameters between 0.5 and 50 μm, with peaks at 1.5–4 and 4.8–14 μm.
In summary, biochars incorporate a larger SSA, whereas the total pore volume range is similar to that of hydrochars. Thus, both char types may display a small SSA combined with a large pore-volume, associated with a large share of macropores appropriate as a microbial habitat. Based on our compilation of literature data, we find that hydrochars contain suitable pore characteristics for PGPR colonization and thus fulfill a vital prerequisite for their use as microbial vectors. This conclusion is reasonable since biochars have been used successfully as a carrier medium, and empirical evidence associated the colonization of bacteria to the appropriate pore sizes. Yet, the mechanisms of colonization and effects of hydrochars on bacteria are crucial likewise on elaborating the efficacy of hydrochars as PGPR vectors, which we cover in the following section.
3. Hydrochars as carriers and vectors for PGPR
One of the significant drawbacks of the practical application of PGPR in agriculture and their potential to replace conventional fertilizer is the reduced survival rate and survival time after soil inoculation (Acea et al., Citation1988). Further, if the liquid inoculum is directly applied to the soil, bacteria adhere to soil particles, impeding their vertical movement towards the roots (Bashan et al., Citation2014; Lehmann et al., Citation2011). Hydrochars and biochars can be used as carriers and vectors to overcome these limitations, as inoculated PGPR in chars can benefit from their physicochemical properties to increase their survival and develop their full effects (Egamberdieva et al., Citation2016, Citation2017, Citation2018). The subsequent sections summarize information on mechanisms involved in bacterial colonization and discuss the role of feedstock and process conditions in engineering hydrochars to provide favorable conditions as a habitat to PGPR (section 3.1.). We further compile observed effects of hydrochars and biochars on the performance of microorganisms with a focus on PGPR. Finally, we collect experimental evidence of chars to function as microbial habitat that improves the survival of PGPR previous to soil application (section 3.2.). Due to the limited availability of data on hydrochars, this section includes, where appropriate, also results from biochar experiments.
3.1. Mechanisms of bacterial char colonization
The stimulation of microbial growth and activity in the presence of hydrochars and biochars encompasses several mechanisms, including the functioning as a habitat, improved nutrient preservation, source of labile organic C, and the supply of electron donors (Bargmann et al., Citation2014; He et al., Citation2014; Lehmann et al., Citation2011). In the following, we briefly review the main mechanisms of bacterial colonization in chars and elaborate on related targets for hydrochar engineering to increase the habitable pore space.
3.1.1. Pore space and optimal pore size for bacterial colonization
In any substrate, including soils, the presence of macropores is critical, not only for hydrological processes, soil aeration, and root growth but also for rhizobacterial colonization. The pore sizes define the ability of PGPR to grow and reproduce and subsequently solubilize nutrients and support plant growth (Albareda et al., Citation2008; Bashan et al., Citation2014; Cattelan et al., Citation1999). Major mechanisms of pore characteristics in bacterial colonization include (1) providing adequate pores that shelter them from predators, (2) creating a favorable surface charge and adsorption of microbial cells, and (3) supplying nutrients and DOC from the biochar, serving as available nutrient and C reservoir (Gul et al., Citation2015). The presence of water as a biological solvent in the pores is a further important aspect that can improve the growth of microbes. Macropores up to a magnitude of 10 µm are capable of retaining a considerable amount of water (Abel et al., 2013), and, as shown in the previous sections, hydrochars contain a large share of pores of this size. Hydrochars even perform superior to biochars in water retention capacity (Gascó et al., Citation2018).
High PGPR survival and activity rates require a specific pore size accessible to bacteria while excluding their larger predators such as mites, collembolans, and most nematodes and protozoans (Lehmann et al., Citation2011). Pores smaller than the size of bacteria are not suited to establish a bacterial community, whereas excessively large pores do not provide adequate shelter from predators (Lehmann et al., Citation2011; Pietikäinen et al., Citation2000; Quilliam et al., Citation2013; Schnee et al., Citation2016; Vanek & Thies, Citation2016). Without a substantial share of compatible pore sizes, bacterial colonization of char is limited, even if other influencing factors provide favorable conditions (Pietikäinen et al., Citation2000). Matching the pore size is a crucial precondition for PGPR to be able to exploit their potential.
Although studies concerning the most favorable pore size are not univocal, they generally show that the pore diameter should moderately exceed that of the organism to be hosted. Because most bacteria, actinomycetes, fungi, and lichens have dimensions between 0.3 and 5 μm, pore sizes below 0.3 μm are generally inadequate for microbial colonization (Lehmann et al., Citation2011; Quilliam et al., Citation2013; Vanek & Thies, Citation2016). Evidence shows that bacteria with a size between 0.3 and 3 μm find shelter in the pores of biochars, thereby protecting themselves from microarthropods (Gul et al., Citation2015). Other studies suggested that pores sized between 3 and 6 μm provide optimal protection as they exclude protozoa (Foster, Citation1988; Hattori, Citation1988), resulting in prolonged survival of bacterial inoculants (Heijnen et al., Citation1991). There is an ecological trade-off of larger pores being less protective but, conversely, stimulate bacterial growth and activity because of improved access to nutrients and the exchange of metabolites. However, the presence of pores sized between 6 and 30 μm can result in a decrease in viable cell concentrations of bacteria compared to smaller pores (3–6 μm) (Wright et al., Citation1995). Experimental evidence indicates that pore sizes exceeding the largest bacterial cell dimension by 2–4 (Messing & Oppermann, Citation1979) or 2–5 times (Samonin & Elikova, Citation2004) support maximum cell abundance as they ensure optimal adhesion in or at the pores. Since the size of bacteria ranges between 0.5–1 × 1–5 μm (Cattelan et al., Citation1999; Rafique et al., Citation2017), we expect the pore size for optimal bacterial colonization in the range between 1 and 20 μm. Note that some studies alternatively suggest pore sizes between 26 and 46 μm in diameter to serve as optimal habitat for bacteria and to increase shelf life (Hale et al., Citation2015).
3.1.2. Other mechanisms
Apart from pore space, hydrochars can provide essential nutrients (e.g., P), electrons, and available carbon.
Available nutrients are fundamental to support bacterial growth in soils (Gul et al., Citation2015). Therefore, depending on the original nutrient content of the raw biomass, concentrations of nutrients that are desorbed or solubilized from the char serve as an essential feed source supporting microbial growth and activity. In addition, char characteristics such as surface charge and porosity can promote transferring nutrients from the bulk soil into the pores, thereby providing favorable conditions for bacterial colonization (Noraini, Citation2015).
Moreover, electron donors are essential for the growth of bacteria (Lu et al., Citation2021). Owing to their large SSA and its aromaticity due to carbonization, chars are redox-active, serving both as an electron donor and acceptor for bacteria during the redox reaction process by directly mediating electron transfer processes that tie microbial cells (Lu et al., Citation2021). Thus, chars can significantly promote microbial electron transport activity and support bacterial growth (Kappler et al., Citation2014).
The presence of labile C is another crucial factor for PGPR to sustain in the pore system. Bacteria require a readily accessible C reservoir as an energy source in their microenvironment to build up biomass (Zimmerman et al., Citation2011). The amount of available labile C determines bacterial feed, growth, and reproduction required to develop a sufficiently large population that endures in the rhizosphere of crops (Ameloot et al., Citation2014).
3.1.3. Related targets for char engineering
Process conditions and feedstock influence the physicochemical features that affect the ability of PGPR to colonize the pore system. As stated in the previous sections, a suitable pore size distribution and the availability of labile C are crucial aspects of bacterial colonization.
Hydrochars are, compared to raw biomass, characterized by a lower H/C and O/C ratio as a result of carbonization. This is because HTC leads to a reduction of hydroxyl groups by dehydration and of carbonyl groups by decarboxylation, the cleavage of ester and ether bonds by hydrolysis, and increased aromaticity by condensation reactions. Consequently, as the polarity decreases, the ratios H/C and O/C decrease accordingly (Fang et al., Citation2018; Funke et al., Citation2013; Libra et al., Citation2011). However, hydrochars have a relatively lower C content than biochars due to lower dehydration and carbonization, and therefore maintain higher amounts of H and O, especially hydrochars derived from vegetal feedstocks (Libra et al., Citation2011). The greater molar O/C and H/C ratios of hydrochars imply lower stability of aromatic formations towards chemical and microbial degradation. As accelerated microbial degradation relates to the less recalcitrant nature of hydrochars compared to biochars (because of their relatively higher O/C and H/C ratios and higher DOC content implying the presence of labile C), the growth of bacterial biomass in hydrochars is therefore typically more pronounced (Bai et al., Citation2013; Bargmann et al., Citation2014; Cárdenas-Aguiar et al., Citation2019; Crombie et al., Citation2013; Fuertes et al., Citation2010).
Because rising HTC temperature increases the recalcitrance of hydrochars (Gajić et al., Citation2012) and narrows the pore size distribution (see section 2.3.), very high process temperatures could worsen the conditions for PGPR colonization (>260 °C). Furthermore, since, owing to its phenolic structure, lignin is relatively stable towards bacterial degradation (Killham & Prosser, Citation2015) and HTC (Borrero-López et al., Citation2018; Wang et al., Citation2018), it is least converted compared to other macromolecular compounds. Moreover, the reactive parts of the lignin molecules form stable aromatic structures after HTC (Wang et al., Citation2018). Consequently, PGPR can less efficiently metabolize hydrochars derived from lignocellulosic feedstocks. Moreover, lignocellulosic biomass is primarily degraded by fungi (Datta et al., Citation2017), indicating that PGPR colonization of hydrochars rich in lignin-derived structures could be less effective. Thus, we conclude that hydrochars from feedstocks high in lignin content generated at high temperatures are unfavorable for bacterial colonization and their use as vectors for PGPR.
Another target of hydrochar engineering relates to the optimization of nutrient supply. This can be achieved by selecting appropriate feedstocks with high contents of essential and beneficial nutrients, in particular, P. Especially waste materials serving as a secondary source for char production vary considerably in their composition and availability regarding mineral nutrients such as P, B, and Si (Duboc et al., Citation2017; Duboc et al., Citation2019; Duboc et al., Citation2019). Selection can be based on analytical data of the total nutrient content in feedstocks and chars and assays of nutrient availability using conventional extraction or infinite-sink approaches that can assess the rate of nutrient release over time (Duboc et al., Citation2017).
In summary, high temperatures during HTC result in increased aromaticity that decreases the labile C pool for PGPR and narrows the pore size distribution of hydrochars with a negative impact on potential bacterial colonization. Because feedstocks high in lignin content are least convertible during HTC compared to other macromolecular compounds and lignin is hardly degradable by microorganisms (Killham & Prosser, Citation2015; Wang et al., Citation2018), hydrochars from lignocellulosic biomass are less suited as a bacterial habitat. Additionally, as stated in section 2.2., high amounts of lignin lead to lower O/C and H/C ratios, associated with lower porosity and less pore space appropriate for bacterial colonization. Further, HTC from secondary waste materials can provide large amounts of nutrients (Duboc et al., Citation2017; Duboc et al., Citation2019; Duboc et al., Citation2019) required for bacterial growth (Gul et al., Citation2015). In order to produce hydrochars as an effective carrier for PGPR, we propose hydrochars produced from, e.g., manure, food waste, or sewage sludge generated at lower temperatures (<260 °C).
The subsequent sections summarize empirical evidence on bacterial colonization and activity as affected by pore space and other physical and chemical properties of hydrochars and biochars concerning their suitability as PGPR vectors.
3.2. Effects of Chars on microorganisms and empirical evidence for successful application of chars as PGPB vectors
To suggest hydrochars as a carrier medium for PGPR, empirical knowledge of their effects on bacteria is indispensable. In the following, we compile studies that analyzed the effects of hydrochars and biochars on the performance of microorganisms observed in inoculation experiments. We include biochars in our compilation as we can draw from a larger body of literature. A separate view on different soil microorganisms (fungi, bacteria, protozoa) is given where possible with an emphasis on bacteria; however, in the available literature, effects are often reported as an increase in overall soil microbial biomass and activity rather than for the performance of the inoculated PGBR.
3.2.1. Effects of hydrochars on soil bacteria and comparison to biochars
compiles information on hydrochars and biochars and their influences on general microbial performance. Note that the chars had been applied without inoculation of specific bacterial strains, i.e., data show the general effect on colonization and soil microorganisms. The effects range from no response (Lanza et al., Citation2016; Rex et al., Citation2015) to strong stimulation (Bamminger et al., Citation2014; Bargmann et al., Citation2014). Some authors observed fungal growth after hydrochar addition due to the acidic milieu in some hydrochars (Lanza et al., Citation2016), while other studies show increased bacterial abundance (Rex et al., Citation2015) or a variation of the relative composition of bacteria and archaea (Andert & Mumme, 2015). Particularly the presence of furfural and organic acids sorbed on hydrochars supports fungal colonization (see section 2.3 on pH influencing pore characteristics). According to the findings of most authors, the labile organic C in hydrochars serves as a readily available energy source for microbial growth, and C mineralization induced by microbial biomass in hydrochars often exceeds that of biochars (e.g., by a factor of 10, see Bargmann et al., Citation2014). Other than for biochars, the pore colonization in hydrochars lacks visual confirmation through SEM. Hence, future research should address the role of pores in microbial colonization of hydrochars by providing a visual analysis of hydrochar colonization by microorganisms. In the following, we, therefore, provide related information available for biochars.
Table 1. Biochar and hydrochar effects on microbial biomass.
Various studies advocate that biochars can serve as a favorable microenvironment (Ezawa et al., Citation2002; Pietikäinen et al., Citation2000), supporting microbial colonization and growth in biochar-amended soils (Rutigliano et al., Citation2014); the porous structure was critical for enhanced bacterial colonization of biochars (Liang et al., Citation2010; Luo et al., Citation2013; ). Biochars generated at higher temperatures are unfavorable for bacterial colonization owing to their larger share of micropores, higher recalcitrance, and the associated declined labile C pool, whereas biochars produced at lower temperatures typically facilitate microbial growth (Gul et al., Citation2015). Empirical evidence demonstrated the establishment of a bacterial population in biochars produced at 350 °C, whereas biochars produced at 700 °C were less suitable. This was due to the formation of a narrower (shift from larger pores to smaller pores) pore size distribution and the reduction of labile C at elevated temperatures. Microbial biomass increased by 20% through colonization of pores and char surfaces, visually confirmed using SEM (Luo et al., Citation2013). Liang et al. (Citation2010) observed a substantial increase of microbial biomass (+125%) in biochar-amended soils compared to the unamended control. They did not link an increase of the microbial biomass to the accessible organic C in biochar-amended soils but emphasized the protective role of biochar in providing shelter and limited access to predators. Other authors visually confirmed that phosphate-solubilizing bacteria (PSB) colonization and improved P solubilization correlated to bacterial cells growth (He et al., Citation2014).
However, other studies indicate no (Quilliam et al., Citation2013) or short-lived (Rutigliano et al., Citation2014) response on microbial biomass in field trials, or less hyphal colonization of fungi in biochars incubated in soils compared to a soilless medium (Jaafar et al., Citation2014). Furthermore, studies show a tendency for reduced microbial biomass in lignocellulosic biochar-amended soils (Gul et al., Citation2015). These partially ambivalent results highlight the requirements of further long-term studies and field trials. Nevertheless, the bulk of literature compiled in emphasizes biochars as having generally positive effects on microbial performance (Domene et al., Citation2015; Ducey et al., Citation2013; Halmi & Simarani, Citation2021; Khadem & Raiesi, Citation2017; Li et al., Citation2016; Lu et al., Citation2015; Teutscherova et al., Citation2018; Zhou et al., Citation2017). Moreover, a meta-analysis that assessed microbial responses to biochar in agricultural soils found a 25% increase in microbial biomass after biochar addition (Zhou et al., Citation2017).
In summary, hydrochars are promising in terms of microbial colonization owing to their lower recalcitrance compared to biochars. Still, the role of pore characteristics remains to be explored in more detail and lacks visual analysis. Nevertheless, empirical evidence for biochars shows that the pore size distribution plays a fundamental role in bacterial colonization, and it is reasonable that this applies to hydrochars likewise. For biochars, studies proved that feedstocks derived from manure or crop residues rather than lignocellulosic materials favor microbial colonization (Gul et al., Citation2015), which is more pronounced in biochars generated at lower temperatures owing to a higher available labile C pool and favorable pore size distribution (Bamminger et al., Citation2014; Bargmann et al., Citation2014; Gul et al., Citation2015; Luo et al., Citation2013). This is consistent with our previous elucidations on the effects of process parameters and feedstocks on hydrochars, where we concluded that lignocellulosic feedstocks and high HTC temperature could be unfavorable for bacterial colonization (see section 3.1.).
Because of significant differences between studies in the laboratory and the field, results are not entirely univocal, emphasizing the need for long-term field trials.
3.2.2. Evidence for successful application of chars as PGPR vectors
The application of biochars as a low-cost microbial carrier has been a successful research subject for almost four decades, originating from pioneering work with an emphasis on mycorrhizal (Ogawa & Okimori, Citation2010; Saito, Citation1990), but also on bacterial inoculation of biochars as vectors (Beck, Citation1991). However, experiments with bacterial inoculants in biochars are still scarce compared to studies with fungal inoculants (Lehmann et al., Citation2011), and studies considering hydrochars as PGPR vectors are hardly available. We, therefore, include evidence from biochar experiments in this section.
Table 4S in the SI compiles evidence for increased microbial survival in biochars and hydrochars as PGPR carriers. The methodical approach of each study is different; some compared the inoculated chars to peat as a microbial carrier, other studies without using a vector. Some work evaluated effects on plant growth and P solubilization and confirmed colonization through SEM. None of the studies witnessed detrimental impacts on bacteria or plant growth. Most studies provide evidence for prolonged bacterial survival compared to the direct application due to the larger share of macropores in biochars produced at lower temperatures (Vanek & Thies, Citation2016). Several authors confirmed bacterial colonization in biochar through SEM, and inoculation with PSB induced higher P solubilisation (He et al., Citation2014; Postma et al., Citation2010). Other work compared biochar to peat as standard reference material and found similar (Hale et al., Citation2015; Sun et al., Citation2015; Vanek & Thies, Citation2016) or even superior performance (Głodowska et al., Citation2016; Sun et al., Citation2016). Several studies show that biochars as a carrier of PSB increased P solubilisation (He et al., Citation2014), fostered disease control (Postma et al., Citation2013), and elevated P uptake in plants incremented plant biomass (Rafique et al., Citation2017). However, Foster et al. (Citation2020) found no effects of biochar as a bacterial vector on nutrient uptake in plants. In summary, experimental evidence finds biochars as an effective carrier for bacteria that can facilitate plant growth and P mobilization through prolonged survival of PGPR. If log10 CFU g−1 values are compared to the initial value, PGPR inoculated in biochars endured without showing significant mortality from 4–11 (initial) to 4.7–10 (biochar inoculation) up to 240 days and can even outperform peat as non-renewable material (3.3–7.6).
At present, trials with hydrochars serving as PGPR vectors are very limited. However, a recent study on Bradyrhizobium inoculated to hydrochars derived from maize silage and biochars from wood and maize feedstocks reported that inoculated hydrochars attained larger CFU counts than biochars after two and six weeks. Furthermore, the PGPR inoculants in the hydrochar also performed better in terms of survival rate, shoot growth, root weight, and content of N and P, especially in drought stress conditions (Egamberdieva et al., Citation2017). Moreover, the same authors found in another study that hydrochars promote plant growth as a result of increased bacterial populations (Egamberdieva et al., Citation2016).
4. Conclusion
Overall, the bulk of literature provides sound evidence for the suitability of the porous structure of hydrochars to serve as a carrier medium for plant-growth-promoting rhizobacteria (PGPR). Their typically large quantity of macropores in the order of several micrometers matches the ideal size (1–20 μm) of colonization through PGPR. Although studies demonstrating the successful use as a microbial carrier are mainly available for biochars, there is also experimental evidence that hydrochars can even outperform biochars. We, therefore, propose HTC as a cost-efficient alternative to pyrolysis to convert especially wet waste materials in carriers/vectors for beneficial microorganisms with numerous potential applications in the fertilizer industry and the agricultural sector.
provides a summary of the engineering options to provide favorable bacterial habitat conditions. Choosing acidic feedstocks with low content of lignocelluloses can optimize the habitable pore space. Moreover, process temperatures of around 240–260 °C provide a suitable pore size distribution. Because of technical, economic, and sustainability concerns, physical and chemical activation are less attractive for engineering pore properties. Instead, adding amendments to feedstocks before HTC holds promise for further improvements of the pore system of hydrochars. Apart from the suitable pore size range (1–20 μm), selecting feedstocks containing reservoirs of essential nutrients such as P can improve the suitability of hydrochars as microbial carriers/vectors. Compared to biochars, hydrochars provide larger amounts of available carbon that microorganisms can utilize as substrate and electron donators.
Figure 6. Summary graph based on the results of this study. Green-shaded boxes relate to the main focus of this review. Information highlighted by red colors provides a summary of the feedstock properties and HTC parameters for obtaining hydrochars optimized for serving as microbial carrier/vector.
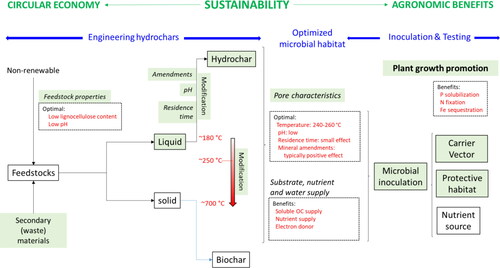
Future research should address the opportunities to engineer the pore characteristics of hydrochars through optimizing feedstocks, process parameters, and innovative use of amendments. In addition, better characterization of the pore systems using methods (e.g., Hg-porosimetry) to capture the pore size range of interest will help select the most appropriate chars. Further, inoculation experiments with PGPR that determine shelf life, survival after application to soil, visual confirmation of colonization, and evidence regarding beneficial effects on plants are required to support products’ development and demonstrate their efficacy.
Supplemental Material
Download Zip (420.9 KB)Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Acea, M. J., Moore, C. R., & Alexander, M. (1988). Survival and growth of bacteria introduced into soil. Soil Biology and Biochemistry, 20(4), 509–515. https://doi.org/10.1016/0038-0717(88)90066-1
- Ajeng, A. A., Abdullah, R., Ling, T. C., Ismail, S., Lau, B. F., Ong, H. C., Chew, K. W., Show, P. L., & Chang, J.-S. (2020). Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environmental Technology & Innovation, 20, 101168. https://doi.org/10.1016/j.eti.2020.101168
- Alaya, M. N., Girgis, B. S., & Mourad, W. E. (2000). Activated Carbon from Some Agricultural Wastes Under Action of One-Step Steam Pyrolysis. Journal of Porous Materials, 7(4), 509–517. https://doi.org/10.1023/A:1009630928646
- Albareda, M., Rodríguez-Navarro, D. N., Camacho, M., & Temprano, F. J. (2008). Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biology and Biochemistry, 40(11), 2771–2779. https://doi.org/10.1016/j.soilbio.2008.07.021
- Ambrosini, A., Souza, R. d., & Luciane, M. P. P. (2016). Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant and Soil, 400(81–2), 193–207. https://doi.org/10.1007/s11104-015-2727-7
- Ameloot, N., Sleutel, S., Case, S. D. C., Alberti, G., McNamara, N. P., Zavalloni, C., Vervisch, B., Vedove, G., Delle, & De Neve, S. (2014). C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biology and Biochemistry, 78, 195–203. https://doi.org/10.1016/j.soilbio.2014.08.004
- Andert, J., & Mumme, J. (2015). Impact of pyrolysis and hydrothermal biochar on gas-emitting activity of soil microorganisms and bacterial and archaeal community composition. Applied Soil Ecology, 96, 225–239.
- Antal, M. J., & Grønli, M. (2003). The Art, Science, and Technology of Charcoal Production. Industrial & Engineering Chemistry Research, 42(8), 1619–1640. https://doi.org/10.1021/ie0207919
- Bai, M., Wilske, B., Buegger, F., Esperschütz, J., Kammann, C. I., Eckhardt, C., Koestler, M., Kraft, P., Bach, M., Frede, H.-G., & Breuer, L. (2013). Degradation kinetics of biochar from pyrolysis and hydrothermal carbonization in temperate soils. Plant and Soil, 372(1–2), 375–387. https://doi.org/10.1007/s11104-013-1745-6
- Baltrėnas, P., Baltrėnaitė, E., & Spudulis, E. (2015). Biochar from Pine and Birch Morphology and Pore Structure Change by Treatment in Biofilter. Water, Air, & Soil Pollution, 226, 69. https://doi.org/10.1007/s11270-015-2295-8
- Bamminger, C., Marschner, B., & Jüschke, E. (2014). An incubation study on the stability and biological effects of pyrogenic and hydrothermal biochar in two soils. European Journal of Soil Science, 65(1), 72–82. https://doi.org/10.1111/ejss.12074
- Bargmann, I., Martens, R., Rillig, M. C., Kruse, A., & Kücke, M. (2014). Hydrochar amendment promotes microbial immobilization of mineral nitrogen. Journal of Plant Nutrition and Soil Science, 177(1), 59–67. https://doi.org/10.1002/jpln.201300154
- Başakçılardan Kabakcı, S., & Baran, S. S. (2019). Hydrothermal carbonization of various lignocellulosics: Fuel characteristics of hydrochars and surface characteristics of activated hydrochars. Waste Management (New York, N.Y.), 100, 259–268. https://doi.org/10.1016/j.wasman.2019.09.021
- Bashan, Y., de-Bashan, L. E., Prabhu, S. R., & Hernandez, J.-P. (2014). Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant and Soil, 378(1–2), 1–33. https://doi.org/10.1007/s11104-013-1956-x
- Bashan, Y., Puente, M. E., Rodriguez-Mendoza, M. N., Toledo, G., Holguin, G., Ferrera-Cerrato, R., & Pedrin, S. (1995). Survival of Azospirillum brasilense in the Bulk Soil and Rhizosphere of 23 Soil Types. Applied and Environmental Microbiology, 61(5), 1938–1945.
- Beck, D. P. (1991). Suitability of charcoal-amended mineral soil as carrier for Rhizobium inoculants. Soil Biology and Biochemistry, 23(1), 41–44. https://doi.org/10.1016/0038-0717(91)90160-L
- Biederman, L. A., & Harpole, W. S. (2013). Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy, 5(2), 202–214. https://doi.org/10.1111/gcbb.12037
- Bird, M. I., Ascough, P. L., Young, I. M., Wood, C. V., & Scott, A. C. (2008). X-ray microtomographic imaging of charcoal. Journal of Archaeological Science, 35(10), 2698–2706. https://doi.org/10.1016/j.jas.2008.04.018
- Borrero-López, A. M., Masson, E., Celzard, A., & Fierro, V. (2018). Modelling the reactions of cellulose, hemicellulose and lignin submitted to hydrothermal treatment. Industrial Crops and Products, 124, 919–930. https://doi.org/10.1016/j.indcrop.2018.08.045
- Brewer, C. E., Chuang, V. J., Masiello, C. A., Gonnermann, H., Gao, X., Dugan, B., Driver, L. E., Panzacchi, P., Zygourakis, K., & Davies, C. A. (2014). New approaches to measuring biochar density and porosity. Biomass and Bioenergy, 66, 176–185. https://doi.org/10.1016/j.biombioe.2014.03.059
- Brown, R. A., Kercher, A. K., Nguyen, T. H., Nagle, D. C., & Ball, W. P. (2006). Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Organic Geochemistry, 37(3), 321–333. https://doi.org/10.1016/j.orggeochem.2005.10.008
- Cárdenas-Aguiar, E., Gascó, G., Paz-Ferreiro, J., & Méndez, A. (2019). Thermogravimetric analysis and carbon stability of chars produced from slow pyrolysis and hydrothermal carbonization of manure waste. Journal of Analytical and Applied Pyrolysis, 140, 434–443. https://doi.org/10.1016/j.jaap.2019.04.026
- Cattelan, A. J., Hartel, P. G., & Fuhrmann, J. J. (1999). Screening for Plant Growth–Promoting Rhizobacteria to Promote Early Soybean Growth. Soil Science Society of America Journal, 63(6), 1670–1680. https://doi.org/10.2136/sssaj1999.6361670x
- Chun, Y., Sheng, G., Chiou, C. T., & Xing, B. (2004). Compositions and Sorptive Properties of Crop Residue-Derived Chars. Environmental Science & Technology, 38(17), 4649–4655. https://doi.org/10.1021/es035034w
- Crombie, K., Mašek, O., Sohi, S. P., Brownsort, P., & Cross, A. (2013). The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy, 5(2), 122–131. https://doi.org/10.1111/gcbb.12030
- Datta, R., Kelkar, A., Baraniya, D., Molaei, A., Moulick, A., Meena, R. S., & Formánek, P. (2017). Enzymatic degradation of lignin in soil: A review. Sustainability, 9(7), 1163. https://doi.org/10.3390/su9071163
- Domene, X., Hanley, K., Enders, A., & Lehmann, J. (2015). Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Applied Soil Ecology, 89, 10–17. https://doi.org/10.1016/j.apsoil.2014.12.005
- Duboc, O., Robbe, A., Santner, J., Folegnani, G., Gallais, P., Lecanuet, C., Zehetner, F., Nagl, P., & Wenzel, W. W. (2019). Silicon Availability from Chemically Diverse Fertilizers and Secondary Raw Materials. Environmental Science & Technology, 53(9), 5359–5368. https://doi.org/10.1021/acs.est.8b06597
- Duboc, O., Santner, J., Golestani Fard, A., Zehetner, F., Tacconi, J., & Wenzel, W. W. (2017). Predicting phosphorus availability from chemically diverse conventional and recycling fertilizers. Science of the Total Environment, 599–600, 1160–1170. https://doi.org/10.1016/j.scitotenv.2017.05.054
- Duboc, O., Steiner, K., Radosits, F., Wenzel, W. W., Goessler, W., & Santner, J. (2019). Functional Recycling of Biobased, Borate-Stabilized Insulation Materials As B Fertilizer. Environmental Science & Technology, 53(24), 14620–14629. https://doi.org/10.1021/acs.est.9b04234
- Ducey, T. F., Ippolito, J. A., Cantrell, K. B., Novak, J. M., & Lentz, R. D. (2013). Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Applied Soil Ecology, 65, 65–72. https://doi.org/10.1016/j.apsoil.2013.01.006
- Egamberdieva, D., Hua, M., Reckling, M., Wirth, S., & Bellingrath-Kimura, S. D. (2018). Potential effects of biochar-based microbial inoculants in agriculture. Environmental Sustainability, 1(1), 19–24. https://doi.org/10.1007/s42398-018-0010-6
- Egamberdieva, D., Reckling, M., & Wirth, S. (2017). Biochar-based Bradyrhizobium inoculum improves growth of lupin (Lupinus angustifolius L.) under drought stress. European Journal of Soil Biology, 78, 38–42. https://doi.org/10.1016/j.ejsobi.2016.11.007
- Egamberdieva, D., Wirth, S., Behrendt, U., Abd Allah, E. F., & Berg, G. (2016). Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Frontiers in Microbiology, 7, 209. https://doi.org/10.3389/fmicb.2016.00209
- Eibisch, N., Schroll, R., Fuß, R., Mikutta, R., Helfrich, M., & Flessa, H. (2015). Pyrochars and hydrochars differently alter the sorption of the herbicide isoproturon in an agricultural soil. Chemosphere, 119, 155–162. https://doi.org/10.1016/j.chemosphere.2014.05.059
- Ezawa, T., Yamamoto, K., & Yoshida, S. (2002). Enhancement of the effectiveness of indigenous arbuscular mycorrhizal fungi by inorganic soil amendments. Soil Science and Plant Nutrition, 48(6), 189–203.
- Fang, J., Zhan, L., Ok, S. Y., & Gao, B. (2018). Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. Journal of Industrial and Engineering Chemistry, 57, 15–21. https://doi.org/10.1016/j.jiec.2017.08.026
- FAO. (2015). World fertilizer trends and outlook to 2018. Rome.
- Foster, E. J., Baas, P., Wallenstein, M. D., & Cotrufo, M. F. (2020). Precision biochar and inoculum applications shift bacterial community structure and increase specific nutrient availability and maize yield. Applied Soil Ecology, 151, 103541. https://doi.org/10.1016/j.apsoil.2020.103541
- Foster, R. C. (1988). Microenvironments of soil microorganisms. Biology and Fertility of Soils, 6, 189–203. https://doi.org/10.1007/BF00260816
- Fu, M.-M., Mo, C.-H., Li, H., Zhang, N.-Y., Huang, W.-X., & Wong, M. H. (2019). Comparison of physicochemical properties of biochars and hydrochars produced from food wastes. Journal of Cleaner Production, 236, 117637. https://doi.org/10.1016/j.jclepro.2019.117637
- Fuertes, A. B., Arbestain, M. C., Sevilla, M., Maciá-Agulló, J. A., Fiol, S., López, R., Smernik, R. J., Aitkenhead, W. P., Arce, F., & Macías, F. (2010). Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Soil Research, 48(7), 618–626. https://doi.org/10.1071/SR10010
- Funke, A., Mumme, J., Koon, M., & Diakité, M. (2013). Cascaded production of biogas and hydrochar from wheat straw: Energetic potential and recovery of carbon and plant nutrients. Biomass and Bioenergy, 58, 229–237. https://doi.org/10.1016/j.biombioe.2013.08.018
- Gai, C., Guo, Y., Liu, T., Peng, N., & Liu, Z. (2016). Hydrogen-rich gas production by steam gasification of hydrochar derived from sewage sludge. International Journal of Hydrogen Energy, 41(5), 3363–3372. https://doi.org/10.1016/j.ijhydene.2015.12.188
- Gajić, A., Ramke, H.-G., Hendricks, A., & Koch, H.-J. (2012). Microcosm study on the decomposability of hydrochars in a Cambisol. Biomass and Bioenergy, 47, 250–259. https://doi.org/10.1016/j.biombioe.2012.09.036
- Gao, Y., Wang, X., Wang, J., Li, X., Cheng, J., Yang, H., & Chen, H. (2013). Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy, 58, 376–383. https://doi.org/10.1016/j.energy.2013.06.023
- Garlapalli, R. K., Wirth, B., & Reza, M. T. (2016). Pyrolysis of hydrochar from digestate: Effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Bioresource Technology, 220, 168–174. https://doi.org/10.1016/j.biortech.2016.08.071
- Gascó, G., Paz-Ferreiro, J., Álvarez, M. L., Saa, A., & Méndez, A. (2018). Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Management (New York, N.Y.), 79, 395–403. https://doi.org/10.1016/j.wasman.2018.08.015
- Giesche, H. (2006). Mercury porosimetry: A general (practical) overview. Particle & Particle Systems Characterization, 23(1), 9–19. https://doi.org/10.1002/ppsc.200601009
- Głodowska, M., Husk, B., Schwinghamer, T., & Smith, D. (2016). Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agronomy for Sustainable Development, 36(1), 21. https://doi.org/10.1007/s13593-016-0356-z
- Gratuito, M. K. B., Panyathanmaporn, T., Chumnanklang, R. A., Sirinuntawittaya, N., & Dutta, A. (2008). Production of activated carbon from coconut shell: Optimization using response surface methodology. Bioresource Technology, 99(11), 4887–4895. https://doi.org/10.1016/j.biortech.2007.09.042
- Gray, M., Johnson, M. G., Dragila, M. I., & Kleber, M. (2014). Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass and Bioenergy, 61, 196–205. https://doi.org/10.1016/j.biombioe.2013.12.010
- Gul, S., Whalen, J. K., Thomas, B. W., Sachdeva, V., & Deng, H. (2015). Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agriculture Ecosystems and Environment, 206, 46–59. https://doi.org/10.1016/j.agee.2015.03.015
- Hale, L., Luth, M., & Crowley, D. (2015). Biochar characteristics relate to its utility as an alternative soil inoculum carrier to peat and vermiculite. Soil Biology and Biochemistry, 81, 228–235. https://doi.org/10.1016/j.soilbio.2014.11.023
- Hale, L., Luth, M., Kenney, R., & Crowley, D. (2014). Evaluation of pinewood biochar as a carrier of bacterial strain Enterobacter cloacae UW5 for soil inoculation. Applied Soil Ecology, 84, 192–199. https://doi.org/10.1016/j.apsoil.2014.08.001
- Halmi, M. F. A., & Simarani, K. (2021). Effect of two contrasting biochars on soil microbiota in the humid tropics of Peninsular Malaysia. Geoderma, 395, 115088. https://doi.org/10.1016/j.geoderma.2021.115088
- Hao, W., Björkman, E., Lilliestråle, M., & Hedin, N. (2013). Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Applied Energy, 112, 526–532. https://doi.org/10.1016/j.apenergy.2013.02.028
- Hardie, M., Clothier, B., Bound, S., Oliver, G., & Close, D. (2014). Does biochar influence soil physical properties and soil water availability? Plant and Soil, 376(1–2), 347–361. https://doi.org/10.1007/s11104-013-1980-x
- Hattori, T. (1988). Soil aggregates as microhabitats of microorganisms. Reports of the Institute for Agricultural Research - Tohoku University (Japan), 37, 23–36.
- He, H., Qian, T.-T., Liu, W.-J., Jiang, H., & Yu, H.-Q. (2014). Biological and chemical phosphorus solubilization from pyrolytical biochar in aqueous solution. Chemosphere, 113, 175–181. https://doi.org/10.1016/j.chemosphere.2014.05.039
- Heijnen, C. E., Hok-A-Hin, C. H., & Veen, J. A. (1991). Protection of Rhizobium by bentonite clay against predation by flagellates in liquid cultures. FEMS Microbiology Ecology, 8(1), 65–71. https://doi.org/10.1111/j.1574-6941.1991.tb01709.x
- Hyväluoma, J., Hannula, M., Arstila, K., Wang, H., Kulju, S., & Rasa, K. (2018). Effects of pyrolysis temperature on the hydrologically relevant porosity of willow biochar. Journal of Analytical and Applied Pyrolysis, 134, 446–453. https://doi.org/10.1016/j.jaap.2018.07.011
- Hyväluoma, J., Kulju, S., Hannula, M., Wikberg, H., Källi, A., & Rasa, K. (2017). Quantitative characterization of pore structure of several biochars with 3D imaging. Environmental Science and Pollution Research, https://doi.org/10.1007/s11356-017-8823-x
- Jaafar, N. M., Clode, P. L., & Abbott, L. K. (2014). Microscopy observations of habitable space in biochar for colonization by fungal hyphae from soil. Journal of Integrative Agriculture, 13(3), 483–490. https://doi.org/10.1016/S2095-3119(13)60703-0
- Jain, A., Balasubramanian, R., & Srinivasan, M. P. (2016). Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chemical Engineering Journal and the Biochemical Engineering Journal, 283, 789–805. https://doi.org/10.1016/j.cej.2015.08.014
- Jeffery, S., Meinders, M. B. J., Stoof, C. R., Bezemer, T. M., van de Voorde, T. F. J., Mommer, L., & van Groenigen, J. W. (2015). Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma, 251–252, 47–54. https://doi.org/10.1016/j.geoderma.2015.03.022
- Jin, J., Li, Y., Zhang, J., Wu, S., Cao, Y., Liang, P., Zhang, J., Wong, M. H., Wang, M., Shan, S., & Christie, P. (2016). Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. Journal of Hazardous Materials, 320, 417–426. https://doi.org/10.1016/j.jhazmat.2016.08.050
- Jones, K., Ramakrishnan, G., Uchimiya, M., & Orlov, A. (2015). New applications of X-ray tomography in pyrolysis of biomass: Biochar imaging. Energy & Fuels, 29(3), 1628–1634. https://doi.org/10.1021/ef5027604
- Kappler, A., Wuestner, M. L., Ruecker, A., Harter, J., Halama, M., & Behrens, S. (2014). Biochar as an Electron Shuttle between Bacteria and Fe(III) Minerals. Environmental Science & Technology Letters, 1(8), 339–344. https://doi.org/10.1021/ez5002209
- Khadem, A., & Raiesi, F. (2017). Responses of microbial performance and community to corn biochar in calcareous sandy and clayey soils. Applied Soil Ecology, 114, 16–27. https://doi.org/10.1016/j.apsoil.2017.02.018
- Killham, K., & Prosser, J. I. (2015). Chapter 3: The bacteria and archaea. In E. A. Paul (Ed.), Soil microbiology, ecology and biochemistry (4th ed., pp. 41–76). Academic Press. https://doi.org/10.1016/B978-0-12-415955-6.00003-7
- Kloss, S., Zehetner, F., Dellantonio, A., Hamid, R., Ottner, F., Liedtke, V., Schwanninger, M., Gerzabek, M. H., & Soja, G. (2012). Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. Journal of Environmental Quality, 41(4), 990–1000. https://doi.org/10.2134/jeq2011.0070
- Laine, J., Simoni, S., & Calles, R. (1991). Preparation of activated carbon from coconut shell in a small scale cocurrent flow rotary kiln. Chemical Engineering Communications, 99(1), 15–23. https://doi.org/10.1080/00986449108911575
- Lanza, G., Rebensburg, P., Kern, J., Lentzsch, P., & Wirth, S. (2016). Impact of chars and readily available carbon on soil microbial respiration and microbial community composition in a dynamic incubation experiment. Soil and Tillage Research, 164, 18–24. https://doi.org/10.1016/j.still.2016.01.005
- Lehmann, J. (2015). Biochar for environmental management: Science, technology and implementation (2nd ed.). Routledge.
- Lehmann, J., Rillig, M. C., Thies, J., Masiello, C. A., Hockaday, W. C., & Crowley, D. (2011). Biochar effects on soil biota—A review. Soil Biology and Biochemistry, 43(9), 1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
- Li, H., Dong, X., da Silva, E. B., de Oliveira, L. M., Chen, Y., & Ma, L. Q. (2017). Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere, 178, 466–478. https://doi.org/10.1016/j.chemosphere.2017.03.072
- Li, M., Liu, M., Li, Z., Jiang, C., & Wu, M. (2016). Soil N transformation and microbial community structure as affected by adding biochar to a paddy soil of subtropical China. Journal of Integrative Agriculture, 15(1), 209–219. https://doi.org/10.1016/S2095-3119(15)61136-4
- Li, Y., Meas, A., Shan, S., Yang, R., & Gai, X. (2016). Production and optimization of bamboo hydrochars for adsorption of Congo red and 2-naphthol. Bioresource Technology, 207, 379–386. https://doi.org/10.1016/j.biortech.2016.02.012
- Liang, B., Lehmann, J., Sohi, S. P., Thies, J. E., O’Neill, B., Trujillo, L., Gaunt, J., Solomon, D., Grossman, J., Neves, E. G., & Luizão, F. J. (2010). Black carbon affects the cycling of non-black carbon in soil. Organic Geochemistry, 41(2), 206–213. https://doi.org/10.1016/j.orggeochem.2009.09.007
- Libra, J. A., Ro, K. S., Kammann, C., Funke, A., Berge, N. D., Neubauer, Y., Titirici, M.-M., Fühner, C., Bens, O., Kern, J., & Emmerich, K.-H. (2011). Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels, 2(1), 71–106. https://doi.org/10.4155/bfs.10.81
- Liu, H., Basar, I. A., Nzihou, A., & Eskicioglu, C. (2021). Hydrochar derived from municipal sludge through hydrothermal processing: A critical review on its formation, characterization, and valorization. Water Research, 199, 117186. https://doi.org/10.1016/j.watres.2021.117186
- Lu, H., Lashari, M. S., Liu, X., Ji, H., Li, L., Zheng, J., Kibue, G. W., Joseph, S., & Pan, G. (2015). Changes in soil microbial community structure and enzyme activity with amendment of biochar-manure compost and pyroligneous solution in a saline soil from Central China. European Journal of Soil Biology, 70, 67–76. https://doi.org/10.1016/j.ejsobi.2015.07.005
- Lu, S., & Zong, Y. (2018). Pore structure and environmental serves of biochars derived from different feedstocks and pyrolysis conditions. Environmental Science and Pollution Research International, 25, 30401–30409. https://doi.org/10.1007/s11356-018-3018-7
- Lu, Y., Hu, Y., Tang, L., Xie, Q., Liu, Q., Zhong, L., Fu, L., & Fan, C. (2021). Effects and mechanisms of modified biochars on microbial iron reduction of Geobacter sulfurreducens. Chemosphere, 283, 130983. https://doi.org/10.1016/j.chemosphere.2021.130983
- Luo, Y., Durenkamp, M., De Nobili, M., Lin, Q., Devonshire, B. J., & Brookes, P. C. (2013). Microbial biomass growth, following incorporation of biochars produced at 350 °C or 700 °C, in a silty-clay loam soil of high and low pH. Soil Biology and Biochemistry, 57, 513–523. https://doi.org/10.1016/j.soilbio.2012.10.033
- Martínez, M. L., Torres, M. M., Guzmán, C. A., & Maestri, D. M. (2006). Preparation and characteristics of activated carbon from olive stones and walnut shells. Industrial Crops and Products, 23(1), 23–28. https://doi.org/10.1016/j.indcrop.2005.03.001
- Méndez, A., Gascó, G., Ruiz, B., & Fuente, E. (2019). Hydrochars from industrial macroalgae “Gelidium Sesquipedale” biomass wastes. Bioresource Technology, 275, 386–393. https://doi.org/10.1016/j.biortech.2018.12.074
- Messing, R. A., & Oppermann, R. A. (1979). Pore dimensions for accumulating biomass. I. Microbes that reproduce by fission or by budding. Biotechnology and Bioengineering, 21(1), 49–58. https://doi.org/10.1002/bit.260210105
- Montes, V., & Hill, J. M. (2018). Activated carbon production: Recycling KOH to minimize waste. Materials Letters, 220, 238–240. https://doi.org/10.1016/j.matlet.2018.03.019
- Nathan, C., & Cunningham-Bussel, A. (2013). Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nature Reviews. Immunology, 13(5), 349–361. https://doi.org/10.1038/nri3423
- Nizamuddin, S., Jaya Kumar, N. S., Narayan Sahu, J., Ganesan, P., Mujawar Mubarak, N., & Mazari, S. A. (2015). Synthesis and characterization of hydrochars produced by hydrothermal carbonization of oil palm shell. The Canadian Journal of Chemical Engineering, 93(11), 1916–1921. https://doi.org/10.1002/cjce.22293
- Noraini, M. J. P. L. C. L. K. A. (2015). Soil microbial responses to biochars varying in particle size, surface and pore properties. Soil Circle: English version, 25, 770–780. https://doi.org/10.1016/S1002-0160(15)30058-8
- Ogawa, M., & Okimori, Y. (2010). Pioneering works in biochar research, Japan. Soil Research, 48(7), 489–500. https://doi.org/10.1071/SR10006
- Oginni, O., Singh, K., Oporto, G., Dawson-Andoh, B., McDonald, L., & Sabolsky, E. (2019). Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresource Technology Reports, 7, 100266. https://doi.org/10.1016/j.biteb.2019.100266
- Parshetti, G. K., Kent Hoekman, S., & Balasubramanian, R. (2013). Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresource Technology, 135, 683–689. https://doi.org/10.1016/j.biortech.2012.09.042
- Pienisch, S. (2018). Nutrient recycling using biowastes from diverse sources. Lincoln University, University of Natural Resources and Life Science.
- Pietikäinen, J., Kiikkilä, O., & Fritze, H. (2000). Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos, 89(2), 231–242. https://doi.org/10.1034/j.1600-0706.2000.890203.x
- Postma, J., Clematis, F., Nijhuis, E. H., & Someus, E. (2013). Efficacy of four phosphate-mobilizing bacteria applied with an animal bone charcoal formulation in controlling Pythium aphanidermatum and Fusarium oxysporum f.sp. radicis lycopersici in tomato. Biological Control, 67(2), 284–291. https://doi.org/10.1016/j.biocontrol.2013.07.002
- Postma, J., Nijhuis, E. H., & Someus, E. (2010). Selection of phosphorus solubilizing bacteria with biocontrol potential for growth in phosphorus rich animal bone charcoal. Applied Soil Ecology, 46(3), 464–469. https://doi.org/10.1016/j.apsoil.2010.08.016
- Postma, J., Schilder, M. T., Bloem, J., & van Leeuwen-Haagsma, W. K. (2008). Soil suppressiveness and functional diversity of the soil microflora in organic farming systems. Soil Biology and Biochemistry, 40(9), 2394–2406. https://doi.org/10.1016/j.soilbio.2008.05.023
- Quilliam, R. S., Glanville, H. C., Wade, S. C., & Jones, D. L. (2013). Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biology and Biochemistry, 65, 287–293. https://doi.org/10.1016/j.soilbio.2013.06.004
- Rafique, M., Sultan, T., Ortas, I., & Chaudhary, H. J. (2017). Enhancement of maize plant growth with inoculation of phosphate-solubilizing bacteria and biochar amendment in soil. Soil Science & Plant Nutrition, 63(5), 460–469. https://doi.org/10.1080/00380768.2017.1373599
- Rex, D., Schimmelpfennig, S., Jansen-Willems, A., Moser, G., Kammann, C., & Müller, C. (2015). Microbial community shifts 2.6 years after top dressing of Miscanthus biochar, hydrochar and feedstock on a temperate grassland site. Plant and Soil, 397(1–2), 261–271. https://doi.org/10.1007/s11104-015-2618-y
- Reza, M. T., Rottler, E., Herklotz, L., & Wirth, B. (2015). Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresource Technology, 182, 336–344. https://doi.org/10.1016/j.biortech.2015.02.024
- Rutigliano, F. A., Romano, M., Marzaioli, R., Baglivo, I., Baronti, S., Miglietta, F., & Castaldi, S. (2014). Effect of biochar addition on soil microbial community in a wheat crop. European Journal of Soil Biology, 60, 9–15. https://doi.org/10.1016/j.ejsobi.2013.10.007
- Saito, M. (1990). Charcoal as a micro-habitat for VA mycorrhizal fungi, and its practical implication. Agriculture Ecosystems and Environment, 29(1–4), 341–344. https://doi.org/10.1016/0167-8809(90)90298-R
- Samonin, V., & Elikova, E. (2004). A study of the adsorption of bacterial cells on porous materials. Microbiology, 73(6), 696–701. https://doi.org/10.1007/s11021-005-0011-1
- Sangeetha, D., & Stella, D. (2012). Survival of plant growth promoting bacterial inoculants in different carrier materials. International Journal of Pharmaceutical and Biological Archive, 3(1), 170–178.
- Saqib, N., Oh, M., Jo, W., Park, S.-K., & Lee, J.-Y. (2015). Conversion of dry leaves into hydrochar through hydrothermal carbonization (HTC). Journal of Material Cycles and Waste Management, 19(1), 111–117. https://doi.org/10.1007/s10163-015-0371-1
- Schnee, L. S., Knauth, S., Hapca, S., Otten, W., & Eickhorst, T. (2016). Analysis of physical pore space characteristics of two pyrolytic biochars and potential as microhabitat. Plant and Soil, 408(1–2), 357–368. https://doi.org/10.1007/s11104-016-2935-9
- Sevilla, M., & Fuertes, A. B. (2009). The production of carbon materials by hydrothermal carbonization of cellulose. Carbon, 47(9), 2281–2289. https://doi.org/10.1016/j.carbon.2009.04.026
- Sevilla, M., Maciá-Agulló, J. A., & Fuertes, A. B. (2011). Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass and Bioenergy, 35(7), 3152–3159. https://doi.org/10.1016/j.biombioe.2011.04.032
- Singh Kambo, H., & Dutta, A. (2015). A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renewable and Sustainable Energy Reviews, 45, 359–378. https://doi.org/10.1016/j.rser.2015.01.050
- Sun, D., Hale, L., & Crowley, D. (2016). Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biology and Fertility of Soils, 52(4), 515–522. https://doi.org/10.1007/s00374-016-1093-9
- Sun, D., Meng, J., Liang, H., Yang, E., Huang, Y., Chen, W., Jiang, L., Lan, Y., Zhang, W., & Gao, J. (2015). Effect of volatile organic compounds absorbed to fresh biochar on survival of Bacillus mucilaginosus and structure of soil microbial communities. Journal of Soils and Sediments, 15(2), 271–281. https://doi.org/10.1007/s11368-014-0996-z
- Teutscherova, N., Lojka, B., Houška, J., Masaguer, A., Benito, M., & Vazquez, E. (2018). Application of holm oak biochar alters dynamics of enzymatic and microbial activity in two contrasting Mediterranean soils. European Journal of Soil Biology, 88, 15–26. https://doi.org/10.1016/j.ejsobi.2018.06.002
- Titirici, M.-M., White, R. J., Falco, C., & Sevilla, M. (2012). Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy & Environmental Science, 5(5), 6796–6822. https://doi.org/10.1039/c2ee21166a
- Tomczyk, A., Sokołowska, Z., & Boguta, P. (2020). Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Reviews in Environmental Science and Bio/Technology, 19(1), 191–215. https://doi.org/10.1007/s11157-020-09523-3
- Tripti, A., Usmani, K., Vipin, Z., & Anshumali, K. (2017). Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. Journal of Environmental Management, 190, 20–27. https://doi.org/10.1016/j.jenvman.2016.11.060
- Vanek, S. J., & Thies, J. (2016). Pore-size and water activity effects on survival of rhizobium tropici in biochar inoculant carriers. Journal of Microbial & Biochemical Technology, 8(4), 296–306. https://doi.org/10.4172/1948-5948.1000300
- Wang, T., Zhai, Y., Zhu, Y., Li, C., & Zeng, G. (2018). A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renewable and Sustainable Energy Reviews, 90, 223–247. https://doi.org/10.1016/j.rser.2018.03.071
- Wildman, J., & Derbyshire, F. (1991). Origins and functions of macroporosity in activated carbons from coal and wood precursors. Fuel, 70(5), 655–661. https://doi.org/10.1016/0016-2361(91)90181-9
- Wilk, M., Magdziarz, A., Jayaraman, K., Szymańska-Chargot, M., & Gökalp, I. (2019). Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass and Bioenergy, 120, 166–175. https://doi.org/10.1016/j.biombioe.2018.11.016
- Wright, D. A., Killham, K., Glover, L. A., & Prosser, J. I. (1995). Role of Pore Size Location in Determining Bacterial Activity during Predation by Protozoa in Soil. Applied and Environmental Microbiology, 61(10), 3537–3543. https://doi.org/10.1128/aem.61.10.3537-3543.1995
- Yu, X., Liu, S., Lin, G., Yang, Y., Zhang, S., Zhao, H., Zheng, C., & Gao, X. (2020). KOH-activated hydrochar with engineered porosity as sustainable adsorbent for volatile organic compounds. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 588, 124372. https://doi.org/10.1016/j.colsurfa.2019.124372
- Yue, Y., Lin, Q., Xu, Y., Li, G., & Zhao, X. (2017). Slow pyrolysis as a measure for rapidly treating cow manure and the biochar characteristics. Journal of Analytical and Applied Pyrolysis, 124, 355–361. https://doi.org/10.1016/j.jaap.2017.01.008
- Zhang, J., Lin, Q., & Zhao, X. (2014). The hydrochar characters of municipal sewage sludge under different hydrothermal temperatures and durations. Journal of Integrative Agriculture, 13(3), 471–482. https://doi.org/10.1016/S2095-3119(13)60702-9
- Zhang, T., Walawender, W. P., Fan, L. T., Fan, M., Daugaard, D., & Brown, R. C. (2004). Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chemical Engineering Journal and the Biochemical Engineering Journal, 105(1–2), 53–59. https://doi.org/10.1016/j.cej.2004.06.011
- Zhou, H., Zhang, D., Wang, P., Liu, X., Cheng, K., Li, L., Zheng, J., Zhang, X., Zheng, J., Crowley, D., van Zwieten, L., & Pan, G. (2017). Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: A meta-analysis. Agriculture Ecosystems and Environment, 239, 80–89. https://doi.org/10.1016/j.agee.2017.01.006
- Zhu, X., Liu, Y., Qian, F., Zhou, C., Zhang, S., & Chen, J. (2015). Role of hydrochar properties on the porosity of hydrochar-based porous carbon for their sustainable application. ACS Sustainable Chemistry & Engineering, 3(5), 833–840. https://doi.org/10.1021/acssuschemeng.5b00153
- Zimmerman, A. R., Gao, B., & Ahn, M.-Y. (2011). Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biology and Biochemistry, 43(6), 1169–1179. https://doi.org/10.1016/j.soilbio.2011.02.005