?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
COVID-19 pandemic has created chaos in almost every walk of life. The harsh impact of the disease is mainly rooted to the rapid and easy spread of SARS-CoV-2 virus through airborne and fomite routes. Thus, disinfection of contaminated surfaces and air is important to hamper COVID-19 disease transmission. Ozone being a potent gaseous disinfectant has been utilized to inactivate a wide-range of viruses and has more recently gained interest in the inactivation of SARS-CoV-2. This article critically reviews the current state-of-knowledge on disinfection of surface-adhered and airborne SARS-CoV-2 by ozone. The transmission and survival characteristics of SARS-CoV-2 alongside the specificity of ozone inactivation process are reviewed. Distinct focus is then given to reviewing the status of ozone inactivation of surface-adhered and airborne SARS-CoV-2 in terms of experimental investigations, kinetics, and influence of the operational factors on the inactivation process. Ozone inactivation of SARS-CoV-2 is compared to other enveloped viruses, and the challenges and future prospects of ozone inactivation of SARS-CoV-2 are also addressed.
Graphical abstract
HANDLING EDITORS:
1. Introduction
The current outbreak of severe acute respiratory syndrome (SARS)-related coronaviruses (CoV) is the third documented havoc introduced into humans via zoonotic-reservoir within the past two decades (Dietz et al., Citation2020; Gorbalenya et al., Citation2020); this is after SARS-CoV-1 in 2003 with 10% mortality rate (Chan-Yeung & Xu, Citation2003) and the Middle East Respiratory Syndrome (MERS) in 2012 with 35% mortality rate (Fisman et al., Citation2014; Korber et al., Citation2020). SARS-CoV-2, the pathogen responsible for COVID-19 was firstly reported in November 2019 in Wuhan, China which dynamically turned into a pandemic with devastating effects globally (Josset et al., Citation2010). Humans are gravely vulnerable to both infection and disease due to the rapidly mutating and transmissible SARS-CoV-2 virus which is estimated to have a basic reproduction number between 2.2–3.9 and mortality rates in the range of 0.8–19.6% with regional variation, mortality analyses of Johns Hopkins University of Medicine (Korber et al., Citation2020; Lv et al., Citation2020). The number of infected cases and/deaths have still not been under controlled limits, with currently 195,266,156 confirmed cases in 224 countries and territories, where 4,180,161 people have died so far (till July 28th, 2021), as per WHO daily report (World Health Organization, Citation2020); these figures are kept rising on a daily-basis urging disease-prevention efforts immensely important.
COVID-19 virus of genus Betacoronavirus belongs to the species SARS-CoV-2 in the family of Coronaviridae and the order Nidovirales as reported by the International Committee on Taxonomy of Viruses. The virus typically has a spherical shape comprising of enveloped positive-polarity, single-stranded ribonucleic acid genomic material (ssRNA (+)). Details on the structure and mode of infection of the virus can be found on the Supporting Information (SI-1). The main transmission route of SARS-CoV-2 virus has been evidenced to be primarily through inhalation of infected droplets, released by coughing/sneezing of the infected person which is capable of transmission over 2 m distance (Wiktorczyk-Kapischke et al., Citation2021). The virus whether being suspended in airborne droplets or adhered to a surface can remain active and possibly infectious over different time durations depending upon the adherent surface, and climatic conditions (SAGE – Environmental and Modelling Group 4th June, Citation2020). This so-called awaken time of the virus is crucial, in a sense that the virus is quietly adherent to the surface awaiting to be uptaken by a host where it can replicate, leading to infection (Hammett, Citation2020). Owing to the longest viable duration in aerosols (1–3 h) and on various surfaces (1–14 days), the indirect transmission route of SARS-CoV2 is the deliberate or unintentional contact with the contaminated surfaces or objects (Morawska & Cao, Citation2020; Setti et al., Citation2020; Van Doremalen et al., Citation2020). Thus, to reduce virus transmission, precautionary measures including respiratory masks and/or gloves together with standard hygiene measures and social distancing have been recommended by WHO as defensive protocols against coronavirus infection across the world (Heinzerling et al., Citation2020). Nonetheless, a total of 3,815,501,716 vaccine doses have been administered (as of July 27th, 2021 WHO report) but so far there is no widely used effective antiviral treatment against SARS-CoV-2, which has been mutating to new variants (Li et al., Citation2021). Henceforth, the contamination of air and surfaces by the infectious virus continues to pose serious implications for outbreak-control strategies and measures.
Ozone has gained hype in the world of disinfection/sanitization technologies due to its outstanding ability to inactivate all kinds of microorganisms in liquid, air, and dry phases (Dubuis et al., Citation2020; Summerfelt & Hochheimer, Citation1997; Vyskocil et al., Citation2020). It shows faster inactivation kinetics compared to other widely used chemical disinfectants and has proven to be a potent disinfectant (or sanitiser) for resistant microorganisms. Being strong oxidizing and diffusible, the microbicidal application of ozone has been utilized in water treatment, food and agriculture, and healthcare (Dennis et al., Citation2020; Hansen et al., Citation2016; Hudson et al., Citation2007, Citation2009; Munter, Citation2001; Quevedo-León et al., Citation2020). Although water disinfection is considered as the cradle of ozonation, coronavirus inactivation by ozone has voiced after it had been successfully used against SARS-CoV-1 (Zhang et al., Citation2004) and theoretically demonstrated as a potential solution against SARS-CoV-2 (Tizaoui, Citation2020). Ozone can damage the lipid membrane, capsid protein, and genome of the virus through oxidation, thereby disrupting its infection mechanism (Kampf et al., Citation2015; Sharma & Graham, Citation2010; Tizaoui, Citation2020). Ozone, a potent virucide, is inexpensive, feasible to administer due to its gaseous nature, and is easy to produce, requiring only an electrical generator and air (Alimohammadi & Naderi, Citation2021). The viral inactivation by ozonation depends upon several factors such as ozone concentration, contact time, virus dose, viral strain, dissolving and/or purification medium of virus, droplet size, nature of adherent surface, contact angle, working temperature, humidity levels, wind speed, etc. The complicated blend of these factors mutually affects the inactivation process which is still immature in research. Though, recently an appreciable volume of work aiming to combat COVID-19 spread by utilizing ozone has started to emerge (Criscuolo et al., Citation2021; Cristiano, Citation2020; De Forni et al., Citation2021; Tizaoui, Citation2020). The present article provides a critical review of the currently reported literature on ozone-mediated disinfection of SARS-CoV-2 adhered to surfaces and airborne. Specifically, the review is aimed to (i) discuss ozone administration and disinfection strategies in both aerosolized and surface applications along with safety issues, (ii) introduce engineering aspects of viral inactivation by ozonation, and (iii) highlight gaps and suggest correlations useful for a growing research and application fields.
2. Overview of SARS-CoV-2 and ozone disinfection
2.1. Sars-CoV-2 transmission and survival
The enormous magnitude and scope of the current pandemic not only mirror the contagious nature of SARS-CoV-2 virus but also exceedingly rapid transmission whereby the exact mechanism of viral spread is still uncertain. The reproduction number of SARS-CoV-2 is estimated to be 2.5, meaning that each infected person can spread infection on average to 2 to 3 other persons (Klompas et al., Citation2020). The virions encapsulated in the respiratory secretions of an infected person may shed during sneezing or coughing and spread to other persons via three potentially significant routes: droplet, contact, and airborne (Mittal et al., Citation2020). Generally, droplets are considered as large particles (>5 µm) that readily drop to the ground due to the gravity effect, usually within 6 feet (∼2 m) of the source. Aerosols, on the other hand, are smaller sized particles (≤5 µm) that readily dry out in the air, leaving behind droplet-nuclei, virions, which is lighter and smaller enough to remain suspended in the air for long period depending upon climatic conditions (Fennelly, Citation2020). There is mounting epidemiological and experimental evidence that most transmission of SARS-CoV-2 is respiratory, in the form of droplet or aerosol (Fennelly, Citation2020; Klompas et al., Citation2020; Lu et al., Citation2020).
Recent studies have also enunciated the link between air pollution and COVID infection rate and mortality (Domingo et al., Citation2020; Nor et al., Citation2021). The particulate matter (PM), air-suspended particles, both fine (PM2.5; particles with diameter ≤ 2.5 μm) and coarse (PM10; diameter ≤ 10 μm), are considered to be a major component of air pollution, whereby, the former ones typically have overwhelming contribution. For instance, Travaglio et al. (Citation2021) reported a 12% rise in COVID-19 infected cases in England associated with only single-unit increase (1 m3) of long-term-average levels of PM2.5 e.g., nitrogen oxide/dioxide. The long-term PM2.5 exposure in 3089 counties of the United Sates, accounting for ∼98% population, has been reported to be linked with rise in COVID-19 mortality rate (11%) (Wu et al., Citation2020). Other publications have also reported analogous findings across various regions of the world, such as nine Asian cities (India, Pakistan, China, and Indonesia) in July 2020 (Gupta et al., Citation2021), 279 Israel towns and cities between March 2020–January 2021 (Levi & Barnett-Itzhaki, Citation2021), Northwest Mexico in February 2020–April 2021 (Páez-Osuna et al., Citation2022), Vienna, Austria in February–April 2020 (Hutter et al., Citation2020), and 110 Italian provinces in March 2020 (Bianconi et al., Citation2020). Generally, prolonged out-door particulate matter exposure causes human airway inflammation which possibly enhances the vulnerability of viral infections (Coker et al., Citation2020). Besides, few other studies have observed an association of air pollution to COVID-19 prevalence without any statistically significant correlation between mortality rate and air pollution, as documented by Salgado et al. (Citation2021) for Chili in March 2020, and Filonchyk et al. (Citation2021) for various Polish provinces between March 2020–Februrary 2021. Albeit, the fine particulate matter air pollution is a front running cause of respiratory infections, but still has not been considered as a sole contributor of SARS-CoV-2 spread, infectivity, and mortality; other co-factors such as population density, air quality index, wind speed, humidity levels etc. undoubtably have significance.
Furthermore, both the low relative humidity (RH) levels and low daily temperature aggravate the viral spread, particularly in closed spaces because small aerosol particles with a higher viral load can spread up to a longer distance of 10 m from the point source (Morawska & Cao, Citation2020; Paules et al., Citation2020). One of the studies conducted on 100 Chinese cities and 1,005 U.S. counties with confirmed COVID-19 infected cases from March 15 to April 25 led by Wang et al. (Citation2021), reported that high temperature (1 °C rise) and high humidity level (1% rise) decreased the reproduction number of the virus. Another study conducted by Santarpia et al. (Citation2020), at the University of Nebraska Medical Center (USA) on airborne viral transmission reported 63% of indoor air samples were viral RNA positive in the range of 2–9 copies/L. The effect of RH and daily temperature on the viability of SARS-CoV-2 is illustrated in Figure SI-2.
Another important feature of COVID-19 disease spread is the survival characteristics of SARS-CoV-2 virus in suspended air and on different kind of surfaces as these contaminated surfaces act as potential vehicles for disease transmission. According to Van Doremalen et al. (Citation2020), SARS-CoV-2 can remain viable in air up to 3 h, with a marked reduction in infectivity titer level from 103.5 to 102.7 TCID50/mL (tissue culture infectious dose) of air. However, there is limited information available on SARS-CoV-2 viability on surfaces and the experimental conditions reported in the literature lack details. This makes comparison of data difficult. Generally, the stability of the virus in the environment is influenced by temperature and RH but also by both the volume of virus inoculation and titer of virus-stock at given temperature and humidity. Besides, the virus has higher stability on smooth, non-porous surfaces such as stainless steel, plastic, glass, etc., contrary to porous, rough surfaces i.e., wood, paper, cloth, etc. (Liu et al., Citation2021).
Chin et al. (Citation2020) reported the survival of SARS-CoV-2 was 3 h on tissue/printing paper, 24 h on cotton cloth, 72 h on glass and banknotes, more than 96 h on stainless steel and plastic, and 96 h on surgical mask under 107.8 TCID50/mL of 5 µL viral stock at 22 °C and 65% RH. Another study conducted by Van Doremalen et al. (Citation2020) reported that SARS-CoV-2 virus was viable for 3 h in air, 4 h on copper surface, 24 h on cardboard wood, 48 h on stainless steel, and more than 72 h on plastic under 105.25TCID50/mL of 5 µL viral stock at 21–23 °C temperature. SARS-CoV-2 viability is sensitive to high humidity and high temperature as reported by Wang et al. (Citation2021). Moreover, Chin et al. (Citation2020) demonstrated high stability of SARS-CoV-2 virus at low temperatures and its sensitivity at high temperatures. They incubated SARS-CoV-2 strains in virus transport medium (106.8 TCID50/mL) for 14 days at 4 °C and 70 °C and observed only ∼0.7-log reduction of infectious titer at 4 °C whereas complete inactivation was achieved in 5 min at 70 °C. In general, coronaviruses survive longer at low temperatures and low relative humidities (Aboubakr et al., Citation2021). Morris et al. (Citation2021) have characterized the stability of SARS-CoV-2 and introduced a mechanistic model, relevant to both fomite and airborne transmission, to predict the effect of temperature and humidity on SARS-CoV-2 survival. They reported half-life times of more than 24 h and 1.5 h at (10 °C, 40% RH) and at (27 °C, 65% RH) respectively. Figure SI-2 also illustrates the review of these findings.
Wearing face masks and observing appropriate social distancing of a minimum of 6 feet apart appear to be effective strategies to combat the disease spread via the respiratory transmission route. However, controlling the disease transmission via the contact route requires increased hand hygiene and surface decontamination using efficient disinfectants. On the other hand, indoor spaces, that are people gathering platforms such as schools, offices, shopping malls, and business centers, are the most likely cause of airborne viral transmission that needs serious consideration. Even though COVID-19 is characterized by the risk of nosocomial transmission, surface contamination is playing a vital role in the disease transmission. One of the studies conducted by Wu et al. (Citation2020) at Wuhan Hospital reported 20% higher vitality of viral RNA on the touchable surfaces inside the hospital compared to surfaces outside the hospital premises such as beepers (50%), water dispenser buttons (50%), elevator buttons (∼43%), computer accessories (40%), and telephones (40%), strongly emphasizing the need for strict surface/air decontamination measures to control virus reproduction number and mitigate disease spread.
2.2. Ozone as a virucidal agent
Ozone with an oxidation potential of +2.07 V, much higher than chlorine (+1.36 V), and its aqueous solution hypochlorous acid (HOCl) (+1.48 V) has outstanding germicidal properties. It can retain its oxidizing capabilities, a key to disinfection, both in water and air application. Ozone is 14 times more soluble in water than oxygen but it forms a metastable aqueous solution. It shows maximum solubility at 0 °C (1.09 kg m−3) which gradually decreases with increasing temperature to 0.14 kg m−3 at 60 °C (Hoff & Akin, Citation1986; Hudson et al., Citation2009; Khadre et al., Citation2001; Loeb et al., Citation2012). Ozone is a potent oxidizing agent and is characterized as a highly rapid and effective microbicide (Roth & Sullivan, 1981). Due to its versatile oxidizing ability, it is known to inactivate almost all kinds of microorganisms such as viruses, bacteria, fungi, spores, protozoa, and algae. Thus ozone has been extensively used globally to disinfect drinking water, wastewater, air, agriculture processing, laundry, and healthcare, as well as enclosed spaces (Hoff & Akin, Citation1986; Hudson et al., Citation2009; Khadre et al., Citation2001; Loeb et al., Citation2012).
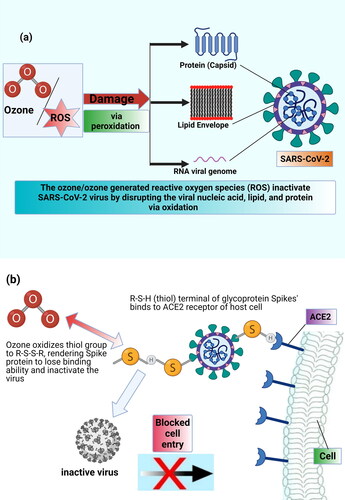
Amongst microorganisms, ozone is particularly proven to be extremely effective against viruses and bacteria as it inactivates them via oxidation of unsaturated aliphatic and aromatic units (Li & Wang, Citation2003; Sharma & Hudson, Citation2008). Viruses are unable to repair the oxidative damages and hence are highly susceptible to ozone than bacteria, fungi, and spores as reported by several groups of researchers concluding the order of microbial vulnerability to ozone follows: virus > bacteria > yeast > spores (Allison et al., Citation2009; Thurston-Enriquez et al., Citation2005). Viral inactivation as peroxidation of its constituents can take place either through direct reaction of molecular ozone with the virus or indirectly through the reaction of a variety of primary reactive oxygen radicals (ROS), produced as a result of ozone decomposition in air or water medium, with viral components. These reactions can produce secondary radicals (RCOO•) which may propagate the oxidation chain reactions to inactivate the virus (as depicted in ).
Figure 1. (a) Targets of oxidative action of ozone for SARS-CoV-2 virus inactivation; (b) possible mechanism of virucidal action of ozone.
Ozone mainly attacks lipids (polyunsaturated fatty acids), antioxidants, glycoproteins, amino acids, carbohydrates, whereby the former ones are highly sensitive to ozone (Murray et al., Citation2008; Tizaoui, Citation2020). The preference of ozone reactivity toward lipids renders enveloped viruses to be least resistant to ozonation compared to non-enveloped viruses (Murray et al., Citation2008; Woolwine & Gerberding, Citation1995) as confirmed by experimental findings of Tseng and Li (Citation2006) work on a comparative study of ozone inactivation of bacteriophage viruses T7, MS2, φX174, and φ6 (surrogate of Norovirus, Adenovirus, Human Immunodeficiency virus HIV, and Influenza). The required values of ozone dose in air (CT value = ozone concentration × contact time) for 2-log (99%) inactivation were 223 mg min/m3, 194 mg min/m3, 72 mg min/m3, and 58 mg min/m3 for T7, MS2, φX174, and φ6, respectively. They inferred enveloped viruses (φX174 and φ6) required nearly half ozone dose for inactivation than non-enveloped viruses (T7 and MS2), thus identifying higher sensitivity of enveloped viruses to ozone.
2.3. Inherent SARS-CoV-2 characteristics during inactivation process
Several studies have reported that ozone impairs the binding ability of virus to the host through oxidation of their lipid envelop and/or protein capsid constituents, thus, the damaged virus ultimately turns inactivated due to lack of self-healing mechanism like living-cells (Jiang et al., Citation2019; Murray et al., Citation2008; Shin & Sobsey, Citation2003; Tseng & Li, Citation2006; Yu et al., Citation2018). Similar findings of ozone-mediated viral denaturation and oxidation of its constituents have been confirmed for coronaviruses (SARS-CoV-1/SARS-CoV-2) (Rowen & Howard, 2020; Volkoff et al., Citation2020; Zhang et al., Citation2004). The structure of coronavirus’ envelop, as described in Supplementary Information (SI-1), is rich in amino acids and lipids, both of them being highly susceptible to ozone attack, make the virus vulnerable to ozonation (Ataei-Pirkooh et al., Citation2021; Tizaoui, Citation2020). Ozone oxidizes the thiol groups (R-S-H) in cysteine units of its spike protein to R-S-S-R which makes spike proteins incapable of binding to the host ACE2 receptors and henceforth penetrate it, as shown in Figure SI-1. Martins et al. (Citation2021) have also postulated that the inactivation of SARS-CoV-2 by ozone proceeds through a mechanism targeting the structure of the virus rather than its genome (0.6 mg/L ozone in water, 1 min exposure time). The mechanism of ozone-induced inactivation for SARS-CoV-2 is well supported by molecular simulation studies reported by Tizaoui (Citation2020), which clearly demonstrates that ozone readily reacts with lipids and proteins of virus’s envelope and spikes, in particular, fatty acids including arachidonic acid, oleic acid, and linoleic acid and the amino acids, methionine, tryptophan, and cysteine. It disrupts the viral envelope and genome by oxidation leading to its inactivation. It was suggested that the oxidized spike proteins lose their binding ability to the ACE2 receptors hence unable to infect the host cell (Tizaoui, Citation2020), as illustrated in b). Based upon the presented literature, we can infer that ozone would likely be lethal to SARS-CoV-2 virus.
2.4. Engineering aspects of ozone disinfection
Investigations on ozone-induced inactivation of airborne and indoor surface (e.g., glass, plastic, wood, fabric, and metal) viruses principally require a closed chamber with built-in units including ozone generator, air, humidifier, timer, scrubber (ozone destructor), and sensors (ozone and RH). Heselton et al. (Citation2008) disclosed a method of disinfecting closed rooms which included gaseous ozone generation up to 30–60 mg/m3 followed by rapidly increasing the RH level (⁓80%) by a humidifier. After attaining the required RH level, removing ozone by passing it through manganese dioxide/activated carbon co-catalyst in the scrubber, and signaling via beep/LED turn-on after attaining a safe ozone concentration.
The concept of CT (concentration × contact time) implies that inactivation rates are affected by the concentration times the exposure time rather than concentration and exposure time separately, meaning that lower concentration for long contact time would likely give the same effect of higher concentration for a shorter time duration if the product CT values were the same. The CT value of disinfectants was originated from the Chick-Watson model, which defines the relationship between inactivation rate and disinfectant concentration and time, assuming that disinfection follows first-order kinetics (Chick, Citation1908; Sivaganesan & Mariñas, Citation2005). Although the model was developed for disinfection in liquid phases, it can also be modified and applied for air or surface disinfection (EquationEq. (1)(1)
(1) ). For this, we assumed in this study that the local intrinsic ozone concentration (qA) (i.e., the ozone concentration effectively in contact with surface or airborne virus) is proportional to the ozone gas concentration in air surrounding the virus medium (CAG) (EquationEq. (2)
(2)
(2) ). With this assumption, the inactivation equation, which relates the virus log10-reduction to ozone gas concentration times time (t) is given by EquationEq. (4)
(4)
(4) . In EquationEq. (4)
(4)
(4) , k’V is a pseudo-first-order rate constant, which depends on the intrinsic ozone/virus rate constant, kV, as well as the conditions under which inactivation takes place including relative humidity, degree of wetting of the virus sample, composition of the virus medium (type and concentration of ozone-reactive interfering substances, inorganic salts), type of surface, air flow, and temperature. These variables affect ozone mass transfer from gas phase to the virus sample, and its reactivity; ultimately affecting the ozone concentration effectively available for disinfection. In addition, upon drying, substances such as inorganic salts crystallize which may occlude the virus thereby preventing ozone attack. Although these variables are significant, studies on their effects on ozone disinfection of SARS-CoV-2 and other viruses in general are lacking.
(1)
(1)
(2)
(2)
Assuming α and CAG are constant over time, by integration, we obtain:
(3)
(3)
EquationEquation (3)(3)
(3) can also be written in the form of log10-reduction as shown in EquationEq. (4)
(4)
(4) .
(4)
(4)
where CTgas is the CT value based on ozone gas concentration, N and N0 are number of virus units (e.g., PFU/mL) at time (t) and initial time t = 0; CAG is the measured ozone concentration in air surrounding the sample (mg/m3); t is time (min); kV is intrinsic virus inactivation rate constant (m3/(mg min)); α (-) is a coefficient relating the intrinsic ozone concentration where inactivation takes place (qA mg/m3 sample) to CAG (mg/m3 air) (i.e., α=qA/CAG); k’V is pseudo-first-order rate constant of virus inactivation by ozone (m3/(mg min)).
3. Inactivation of surface-adhered SARS-CoV-2
3.1. Effect of CT-value and relative humidity
A summary of literature on SARS-CoV-2 inactivation by ozone is presented in Table SI-1. Surface-adhered SARS-CoV-2 inactivation was reported by Yano et al. (Citation2020) highlighting the observation that the spread of SARS-CoV-2 was reduced by increasing ozone concentrations in the experimental chamber. They deposited a 50 µL (8.5 × 105 pfu) viral suspension of SARS-CoV-2 on 3 cm2 stainless steel plate which were allowed to dry. The ozone concentration was 1 ppm and exposure time was 60 min and 6 ppm for 55 min (CTgas values of 120 and 660 mg min/m3) at 25 °C and 60% to 80% RH. The virus reductions were 97.07% and 99.95% at CTgas values of 120 and 660 mg min/m3 respectively. The virus medium was filtered by ultrafiltration and washed three times with phosphate-buffered saline solution. They claimed it to be World’s first successful study on SARS-CoV-2 viral inactivation by ozone as they obtained efficient inactivation results. This is in contrast to an ozone CTgas value of 330 mg min/m3 reported for disinfecting medical device certification by the Pharmaceuticals and Medical Devices Agency of the Ministry of Health, Labor, and Welfare, Japan (Beck et al., Citation1998). SARS-CoV-2 inactivation was investigated in more details by Blanchard et al. (Citation2020), on its surrogate Influenza Virus A (RSV), by finding the effects of RH, temperature, and ozone dose on inactivation efficacy. They reported a 4-log (99.99%) reduction of viral activity by 20 ppm ozone dose exposure for 40 in (i.e., CTgas = 1600 mg min/m3) at >70% RH and ambient temperatures (21–24 °C) on the surface of PPE’s, including N95 respirators, gowns, and face shields. They noticed that 50–70% RH gave maximum efficacy, with <40% ineffective and >70–80% provided no significant improvement. Similarly, increasing ozone concentration from 20 to 50 ppm at ambient temperature and 50% RH enhanced the inactivation efficacy. On the other hand, a rise in temperature from ambient temperature to 48 °C under 50–70% humidity caused viral inactivation within 5 min while the same rise in temperature at a relatively low RH level did not increase the performance.
Another study on a SARS-CoV-2 surrogate (Human Coronavirus HCoV-229E) led by Lee et al. (Citation2021), confirmed total viral inactivation within 1 min by exposure of 120 ppm gaseous ozone (i.e., CTgas = 240 mg min/m3) directly produced on the surface of N95 and KF94 by a dielectric barrier discharge plasma generator in a ventilated room. Results of scanning electron microscopy and particulate filtration efficiency confirmed the structural and functional integrity of masks for 5 cycles (5 times; 1-min exposure). Zucker et al. (Citation2021) tested the ozone disinfection of model pseudo-viruses (HuCoV-229E), with structural and genomic resemblance to SARS-CoV-2, on different surfaces (glass, aluminum, copper, nickel, stainless steel, and brass) at ozone concentrations of 30, 100, and 1000 ppm. They observed at ∼60% RH, 26 °C and exposure time of 30 min, 1-log10, 1.2-log10, and 2-log10 reduction of viral infectivity of concentrated pseudoviruses-containing supernatant at 30 ppm (CTgas = 1,800 mg min/m3), 100 ppm (CTgas = 6,000 mg min/m3), and 1000 ppm ozone (CTgas = 60,000 mg min/m3) respectively. They also observed similar viral disinfection results in various directions on the studied surfaces i.e., top, interior, sides, and bottom, revealing effective penetration of ozone gas and its efficacy over liquid disinfectants. Clavo et al. (Citation2020) investigated the viricidal efficacy of ozone against heat-inactivated SARS-CoV-2 contaminated PPE gowns and filtering facepiece (FFP2) face masks with a minimum efficiency of >92% under different ozone concentrations and RH conditions by measuring the viral gene amplification using Real-Time Polymerase Chain Reaction (RT-PCR) method on 20 × 10 mm sample contaminated with a drop of 10 µL of a viral strain having 1000 copies/µL. They conducted their study in two sets; low ozone concentration (4–12 ppm) exposure for longer duration (30–50 min) under relative humidity of 62–63% and 99% RH in an ozonation chamber and short-term (0.5–10 min) high ozone concentration (500–40,000 ppm) exposure at 53–65% RH in a hospital. They observed that high ozone concentration (10,000 ppm) favored the viricidal action of ozone on PPE gowns in a minimum time as 30 s (CTgas = 10,000 mg min/m3), 5 min at 4000 ppm (CTgas=40,000 mg min/m3), and 10 min at 2000 ppm (CTgas=40,000 mg min/m3). Whereas lower dose ozone exposure at 4–6.5 ppm under 99% RH level has eliminated the SARS-CoV-2 from PPE gowns within 30–50 min (CTgas ∼315 mg min/m3), however, face masks showed gene amplification under those conditions. Overall, their results showed that increasing RH from 63 to 99% reduced CTgas dose from ∼10,000 to ∼300 mg min/m3, respectively. Their findings validated the viricidal activity of ozone against SARS-CoV-2 for potential application in the management and/or utilization of PPEs. Nevertheless, the virus analysis was made by RT-PCR, thus the results do not differentiate between inactive and active (i.e., infective) virus after treatment and may therefore underestimate the effectiveness of ozone (Hudson et al., Citation2007).
Criscuolo et al. (Citation2021) investigated the sanitization of five SARS-CoV-2 infected (1.5 × 106TCID50/mL) surfaces (fleece, gauze, plastic, wood, and glass) with two different exposure modes; low ozone gas dose (0.2 ppm) exposure which is nontoxic to human in a non-evacuated place and quick sanitization at high ozone dose (4 ppm) in a closed, evacuated room and then compared their ozonation results with the effect of short-wavelength UVC irradiation exposure. Back-titration was performed to evaluate the viral titer reduction. Results obtained from 2 h exposure of 0.2 ppm gaseous ozone (CTgas=48 mg min/m3) showed complete inactivation only at fleece (>3-log), while fewer viral titer reductions were obtained for gauze (96.8%), wood (93.3%), glass (90%), and the least one on plastic (82.2%). Similarly, quick sanitization resulted in viral titer reduction of 98.2% (glass), 99.8% (gauze) within 90 min of ozone gas exposure while 120 min exposure caused 99.8% at fleece and only 90% at plastic surface. Overall, this study showed that CTgas doses of 240 mg min/m3 could achieve at least 90% virus reduction on all studied surfaces. On the other hand, rapid viral titer reduction was achieved on exposed surfaces by 1.8 mW/cm2 UVC irradiation for 15 min at a distance of 20 cm from the lamp; the UVC was, however, highlighted as ineffective to disinfect difficult surfaces such as wood. Their study findings suggested the use of ozone generators and/or devices with in-built UVC lamps for rapid sanitization of highly populated indoor environments such as nosocomial areas that are hard to disinfect thoroughly, to mitigate spread of the disease.
The fumigation of eight different SARS-CoV-2 contaminated surfaces (stainless steel, painted and not-painted aluminum, Plexiglas, glass, plastic, FFP2 mask and surgical gown) was investigated by Percivalle et al. (Citation2021) using three different concentrations of ozone (0.5, 1, and 2 ppm) for 40 and 60 min, respectively. They spotted 50 µL droplets on 3 × 8 cm surface and exposed to gaseous ozone at 0.5, 1 and 2 ppm for 40 min and 60 min (∼120 mg min/m3) at 55% RH and 24 °C; the droplets were not dried at the beginning of experiment. The study showed that the virucidal efficacy of ozone was not dependent on surface type. However, when they dried the droplets, no viable virus was measured in the treated and control samples. The authors suggested that hydration is essential for the survival of virus. Overall, their results demonstrated approximately 80% viral titer reduction after ozone exposure of about 1 ppm for 40 min (CTgas=80 mg min/m3) with a negligible variation among various surfaces and no apparent differences between 40- and 60-minutes exposure times. This leads to suggest that the CTgas for this study may be an overestimation of the CT value for the reported level of virus viability reduction since no shorter times than 40 min were studied. The efficacy of fully automatic room decontamination was investigated by Franke et al. (Citation2021) against bacteriophage φ6 and bovine coronavirus L9 as highly active surrogates for SARS-CoV-2. Three different surfaces (furniture board, ceramic tile, and stainless steel) were contaminated with Φ6 with a titer of 1.4–3.2 × 107 plaque-forming units (pfu)/mL and 2.5–6.4 × 105 pfu/mL and were placed in a 6 m3 wide gas-tight ozone-decontamination device at two different positions (high at 1.69 m and low at 0.07 m at 90% RH level and 22 °C). More than 4-log inactivation was achieved against both viral strains regardless of surface material type within 60 min at 80 ppm ozone gas exposure (CTgas=9600 mg min/m3). After completion of the disinfection experiment, the ozone was converted back to oxygen and room ozone concentration was reached 0.1 ppm implying the feasibility of ozone-decontamination procedure for high-risk indoor places. Another group, Uppal et al. (Citation2021) tested the virucidal activity of ozone-based dry-sanitizing device (FATHHOME’s) against inactivation of human coronavirus (HCoV-OC43), model of SARS-CoV-2 on N95 facepiece filter respirators (FRR) and a glass surface. The virus was in a culture medium containing FBS and was exposed to ozone without being dried. A 3-log inactivation was obtained using 20 ppm ozone exposure for 10 min (CTgas=400 mg min/m3) and over 6-log (complete) inactivation was achieved at 25 ppm exposure for 15 min (CTgas=750 mg min/m3) on both surfaces, whereas faster and complete inactivation resulted within 10 min using a higher ozone concentration of 50 ppm (CTgas=1000 mg min/m3). Interestingly, their study used Reverse Transcriptase qPCR (qRT-PCR) and infectivity assay to quantify the viral genomes. The results, generally, showed higher infectivity reduction in comparison to reduction of genomes quantified by qRT-PCR. Volkoff et al. (Citation2020) reported the viral inactivation efficacy of ozone against SARS-CoV-2 obtained from municipal wastewater by RT-PCR method, where 60 µL of wastewater having 140 viral copies/µL were dosed on hard-surfaced laminated strips for testing. They found complete inactivation under ozone exposure of 4.5 ppm for 60 min (CTgas=540 mg min/m3) at RH 47–58% and they suggested that this dose could represent a viable target concentration for SARS-CoV-2 disinfection. The same authors investigated the ozone gas diffusion and its compatibility with common indoor surface materials, such latex gloves, nitrile gloves, paper, laptop, computer monitor, mobile phone, hardware flooring, ceramic floor tiles, stainless steel, iron fillings, plastic items, and butyl rubber. Their results demonstrated complete compatibility of all the items in terms of color, function, material stability at 12 ppm ozone exposure for 30 min, except latex gloves that developed discoloration and degradation. The virucidal effect of ozone gas on SARS-CoV-2 infected stainless steel surface was also investigated by Murata et al. (Citation2021). They used 50 µL of virus solution dropped and spread onto a 2 cm stainless steel disk carrier and air-dried. Virus was collected by suspending the carriers in DMEM containing 2% FBS (500 μL). The study showed that at ambient humidity (RH 55%), a 0.1 ppm ozone gas applied for 10 h or 0.05 ppm for 20 h, both conditions have the same CTgas dose (120 mg min/m3), resulted in only 68.4% infectivity reduction measured by viral titer. However, exposure of virus at RH 80% for 10 h to 0.1 ppm or for 20 h at 0.05 ppm (i.e., CTgas=120 mg min/m3) resulted in approximately 95% viral reduction. The authors concluded that humidity plays a significant role in reducing SARS-CoV-2 infectivity. The study also showed that there was no difference in infectivity reduction for the same CTgas value obtained by concentration doubling but time halving.
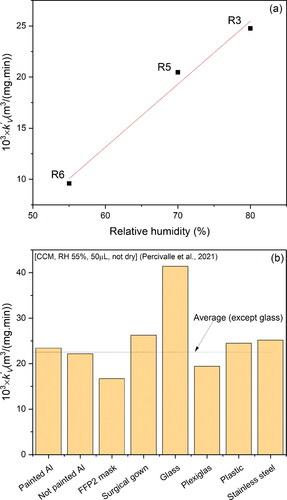
3.2. Kinetic modeling
In the current study, we determined the values of k’V (EquationEq. (4)(4)
(4) ) from virus inactivation data reported in the reviewed studies (). According to , the values of k’V varied between 6.1 × 10−4 to 38.4 × 10−3 m3/(mg min) depending on the experimental conditions. This range of k’V values signifies that to obtain, for example, a 3-log10 virus reduction, the range of CTgas values vary widely between 11,324 to only 180 mg min/m3 for the low and high k’V, respectively. This indicates that not only the CTgas alone influences the inactivation process, but as previously highlighted, other factors related to the experimental conditions (e.g., RH, composition of virus solution, etc.) are also significant. The highest k’V value was obtained for the study conducted by De Forni et al. (Citation2021) (R1 in ) possibly due to the state of the sample being wet. The presence of water in the virus sample during ozone disinfection facilitates ozone mass transfer and consequently increases the effective ozone concentration in contact with the virus as opposed to a dried sample (M. Sharma & Hudson, Citation2008). Water also leads to the formation of hydroxyl radicals which are potent oxidizing agents against the virus. Besides the state of the virus sample, the volume of the virus solution in study R1 was the smallest in comparison to other studies, which results in higher specific surface area available for ozone mass transfer. All these experimental conditions favor better ozone/virus contact and reactivity resulting in a high inactivation rate. The studies R2, R3 and R4 () gave similar k’V value (∼24.8 × 10−3 m3/(mg min)) that could be explained by the similarity of their experimental conditions (50 µL sample volume and either wet or high RH samples). However, as the sample was dried before exposure to ozone and as RH was reduced from 70% (R5) to 55% (R6), the k’V values reduced to 20.5 × 10−3 and 9.6 × 10−3 m3/(mg min), respectively (). This clearly demonstrates the positive influence of high RH on ozone gas disinfection, which could be represented by a linear function with a slope 0.062 m3/mg min/1% RH as illustrated in , using data from R3, R5 and R6. The value of k’V obtained for the study conducted by Criscuolo et al. (Citation2021) (R7) () was about half of that obtained by Murata et al. (Citation2021) (R6) but since no information was given in terms of RH and state of the virus sample used by Criscuolo et al. (Citation2021), a plausible justification for this difference cannot be made. In the study conducted by Volkoff et al. (Citation2020) (R8), k’V was about 15 times lower than that obtained by Murata et al. (Citation2021) (R6) who used similar RH (52%–55%). Although, both the volume and state of the virus sample used in Volkoff et al. (Citation2020) were reported, the magnitude of k’V is significantly low and could be explained by shedding of the virus from ozone attack by the porous filter paper used as the support surface in their study. Besides, the virus concentration was determined by RNA measurement, a method that cannot differentiate between infective and non-infective viral concentrations, thus the calculated reduction of infective virus could be underestimated leading to low k’V value. The calculated rate constant k’V obtained from the data reported in Zucker et al. (Citation2021) (study R9) was the lowest among all of the reviewed studies 6.1 × 10−4 m3/(mg min) despite the experimental conditions (wet sample and small volume) being favorable for higher inactivation; this is in comparison to similar conditions in other studies such as study R4. The extremely low k’V calculated for Zucker et al. (Citation2021) (∼40 times lower than k’V for R4) could be due to the complex cell culture medium used to synthesize the pseudovirus model for SARS-CoV-2 (Zucker et al., Citation2021). In addition, the ozone concentrations used by Zucker et al. (Citation2021) were much higher which could decrease the diffusivity of ozone because the diffusion coefficient is a decreasing function of concentration, while high ozone concentrations increase the rate of side reactions. Thus, the overall resulting effect is reduction in ozone rate as manifested by low observed rate constant k’V.
Figure 2. (a) Pseudo-first-order rate constants, k’V, of ozone inactivation of surface SARS-CoV-2 obtained for different experimental conditions; (b) Effect of ozone gas concentration on required time to attain ∼99% virus inactivation (RH∼50–60%). Experimental conditions—(Support material, virus solution, RH, volume of virus solution spread on the support material, physical state of the sample at the start of ozonation): R1: (Plastic, CCM, RH 50%, 0.5 µL, not dry) (De Forni et al., Citation2021); R2: (SS, CCM, RH 55%, 50 µL, not dry) (Percivalle et al., Citation2021); R3: (SS, CCM centrifuged at low speed and filtered, RH 80%, 50 µL, dry) (Murata et al., Citation2021); R4: (Plastic, CCM, RH 55%, 50 µL, not dry) (Percivalle et al., Citation2021); R5: (SS, CCM filtered and washed with phosphate solution, RH 70%, 50 µL, dry) (Yano et al., Citation2020); R6: (SS, CCM centrifuged at low speed and filtered, RH 55%, 50 µL, dry) (Murata et al., Citation2021); R7: (Plastic, CCM, RH ?%, 50 µL, ?) (Criscuolo et al., Citation2021); R8: (Filter paper, CCM, RH 52%, ? µL, ?) (Volkoff et al., Citation2020)—SARS-CoV-2 RNA; R9: (Plastic, complex mixture of CCM, RH 60%, 13 µL, not dry) (Zucker et al., Citation2021)—pseudovirus; CCM: cell culture medium; SS: stainless steel; RH: relative humdity.
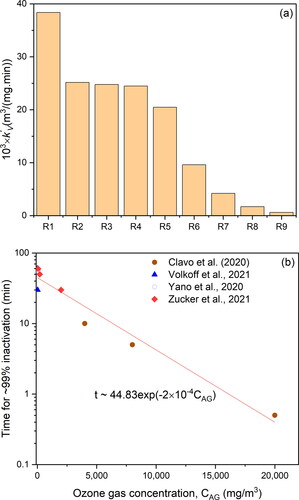
3.3. Effect of ozone gas concentration (CAG)
To illustrate the effect of ozone gas concentration (CAG) on the disinfection efficiency, we reviewed the time required for attaining >99% inactivation at RH∼60–70%. shows that for low ozone concentrations (∼10 mg/m3), around 45 min (CTgas ∼450 mg min/m3) are required for this level of virus inactivation. However, as disinfection times are reduced to around 10 min, the ozone concentration was substantially increased in nonlinear fashion to ∼6000 mg/m3 (CTgas ∼60,000 mg min/m3). Based on data reported by the references in , an empirical decaying exponential equation that correlates the time required for ∼99% inactivation (t∼99%) and gas concentration (CAG) at ∼60–70% RH can be represented by t∼99% = 44.83 exp (−2 × 10−4 CAG), where time in minutes and CAG in mg/m3. Thus, if time is not a crucial factor for a given disinfection application, low ozone concentrations (<10 mg/m3 or 5 ppm) applied for about one hour at RH ∼70% could be sufficient to disinfect an environment while rapid disinfection would require significantly high ozone concentrations (e.g., CAG(mg/m3)/t∼99%(min) are 2000/30;7500/15;11000/5).
3.4. Effect of support material
The effect of support material was evaluated using data from the study conducted by Percivalle et al. (Citation2021); the results are plotted in . Apart from glass, the values of k’V do not appear to significantly vary with the type of materials used (painted aluminum, not painted aluminum, surgical gown, plexiglass, plastic, and stainless steel). The slightly higher k’V for glass (∼1.8 times higher than the average k’V for the other materials) is possibly due to its better wettability (contact angle ∼30°) in comparison to the other materials (contact angle ∼70°). Lower contact angle means that the liquid virus solution spreads over a larger surface area, thus establishes better contact between ozone and the virus, which enhances the virucidal effect (Huhtamäki et al., Citation2018). The area, A, that a droplet occupies once deposited on a surface depends on the contact angle, θ, and can be calculated by A=π(R2+H2); where H and R are the droplet height and wetted radius, respectively, with the ratio determined by
and R determined from the volume of the droplet by
1/3. Based on a volume of 50 µL droplet, as used in the study of Percivalle et al. (Citation2021), the ratio of droplet areas deposited on materials of contact angles of 30° and 70° was found to be equal to 1.5. This ratio of areas is in the same order of magnitude to the ratio of k’V obtained for glass and the other materials, which further supports the explanation provided. This suggests that hydrophilicity of the surface (or contact angle) plays an important role in enhancing ozone inactivation of surface-adhered viruses. However, this surface property is rarely reported in virus inactivation studies, which makes comparison of studies difficult.
This review of literature, and as summarized in Figure SI-3, demonstrates that ozone can be applied in its gaseous form to achieve high disinfection of SARS-CoV-2 on surfaces. However, there is a glaring discrepancy between the experimental results, rightly due to the ozone inactivation ability being prone to the effect of several experimental variables and not only to the ozone concentration and exposure time. The high CTgas reported by Clavo et al. (Citation2020) and Zucker et al. (Citation2021) for disinfection of surface-adhered viruses tend to be exceptional which only account for rapid disinfection in shortest possible time exposure (fraction of seconds), whereas, ordinary ozone exposure is required at ambient conditions i.e., ∼10 mg/m3 ozone dose exposure for ∼40–60 min (CTgas ∼450 mg min/m3) at ∼70% RH and ambient temperature (21–25 °C) produces ≥99% inactivation of surface-adhered SARS-CoV-2 virus and a much lower ozone CTgas values (<120 mg min/m3) are sufficient to disinfect aerosolised virus. Many gaps in knowledge remain including the factors that influence inactivation and how to control them. We therefore, recommend that investigations should be carried out under standardized conditions to understand how inactivation takes place across different settings and be able to compare studies. However, before standard experimental protocols are set and agreed by the community, research studies should report all details of the experimental conditions they used and present the information clearly to avoid misinterpretation or guessing.
4. Inactivation of airborne SARS-CoV-2
Studies on ozone inactivation of airborne and aerosolized SARS-CoV-2 are scarce. Blanco et al. (Citation2021) showed that in most cases, CTgas values <200 mg min/m3 are required for a 99% reduction of aerosolised viruses and based on the similitude between SARS-CoV-2 and murine norovirus, the authors suggested that a CTgas of 20 mg min/m3 could be sufficient to inactivate SARS-CoV-2 under high RH. Recent research studies have linked outdoor ambient ozone concentrations to a reduction in the half-life time of SARS-CoV-2, thereby, a reduction in infection cases (Semple & Moore, Citation2020; Yao et al., Citation2020). Yao et al. (Citation2020) reported that the spread of SARS-CoV-2, potentially through the airborne route, was reduced by increased ambient ozone concentrations from ∼49 to 95 μg/m3 at a rate of 0.0623 log10 confirmed cases for every 1 μg/m3 increase in ambient ozone concentration. Recently, one group of researchers Albert et al. (Citation2021) investigated the efficacy, safety and efficiency of aqueous ozone spraying using unmanned aerial vehicle (UAV) to produce atmospheric ozone concentrations of ∼ 0.04 ppm. The authors reported that >4.5-log inactivation of two different SARS-CoV-2 strains in aqueous solutions were achieved using 0.75 mg/L aqueous ozone within 5 min of incubation and 3-log inactivation was obtained using half of this aqueous ozone concentration within the same time duration. Using a non-dried droplet (volume 0.5 µL), De Forni et al. (Citation2021) found that exposure of SARS-CoV-2 to 3 ppm ozone for 20 min (CTgas=120 mg min/m3) at 21 °C and 50% RH resulted in 99% inactivation of the virus. Their results show that as droplet size increased to 3 and 10 µL, the virus reduction after exposure to ozone (120 mg min/m3) was reduced to 58% and 37%, respectively. We used these results reported in De Forni et al. (Citation2021) and found that the log10 reduction at (120 mg min/m3, 50% RH, 21 °C, non-dried droplet) could be correlated by a power function to the droplet volume as: LR = 1.0219×V−0.754; where V is the droplet volume in µL and LR is the log10-reduction. Thus, by extrapolation, the LR of a 5 µm droplet is almost an infinite number indicating that ozone is highly effective against small droplets such as those originating from human coughing, sneezing or breathing (<5 µm) as also suggested by De Forni et al. (Citation2021). The reduction of droplet size enhances the absorption of ozone in the fluid due to increased specific surface area available for ozone mass transfer (i.e., the specific area of a droplet is inversely proportional to its diameter). Thus, the inactivation of airborne SARS-CoV-2 could be achieved rapidly without the need for high ozone concentrations. This is particularly beneficial in applications where high ozone concentration could be an issue (e.g., damage to rubber materials).
5. Comparison to other enveloped viruses
SARS-CoV-2 belongs to the family of enveloped viruses, which are viruses whose genome (i.e., either deoxyribonucleic acid (DNA) or Ribonucleic acid (RNA), generally enclosed in a capsid protein) is shielded by an external lipid-envelope layer; examples of such viruses include Corona viruses, Ebola virus, Influenza virus, etc. (Torrey et al., Citation2019). Generally, this external lipid-layer envelope makes virus more susceptible to ozone attack (Alimohammadi & Naderi, Citation2021). Tseng and Li (Citation2008) performed a set of studies on the gelatin-based plastic plate surface and quantified the inactivation of enveloped virus bacteriophage φ6 and three non-enveloped phage viruses (MS2, φX174, and T7) by plaque assay. They observed an exposure to ∼0.6 ppm ozone at 55% RH resulted in 1-log (90%) inactivation of φ6 in 22 min (CTgas∼25 mg min/m3) and found 20–25% reduction in viral inactivation time by 50% rise in ozone dose. Similarly, a 1.2–2.4-fold decrease in inactivation time was observed by increasing RH level from 55 to 85%. Ozone dose requirement at 85% RH level was found to be 2-fold lower than at 55% for the same 1-log and 2-log inactivation. They inferred that ozone can be used as a disinfectant for surfaces and indoor places using low ozone doses for a longer duration to avoid the harmful effects caused by ozone exposure.
Hudson et al. (Citation2009) investigated the factors influencing the inactivation efficacy of gaseous ozone against viruses attached to surfaces and developed a practical method for disinfecting indoor spaces. They evaluated the inactivation of a group of enveloped viruses including Influenza A, Rhinovirus, Herpes Simplex Virus Type 1, Sindbis Virus, Yellow Fever Virus, And Vesicular Stomatitis Virus and a pair of non-enveloped viruses (Adenovirus and Poliovirus) using different hard and porous surfaces (stainless steel, plastic, cotton, and glass). They observed 2-log inactivation by exposure to 50 mg/m3 for 50 min (CTgas =2500 mg min/m3) at 40% RH. However, at high RH levels ≥90%, ⁓3-log inactivation of all 12 viruses were achieved by exposure to 40–50 mg/m3 for only a short time (⁓25 min) (CTgas∼1000–1250 mg min/m3), in the laboratory and in their simulated field trials. These findings show that ozone is effective against enveloped viruses deposited on surfaces whilst highlighting the important positive effect of high RH on inactivation as also outlined for SARS-CoV-2.
The effects of ozone dose, exposure time, viral strain type, and RH on inactivation efficacy of airborne enveloped virus bacteriophage φ6 (surrogate of HIV) and three non-enveloped phage viruses (MS2, φX174, and T7) were investigated by Tseng and Li (2006). In their experiments, they used a laboratory-made test chamber consisting of an aerodynamic particle sizer to measure the real-time number-concentration and size distribution of virus-laden aerosols. The predicted CTgas values of the tested virus-containing aerosols of size <2.1 μm were in the range of 0.34–1.98 and 1.05–4.19 mg min/m3 for 1-log (90%) and 2-log (99%) inactivation, respectively, for all four viruses tested in their study. The required ozone concentration at 55% RH was found to be 1.2–1.7 times higher than those at 85% RH levels at the same 1-log and 2-log viral inactivation possibly due to more hydroxyl radicals being generated at high RH levels. Their findings suggested the lethal potency of ozone to airborne viruses depending upon ozone dose, contact time, and RH level. Dubuis et al. (Citation2020) tested the ozone inactivation efficacy in hospitals for airborne enveloped virus φ6 and non-enveloped bacteriophages (φX174, PR772, and MS2) and Murine Norovirus MNV-1. They observed 2-log inactivation of φ6 at low ozone exposure 2.46 mg/m3 for 10 min (CTgas∼25 mg min/m3) at 20% RH while similar log10 reduction was obtained for non-enveloped viruses but within a much longer exposure time (40 min) and at high RH 80%, which highlights the fragility of enveloped viruses for ozone attack. In their recent review, Bayarri et al. (Citation2021) proposed for aerosols much higher CTgas values between 100 to 400 mg min/m3 to achieve 3–4 log10 virus reductions whilst they suggested CTgas values of 1000 to 4000 mg min/m3 to guarantee an inactivation of 3–4 log10 on surfaces. This suggests that the CTgas values required for airborne viruses are approximately 10 times lower than for surface-adhered viruses.
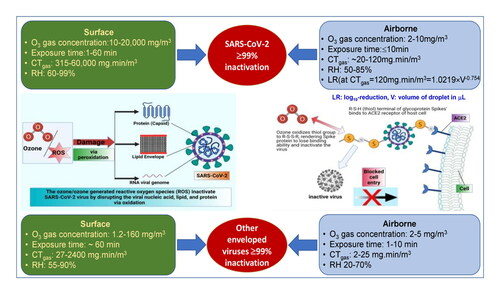
In summary, the variation among airborne findings of various researchers could be rooted in several factors including methods of aerosol generation, sampling procedures, and methods of viral infectivity measurements. Aerosol sampler and their collection efficiency greatly affect the estimation of viral infectivity in a disinfection procedure (Hudson et al., Citation2009). The successful inactivation of SARS-CoV-2 on tested surfaces (stainless steel, surgical masks, N95 respirators, gowns, and face shields) has proven to be a suitable option against the prevention of this disease transmission. Key working conditions required for >99% ozone-mediated inactivation of SARS-CoV-2 and other enveloped viruses are summarized on the Graphical Abstract. Based on reported evidence, generally, 2–12 mg/m3 ozone dose exposure for ∼10 min (CTgas∼20–120 mg min/m3) at 50–85% RH and ambient temperature (21–25 °C) causes >99% inactivation of airborne viruses while 10–20,000 mg/m3 for 1–60 minutes (CTgas⁓315–60,000 mg min/m3) inactivates viruses adhered to common surfaces at 60–99% RH and ambient temperature (21–25 °C). However, care should be taken when using these CTgas values since there is greater diversity in the data reported due to diversity in experimental conditions and the effect of ozone concentration is not linear.
6. Limitations of gaseous ozone
Being a gaseous potent oxidant, made up of oxygen only, ozone-based disinfection technology is practical and beneficial compared to other counterparts such as chlorine dioxide, hydrogen peroxide vapors, cold plasma, etc. (Alimohammadi & Naderi, Citation2021). Ozone is a highly effective disinfectant for almost all kinds of viruses and its ability for disinfection is significantly higher than other disinfectants such as hypochloric acid (25 times), hypochlorite (∼3000 times) (Alimohammadi & Naderi, Citation2021; Moccia et al., Citation2020; Rojas-Valencia, 2011) and so for hydrogen peroxide (Filippi, Citation2000). Besides inherent disinfectant characteristics, the interest in using ozone lies in its ability to penetrate spaces easily and revert back into oxygen after use, without releasing toxic by-products (Britton et al., Citation2020). The ozone technology is relatively cheap and could, through optimized operating conditions, be tailored for rapid disinfection demands. However, three major impediments arise in using ozone as a disinfectant, rendering its use to be limited, which are related to (i) the health risks linked to ozone exposure, (ii) deleterious effects to surfaces/material composition, and (iii) the oxidation products that may form because of ozone reactions with materials.
The permissible exposure limit of ozone by the U.S. Environmental Protection Agency and National Ambient Air Quality Standard for outdoor ozone exposure is 0.08 ppm over 8 hours average (EPA 7, Citation2005; McCarthy, Citation2014) and the National Institute for Occupational Safety and Health has set the Threshold Limit Value for ozone as 0.10 ppm and the Immediately Dangerous to Life and Health value as 5 ppm (National Institute for Occupational Safety and Health, Citation2007). Ozone inhalation, as low as 0.5 ppm, may induce asthma, throat irritation, chest pain, shortness of breath, and lung tissue inflammation and 50 ppm exposure for 30 min may cause death (Health and Safety Executive, Citation2001). The susceptibility of personnel to ozone exposure may vary among healthy or individuals with underlying respiratory difficulties.
Ozone at high concentrations can also damage materials containing dyes and pigments such as latex, rubber, fabric, electrical wire coating, artwork, etc. In addition, it can harmfully affect indoor plants by catalyzed surface reactions producing a variety of carbonyl compounds (aldehydes, ketones, and carboxylic acid); especially formic acid is an issue since it causes lung irritation and is corrosive (Poppendieck et al., Citation2007). These facts make air quality monitoring an essential safety measure.
Though by-product concentration can be reduced by catalysts (MnO2/activated carbon) integrated in the ozone generator.
Therefore, virucidal application of gaseous ozone should never be practised in inhabited areas. It is mandatory to ensure the room to be treated is free of human, pets, indoor plants, and/or susceptible surfaces (Cristiano, Citation2020). Further, the working place should be sealed or quarantined for the duration of treatment to prevent ozone exposure of nearby environments while specific risk assessment should be done for each space depending on its specificities.
7. Conclusions
Fomite and aerosol transmission of infectious pathogens poses a great danger not only in spreading the disease but also in its eradication. SARS-CoV-2, being a respiratory virus, has proven to be highly infectious and lethal. Combatting this virus, thus far, primarily requires controlling the spread of the virus which undoubtedly demands efficient disinfection methods to sanitize the infected surfaces and air. Ozone has been evidenced, both in the lab and in the field (real-time), to be a highly efficient virucidal agent and offers a wide range of benefits over conventional physical and chemical disinfectants. Being gaseous, it dominates the liquid disinfectants for penetration, does not need storage and can be inexpensively generated in-situ from air using electrical generators while it can be converted back to oxygen within a short period of time using catalysts. Based on the reviewed literature, 10–20,000 mg/m3 ozone applied for 1–60 min (CTgas⁓315–60,000 mg min/m3) at 60–99% relative humidity (RH) and ambient temperature inactivate SARS-CoV-2 adhered to common surfaces by ≥99%. When rapid disinfection is required (e.g., ambulances and medical facilities), time of less than 10 min could be employed but at the expense of extremely high ozone concentrations. Nevertheless, a much lower ozone CTgas values (<120 mg min/m3) are sufficient to disinfect aerosolised virus. Thus, under controllable conditions of humidity, exposure time, and ozone gas concentration, ozone disinfection is a robust, scalable, flexible, and controllable methodology for the abatement of airborne and surface-borne SARS-CoV-2.
8. Future scope of work
This review highlighted discrepancies between studies in terms of the requirements for ozone disinfection due to differences in experimental conditions and laboratory protocols used. Of particular significance is the nature of the virus matrix, which could contain substances (e.g., amino acids, inorganics) that reduce ozone’s virucidal effect, yet this is rarely considered in the literature. Nevertheless, the reviewed data and correlations developed in this article can be utilized to advance the use of ozone as a mitigation strategy in combating COVID-19. Research studies are therefore required to fill in the gaps in knowledge to understand the impact of factors that influence the inactivation kinetics and how to control them. There is also a need to standardize the experimental methods of inactivation procedures taking place across different environmental settings so comparisons between studies can be made. Prior to such standards are set and agreed by the research community, it is highly recommended that current and future research studies should report all details of their experiments including virus strain, virus medium and purification method used, initial virus concentration, volume of the virus sample, method used to analyze the virus concentration before and after inactivation, surface area of the sample, its physical state pre- and post-ozonation (i.e., liquid or dried), ozone gas concentration and gas flow rate, the method used to produce ozone gas, air mixing (e.g., stagnant, flowing or internal circulation), experimental chamber sizes and content including material, type of surface used and its properties (at least the study should report whether the material was porous or non-porous, and the contact angle between the virus medium and the surface), properties of the substrate surface before and after ozonation (any change in morphology, etc.), working temperature, humidity level, exposure time, rate constant of inactivation, and inactivation percentage with log 10-reduction. These details are mandatory to be reported to provide clear understanding of procedures used and avoid misinterpretation of results.
| Abbreviations | ||
| CCM | = | cell culture medium |
| CoV | = | coronavirus |
| CT | = | concentration × contact time |
| ppm | = | parts per million |
| RH | = | relative humidity |
| ROS | = | Reactive oxygen species |
| RT-PCR | = | Real-Time Polymerase Chain Reaction |
| FBS | = | Fetal Bovine serum |
| SARS | = | Severe Acute Respiratory Syndrome |
| SS | = | stainless steel |
| ssRNA | = | single-stranded ribonucleic acid genomic material |
| WHO | = | World Health Organization. |
Supplemental Material
Download MS Word (801.5 KB)Disclosure statement
There is no conflict of interest to be addressed.
References
- Aboubakr, H. A., Sharafeldin, T. A., & Goyal, S. M. (2021). Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transboundary and Emerging Diseases, 68(2), 296–312. https://doi.org/10.1111/tbed.13707
- Albert, S., Amarilla, A. A., Trollope, B., Sng, J. D. J., Setoh, Y. X., Deering, N., Modhiran, N., Weng, S. H., Melo, M. C., Hutley, N., Nandy, A., Furlong, M. J., Young, P. R., Watterson, D., Grinham, A. R., & Khromykh, A. A. (2021). Assessing the potential of unmanned aerial vehicle spraying of aqueous ozone as an outdoor disinfectant for SARS-CoV-2. Environmental Research, 196, 110944. https://doi.org/10.1016/j.envres.2021.110944
- Alimohammadi, M., & Naderi, M. (2021). Effectiveness of ozone gas on airborne virus inactivation in enclosed spaces: A review study. Ozone: Science & Engineering, 43(1), 21–31. https://doi.org/10.1080/01919512.2020.1822149
- Allison, K., Hook, J., Cardis, D., & Rice, R. G. (2009). Quantification of the bactericidal, fungicidal, and sporicidal efficacy of the JLA Ltd. ozone laundering system. Science and Engineering. https://doi.org/10.1080/01919510903155816
- Ataei-Pirkooh, A., Alavi, A., Kianirad, M., Bagherzadeh, K., Ghasempour, A., Pourdakan, O., Adl, R., Kiani, S. J., Mirzaei, M., & Mehravi, B. (2021). Destruction mechanisms of ozone over SARS-CoV-2. Scientific Reports, 11, 18851. https://doi.org/10.1038/s41598-021-97860-w
- Bayarri, B., Cruz-Alcalde, A., López-Vinent, N., Micó, M. M., & Sans, C. (2021). Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. Journal of Hazardous Materials, 415, 125658. https://doi.org/10.1016/j.jhazmat.2021.125658
- Beck, E. G., Wasser, G., & Viebahn-Hansler, R. (1998). The current status of ozone therapy - Empirical developments and basic research. Forschende Komplementarmedizin, 5, 61–75.
- Bianconi, V., Bronzo, P., Banach, M., Sahebkar, A., Mannarino, M. R., & Pirro, M. (2020). Particulate matter pollution and the Covid-19 outbreak: Results from Italian regions and provinces. Archives of Medical Science: AMS, 16(5), 985–992. https://doi.org/10.5114/aoms.2020.95336
- Blanchard, E. L., Lawrence, J. D., Noble, J. A., Xu, M., Joo, T., Ng, N. L., Schmidt, B. E., Santangelo, P. J., & Finn, M. G. (2020). Enveloped virus inactivation on personal protective equipment by exposure to ozone. MedRxiv: The Preprint Server for Health Sciences. https://doi.org/10.1101/2020.05.23.20111435
- Blanco, A., Ojembarrena, F., de, B., Clavo, B., & Negro, C. (2021). Ozone potential to fight against SAR-COV-2 pandemic: Facts and research needs. Environmental Science and Pollution Research International, 28(13), 16517–16531. https://doi.org/10.1007/s11356-020-12036-9
- Britton, H. C., Draper, M., & Talmadge, J. E. (2020). Antimicrobial efficacy of aqueous ozone in combination with short chain fatty acid buffers. Infection Prevention in Practice, 2(1), 100032. https://doi.org/10.1016/j.infpip.2019.100032
- Chan-Yeung, M., & Xu, R. H. (2003). SARS: Epidemiology. Respirology, 8(s1), S9–S14. https://doi.org/10.1046/j.1440-1843.2003.00518.x
- Chick, H. (1908). An investigation of the laws of disinfection. Journal of Hygiene, 8(1), 92–158. https://doi.org/10.1017/S0022172400006987
- Chin, A. W. H., Chu, J. T. S., Perera, M. R. A., Hui, K. P. Y., Yen, H.-L., Chan, M. C. W., Peiris, M., & Poon, L. L. M. (2020). Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe, 1(1), e10. https://doi.org/10.1016/s2666-5247(20)30003-3
- Clavo, B., Córdoba-Lanús, E., Rodríguez-Esparragón, F., Cazorla-Rivero, S. E., García-Pérez, O., Piñero, J. E., Villar, J., Blanco, A., Torres-Ascensión, C., Martín-Barrasa, J. L., González-Martin, J. M., Serrano-Aguilar, P., & Lorenzo-Morales, J. (2020). Effects of ozone treatment on personal protective equipment contaminated with sars-cov-2. Antioxidants, 9(12), 1222. https://doi.org/10.3390/antiox9121222
- Coker, E. S., Cavalli, L., Fabrizi, E., Guastella, G., Lippo, E., Parisi, M. L., Pontarollo, N., Rizzati, M., Varacca, A., & Vergalli, S. (2020). The effects of air pollution on COVID-19 related mortality in Northern Italy. Environmental & Resource Economics, 76(4), 611–634. https://doi.org/10.1007/s10640-020-00486-1
- Criscuolo, E., Diotti, R. A., Ferrarese, R., Alippi, C., Viscardi, G., Signorelli, C., Mancini, N., Clementi, M., & Clementi, N. (2021). Fast inactivation of SARS-CoV-2 by UV-C and ozone exposure on different materials. Emerging Microbes and Infections, 10, 206–209. https://doi.org/10.1080/22221751.2021.1872354
- Cristiano, L. (2020). Could ozone be an effective disinfection measure against the novel coronavirus (SARS-CoV-2)? Journal of Preventive Medicine and Hygiene, 61, E301–E303. https://doi.org/10.15167/2421-4248/jpmh2020.61.3.1596
- De Forni, D., Poddesu, B., Cugia, G., Gallizia, G., Licata, M. L., Lisziewicz, J., Chafouleas, J. G., & Lori, F. (2021). Low ozone concentration and negative ions for rapid SARS-CoV-2 inactivation. BioRxiv. https://doi.org/10.1101/2021.03.11.434968
- Dennis, R., Cashion, A., Emanuel, S., & Hubbard, D. (2020). Ozone gas: Scientific justification and practical guidelines for improvised disinfection using consumer-grade ozone generators and plastic storage boxes. The Journal of Science and Medicine, 2(1). https://doi.org/10.37714/josam.v2i1.35
- Dietz, L., Horve, P. F., Coil, D., Fretz, M., Eisen, J., & Wymelenberg, K. V. D. (2020). 2019 novel coronavirus (COVID-19) pandemic: Built environment considerations to reduce transmission. mSystems, 5(2), e00245–20. https://doi.org/10.1128/mSystems.00245-20
- Domingo, J. L., Marquès, M., & Rovira, J. (2020). Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Environmental Research, 188, 109861. https://doi.org/10.1016/j.envres.2020.109861
- Dubuis, M. E., Dumont-Leblond, N., Laliberté, C., Veillette, M., Turgeon, N., Jean, J., & Duchaine, C. (2020). Ozone efficacy for the control of airborne viruses: Bacteriophage and norovirus models. PLoS One, 15(4), e0231164. https://doi.org/10.1371/journal.pone.0231164
- EPA 7. (2005). Evaluating ozone control programs in the Eastern United States: Focus on the NOx Budget Trading Program, 2004. Environmental Protection Agency.
- Fennelly, K. P. (2020). Particle sizes of infectious aerosols: Implications for infection control. The Lancet Respiratory Medicine, 8(9), 914–924. https://doi.org/10.1016/S2213-2600(20)30323-4
- Filippi, A. (2000). The disinfecting action of ozonated water and of hydrogen peroxide/silver ions in vitro. Ozone: Science and Engineering, 22, 441–445. https://doi.org/10.1080/01919510009408786
- Filonchyk, M., Hurynovich, V., & Yan, H. (2021). Impact of Covid-19 lockdown on air quality in the Poland, Eastern Europe. Environmental Research, 198, 110454. https://doi.org/10.1016/j.envres.2020.110454
- Fisman, D., Rivers, C., Lofgren, E., & Majumder, M. S. (2014). Estimation of MERS-coronavirus reproductive number and case fatality rate for the Spring 2014 Saudi Arabia outbreak: Insights from publicly available data. PLoS Currents, 6. https://doi.org/10.1371/currents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c
- Franke, G., Knobling, B., Brill, F. H., Becker, B., Klupp, E. M., Belmar Campos, C., Pfefferle, S., Lütgehetmann, M., & Knobloch, J. K. (2021). An automated room disinfection system using ozone is highly active against surrogates for SARS-CoV-2. The Journal of Hospital Infection, 112, 108–113. https://doi.org/10.1016/j.jhin.2021.04.007
- Gorbalenya, A. E., Baker, S. C., Baric, R. S., de Groot, R. J., Drosten, C., Gulyaeva, A. A., Haagmans, B. L., Lauber, C., Leontovich, A. M., Neuman, B. W., Penzar, D., Perlman, S., Poon, L. L. M., Samborskiy, D. V., Sidorov, I. A., Sola, I., & Ziebuhr, J. (2020). The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology, 5, 536–544. https://doi.org/10.1038/s41564-020-0695-z
- Gupta, A., Bherwani, H., Gautam, S., Anjum, S., Musugu, K., Kumar, N., Anshul, A., & Kumar, R. (2021). Air pollution aggravating COVID-19 lethality? Exploration in Asian cities using statistical models. Environment, Development and Sustainability, 23(4), 6408–6417. https://doi.org/10.1007/s10668-020-00878-9
- Hammett, E. (2020). How long does Coronavirus survive on different surfaces? BDJ Team, 7(5), 14–15. https://doi.org/10.1038/s41407-020-0313-1
- Hansen, K. M. S., Spiliotopoulou, A., Chhetri, R. K., Escolà Casas, M., Bester, K., & Andersen, H. R. (2016). Ozonation for source treatment of pharmaceuticals in hospital wastewater - Ozone lifetime and required ozone dose. Chemical Engineering Journal, 290, 507–514. https://doi.org/10.1016/j.cej.2016.01.027
- Health and Safety Executive. (2001). A guide to measuring health & safety. HSE.
- Heinzerling, A., Stuckey, M. J., Scheuer, T., Xu, K., Perkins, K. M., Resseger, H., Magill, S., Verani, J. R., Jain, S., Acosta, M., & Epson, E. (2020). Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient—Solano County, California, February 2020. MMWR. Morbidity and Mortality Weekly Report, 69(15), 472–476. https://doi.org/10.15585/mmwr.mm6915e5
- Heselton, D., Boast, N., Hudson, J., & Esplin, G. (2008). Apparatus and method for using ozone as a disinfectant. U.S. Patent Application 11/605,311.
- Hoff, J. C., & Akin, E. W. (1986). Microbial resistance to disinfectants: Mechanisms and significance. Environmental Health Perspectives, 69, 7–13. https://doi.org/10.1289/ehp.86697
- Hudson, J. B., Sharma, M., & Petric, M. (2007). Inactivation of Norovirus by ozone gas in conditions relevant to healthcare. The Journal of Hospital Infection, 66(1), 40–45. https://doi.org/10.1016/j.jhin.2006.12.021
- Hudson, J. B., Sharma, M., & Vimalanathan, S. (2009). Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone: Science and Engineering, 31, 216–223. https://doi.org/10.1080/01919510902747969
- Huhtamäki, T., Tian, X., Korhonen, J. T., & Ras, R. H. A. (2018). Surface-wetting characterization using contact-angle measurements. Nature Protocols, 13(7), 1521–1538. https://doi.org/10.1038/s41596-018-0003-z
- Hutter, H. P., Poteser, M., Moshammer, H., Lemmerer, K., Mayer, M., Weitensfelder, L., Wallner, P., & Kundi, M. (2020). Air pollution is associated with covid-19 incidence and mortality in Vienna, Austria. International Journal of Environmental Research and Public Health, 17(24), 9275. https://doi.org/10.3390/ijerph17249275
- Jiang, H. J., Chen, N., Shen, Z. Q., Yin, J., Qiu, Z. G., Miao, J., Yang, Z. W., Shi, D. Y., Wang, H. R., Wang, X. W., Li, J. W., Yang, D., & Jin, M. (2019). Inactivation of poliovirus by ozone and the impact of ozone on the viral genome. Biomedical and Environmental Sciences, 32(5), 324–333. https://doi.org/10.3967/bes2019.044
- Josset, S., Taranto, J., Keller, N., Keller, V., & Lett, M. C. (2010). Photocatalytic treatment of bioaerosols: Impact of the reactor design. Environmental Science and Technology, 44, 2605–2611. https://doi.org/10.1021/es902997v
- Kampf, C. J., Liu, F., Reinmuth-Selzle, K., Berkemeier, T., Meusel, H., Shiraiwa, M., & Pöschl, U. (2015). Protein cross-linking and oligomerization through dityrosine formation upon exposure to ozone. Environmental Science and Technology, 49, 10859–10866. https://doi.org/10.1021/acs.est.5b02902
- Khadre, M. A., Yousef, A. E., & Kim, J. G. (2001). Microbiological aspects of ozone applications in food: A review. Journal of Food Science, 66(9), 1242–1252. https://doi.org/10.1111/j.1365-2621.2001.tb15196.x
- Klompas, M., Baker, M. A., & Rhee, C. (2020). Airborne transmission of SARS-CoV-2: Theoretical considerations and available evidence. JAMA, 324(5), 441. https://doi.org/10.1001/jama.2020.12458
- Korber, B., Fischer, W. M., Gnanakaran, S., Yoon, H., Theiler, J., Abfalterer, W., Hengartner, N., Giorgi, E. E., Bhattacharya, T., Foley, B., Hastie, K. M., Parker, M. D., Partridge, D. G., Evans, C. M., Freeman, T. M., de Silva, T. I., McDanal, C., Perez, L. G., Tang, H., … Wyles, M. D. (2020). Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell, 182(4), 812–827.e19. https://doi.org/10.1016/j.cell.2020.06.043
- Lee, J., Bong, C., Lim, W., Bae, P. K., Abafogi, A. T., Baek, S. H., Shin, Y.-B., Bak, M. S., & Park, S. (2021). Fast and easy disinfection of coronavirus-contaminated face masks using ozone gas produced by a dielectric barrier discharge plasma generator. Environmental Science & Technology Letters, 8, 339–344. https://doi.org/10.1021/acs.estlett.1c00089
- Levi, A., & Barnett-Itzhaki, Z. (2021). Effects of chronic exposure to ambient air pollutants, demographic, and socioeconomic factors on COVID-19 morbidity: The Israeli case study. Environmental Research, 202, 111673. https://doi.org/10.1016/j.envres.2021.111673
- Li, C. S., & Wang, Y. C. (2003). Surface germicidal effects of ozone for microorganisms. American Industrial Hygiene Association Journal, 64, 533–537. https://doi.org/10.1080/15428110308984851
- Li, Y., Cheng, P., & Qian, H. (2021). Dominant transmission route of SARS-CoV-2 and its implication to indoor environment. Kexue Tongbao/Chinese Science Bulletin, 66, 417–423. https://doi.org/10.1360/TB-2020-1532
- Liu, Y., Li, T., Deng, Y., Liu, S., Zhang, D., Li, H., Wang, X., Jia, L., Han, J., Bei, Z., Li, L., & Li, J. (2021). Stability of SARS-CoV-2 on environmental surfaces and in human excreta. Journal of Hospital Infection, 107, 105–107. https://doi.org/10.1016/j.jhin.2020.10.021
- Loeb, B. L., Thompson, C. M., Drago, J., Takahara, H., & Baig, S. (2012). Worldwide ozone capacity for treatment of drinking water and wastewater: A review. Ozone: Science & Engineering, 34(1), 64–77. https://doi.org/10.1080/01919512.2012.640251
- Lu, J., Gu, J., Gu, J., Li, K., Xu, C., Su, W., Lai, Z., Zhou, D., Yu, C., Xu, B., & Yang, Z. (2020). COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerging Infectious Diseases, 26(11), 2789–2791. https://doi.org/10.3201/eid2607.200764
- Lv, M., Luo, X., Estill, J., Liu, Y., Ren, M., Wang, J., Wang, Q., Zhao, S., Wang, X., Yang, S., Feng, X., Li, W., Liu, E., Zhang, X., Wang, L., Zhou, Q., Meng, W., Qi, X., Xun, Y., Yu, X., & Chen, Y. L. (2020). Coronavirus disease (COVID-19): A scoping review. Eurosurveillance, 25(15), 14–26. https://doi.org/10.2807/1560-7917.ES.2020.25.15.2000125
- Martins, R. B., Castro, I. A., Pontelli, M., Souza, J. P., Lima, T. M., Melo, S. R., Siqueira, J. P. Z., Caetano, M. H., Arruda, E., & de Almeida, M. T. G. (2021). SARS-CoV-2 inactivation by ozonated water: A preliminary alternative for environmental disinfection. Ozone: Science & Engineering, 43(2), 108–111. https://doi.org/10.1080/01919512.2020.1842998
- McCarthy, J. E. (2014). Ozone air quality standards: EPA’S 2013 revision. In Air quality observation in the U.S.: Systems, needs, and standards. Nova Science Publishers.
- Mittal, R., Ni, R., & Seo, J. H. (2020). The flow physics of COVID-19. Journal of Fluid Mechanics, 894, F2.1–F2.14. https://doi.org/10.1017/jfm.2020.330
- Moccia, G., De Caro, F., Pironti, C., Boccia, G., Capunzo, M., Borrelli, A., & Motta, O. (2020). Development and improvement of an effective method for air and surfaces disinfection with ozone gas as a decontaminating agent. Medicina, 56(11), 578. https://doi.org/10.3390/medicina56110578
- Morawska, L., & Cao, J. (2020). Airborne transmission of SARS-CoV-2: The world should face the reality. Environment International, 139, 105730. https://doi.org/10.1016/j.envint.2020.105730
- Morris, D. H., Yinda, K. C., Gamble, A., Rossine, F. W., Huang, Q. S., Bushmaker, T., Fischer, R. J., Matson, M. J., van Doremalen, N., Vikesland, P. J., Marr, L. C., Munster, V. J., & Lloyd-Smith, J. O. (2021). Mechanistic theory predicts the effects of temperature and humidity on inactivation of SARS-CoV-2 and other enveloped viruses. eLife, 10, e65902. https://doi.org/10.7554/eLife.65902
- Munter, R. (2001). Advanced oxidation processes - Current status and prospect. Proceedings of the Estonian Academy of Sciences Chemistry, 50(2), 59–80. https://doi.org/10.1002/9780470561331.ch18
- Murata, T., Komoto, S., Iwahori, S., Sasaki, J., Nishitsuji, H., Hasebe, T., Hoshinaga, K., & Yuzawa, Y. (2021). Reduction of severe acute respiratory syndrome coronavirus-2 infectivity by admissible concentration of ozone gas and water. Microbiology and Immunology, 65(1), 10–16. https://doi.org/10.1111/1348-0421.12861
- Murray, B. K., Ohmine, S., Tomer, D. P., Jensen, K. J., Johnson, F. B., Kirsi, J. J., Robison, R. A., & O’Neill, K. L. (2008). Virion disruption by ozone-mediated reactive oxygen species. Journal of Virological Methods, 153(1), 74–77. https://doi.org/10.1016/j.jviromet.2008.06.004
- National Institute for Occupational Safety and Health. (2007). Pocket guide to chemical hazards. In DHHS (NIOSH) Publication No. 2005-149. https://doi.org/10.1109/icnn.1993.298588
- Nor, N. S. M., Yip, C. W., Ibrahim, N., Jaafar, M. H., Rashid, Z. Z., Mustafa, N., Hamid, H. H. A., Chandru, K., Latif, M. T., Saw, P. E., Lin, C. Y., Alhasa, K. M., Hashim, J. H., & Nadzir, M. S. M. (2021). Particulate matter (PM2.5) as a potential SARS-CoV-2 carrier. Scientific Reports, 11 (1), 2508. https://doi.org/10.1038/s41598-021-81935-9
- Páez-Osuna, F., Valencia-Castañeda, G., & Rebolledo, U. A. (2022). The link between COVID-19 mortality and PM2.5 emissions in rural and medium-size municipalities considering population density, dust events, and wind speed. Chemosphere, 286, 131634. https://doi.org/10.1016/j.chemosphere.2021.131634
- Paules, C. I., Marston, H. D., & Fauci, A. S. (2020). Coronavirus infections-more than just the common cold. JAMA, 323(8), 707. https://doi.org/10.1001/jama.2020.0757
- Percivalle, E., Clerici, M., Cassaniti, I., Vecchio Nepita, E., Marchese, P., Olivati, D., Catelli, C., Berri, A., Baldanti, F., Marone, P., Bruno, R., Triarico, A., & Lago, P. (2021). SARS-CoV-2 viability on different surfaces after gaseous ozone treatment: A preliminary evaluation. Journal of Hospital Infection, 110, 33–36. https://doi.org/10.1016/j.jhin.2021.01.014
- Poppendieck, D., Hubbard, H., Ward, M., Weschler, C., & Corsi, R. L. (2007). Ozone reactions with indoor materials during building disinfection. Atmospheric Environment, 41(15), 3166–3176. https://doi.org/10.1016/j.atmosenv.2006.06.060
- Quevedo-León, R., Bastías-Montes, J. M., Espinoza-Tellez, T., Ronceros, B., Balic, I., & Muñoz, O. (2020). Inactivation of coronaviruses in food industry: The use of inorganic and organic disinfectants, ozone, and UV radiation. Scientia Agropecuaria, 11(2), 257–266. https://doi.org/10.17268/sci.agropecu.2020.02.14
- Rojas-Valencia, M. N. (2011). Research on ozone application as disinfectant and action mechanisms on wastewater microorganisms. In Science against microbial pathogens: Communicating current research and technological advances. Formatex Research Center.
- Roth, J. A., & Sullivan, D. E. (1981). Solubility of ozone in water. Industrial and Engineering Chemistry Fundamentals, 20, 137–140. https://doi.org/10.1021/i100002a004
- Rowen, R. J., & Howard, R. (2020). A plausible “penny” costing effective treatment for corona virus - Ozone therapy. Journal of Infectious Diseases and Epidemiology, 6(2), 113. https://doi.org/10.23937/2474-3658/1510113
- SAGE – Environmental and Modelling Group 4th June. (2020). Transmission of SARS-CoV-2 and mitigating measures. Sage, 40.
- Salgado, M. V., Smith, P., Opazo, M. A., & Huneeus, N. (2021). Long-term exposure to fine and coarse particulate matter and covid-19 incidence and mortality rate in Chile during 2020. International Journal of Environmental Research and Public Health, 18(14), 7409. https://doi.org/10.3390/ijerph18147409
- Santarpia, J., Rivera, D., Herrera, V., Morwitzer, M. J., Creager, H., Santarpia, G., Crown, K., Brett-Major, D., Schnaubelt, E., Broadhurst, M. J., Lawler, J., Reid, S., & Lowe, J. (2020). Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Scientific Reports, 10(1), 12732. https://doi.org/10.1038/s41598-020-69286-3
- Semple, J. L., & Moore, G. W. K. (2020). High levels of ambient ozone (O3) may impact COVID-19 in high altitude mountain environments. Respiratory Physiology & Neurobiology, 280, 103487. https://doi.org/10.1016/j.resp.2020.103487
- Setti, L., Passarini, F., De Gennaro, G., Barbieri, P., Perrone, M. G., Borelli, M., Palmisani, J., Di Gilio, A., Piscitelli, P., & Miani, A. (2020). Airborne transmission route of covid-19: Why 2 meters/6 feet of inter-personal distance could not be enough. International Journal of Environmental Research and Public Health, 17(8), 2932. https://doi.org/10.3390/ijerph17082932
- Sharma, M., & Hudson, J. B. (2008). Ozone gas is an effective and practical antibacterial agent. American Journal of Infection Control, 36(8), 559–563. https://doi.org/10.1016/j.ajic.2007.10.021
- Sharma, V. K., & Graham, N. J. D. (2010). Oxidation of amino acids, peptides and proteins by ozone: A review. Ozone: Science & Engineering, 32(2), 81–90. https://doi.org/10.1080/01919510903510507
- Shin, G. A., & Sobsey, M. D. (2003). Reduction of Norwalk virus, poliovirus 1, and bacteriophage MS2 by ozone disinfection of water. Applied and Environmental Microbiology, 69(7), 3975–3978. https://doi.org/10.1128/AEM.69.7.3975-3978.2003
- Sivaganesan, M., & Mariñas, B. J. (2005). Development of a Ct equation taking into consideration the effect of lot variability on the inactivation of Cryptosporidium parvum oocysts with ozone. Water Research, 39(11), 2429–2437. https://doi.org/10.1016/j.watres.2005.04.028
- Summerfelt, S. T., & Hochheimer, J. N. (1997). Review of ozone processes and applications as an oxidizing agent in aquaculture. The Progressive Fish-Culturist, 59(2), 94–105. https://doi.org/10.1577/1548-8640(1997)059<0094:ROOPAA>2.3.CO;2
- Thurston-Enriquez, J. A., Haas, C. N., Jacangelo, J., & Gerba, C. P. (2005). Inactivation of enteric adenovirus and feline calicivirus by ozone. Water Research, 39(15), 3650–3656. https://doi.org/10.1016/j.watres.2005.06.006
- Tizaoui, C. (2020). Ozone: A potential oxidant for COVID-19 virus (SARS-CoV-2). Ozone: Science and Engineering, 42(5), 378–385. https://doi.org/10.1080/01919512.2020.1795614
- Torrey, J., von Gunten, U., & Kohn, T. (2019). Differences in viral disinfection mechanisms as revealed by quantitative transfection of echovirus 11 genomes. Applied and Environmental Microbiology, 85(14), e00961-19. https://doi.org/10.1128/AEM.00961-19
- Travaglio, M., Yu, Y., Popovic, R., Selley, L., Leal, N. S., & Martins, L. M. (2021). Links between air pollution and COVID-19 in England. Environmental Pollution (Barking, Essex: 1987), 268, 115859. https://doi.org/10.1016/j.envpol.2020.115859
- Tseng, C. C., & Li, C. S. (2006). Ozone for inactivation of aerosolized bacteriophages. Aerosol Science and Technology, 40(9), 683–689. https://doi.org/10.1080/02786820600796590
- Tseng, C., & Li, C. (2008). Inactivation of surface viruses by gaseous ozone. Journal of Environmental Health, 70, 56–62.
- Uppal, T., Khazaieli, A., Snijders, A. M., & Verma, S. C. (2021). Inactivation of human coronavirus by fathhome’s dry sanitizer device: Rapid and eco-friendly ozone-based disinfection of sars-cov-2. Pathogens, 10(3), 339. https://doi.org/10.3390/pathogens10030339
- Van Doremalen, N., Bushmaker, T., Morris, D. H., Holbrook, M. G., Gamble, A., Williamson, B. N., Tamin, A., Harcourt, J. L., Thornburg, N. J., Gerber, S. I., Lloyd-Smith, J. O., De Wit, E., & Munster, V. J. (2020). Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine, 382(16), 1564–1567. https://doi.org/10.1056/NEJMc2004973
- Volkoff, S. J., Carlson, T. J., Leik, K., Smith, J. J., Graves, D., Dennis, P., Aris, T., Cuthbertson, D., Holmes, A., Craig, K., Marvin, B., & Nesbit, E. (2020). Demonstrated SARS-CoV-2 surface disinfection using ozone. Ozone: Science and Engineering, 43(4), 296–305. https://doi.org/10.1080/01919512.2020.1863770
- Vyskocil, J. M., Turgeon, N., Turgeon, J. G., & Duchaine, C. (2020). Ozone treatment in a wind tunnel for the reduction of airborne viruses in swine buildings. Aerosol Science and Technology, 54(12), 1471–1478. https://doi.org/10.1080/02786826.2020.1790495
- Wang, J., Tang, K., Feng, K., Lin, X., Lv, W., Chen, K., & Wang, F. (2021). Impact of temperature and relative humidity on the transmission of COVID-19: A modelling study in China and the United States. BMJ Open, 11, e043863. https://doi.org/10.1136/bmjopen-2020-043863
- Wiktorczyk-Kapischke, N., Grudlewska-Buda, K., Wałecka-Zacharska, E., Kwiecińska-Piróg, J., Radtke, L., Gospodarek-Komkowska, E., & Skowron, K. (2021). SARS-CoV-2 in the environment—Non-droplet spreading routes. The Science of the Total Environment, 770, 145260. https://doi.org/10.1016/j.scitotenv.2021.145260
- Woolwine, J. D., & Gerberding, J. L. (1995). Effect of testing method on apparent activities of antiviral disinfectants and antiseptics. Antimicrobial Agents and Chemotherapy, 39(4), 921–923. https://doi.org/10.1128/AAC.39.4.921
- World Health Organization. (2020). WHO coronavirus disease dashboard. WHO.Int.
- Wu, S., Wang, Y., Jin, X., Tian, J., Liu, J., & Mao, Y. (2020). Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. American Journal of Infection Control, 48(8), 910–914. https://doi.org/10.1016/j.ajic.2020.05.003
- Wu, X., Nethery, R. C., Sabath, M. B., Braun, D., & Dominici, F. (2020). Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Science Advances, 6(45), eabd4049. https://doi.org/10.1126/sciadv.abd4049
- Yano, H., Nakano, R., Suzuki, Y., Nakano, A., Kasahara, K., & Hosoi, H. (2020). Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. Journal of Hospital Infection, 106(4), 837–838. https://doi.org/10.1016/j.jhin.2020.10.004
- Yao, Y., Tian, Y., Zhou, J., Ma, X., Yang, M., & Wang, S. Y. (2020). Epidemiological characteristics of SARS-CoV-2 infections in Shaanxi, China by 8 February 2020. European Respiratory Journal, 55, 2000310. https://doi.org/10.1183/13993003.00310-2020
- Yu, H., Chen, P., Chen, J., Dai, W., Wu, Z., & Guo, Y. (2018). Efficacy and safety of ozone therapy for patients with chronic hepatitis b: A multicenter, randomized clinical trial. Journal of Ozone Therapy, 2(2). https://doi.org/10.7203/jo3t.2.2.2018.11131
- Zhang, J., Zheng, C., Xiao, G., Zhou, Y., & Gao, R. (2004). Examination of the efficacy of ozone solution disinfectant in in activating sars virus. Chinese Journal of Disinfection. http://en.cnki.com.cn/Journal_en/E-E055-ZGXD-2004-01.htm
- Zucker, I., Lester, Y., Alter, J., Werbner, M., Yecheskel, Y., Gal-Tanamy, M., & Dessau, M. (2021). Pseudoviruses for the assessment of coronavirus disinfection by ozone. Environmental Chemistry Letters, 19(2), 1779–1785. https://doi.org/10.1007/s10311-020-01160-0