Abstract
Birds are considered as good bio-monitors and they can provide highly valuable data about the level of contamination in their habitat. During the design of biomonitoring studies one of the first issues after choosing species is the choice of biological material. Non-lethally collected samples have recently been gaining greater attention as they offer several ethical and practical advantages. However, not all sample matrices are suitable for biomonitoring of certain compounds. The main aim of this review is to bring to closer attention the utility of non-lethally collected samples from avian species, based on recent literature. The selected samples are feathers, blood, preen oil and eggs, as these are the types of biological materials most often chosen and may reveal birds’ exposure from their diet. It is not my intention to single out one of them as the ultimate tool for organic compound analysis, but rather to present their utility in order to support or advise on future choices, as a single matrix might not be sufficient to fully evaluate birds’ exposure. Therefore, this paper presents the current status of the non-lethal approach in avian species for determination of PCBs, OCPs, PBDEs and PFASs.
Graphical abstract
HANDLING EDITORS:
1. Introduction
Comprehensive quantitative analyses of pollutant presence in tissues and other biological samples collected from representative species provide valuable information about the delivery and transfer of pollutants into the trophic network and their impact on biota. Knowing their accumulation profile, concentrations and distribution within tissues enables us to make better predictions about habitat quality and future population status, which is especially important for threatened species.
A multitude of contaminants emitted to the environment ends up being introduced to the food chain. Compounds such as persistent organic pollutants (POPs) are of highest concern due to their high toxicity, low potential to biodegradation and easy bioaccumulation and sometimes biomagnification within the food web (UNEP; https://www.unep.org/). For many compounds, such as organochlorine pesticides (OCPs) or polychlorinated biphenyls (PCBs), global regulation restricts the use and production of the substances with the highest toxicity potential (Espín et al., Citation2016). However, they are still ubiquitous and can be found in abiotic components as well as biota, even in the polar areas (UNEP; Souza et al., Citation2021; Svendsen et al., Citation2018). The Stockholm Convention list of legacy POPs causing adverse effects on humans and the ecosystem originally included 12 compounds (pesticides, industrial chemicals and by-products). In 2021, this list, only for the group of compounds subject to elimination (Annex A), has been updated to 26 chemicals (UNEP; http://chm.pops.int/, accessed September 27, 2021).
Emerging organic pollutants (EOPs) can also constitute a high threat, as they continue to be introduced into the environment. Many of these, too, are not regulated and not enough ecotoxicological data on these compounds are available (Tartu et al., Citation2014). Among the best known examples of EOPs are poly- and perfluorinated alkyl substances (PFASs), which are used in a variety of products, e.g. fire-fighting foam, and impregnation agents for carpets and textiles (Tartu et al., Citation2014). PFASs are thermally and chemically stable and may affect body condition and health (Ask et al., Citation2021; Tartu et al., Citation2014). They also have very high surface tension lowering potential, and thus would behave differently in the marine ecosystem than do classical POPs (Herzke et al., Citation2009). The best known PFASs are perfluorooctane sulfonic acid (PFOS) and perfluooctanoic acid (PFOA) (Pereira et al., Citation2021). In 2009, PFOS, which is one of the most persistent and bioaccumulative PFASs, was declared one of the legacy POPs by the Stockholm Convention (UNEP). As it is a stable end product of the degradation of PFASs, is often found in both the environment and biota (Dauwe et al., Citation2007). In 2019 also PFOA was added to the Stockholm Convention list (UNEP), and PFHxS (perfluorohexane sulfonic acid) is under consideration (UNEP). These features, and their wide applicability, make PFASs especially interesting for ecotoxicological studies.
Before designing biomonitoring studies, one of the most important things is the choice of species and matrix. Contaminant levels may be reported as concentrations in internal tissues from collected dead or randomly selected sacrificed individuals. In all vertebrates the majority of detoxification and excretion of metabolites and parent compounds happens in the liver and kidneys, where the compounds may also be accumulated (Lehman-McKeeman, Citation2008). Substances are thus often determined in the main target tissues for toxic effects: liver, brain, muscle, kidney or in subcutaneous fat (e.g. Gebbink & Letcher, Citation2012; Sagerup et al., Citation2009). Non-polar compounds in particular, such as PCBs, would be accumulated in tissues rich in triglycerides, such as liver or fat (Espín et al., Citation2016). The selection of internal tissues for analysis allows the individual body burden to be assessed (Bustnes et al., Citation2001) and also to be connected with individual health (e.g. Tartu et al., Citation2014) and population declines. But apart from ethical implications and legal regulation in the case of endangered species, such a sample collection may carry potentially unquantified biases. Samples collected from stranded individuals are often of unknown provenance and cause of death. Individuals may not be representative of a random cross-section of the whole population, and may have an altered lipid content in a starved organ (Finger et al., Citation2015). Healthy individuals sacrificed for a study cannot be further monitored, as they are eliminated from the population, which may have deleterious effects on the species population (Cobb et al., Citation2003).
Non-lethally gained samples enable individual release after sample collection and in some cases do not require direct contact with an individual. Consequently it is possible to collect samples from the same individuals over seasons and track temporal changes of pollutant levels. It also allows samples to be collected from endangered species while omitting many ethical issues (still the collection may require a special permit, depending on the country). Therefore, the usage of non-lethally collected samples has recently become a crucial part of environmental studies of vertebrates such as mammals, reptiles and birds. However, there are some flows connected with its usage. The profiles of a compound can differ among tissues and thus the information gained can vary between them. Not all sample matrices are suitable for biomonitoring, as the information they provide about exposure would vary. It is important to use samples were it is expected that significant proportions would show levels above the limit of detection and that are widely available (Espín et al., Citation2016).
Birds can provide valuable data, as they adapt to live in almost all kinds of environment. They are generally considered as good bio-monitors as they integrate pollutants over wide areas, time-scale or food webs, and may transfer contaminants between different habitats, like from sea to land (Eulaers et al., Citation2011a, Citation2011b). Birds are ubiquitous, long-lived creatures, with variable diets and inhabiting all kinds of habitats, and are consequently often chosen in ecotoxicological studies. Also, the number of tissues that can be collected non-lethally is high and includes feathers, blood, preen oil, pellets, guano and eggs.
The main aim of this paper is to bring to closer attention the utility of non-lethally collected samples from avian species, based on critical review of the recent literature. The chosen samples are feathers, blood, preen oil and eggs, as these are the most frequently chosen types (). Other types of biological materials were not discussed mainly due to their limited applicability and limited data available. Pellets can only be obtained for certain species (e.g. owls), and they contain mainly undigested parts of food. Claws can be collected non-lethally, but collection can affect the bird’s well-being (e.g., in the case of birds of prey, it can affect their hunting), and for feces the collection is not always easy and the information is from a very short period of time. In addition, I will not discuss the properties, behavior in the environment and toxicity of chosen compounds, as it has already been done for legacy POPs and EOPs (for the references see e.g. Lohmann et al., Citation2007, Letcher et al., Citation2010). Biological material characteristics are presented together with advantages and flows of use and chosen examples from the field representing both legacy and emerging compounds: OCPs (organochlorine pesticides), PCBs (polychlorinated biphenyls), PBDEs (polybrominated diphenyl ethers) and PFASs (poly- and perfluorinated alkyl substances). These groups of contaminants are commonly used in ecotoxicological studies, including those that use bird as a model species.
Figure 1. Number of papers found using the given keywords published between 2010 and 2021 (using Web of Science searches only).
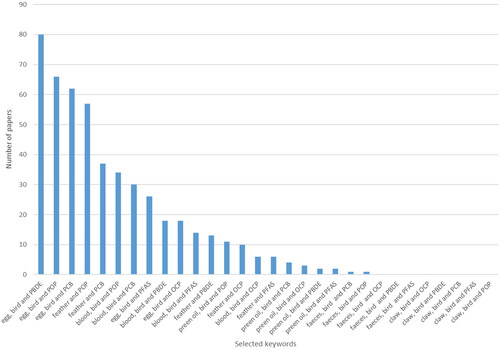
An extensive data search was conducted using Web of Science and Google Scholar search engines to find relevant data. However, the reader should be aware that the papers presented here are not the only ones that can be found on this topic (see ). More studies on exposure to organic compounds can be found under selected keywords. I chose the papers that were most relevant for the purposes of this publication, and if they did not provide any additional information beyond reporting compound levels, they might not be included. The main keywords used for the search were “contaminants,” “birds” and various combinations of contaminant names and/or matrix names (feather, blood, plasma, blood, eggs, pellet, faeces, claws). Additionally references in both review and research papers were screened for relevant publications.
2. Feathers
2.1. Tissue characterization
The feather is a complex epidermal structure of keratin, which provides function in flight, communication and thermoregulation. Keratin is rich in amino acids with sulfhydryl groups that form disulfide bonds, providing strength and durability to the protein (Burger & Gochfeld, Citation1997). The number of feathers varies and depends on the species. In the case of adult birds, feathers go through regenerative cycling phases. The changes in feather morphology that happen during each of the phases are described in the review by Chen et al. (Citation2015).
Organic contaminants are deposited in feathers during their growth time, via blood connection. In fully mature feathers, vascular connections undergo atrophy, and compounds can no longer reach the feather’s interior (Jaspers et al., Citation2008; Van den Steen et al., Citation2007). As such, feathers can give information on compound concentration in the blood during the time of feather growth (app. 2–3 weeks) (Burger & Gochfeld, Citation1997). This is important as fully grown feathers sampled at one place may give information about bird exposure during a bird’s stay in a completely different area. Adult birds are often migratory, and if their feathers are grown during their stay at overwintering areas, they would not reflect local contamination at breeding sites. This difference between time of formation and collection often results in a lack of correlation for organic compounds between plasma and feathers (Svendsen et al., Citation2018) or internal tissues and feathers (Jaspers et al., Citation2019). If the body feathers of nestlings are sampled, they may still have an active blood connection, so the exposure would be more recent. Due to an active blood connection, also the correlation of organic contaminants between body feathers and blood plasma tends to be higher in nestlings than in adults (Eulaers et al., Citation2011b; Jaspers et al., Citation2006, Citation2007).
The first crucial step is choosing the species and type of feathers for further analysis. Knowledge of the species migratory habits and molting pattern is essential to proper result interpretation. High differences in the concentrations of organic compounds may occur between long-distance migrants, local migrants and strict resident birds (Behrooz et al., Citation2009). Species with large foraging areas will not be suitable for monitoring local pollution, e.g., for local terrestrial environments, species with more restricted ranges should be considered (Jaspers et al., Citation2019). Some birds have a sequential molt, replacing contour and flight feathers prior to migration during a post-nuptial molt, and the head and throat feathers during a pre-nuptial molt (e.g. razorbills Alca torda or little auks Alle alle; Espín et al., Citation2012). For large predatory birds, the molting period can last over two or more years, whereas most small birds, like songbird species, molt within one year (Groffen et al., Citation2020). This will thus result in differences in exposure duration. Also, molts do not always proceed simultaneously in different individuals, so the results derived from them may not be related to the exact same period of time (García-Fernandez et al., Citation2013).
Although the specific chemical structure of organic pollutants and their binding capacities to feathers are still poorly understood, contaminants incorporated into the feather’s internal structure seem to be permanently retained, as even non-persistent pollutants such as lower chlorinated PCBs or ɣ-HCH (lindane) can be effectively detected (Adrogué et al., Citation2019; Espín et al., Citation2012; García-Fernandez et al., Citation2013; Souza et al., Citation2021). In energy-demanding periods (time of migration, breeding and molt), lipophilic organochlorines can be additionally mobilized from stored fat and re-distributed to the blood stream. Thus, during molt, when contaminants are transported to the growing feathers, the concentrations detected in them may also be affected by previous exposure (Espín et al., Citation2012; Perkins & Barclay, Citation1997). The correlations between levels in feathers and in internal tissues tend to be lower in starved birds (Jaspers et al., Citation2006, Citation2007). The reason for this may include altered lipid content in starved organs (Finger et al., Citation2015). For examples of correlation studies see .
Table 1. Examples of correlations or associations between POPs concentration in the different feather types and other biological materials (in studies after 2005); for more studies on correlation, see the review by García-Fernandez et al. (Citation2013).
The more factors we know, the more detailed information regarding bird exposure we can get by feather analysis. For this reason, using molted feathers provides limited data, as an external contamination may be significant and the interpretation of birds’ exposure has some uncertainties (Jaspers et al., Citation2019). Still, it may be a useful way to analyze samples from rare and endangered species without causing unnecessary stress. Freshly molted feathers can be collected as part of routine monitoring studies.
2.2. Advantages and disadvantages of use
There are many advantages to using feathers: they can be collected from living birds and are metabolically inactive. Feathers can be sampled irrespective of age, sex and season. They can be used to monitor remote populations while birds are in a location that was previously very difficult to study. For example, feathers of seabirds grown during their wintering in the open ocean can be collected when the birds are on land during the breeding period (Pacyna et al., Citation2019; Pacyna-Kuchta et al., Citation2020).
Levels in feathers usually do not show point source pollution, but a larger foraging area (Burger & Gochfeld, Citation1997). The amount of feathers needed for organic contaminants analysis may be problematic. It is advised to use at least 200 mg of feathers (Dauwe et al., Citation2005), but for small species it may not be possible to collect this much from living individuals without affecting the birds’ thermoregulation and health or survival chances. For larger birds, even one tail feather can be sufficient (Jaspers et al., Citation2006, Citation2008). It should be noted that some authors used much smaller amounts of feathers and were able to show detectable amount of contaminants. Groffen et al. (Citation2020) used a single great tit (Parus major) tail feather that weighed approximately 8–10 mg, yet they were able to detect most of the PFASs analyzed. However, this was most likely due to the fact that great tits are resident birds with limited foraging areas, and the birds selected for this study lived in close proximity to a fluorochemical plant, which is a known PFASs hotspot. The authors also used unwashed feathers, which may have increased compound levels due to external contamination.
Feathers are not always appropriate for selected analysis. As feathers are connected to the blood stream only during feather growth, they may have a limited storage capacity (Meyer et al., Citation2009). Feather physiology may directly affect compounds deposition, as some compounds are incorporated in proportion to their abundance in the bloodstream, while others are incorporated as part of the keratin blocks (Bortolotti, Citation2010). There are many other factors that may affect compound concentration, including seasonal variations in diet, external contamination, metabolic rate and time of exposition (Behrooz et al., Citation2009; Dauwe et al., Citation2005; Eulaers et al., Citation2011a). In nestlings, factors such as trophic level, dietary carbon source (food chain discrimination), local habitat residence, and nest location may influence the variability in contaminant exposure during early life stage (Eulaers et al., Citation2013). Feather effectiveness and applicability as a biomonitoring tool has been confirmed only for certain POPs, while for many of them it is unconfirmed and more research is needed. The usefulness of feathers for analyses of legacy compound such as PCBs or OCPs has already been summarized in previous reviews such as García-Fernandez et al. (Citation2013), Espín et al. (Citation2016) or Jaspers et al. (Citation2019).
Feathers can be transported and stored at room temperature for many years, e.g. in an envelope or aluminum foil further closed in string bags (Burger & Gochfeld, Citation1997; Jaspers et al., Citation2019). Freezing should be used to store dirty or wet feathers. Long-term storing should always be performed under controlled conditions, with feathers protected from external contaminants, moisture and UV-light action (Jaspers et al., Citation2019).
Feathers can be collected not only from rare and protected species, but museum specimens, too, may be used to track changes in exposure over time. For example, archived body feathers from 1968–2015 were successfully used to evaluate long-term spatio-temporal trends of PFASs in a top predator, the white-tailed eagle (Haliaeetus albicilla) (Sun et al., Citation2019). But although archived feathers are a valuable tool to assess long-term trends, there are some limitations when using samples from bank specimen or museums, such as possibly unknown bird parameters (e.g. age and sex) and differences in storage conditions between places that may affect compound degradation or transformation. External contamination and preservation or pretreatment methods also may affect the results (Sun et al., Citation2019).
Feathers are often contaminated externally by birds’ own secretions from preen and salt glands or by the direct contact with water, air or mud (Goede & De Bruin, Citation1986). Choosing the right solvent and washing procedure is very important for relevant analysis. In general, a limited number of studies have examined the effectiveness of removing external contamination from feather surface. For lipophilic organic compounds, external contamination mostly comes from preening (Jaspers et al., Citation2008). Even 85% of feather lipids may come from uropygial secretions. Uropygial oil acts also as an adhesive layer, allowing pollution particles originating from the air to be deposited on the feather surface (Mardon et al., Citation2011). External contamination with preen oil may vary over life stages and with the age of the feather (Eulaers et al., Citation2011a). To remove preen oil from feathers a strong solvent must be used, for instance acetone has proven to be very effective (Jaspers et al., Citation2008). But this can also affect weakly bounded compounds deposited inside the feather structure. It has been discussed whether it is necessary to remove preen oil from feathers, as its addition increases correlations with internal concentrations (Eulaers et al., Citation2011a; Jaspers et al., Citation2008). So for lipophilic compounds analyses studies suggested thorough washing with de-ionized water and using stainless steel tweezers to separate barbs. This allows to remove only airborne particles and dust (Jaspers et al., Citation2008). Majority of authors follow this instruction with minor modifications, such as using distilled water (e.g. Behrooz et al., Citation2009; Eulaers et al., Citation2013; Monclús et al., Citation2018). Washing solely with water will not be appropriate for feathers heavily contaminated with, for example, blood or fat. In the case of non-lipophilic PFOS, such as PFOA, external contamination more likely to originate from wet or dry depositions (Jaspers et al., Citation2013). No standardized procedure is currently available for washing feathers prior to PFASs analysis. Hexane is often chosen as a cleaning solvent (Briels et al., Citation2019; Jaspers et al., Citation2013; Løseth et al., Citation2019). The procedure may involve washing the feathers first in ultrapure water and, after drying and cutting them into <5 mm pieces, immersing them in hexane and placing in an ultrasonic bath for 10 min (Gómez-Ramírez et al., Citation2017). Other cleaning solvents include e.g. acetonitrile (Meyer et al., Citation2009). However, as even hexane did not remove all contaminants from the feather surface, further tests were encouraged (Jaspers et al., Citation2013). The impact of external contamination can sometimes be minimized by collecting feathers from nestlings, as they live in a restricted area and their feathers are less prone to external contamination by preen oil. It is also often easier to monitor nestlings than adult individuals and there are fewer influences of factors like molt, age, foraging and migration behavior (Eulaers et al., Citation2011b). But it should be noted, that for species nesting on the ground, external contamination with dust and soil particles can be still significant.
2.3. Examples of the use of feathers in field research
Although birds have been used as biomonitors for an extensive period of time (e.g. in the late 1960s and early 1970s, research on birds focused on the effects of OCPs and PCBs on eggshell thinning; Ohlendorf & Fleming, Citation1988), feather usage for organic pollutant analysis is still relatively new. One of the first studies where feathers of wild birds were used to assess POP concentrations in adult birds was a study by Dauwe et al. (Citation2005) on passerines, namely great tits. In this pilot study, the concentrations of organic contaminants (PCBs, PBBs, OCPs and PBDEs) were determined in both feathers and fat tissue. Of the examined compounds, the majority of PCB congeners and DDTs (dichlorodiphenyltrichloroethanes) could be quantified in feathers, while other pesticides and brominated flame retardants were not detected. Concentrations found in feathers (expressed in ng/g) were significantly lower than concentrations in fat (ng/g lipid weight), and there were no strict correlations for quantified compounds in the examined tissues. But this study initiated further research, suggesting that, for some POPs, feathers may be a good nondestructive alternative. Passerines can be problematic for determining concentrations of organic compound, both legacy and emerging, due to the low bird mass as opposed to the high feather mass required for a compound detection. This problem can be solved by pooling data from individuals or choosing a bigger species. For this reason, large predatory birds are often chosen for POPs analysis, with multiple examples such as Jaspers et al. (Citation2006, Citation2007); Eulaers et al. (Citation2011a, Citation2011b); Sun et al. (Citation2019). In fact, for birds of prey in Europe, feathers, plasma and eggs are the most common type of samples collected for contaminant determination (Espín et al., Citation2016; González-Rubio et al., Citation2021). Beside large predatory birds, seabirds are another frequently examined class of birds. Many of them are endangered, so the use of feathers is advantageous. Examples of using seabird feathers for organic compound determination include Espín et al. (Citation2012); Svendsen et al. (Citation2018), Souza et al. (Citation2021).
It is important to recognize congener profile differences between feathers and other tissues. A feather profile might be modified due to external contamination with preen oil or atmospheric deposition (García-Fernandez et al., Citation2013). For instance, it has been shown that feathers are often characterized by higher contributions of lighter PCBs (Adrogué et al., Citation2019; Dauwe et al., Citation2005; Jaspers et al., Citation2007; Briels et al., Citation2019; Souza et al., Citation2021). However, that is not the case in all studies; for example, in Eulaers et al. (Citation2011a, Citation2011b) study the most abundant were highly persistent hexa-CBs, such as CB 138 and CB 153 (in blood plasma and nestling body feathers). In the study of González-Gómez et al. (Citation2020) penta- and hexa-CBs dominated the PCB profile in body feathers of feral pigeons (mainly PCB 126, 138 and 180), although in general the concentration of PCBs was low (0.2–4.6 ng/g). Several studies found low brominated congeners such as BDE-47 to be one of the dominant congeners in feathers (Adrogué et al., Citation2019; Eulaers et al., Citation2011b; González-Gómez et al., Citation2020; Jaspers et al., Citation2006, Citation2007; Løseth et al., Citation2019). It is good to provide comparison for the same species or one with a similar feeding profile, as differences may also be based on the ecology of the studied species (such as diet specialization and feeding on different trophic levels).
The suitability of choosing feathers in the biomonitoring of PFASs has been discussed in a number of papers, as the deposition mechanism would be different than in the case of legacy pollutants (e.g. Jaspers et al., Citation2019; Meyer et al., Citation2009). There are differences in different affinities of PFASs for proteins, which mainly depend on the carbon-chain length, functional groups and hydrophobicity of the chemical (Groffen et al., Citation2020). Correlations between the PFASs concentrations for feathers and for other tissues found in the literature are presented in . For some compounds in this group, like PFOA, it is possible that levels in feathers reflects not ingestion through diet, but rather environmental contamination and are accumulated on the external surface of the feathers (other possible explanations are excretion or quick compound transformation) (Jaspers et al., Citation2013; Løseth et al., Citation2019). Thus, some authors suggest the complementary use of feathers and other biological material, e.g. blood to provide representation for different time lines (Groffen et al., Citation2020).
Table 2. Correlation studies between feathers and other sample types for PFAS.
Feathers can be collected not only from adults, but also from chicks and nestlings, which have two types of feathers: down and body. Down feathers are formed in the egg from maternal nutrients and generally represent maternal input, whereas body feathers represent the nesting period and input from the environment (as food/water intake) (Ackerman et al., Citation2016; Pacyna et al., Citation2019). Nestling body feathers sampled from predatory bird species have been successfully used to determine several legacy and emerging contaminants (e.g. Briels et al., Citation2019; Eulaers et al., Citation2011b, Citation2013; Gómez-Ramírez et al., Citation2017; Løseth et al., Citation2019). But the suitability of down feathers to detect POPs is still poorly investigated. Monclús et al. (Citation2018) performed a study on down feathers of nestlings of the Spanish cinereous vulture (Aegypius monachus). When comparing to contour feathers, the down feathers (second natal down feathers grown 15 to 25 days posthatching) showed a higher accumulation of the most persistent studied POPs, with a contamination profile similar to that previously described in predator eggs (approx. 140 mg was sufficient to quantify fourteen different POPs). Down feathers also had a higher detection frequency of PCBs. Nestling contour feathers (mean 220 mg per bird) had higher contribution of the more volatile compounds- lindane and lower chlorinated PCBs (Monclús et al., Citation2018).
3. Preen oil
3.1. Tissue characterization
Preen oil is a secretion from the preen gland at the base of a tail. It appears in the majority of species, is largest in aquatic birds, and is mostly composed of wax esters, triacylglycerols, phosphatidylcholines and volatile organic compounds (Soini et al., Citation2013; Solheim et al., Citation2016). It also has a non-lipoidal fraction that contain proteins, inorganic salts and cell fragments (Rawles, Citation1960; King & McLelland, Citation1975). The composition differs between species and may also change with age, sex and diet (Sandilands et al., Citation2004). Preen oil is spread by the bill, it serves to protect the body from water by waterproofing the feathers and improving their appearance. It also provides antibacterial and antiparasitic protection and may be used to chemosygnaling (Mardon et al., Citation2011; Soini et al., Citation2013). It can be collected from either alive or dead birds (Yamashita et al., Citation2007). Preen oil is known to contain high levels of lipophilic compounds (e.g. PCBs; Jaspers et al., Citation2008), but due to its specific composition it may also accumulate some non-lipophilic substances, like PFOS (Campagna et al., Citation2012, Jaspers et al., Citation2013).
The amounts of oil excreted vary according to species (Solheim et al., Citation2016). Some studies have found correlations between levels of organic contaminants in preen oil and other biological materials, including blood (Eulaers et al., Citation2011a) and adipose tissue (Yamashita et al., Citation2007).
3.2. Advantages and disadvantages of use
Using preen oil has many advantages: samples might be obtained from both sexes and from nestlings (Eulaers et al., Citation2011a); due to its high lipid content it can be used for determining many lipophilic compounds; due to inseparable blood connection the sequestration time frame is close to this in blood and in comparison to feathers there is little or no external contamination (Eulaers et al., Citation2011a; Yamashita et al., Citation2007). However, sequestration time frame integrates a short period of time (Ito et al., Citation2013). Also, the metabolization of compounds is possible (Eulaers et al., Citation2011a). The concentration of organic contaminants is species specific and depends on dietary behavior (Yamashita et al., Citation2018).
Preen oil is quite easy to obtain, although this should be done in such a way as to minimize the possibility of external contamination. It can be done by, for example, pressing the preen gland with a pre-cleaned glass rod, paper wiper, surgical gauze, or glass fiber filter (Briels et al., Citation2019; Ito et al., Citation2013; Yamashita et al., Citation2018). Briels et al. (Citation2019) also collected the oil-covered feather tuft that surrounds the preen gland. The same individual may be sampled repeatedly over a short period of time (Yamashita et al., Citation2018). Around 30- 50 mg was enough to determine several PCBs, PBDEs, DDTs and HCHs (Eulaers et al., Citation2011a; Ito et al., Citation2013; Yamashita et al., Citation2018).
Preen oil sampling is less invasive than blood collection (Ito et al., Citation2013). Its transport and storage requires cold conditions (around −20 °C; Eulaers et al., Citation2011a). For some species, collecting samples from juveniles and adult birds may be troublesome and the required amount could be problematic to obtain (Jaspers et al. 2011).
3.3. Examples of the use of preen oil in field research
Due to its high lipid content, preen oil may contain high concentrations of some lipophilic compounds. For example, average concentrations of PCBs in adult black-footed albatross preen oil samples were approx. 74–78 times higher than concentrations found in plasma samples (Wang et al., Citation2015). Levels of several POPs (PCBs, PBDEs and OCPs) were ten to hundredfold higher in preen oil comparing to their levels in blood or body feathers of nestling white-tailed eagles (Eulaers et al., Citation2011a). Also in a study by Briels et al. (Citation2019), preen oil was characterized with a much higher POP concentration as compared to feathers and plasma, the dominating compounds were OCPs (169–3560 ng/g). Solheim et al. (Citation2016) found the highest concentrations of analyzed POPs in preen glands (ƩPOP for feathers 79.3 ± 47.9 ng/g; for livers 711 ± 462 ng/g wet weight; for preen glands 2739 ± 1260 ng/g wet weight). They also found small, but significant differences for POP profiles in feathers, preen oil and livers of adult kittiwakes, with preen gland and feather profiles being the most closely related (Solheim et al., Citation2016). POP profiles were very similar between preen oil and plasma in a study by Briels et al. (Citation2019). Strong correlations found between blood and preen oil concentrations in nestlings suggest that preen oil very well reflects internal contamination (Eulaers et al., Citation2011a; Løseth et al., Citation2019).
A study performed on adult birds of 24 species examined the utility of preen gland oil to monitor PCBs, DDTs and HCHs (Yamashita et al., Citation2018). High differences between species were found: the Σ20PCBs concentrations found in birds was from 1.9 to 60 700 ng/g-lipid; ΣDDT concentrations ranged from 1.4 to 22 899 ng/g-lipid; concentrations of ΣHCHs were from 0.2 to 506 ng/g-lipid. The factors most important for level of chosen POPs in preen oil were geographic concentration patterns and species dietary behavior (Yamashita et al., Citation2018).
Yamashita et al. (Citation2007) found higher proportion of lower-chlorinated PCB congeners in the preen oil as compared to abdominal adipose, suggesting less time to undergo metabolization before they were secreted from the preen gland. Also, a weak but significant correlation was observed between the PCB levels in the oil and the adipose tissue, with even higher correlation when correcting for the metabolic loss of PCBs. Wang et al. (Citation2015) detected mainly highly chlorinated congeners in preen oil. Also in the studies done by Eulaers et al. (Citation2011a) more higher chlorinated PCB congeners were detected in preen oil.
The degree of PFASs excretion via the preen gland is still barely understood, but high levels for PFOS and low levels for PFOA and PFHxS were found in preen oil collected from the barn owl (median PFOS 431.2 ng/g wet weight; Jaspers et al., Citation2013).
4. Blood
4.1. Tissue characterization
Blood is a widely used matrix as it circulates through the whole body transporting nutrients and also contaminants. Factors like high tissue perfusion rates, enable circulating blood and internal tissues to very quickly attain equilibrium with each other for lipophilic and persistent POPs such as OCPs. However, this is not the case for quickly metabolized or eliminated compounds (Bustnes et al., Citation2001). The half-life of contaminants is usually shorter in blood than in internal tissues (Espín et al., Citation2016). Low lipid content in blood also means that lipophilic contaminant concentrations are lower, meaning that a relatively high volume of blood should be collected to exceed analytical limits and provide enough samples for repetitions (Espín et al., Citation2016). However, to ensure bird well-being, the volume of sampled blood should not exceed 1% of the bird’s body weight, and even less during energetically demanding time periods (Fair et al., Citation2010). Thus, analytical problems with too low a sample amount may occur for small species, such as passerines. In larger species, such as birds of prey, both adults and nestlings can be sampled (e.g. Ortiz-Santaliestra et al., Citation2015; Pittman et al., Citation2015).
Sampling time is essential when using blood for monitoring purposes (Bustnes et al., Citation2017). Species differences may be caused by differences in breeding strategy. For instance, common eiders (Somateria mollissima) are fasting during incubation, using stored fat reserves and remobilizing contaminants from the fat to the blood stream. Thus, contaminant levels in blood collected during incubation might not reflect only the recent diet, but also contaminants remobilized from fat (Thorstensen et al., Citation2021).
Lipid percentage and quality and the concentration gradient may impact the partition of POPs between blood and internal tissue (Bustnes et al., Citation2001). Several factors may affect concentrations of POPs in birds’ blood, including altered transport during a season, diet, remobilization of lipid stores and sex, as some POPs may be eliminated from the body through egg production (Bustnes et al., Citation2017).
Information about bird exposure can be gained using whole blood, plasma, serum or red blood cells (RBC) (e.g. Gebbink & Letcher, Citation2012; Jaspers et al., Citation2008; Olsson et al., Citation2000; Pittman et al., Citation2015). Serum can be obtained by allowing whole blood without anticoagulants to clot at room temperature. RBC and plasma are usually isolated by centrifugation (Ehresman et al., Citation2007). For whole blood and plasma, anticoagulants such as heparin or EDTA are needed to prevent coagulation (Espín et al., Citation2016; Wang et al., Citation2015). Whole blood is a complex matrix and proper frozen storage conditions are necessary to maintain its stability (Ehresman et al., Citation2007). Using whole blood prevents potential loss of compounds in the cellular fraction and a smaller volume is needed for the analysis (Espín et al., Citation2016). Unless samples are stored for an extended period of time under frozen conditions, during which lysis of cellular components can occur (Ehresman et al., Citation2007). For compounds such as PFASs (including PFOS, and PFOA), which are not attached to cellular components, levels in plasma or serum can be twice the level in whole blood (Ehresman et al., Citation2007).
4.2. Advantages and disadvantages of use
Blood can be sampled from adults and nestlings. It can be collected during egg incubation and the early chick-rearing period to investigate relationships between contaminant concentration and parental care, including plasma hormone levels and egg-turning frequency (Blévin et al., Citation2020; Tartu et al., Citation2014). It also allows study of the association between concentrations of endocrine‐disrupting chemicals and hormone balance in the body, including stress hormone or plasma carotenoid concentrations (Ask et al., Citation2021; Bortolotti et al., Citation2003; Tartu et al., Citation2014). Blood is a source of information regarding birds’ immune or inflammatory response, and blood clinical-chemical parameters (BCCPs) associated with liver and kidney damage, bone diseases and metabolic disorders (e.g. Sonne et al., Citation2010, Citation2013). Blood may also be used to study the influence of recent food intake and regional site-specific differences on contaminant concentrations in distant populations (Carravieri et al., Citation2017; Olsson et al., Citation2000).
POPs levels in blood may be affected by intra-individual temporal variation (Bustnes et al., Citation2001), migration strategies and diet specialization which may be influenced by individual behavior resulting in inter-colony variation (Leat et al., Citation2013, Citation2019). During the breeding season, females may have lower blood levels of POPs as a result of compound deposition in eggs. However, some studies have revealed inconclusive patterns—e.g. differences in OCPs not found between sexes, but found for part of the PFAS congeners, suggesting that they may be deposited into eggs (Bustnes et al., Citation2008).
Sampling may carry a risk of disease transmission. The amount of the sample must be appropriate, otherwise it could be difficult to approach analytical limits (Jaspers et al., Citation2008; Yamashita et al., Citation2007). It requires some technical skills and may cause stress in birds. Blood samples should be preserved in refrigerator or buffer solution.
To overcome challenges with sample collection, dried blood spots may in a certain cases be a better alternative for plasma sampling (Perkins & Basu, Citation2018). Dried blood spots are a method for collecting blood samples by applying it to a specialized filter paper and then air-drying (Perkins & Basu, Citation2018). This method was first developed to detect metabolic diseases in newborns (Guthrie & Susi, Citation1963). In the case of birds it was used mostly for toxic metal exposure assessment (Lehner et al., Citation2013; Perkins & Basu, Citation2018), but also for several organic pollutants (Shlosberg et al., Citation2012). For a single dried blood spot, less than 60 µl of blood is needed (Perkins & Basu, Citation2018; Shlosberg et al., Citation2012). Also, anticoagulant is unnecessary (Espín et al., Citation2016). This method includes the need for the right protocol and there is also a high potential for external contamination. Not all laboratories can provide sufficiently sensitive methods (Espín et al., Citation2016).
4.3. Examples of the use of blood in field research
One of the most beneficial things about using blood is the possibility to simultaneously study other factors, such as possible association with reduced body condition and lower triglyceride levels (Ortiz-Santaliestra et al., Citation2015). It can also be used to determine temporal trends in exposure, e.g., in a study conducted on bald eagle (Haliaeetus leucocephalus) nestlings, plasma collected between 1997 and 2010 showed decreasing concentrations of PCBs and DDT, with the opposite trend for dieldrin (Pittman et al., Citation2015). Studies performed on the birds of prey from Norway suggested that some POPs may impact blood plasma biochemistry markers, including liver and renal parameters (Sonne et al., Citation2010).
Based on analysis of plasma from bald eagles, the researchers found that in this species, ΣPCBs and DDE concentrations increased with trophic level and marine input, while ΣPBDEs concentrations were not significantly dependent on trophic level (Elliott et al., Citation2009). Plasma of fledgling sea eagles was characterized by significantly lower concentration of PBDEs comparing to body feathers, no significant difference was found for ΣPCBs, and OCPs (Eulaers et al., Citation2011a). Blood of the southern giant petrels (Macronectes giganteus) living in Antarctica was sampled to compare organic contamination levels between adults and chicks, with the levels being significantly higher in adults for PCBs and OCPs most likely resulting from bioaccumulation during breeding and wintering time, with almost no sex differences reported (Colabuono et al., Citation2016).
Blood is broadly used to determine PFASs concentration in birds. Gebbink and Letcher (Citation2012) found that PFASs plasma/RBC pattern in herring gulls (Larus argentatus) was dominated by PFOS and also contained high percentages of C8-C11 PFCAs. When comparing the concentrations of C4-C15 perfluorinated sulfonates (ƩPFSAs) and carboxylates (ƩPFCAs) between plasma and red blood cells, they were significantly higher in plasma (Gebbink & Letcher, Citation2012), which contains protein such as albumin to which PFASs may bind (Bustnes et al., Citation2008; Jones et al., Citation2003; Verreault et al., Citation2005). Plasma had the highest concentration of linear PFOS and high detection frequency, in contrast to feathers (DF = 100% vs DF: 48.9%) in study by Løseth et al. (Citation2019). Also in plasma the highest level of PFAS was found (1.37–36.0 ng/mL) in the study by Briels et al. (Citation2019).
In Herzke et al. (Citation2009) study, PFOS was prevalent in seabird egg, plasma and liver samples, with similar median concentrations (27–32 ng/g ww). Other congeners varied between the sample types; e.g. PFOA was detected at low concentration in birds’ plasma (2–6 ng/g ww), but was undetectable in eggs and rarely found in liver (Herzke et al., Citation2009). Of all analyzed compounds in plasma and liver (lipophilic POPs, PFAS), PFASs were the major compound group (70–80% of the pollutant burden [Herzke et al., Citation2009]). In the biological materials collected from glaucous gulls (Larus hyperboreus) living in the Norwegian Arctic, PFOS was also the predominant PFAS in all studied samples (plasma, liver, brain, eggs), being the highest in plasma (48.1–349 ng/g ww) followed by liver, eggs and brain (Verreault et al., Citation2005). Perfluorocarboxylic acids (PFCAs) with 8–15 carbon had the highest concentrations in plasma (sum PFCA: 41.8–262 ng/g ww) (Verreault et al., Citation2005).
Tartu et al. (Citation2014) showed that hatching success may be affected by high concentrations of PFDoA. The longer-chained PFCAs were the dominant compounds found in adult chick-rearing kittiwakes (Tartu et al., Citation2014). Studies on small passerines found that great tits living close to a large fluorochemical plant had extremely high PFOS concentrations: in liver these were 553–11 359 ng/g and 24–1625 ng/ml in blood; the concentrations were highly correlated between liver and blood (Dauwe et al., Citation2007). Another study showed that blood PFOS concentration decreased with increasing distance from the plant, but for other PFASs no clear distance gradient was observed (Groffen et al., Citation2020).
PFASs were also analyzed in the plasma of the white-tailed eagle and northern goshawk (Accipiter gentilis) nestlings. Significant differences in exposure were found, with the higher levels detected in the white-tailed eagle (ΣPFASs Median = 45.83 vs 17.02 ng/mL) (Gómez-Ramírez et al., Citation2017). Differences were most likely caused by the dietary input, as the northern goshawk is associated with woodland areas, and the white-tailed eagle with the marine food web (Gómez-Ramírez et al., Citation2017).
5. Eggs
5.1. Tissue characterization
Breeding time is a demanding period for any birds, and during egg formation contaminants are transferred into the egg from female lipid deposits. Thus eggs indicate the contamination of the area where the female birds were feeding during egg formation, and may include exposure in the areas used before nesting (Martínez-López et al., Citation2007; Zhao et al., Citation2019).
Eggs represent the earliest life stage, when birds are highly sensitive to contaminants. The size of the clutch, as well as the size of the egg and the embryo development strategy, affect how much lipid and protein is mobilized and transferred to the single egg (Van den Steen et al., Citation2009). But in general, they have a high fat content and both lipophilic POPs and PFASs can be transferred to them from the female body (Dauwe et al., Citation2006; Gebbink & Letcher, Citation2012). Some POPs are eliminated more easily to the developing eggs (Bustnes et al., Citation2017). In fact several factors may affect which compound would be effectively transferred to eggs, and especially compound chain length, but also hydrophobicity, persistence and recalcitrant characteristics (Gebbink & Letcher, Citation2012; Holmström & Berger, Citation2008; Verreault et al., Citation2006; Vicente et al., Citation2015). When sampling eggs, little intervention should be made and the number of visits to a single nest should be limited. To ensure this, sampling only a single egg from multiple clutches instead of repeatedly returning to the same few clutches is preferred when possible (Morganti et al., Citation2021).
For biomonitoring purposes contaminant burden may be determined in full eggs (the most common) or in egg compartments left after hatching, such as eggshells or extra-embryonic membrane. Post-hatching eggshells are relatively rarely used in environmental monitoring studies. The record of contaminants in eggshell represents relatively a short time of pre-laying, and exposure comes from various areas depending on the species foraging strategy (Becker, Citation2003). Some compounds may be adsorbed by the developing embryo. Therefore, the information obtained from egg shell analysis alone is limited. Extra-embryonic membranes can also be used in some cases, e.g. those from peregrine falcon eggshells were used as an indicator of chemical exposure (p,p′-DDE) by Peakall (Citation1974) to demonstrate an increase in DDT exposure in a top predator. The chorioallantoic membranes of the eggshells can be used as a direct indicator of the DDT level in the egg, through a calculated correlation index (Cobb et al., Citation2003).
Eggs comprise yolk and albumen. They are often homogenized and then analyzed for compound concentrations (e.g. Ahrens et al., Citation2011; Braune et al., Citation2015; Holmström et al., Citation2010; Martínez-López et al., Citation2007). Recently some reports have stated that, if eggs were embryonated, it is better to use whole homogenized eggs (Morganti et al., Citation2021). But a study done by Vicente et al. (Citation2015) showed that for some compounds partitioning between those components may occur. The authors found that only yolk samples contained detectable levels of PFASs (with PFOS being the main compound), which was probably caused by differences in composition (yolk has more lipids and proteins than albumen, which is 88.5% water; study on the Audouin’s gull [Larus audouinii]) (Vicente et al., Citation2015). Also Gebbink and Letcher (Citation2012), could not detect any PFASs in the albumen of herring gulls, while they were present in the yolk. Similarly, Wang et al. (Citation2019) found significantly higher concentrations of PFASs in yolks than in albumen in chicken eggs collected around a fluoro-chemical plant. Thus, using whole egg homogenates to monitor those compounds may result in diluted detected concentration (Gebbink & Letcher, Citation2012).
5.2. Advantages and disadvantages of use
Contaminants deposited in eggs may be assimilated from different locations (especially for long-distance migrants) and a relatively long time period as it comes from food ingested and the mobilization of female body stores (Burger & Gochfeld, Citation1997; Martínez-López et al., Citation2007; Zhao et al., Citation2019).
Eggs can usually be easily obtained and transported, and their collection allows the invasive sampling of adult individuals to be avoided. One egg typically provides enough material for organic compound analysis to proceed (Morganti et al., Citation2021). They should be kept in the cold conditions to prevent decay. It is often assumed that for birds, with a number of eggs laid by a female, a single egg can represent the contamination levels of a whole clutch (Van den Steen et al., Citation2006). However, during a laying period, variable factors may cause within-clutch differences between capital and income breeders. For capital breeders, maternal resources are used for production of the first eggs, so the contaminants accumulated in lipids may be transferred into them. Daily diet is used to produce later eggs, so within-clutch variation caused by laying sequence is expected (Espín et al., Citation2016; Lasters et al., Citation2019). Income breeders, such as passerines, mostly use exogenous resources (diet) for the egg formation, and endogenous reserves are usually used, if needed, for the last eggs. So in this case the last eggs would show the female charge of pollutants (Vicente et al., Citation2015). Thus, income breeders would be useful to expose local contamination (Morganti et al., Citation2021), although it may also have a minor contribution of the previous winter intake (Pereira et al., Citation2021).
The egg-laying order may affect the concentration of organic compounds in eggs, although the data are inconclusive. Some studies find a clear decrease in the concentration of organic compounds in later eggs (a decrease according to egg-laying order of 36% for the sum PCBs, 31% for the sum OCPs and 45% for the sum PBDEs in Van den Steen et al., Citation2009) or no clear effect of laying order (Reynolds et al., Citation2004; Van den Steen et al., Citation2006; Verreault et al., Citation2006). Interestingly, differences can be found even in two species of income breeders: an effect of egg-laying order for PCBs, OCPs, PBDEs was found in the blue tits (Cyanistes caeruleus), while no such effect was found in earlier studies on the great tits (Van den Steen et al., Citation2006, Citation2009). These differences may be due to species differences such as clutch size, female size, physiological processes of lipid uptake, metabolism, percentage of energy to body weight invested in eggs, or differences in study methodology (Van den Steen et al., Citation2009). For PCBs and PBDEs, but not for OCPs, the variance in concentrations was greater among clutches than within clutches, suggesting that for these two groups a single randomly collected egg from a clutch can be useful as a biomonitoring tool. For OCPs consistent collection of the same egg from the laying sequence was suggested (Van den Steen et al., Citation2009).
For PFASs a decreasing concentration of PFOS was found according to laying sequence (Audouin’s gull clutch [Larus audouini]; Vicente et al., Citation2015). Other PFASs were mostly detected in the first eggs, and in this case using the last one would lead to underestimated exposure (clutch of three eggs). The authors’ suggestion was to collect and analyze the first egg, as in general it may contain the highest level of PFASs. For species with larger clutches (e.g. passerines) large variations in PFAS concentrations were also found, with the authors suggesting the collection of the same egg along the laying sequence (e.g. for great tits, the first and third egg were preferred) (Lasters et al., Citation2019).
Eggs can be collected only in a specific period of time and from just one section of the population (adult females). Obtaining a complete picture of exposure can be particularly problematic if there are significant differences in feeding behavior between females and males. Also, some birds start having chicks very late in life (e.g. albatrosses), so the young generation cannot be bio-monitored using eggs or eggshells. Biomonitoring of individuals that skipped breeding would also not be possible.
Egg collection is expected to have a minor impact on population (for highly dense populations), but for species producing limited numbers of eggs, this method is not applicable (Jaspers et al., Citation2008). In particular, species that produce a single egg per season are the most vulnerable to this type of biomonitoring. Egg sampling is also not suitable for rare, endangered species or for species with a low breeding success. The exception is to use unhatched eggs (unfertilized or addled eggs), but in this case the number of available samples may be low. Also, in unhatched eggs, concentrations may not be stable due to posthatch microbiological degradation of organic contaminants. It can be especially significant for less persistent transformation products, such as oxychlordane, which can be detected as a result of microbiological degradation (Dauwe et al., Citation2005; Espín et al., Citation2016; Morganti et al., Citation2021). Also failed/addled eggs are nonrandom and represent only failed breeding outcome, and thus are more likely to have accumulated sufficiently high concentrations of contaminants to have affected hatchability (Espín et al., Citation2016). Post-laying diffusive loss of water may result in weight loss of eggs, so correction/normalization of results for lipid or water content may be needed (Espín et al., Citation2016).
5.3. Examples of the use of eggs in field research
Transfer of contaminants to the eggs can be species dependent, with differences caused by variable diet or exposure pathways (Ahrens et al., Citation2011; Van den Steen et al., Citation2009). For instance, when comparing contaminant concentrations in herring gulls and common eiders authors have found higher maternal transfer ratios of lipophilic OHCs in gulls. Authors have also found higher numbers of lipophilic OHCs in herring gull eggs (Thorstensen et al., Citation2021). Braune et al. (Citation2015) found a dominance of BDE-47 and -99 in BDE congener profiles in eggs collected from North American seabird species. Temporal trends showed a changing pattern of use of products containing flame retardants.
Eggs of snow buntings (Plectrophenax nivealis) feeding solely on the terrestrial food sources from Arctic areas were used to determine legacy and emerging organic contaminants levels in colonies from three different settlements in Svalbard. The relatively narrow foraging ranges of these birds during breeding season allowed assessment of potential point sources for contaminants. Significant differences were found between places for a number of compounds, e.g. PCBs or PFOS and PFCAs. High differences were sometimes found between closely located nests (Warner et al., Citation2019). Dauwe et al. (Citation2006) compared the concentrations and profile of the PCBs, PBDEs, and OCPs between the great tit eggs and nestlings. The concentrations found in the eggs were 4–6 times higher, and as the nestlings were getting older the profile changed as the proportion of many POPs decreased.
Eggs are seen as a good proxy for contamination by environmental PFASs via maternal exposure (Morganti et al., Citation2021). Bertolero et al. (Citation2015) intended to determine the maternal transfer of PFASs from female blood to eggs in seabirds living in Ebro Delta Natural Park in Spain. As a result, the authors assessed the transfer capacity from blood to first eggs of two gull species, yellow-legged gulls (Larus michahellis) and Audouin’s gull (Larus audouinii), to be around 6700 ± 2700 ng per egg and 5500 ± 1600 ng per egg, respectively.
Of all PFASs, PFOS is often a predominant compound in bird eggs (Ahrens et al., Citation2011; Holmström & Berger, Citation2008; Holmström et al., Citation2010; Morganti et al., Citation2021; Pereira et al., Citation2021). The PFOS concentration in eggs from the common guillemot (Uria aalge) approximately three times higher than in adult female livers, which may suggest efficient transfer during egg formation (Holmström & Berger, Citation2008). Ahrens et al. (Citation2011) assessed long-term exposure to PFASs in unhatched tawny owl (Strix aluco) eggs over a period of 24 years (up until 2009). Over this time, PFOS was the dominating congener in the egg profile, but with a decreasing trend of 1.6% annually. On the other hand, the contribution of C10-C13 PFCA increased over this period by 4.2–12% annually.
Unhatched eggs were used in numerous studies e.g. in predatory bird species to analyze temporal trends in PFASs concentration (Holmström et al., Citation2010), or to measure residues of OCs and PCBs (Martínez-López et al., Citation2007). PBDEs were analyzed in failed eggs from two colonies of white stork (Ciconia ciconia) in Spain, and higher brominated BDEs dominated the congener profiles (Muñoz-Arnanz et al., Citation2011).
Studies performed by Zhao et al. (Citation2019) showed that the major pesticides accumulated in eggshells were chlordanes and HCHs, possibly from soil. Although they found bioaccumulation potential to be higher for feathers than eggshells, the latter were still proven to be useful for predicting POPs pollution in the study area (Zhao et al., Citation2019). Matache et al. (Citation2016) used chorioallantoid membrane to determine several OCPs. The prevalent contributors to the total organochlorine were DDT congeners, and gamma-HCH was the main congener from the HCH isomers. Also, concentrations were mostly higher than those from feathers, suggesting significant maternal transfer of pollutants to embryo. However, the difference was species dependent; for example, in Anas sp. the OC concentration was up to ten times higher in eggshells, whilst in a top predator, the seagull (Larus argentatus), feathers had higher concentrations of OCPs ().
Table 3. Summary of key factors relevant to the preparatory phase that may influence the decision-making process (based on references provided in the review).
6. Conclusion and perspectives
Non-lethal sampling becomes an important part of environmental studies. It offers more ethical choices by leaving studied animals alive and often has practical advantages, including storage and collection of samples. Following the recommendation made by previous studies, the benefits and drawbacks of using four nondestructively collected biological materials from birds were presented. The choice should be made with an informed decision, knowing the factors that can affect the concentration of compounds in them and also how the collection can affect birds.
The decision of whether it is feather, blood, preen oil, or egg should be made based on the species ecology, habitat, breeding tactics and biological sample characteristics. It should be noticed that for newer emerging compounds, often little data exist. But this is changing; for instance, an organophosphate insecticide chlorpyfiros was found in feathers (Adrogué et al., Citation2019; González-Gómez et al., Citation2020). Briels et al. (Citation2019) found that the dominant compounds in goshawk nestling feathers were phosphorus flame retardants. Another example is the use of blood to detect novel contaminants, confirming the presence of novel flame retardant HBB (hexabromobenzene) in six individuals (per 30 studied in total) (Lewis et al., Citation2020). Non-PBDEs halogenated flame retardants and organophosphate ester flame retardants were analyzed in eggs collected from the herring gulls (Su et al., Citation2015). These compounds are not the focus of the presented publication, but this indicates that interest in non-lethal approaches is growing. In the future, we should see more studies using the non-lethally collected samples, and those are needed to make a clear division as to which matrix is reliable for which compound.
Acknowledgements
I would like to thank the anonymous reviewers for their valuable comments that helped me improve this manuscript.
References
- Ackerman, J. T., Eagles-Smith, C. A., Herzog, M. P., & Hartman, C. A. (2016). Maternal transfer of contaminants in birds: Mercury and selenium concentrations in parents and their eggs. Environmental Pollution, 210, 145–154. https://doi.org/10.1016/j.envpol.2015.12.016
- Adrogué, A. Q., Miglioranza, K. S., Copello, S., Favero, M., & Pon, J. P. S. (2019). Pelagic seabirds as biomonitors of persistent organic pollutants in the Southwestern Atlantic. Marine Pollution Bulletin, 149, 110516. https://doi.org/10.1016/j.marpolbul.2019.110516
- Ahrens, L., Herzke, D., Huber, S., Bustnes, J. O., Bangjord, G., & Ebinghaus, R. (2011). Temporal trends and pattern of polyfluoroalkyl compounds in tawny owl (Strix aluco) eggs from Norway, 1986–2009. Environmental Science & Technology, 45(19), 8090–8097. https://doi.org/10.1021/es103473v
- Ask, A. V., Jenssen, B. M., Tartu, S., Angelier, F., Chastel, O., & Gabrielsen, G. W. (2021). Per- and polyfluoroalkyl substances are positively associated with thyroid hormones in an arctic seabird. Environmental Toxicology and Chemistry, 40(3), 820–831. https://doi.org/10.1002/etc.4978
- Becker, P. H. (2003). Biomonitoring with birds. In B. Markert, T. Breure, & H. Zechmeister (Eds.), Bioindicators and biomonitors: Principles, concepts and applications (pp. 677–736). Elsevier Sciences Ltd.
- Behrooz, R. D., Esmaili-Sari, A., Ghasempouri, S. M., Bahramifar, N., & Hosseini, S. M. (2009). Organochlorine pesticide and polychlorinated biphenyl in feathers of resident and migratory birds of South-West Iran. Archives of Environmental Contamination and Toxicology, 56(4), 803–810. https://doi.org/10.1007/s00244-008-9211-9
- Bertolero, A., Vicente, J., Meyer, J., & Lacorte, S. (2015). Accumulation and maternal transfer of perfluorooctane sulphonic acid in yellow-legged (Larus michahellis) and Audouin’s gull (Larus audouinii) from the Ebro Delta Natural Park. Environmental Research, 137, 208–214. https://doi.org/10.1016/j.envres.2014.12.018
- Blévin, P., Shaffer, S. A., Bustamante, P., Angelier, F., Picard, B., Herzke, D., Moe, B., Gabrielsen, G. W., Bustnes, J. O., & Chastel, O. (2020). Contaminants, prolactin and parental care in an Arctic seabird: Contrasted associations of perfluoroalkyl substances and organochlorine compounds with egg-turning behavior. General and Comparative Endocrinology, 291, 113420. https://doi.org/10.1016/j.ygcen.2020.113420
- Bortolotti, G. R. (2010). Flaws and pitfalls in the chemical analysis of feathers: Bad news-good news for avian chemoecology and toxicology. Ecological Applications, 20(6), 1766–1774. https://doi.org/10.1890/09-1473.1
- Bortolotti, G. R., Fernie, K. J., & Smits, J. E. (2003). Carotenoid concentration and coloration of American Kestrels (Falco sparverius) disrupted by experimental exposure to PCBs. Functional Ecology, 17(5), 651–657. https://doi.org/10.1046/j.1365-2435.2003.00778.x
- Braune, B. M., Letcher, R. J., Gaston, A. J., & Mallory, M. L. (2015). Trends of polybrominated diphenyl ethers and hexabromocyclododecane in eggs of Canadian Arctic seabirds reflect changing use patterns. Environmental Research, 142, 651–661. https://doi.org/10.1016/j.envres.2015.08.010
- Briels, N., Torgersen, L. N., Castaño-Ortiz, J. M., Løseth, M. E., Herzke, D., Nygård, T., Bustnes, J. O., Ciesielski, T. M., Poma, G., Malarvannan, G., Covaci, A., & Jaspers, V. L. B. (2019). Integrated exposure assessment of northern goshawk (Accipiter gentilis) nestlings to legacy and emerging organic pollutants using non-destructive samples. Environmental Research, 178, 108678. https://doi.org/10.1016/j.envres.2019.108678
- Burger, J., & Gochfeld, M. (1997). Risk, mercury levels, and birds: Relating adverse laboratory effects to field biomonitoring. Environmental Research, 75(2), 160–172. https://doi.org/10.1006/enrs.1997.3778
- Bustnes, J. O., Skaare, J. U., Erikstad, K. E., Bakken, V., & Mehlum, F. (2001). Whole blood concentrations of organochlorines as a dose metric for studies of the glaucous gull (Larus hyperboreus). Environmental Toxicology and Chemistry, 20(5), 1046–1052.
- Bustnes, J. O., Bårdsen, B.-J., Moe, B., Herzke, D., Hanssen, S. A., Sagerup, K., Bech, C., Nordstad, T., Chastel, O., Tartu, S., & Gabrielsen, G. W. (2017). Temporal variation in circulating concentrations of organochlorine pollutants in a pelagic seabird breeding in the high Arctic. Environmental Toxicology and Chemistry, 36(2), 442–448. https://doi.org/10.1002/etc.3560
- Bustnes, J. O., Borgå, K., Erikstad, K. E., Lorentsen, S.-H., & Herzke, D. (2008). Perfluorinated, brominated, and chlorinated contaminants in a population of lesser black-backed gulls (Larus fuscus). Environmental Toxicology and Chemistry, 27(6), 1383–1392. https://doi.org/10.1897/07-473.1
- Campagna, S., Mardon, J., Celerier, A., & Bonadonna, F. (2012). Potential semiochemical molecules from birds: A practical and comprehensive compilation of the last 20 years studies. Chemical Senses, 37(1), 3–25. https://doi.org/10.1093/chemse/bjr067
- Carravieri, A., Cherel, Y., Brault-Favrou, M., Churlaud, C., Peluhet, L., Labadie, P., Budzinski, H., Chastel, O., & Bustamante, P. (2017). From Antarctica to the subtropics: Contrasted geographical concentrations of selenium, mercury, and persistent organic pollutants in skua chicks (Catharacta spp.). Environmental Pollution, 228, 464–473. https://doi.org/10.1016/j.envpol.2017.05.053
- Chen, C.-F., Foley, J., Tang, P.-C., Li, A., Jiang, T. X., Wu, P., Widelitz, R. B., & Chuong, C. M. (2015). Development, regeneration, and evolution of feathers. Annual Review of Animal Biosciences, 3(1), 169–111.27. https://doi.org/10.1146/annurev-animal-022513-114127
- Cobb, G. P., Bargar, T. A., Pepper, C. B., Norman, D. M., Houlis, P. D., & Anderson, T. A. (2003). Using chorioallantoic membranes for non-lethal assessment of persistent organic pollutant exposure and effect in oviparous wildlife. Ecotoxicology, 12(1/4), 31–45. https://doi.org/10.1023/A:1022532711353
- Colabuono, F. I., Vander Pol, S. S., Huncik, K. M., Taniguchi, S., Petry, M. V., Kucklick, J. R., & Montone, R. C. (2016). Persistent organic pollutants in blood samples of Southern Giant Petrels (Macronectes giganteus) from the South Shetland Islands, Antarctica. Environmental Pollution, 216, 38–45. https://doi.org/10.1016/j.envpol.2016.05.041
- Dauwe, T., Jaspers, V., Covaci, A., Schepens, P., & Eens, M. (2005). Feathers as a nondestructive biomonitor for persistent organic pollutants. Environmental Toxicology and Chemistry, 24(2), 442–449. https://doi.org/10.1897/03-596.1
- Dauwe, T., Jaspers, V. L., Covaci, A., & Eens, M. (2006). Accumulation of organochlorines and brominated flame retardants in the eggs and nestlings of great tits, Parus major. Environmental Science & Technology, 40(17), 5297–5303. https://doi.org/10.1021/es060747a
- Dauwe, T., Van de Vijver, K., De Coen, W., & Eens, M. (2007). PFOS levels in the blood and liver of a small insectivorous songbird near a fluorochemical plant. Environment International, 33(3), 357–361. https://doi.org/10.1016/j.envint.2006.11.014
- Ehresman, D. J., Froehlich, J. W., Olsen, G. W., Chang, S.-C., & Butenhoff, J. L. (2007). Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environmental Research, 103(2), 176–184. https://doi.org/10.1016/j.envres.2006.06.008
- Elliott, K. H., Cesh, L. S., Dooley, J. A., Letcher, R. J., & Elliott, J. E. (2009). PCBs and DDE, but not PBDEs, increase with trophic level and marine input in nestling bald eagles. The Science of the Total Environment, 407(12), 3867–3875. https://doi.org/10.1016/j.scitotenv.2009.02.027
- Espín, S., Martínez-López, E., María-Mojica, P., & García-Fernández, A. J. (2012). Razorbill (Alca torda) feathers as an alternative tool for evaluating exposure to organochlorine pesticides. Ecotoxicology, 21(1), 183–190. https://doi.org/10.1007/s10646-011-0777-z
- Espín, S., García-Fernández, A. J., Herzke, D., Shore, R. F., van Hattum, B., Martínez-López, E., Coeurdassier, M., Eulaers, I., Fritsch, C., Gómez-Ramírez, P., Jaspers, V. L. B., Krone, O., Duke, G., Helander, B., Mateo, R., Movalli, P., Sonne, C., & van den Brink, N. W. (2016). Tracking pan-continental trends in environmental contamination using sentinel raptors—What types of samples should we use? Ecotoxicology, 25(4), 777–801. https://doi.org/10.1007/s10646-016-1636-8
- Eulaers, I., Covaci, A., Hofman, J., Nygård, T., Halley, D. J., Pinxten, R., Eens, M., & Jaspers, V. L. B. (2011a). A comparison of non-destructive sampling strategies to assess the exposure of white-tailed eagle nestlings (Haliaeetus albicilla) to persistent organic pollutants. The Science of the Total Environment, 410-411, 258–265. https://doi.org/10.1016/j.scitotenv.2011.09.070
- Eulaers, I., Covaci, A., Herzke, D., Eens, M., Sonne, C., Moum, T., Schnug, L., Hanssen, S. A., Johnsen, T. V., Bustnes, J. O., & Jaspers, V. L. B. (2011b). A first evaluation of the usefulness of feathers of nestling predatory birds for non-destructive biomonitoring of persistent organic pollutants. Environment International, 37(3), 622–630. https://doi.org/10.1016/j.envint.2010.12.007
- Eulaers, I., Jaspers, V. L. B., Bustnes, J. O., Covaci, A., Johnsen, T. V., Halley, D. J., Moum, T., Ims, R. A., Hanssen, S. A., Erikstad, K. E., Herzke, D., Sonne, C., Ballesteros, M., Pinxten, R., & Eens, M. (2013). Ecological and spatial factors drive intra- and interspecific variation in exposure of subarctic predatory bird nestlings to persistent organic pollutants. Environment International, 57-58, 25–33. https://doi.org/10.1016/j.envint.2013.03.009
- Eulaers, I., Jaspers, V. L. B., Pinxten, R., Covaci, A., & Eens, M. (2014). Legacy and current-use brominated flame retardants in the Barn Owl. The Science of the Total Environment, 472, 454–462. https://doi.org/10.1016/j.scitotenv.2013.11.054
- Fair, J., Paul, E., Jones, J., Clark, A. B., Davie, C., & Kaiser, G. (2010). Chapter 6: Minor manipulative procedures. In J. Fair, E. Paul, & J. Jones (Eds.), Guidelines to the use of wild birds in research (pp. 131–167). The Ornithological Council.
- Finger, A., Lavers, J. L., Dann, P., Nugegoda, D., Orbell, J. D., Robertson, B., & Scarpaci, C. (2015). The little penguin (Eudyptula minor) as an indicator of coastal trace metal pollution. Environmental Pollution, 205, 365–377. https://doi.org/10.1016/j.envpol.2015.06.022
- García-Fernandez, A. J., Espín, S., & Martínez-López, E. (2013). Feathers as a biomonitoring tool of polyhalogenated compounds: A review. Environmental Science & Technology, 47(7), 3028–3043. https://doi.org/10.1021/es302758x
- Gebbink, W. A., & Letcher, R. J. (2012). Comparative tissue and body compartment accumulation and maternal transfer to eggs of perfluoroalkyl sulfonates and carboxylates in Great Lakes herring gulls. Environmental Pollution, 162, 40–47. https://doi.org/10.1016/j.envpol.2011.10.011
- Goede, A. A., & De Bruin, M. (1986). The use of bird feathers for indicating heavy metal pollution. Environmental Monitoring and Assessment, 7(3), 249–256. https://doi.org/10.1007/BF00418017
- Gómez-Ramírez, P., Bustnes, J. O., Eulaers, I., Herzke, D., Johnsen, T. V., Lepoint, G., Pérez-García, J. M., García-Fernández, A. J., & Jaspers, V. L. B. (2017). Per- and polyfluoroalkyl substances in plasma and feathers of nestling birds of prey from northern Norway. Environmental Research, 158, 277–285. https://doi.org/10.1016/j.envres.2017.06.019
- González-Gómez, X., Simal-Gándara, J., Fidalgo Alvarez, L. E., López-Beceiro, A. M., Pérez-López, M., & Martínez-Carballo, E. (2020). Non-invasive biomonitoring of organic pollutants using feather samples in feral pigeons (Columba livia domestica). Environmental Pollution, 267, 115672. https://doi.org/10.1016/j.envpol.2020.115672
- González-Rubio, S., Ballesteros-Gómez, A., Asimakopoulos, A. G., & Jaspers, V. L. B. (2021). A review on contaminants of emerging concern in European raptors (2002–2020). The Science of the Total Environment, 760, 143337. https://doi.org/10.1016/j.scitotenv.2020.143337
- Groffen, T., Lasters, R., Bervoets, L., Prinsen, E., & Eens, M. (2020). Are feathers of a songbird model species (The Great Tit, Parus major) suitable for monitoring perfluoroalkyl acids (PFAAs) in blood plasma? Environmental Science & Technology, 54(15), 9334–9344. https://doi.org/10.1021/acs.est.0c00652
- Guthrie, R., & Susi, A. (1963). A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics, 32, 338–343.
- Herzke, D., Nygård, T., Berger, U., Huber, S., & Røv, N. (2009). Perfluorinated and other persistent halogenated organic compounds in European shag (Phalacrocorax aristotelis) and common eider (Somateria mollissima) from Norway: A suburban to remote pollutant gradient. The Science of the Total Environment, 408(2), 340–348. https://doi.org/10.1016/j.scitotenv.2009.08.048
- Holmström, K. E., & Berger, U. (2008). Tissue distribution of perfluorinated surfactants in common guillemot (Uria aalge) from the Baltic Sea. Environmental Science & Technology, 42(16), 5879–5884. https://doi.org/10.1021/es800529h
- Holmström, K. E., Johansson, A. K., Bignert, A., Lindberg, P., & Berger, U. (2010). Temporal trends of perfluorinated surfactants in Swedish peregrine falcon eggs (Falco peregrinus), 1974-2007. Environmental Science & Technology, 44(11), 4083–4088. https://doi.org/10.1021/es100028f
- Ito, A., Yamashita, R., Takada, H., Yamamoto, T., Shiomi, K., Zavalaga, C., Abe, T., Watanabe, S., Yamamoto, M., Sato, K., Kohno, H., Yoda, K., Iida, T., & Watanuki, Y. (2013). Contaminants in Tracked Seabirds Showing Regional Patterns of Marine Pollution. Environmental Science & Technology, 47(14), 7862–7867. https://doi.org/10.1021/es4014773
- Jaspers, V. L. B., Voorspoels, S., Covaci, A., & Eens, M. (2006). Can predatory bird feathers be used as a non-destructive biomonitoring tool of organic pollutants? Biology Letters, 2(2), 283–285. https://doi.org/10.1098/rsbl.2006.0450
- Jaspers, V. L. B., Voorspoels, S., Covaci, A., Lepoint, G., & Eens, M. (2007). Evaluation of the usefulness of bird feathers as a non-destructive biomonitoring tool for organic pollutants: A comparative and meta-analytical approach. Environment International, 33(3), 328–337. https://doi.org/10.1016/j.envint.2006.11.011
- Jaspers, V. L. B., Covaci, A., Deleu, P., Neels, H., & Eens, M. (2008). Preen oil as the main source of external contamination with organic pollutants onto feathers of the common magpie (Pica pica). Environment International, 34(6), 741–748. https://doi.org/10.1016/j.envint.2007.12.002
- Jaspers, V. L. B., Covaci, A., Herzke, D., Eulaers, I., & Eens, M. (2019). Bird feathers as a biomonitor for environmental pollutants: Prospects and pitfalls. Trends in Analytical Chemistry, 118, 223–226. https://doi.org/10.1016/j.trac.2019.05.019
- Jaspers, V. L. B., Herzke, D., Eulaers, I., Gillespie, B. W., & Eens, M. (2013). Perfluoroalkyl substances in soft tissues and tail feathers of Belgian barn owls (Tyto alba) using statistical methods for left-censored data to handle non-detects. Environment International, 52, 9–16. https://doi.org/10.1016/j.envint.2012.11.002
- Jones, P. D., Hu, W., De Coen, W., Newsted, J. L., & Giesy, J. P. (2003). Binding of perfluorinated fatty acids to serum proteins. Environmental Toxicology and Chemistry, 22(11), 2639–2649. https://doi.org/10.1897/02-553
- King, A. S., & McLelland, J. (1975). Uropygial gland (preen gland or oil gland) and other cutaneous glands. In Outlines of avian anatomy (pp. 6–7). Balliere Tindall.
- Lasters, R., Groffen, T., Lopez-Antia, A., Bervoets, L., & Eens, M. (2019). Variation in PFAA concentrations and egg parameters throughout the egg-laying sequence in a free-living songbird (the great tit, Parus major): Implications for biomonitoring studies. Environmental Pollution, 246, 237–248. https://doi.org/10.1016/j.envpol.2018.12.014
- Leat, E. H. K., Bourgeon, S., Hanssen, S. A., Petersen, A., Strøm, H., Bjørn, T. H., Gabrielsen, G. W., Bustnes, J. O., Furness, R. W., Haarr, A., & Borgå, K. (2019). The effect of long-range transport, trophic position and diet specialization on legacy contaminant occurrence in great skuas, Stercorarius skua, breeding across the Northeast Atlantic. Environmental Pollution, 244, 55–65. https://doi.org/10.1016/j.envpol.2018.10.005
- Leat, E. H. K., Bourgeon, S., Magnusdottir, E., Gabrielsen, G. W., Grecian, W. J., Hanssen, S. A., Olafsdottir, K., Petersen, A., Phillips, R. A., Strøm, H., Ellis, S., Fisk, A. T., Bustnes, J. O., Furness, R. W., & Borgå, K. (2013). Influence of wintering area on persistent organic pollutants in a breeding migratory seabird. Marine Ecology Progress Series, 491, 277–293. https://doi.org/10.3354/meps10455
- Letcher, R. J., Bustnes, J. O., Dietz, R., Jenssen, B. M., Jørgensen, E. H., Sonne, C., Verreault, J., Vijayan, M. M., & Gabrielsen, G. W. (2010). Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. The Science of the Total Environment, 408(15), 2995–3043. https://doi.org/10.1016/j.scitotenv.2009.10.038
- Lehner, A. F., Rumbeiha, W., Shlosberg, A., Stuart, K., Johnson, M., Domenech, R., & Langner, H. (2013). Diagnostic analysis of veterinary dried blood spots for toxic heavy metals exposure. Journal of Analytical Toxicology, 37(7), 406–422. https://doi.org/10.1093/jat/bkt048
- Lehman-McKeeman, L. D. (2008). Absorption, distribution, and excretion of toxicants. In C. D. Klaassen (Ed.), Casarett & Doull’s toxicology: The basic science of poisons (pp. 131–159). McGraw Hill Medical.
- Lewis, P. J., McGrath, T. J., Chiaradia, A., McMahon, C. R., Emmerson, L., Allinson, G., & Shimeta, J. (2020). A baseline for POPs contamination in Australian seabirds: Little penguins vs. short-tailed shearwaters. Marine Pollution Bulletin, 159, 111488. https://doi.org/10.1016/j.marpolbul.2020.111488
- Lohmann, R., Breivik, K., Dachs, J., & Muir, D. (2007). Global fate of POPs: Current and future research directions. Environmental Pollution, 150(1), 150–165. https://doi.org/10.1016/j.envpol.2007.06.051
- Løseth, M. E., Briels, N., Flo, J., Malarvannan, G., Poma, G., Covaci, A., Herzke, D., Nygård, T., Bustnes, J. O., Jenssen, B. M., & Jaspers, V. L. B. (2019). White-tailed eagle (Haliaeetus albicilla) feathers from Norway are suitable for monitoring of legacy, but not emerging contaminants. The Science of the Total Environment, 647(647), 525–533. https://doi.org/10.1016/j.scitotenv.2018.07.333
- Mardon, J., Saunders, S. M., & Bonadonna, F. (2011). From preen secretions to plumage: The chemical trajectory of blue petrels’ Halobaena caerulea social scent. Journal of Avian Biology, 42(1), 29–38. https://doi.org/10.1111/j.1600-048X.2010.05113.x
- Martínez-López, E., María-Mojica, P., Martínez, J. E., Calvo, J. F., Wright, J., Shore, R. F., Romero, D., & García-Fernández, A. J. (2007). Organochlorine residues in booted eagle (Hieraaetus pennatus) and goshawk (Accipiter gentilis) eggs from southeastern Spain. Environmental Toxicology and Chemistry, 26(11), 2373–2378. https://doi.org/10.1897/07-057R.1
- Matache, M. L., Hura, C., & David, I. G. (2016). Non-invasive monitoring of organohalogen compounds in eggshells and feathers of birds from the Lower Prut Floodplain Natural Park in Romania. Procedia Environmental Sciences, 32, 49–58. https://doi.org/10.1016/j.proenv.2016.03.011
- Meyer, J., Jaspers, V. L. B., Eens, M., & de Coen, W. (2009). The relationship between perfluorinated chemical levels in the feathers and livers of birds from different trophic levels. Science of the Total Environment, 407(22), 5894–5900. https://doi.org/10.1016/j.scitotenv.2009.07.032
- Monclús, L., Lopez-Bejar, M., De la Puente, J., Covaci, A., & Jaspers, V. L. B. (2018). First evaluation of the use of down feathers for monitoring persistent organic pollutants and organophosphate ester flame retardants: A pilot study using nestlings of the endangered cinereous vulture (Aegypius monachus). Environmental Pollution, 238, 413–420. https://doi.org/10.1016/j.envpol.2018.03.065
- Morganti, M., Polesello, S., Pascariello, S., Ferrario, C., Rubolini, D., Valsecchi, S., & Parolini, M. (2021). Exposure assessment of PFAS-contaminated sites using avian eggs as a biomonitoring tool: A frame of reference and a case study in the Po River valley (Northern Italy). Integrated Environmental Assessment and Management, 17(4), 733–745. https://doi.org/10.1002/ieam.4417
- Muñoz-Arnanz, J., Sáez, M., Aguirre, J. I., Hiraldo, F., Baos, R., Pacepavicius, G., Alaee, M., & Jiménez, B. (2011). Predominance of BDE-209 and other higher brominated diphenyl ethers in eggs of white stork (Ciconia ciconia) colonies from Spain. Environment International, 37(3), 572–576. https://doi.org/10.1016/j.envint.2010.11.013
- Ohlendorf, H. M., & Fleming, W. J. (1988). Birds and environmental contaminants in San Francisco and Chesapeake Bays. Marine Pollution Bulletin, 19(9), 487–495. https://doi.org/10.1016/0025-326X(88)90405-5
- Olsson, A., Ceder, K., Bergman, A., & Helander, B. (2000). Nestling blood of the white-tailed sea eagle (Haliaeetus albicilla) as an indicator of territorial exposure to organohalogen compounds: An evaluation. Environmental Science & Technology, 34(13), 2733–2740. https://doi.org/10.1021/es991426k
- Ortiz-Santaliestra, M. E., Resano-Mayor, J., Hernández-Matías, A., Rodríguez-Estival, J., Camarero, P. R., Moleón, M., Real, J., & Mateo, R. (2015). Pollutant accumulation patterns in nestlings of an avian top predator: Biochemical and metabolic effects. The Science of the Total Environment, 538, 692–702. https://doi.org/10.1016/j.scitotenv.2015.08.053
- Pacyna, A. D., Jakubas, D., Ausems, A. N. M. A., Frankowski, M., Polkowska, Ż., & Wojczulanis-Jakubas, K. (2019). Storm petrels as indicators of pelagic seabird exposure to chemical elements in the Antarctic marine ecosystem. The Science of the Total Environment, 692, 382–392. https://doi.org/10.1016/j.scitotenv.2019.07.137
- Pacyna-Kuchta, A. D., Jakubas, D., Frankowski, M., Polkowska, Ż., & Wojczulanis-Jakubas, K. (2020). Exposure of a small Arctic seabird, the little auk (Alle alle) breeding in Svalbard, to selected elements throughout the course of a year. The Science of the Total Environment, 732, 139103. https://doi.org/10.1016/j.scitotenv.2020.139103
- Peakall, D. B. (1974). DDE: Its presence in Peregrine eggs in 1948. Science, 183(4125), 673–674. https://doi.org/10.1126/science.183.4125.673
- Pereira, M. G., Lacorte, S., Walker, L. A., & Shore, R. F. (2021). Contrasting long term temporal trends in perfluoroalkyl substances (PFAS) in eggs of the northern gannet (Morus bassanus) from two UK colonies. The Science of the Total Environment, 754, 141900. https://doi.org/10.1016/j.scitotenv.2020.141900
- Perkins, C. R., & Barclay, J. S. (1997). Accumulation and mobilization of organochlorine contaminants in wintering greater scaup. Journal of Wildlife Management, 61(2), 444–449. https://doi.org/10.2307/3802602
- Perkins, M., & Basu, N. (2018). Dried blood spots for estimating mercury exposure in birds. Environmental Pollution, 236, 236–246. https://doi.org/10.1016/j.envpol.2018.01.036
- Pittman, H. T., Bowerman, W. W., Grim, L. H., Grubb, T. G., Bridges, W. C., & Wierda, M. R. (2015). Using nestling plasma to assess long-term spatial and temporal concentrations of organochlorine compounds in bald eagles within Voyageurs National Park, Minnesota, USA. Chemosphere, 123, 79–86. https://doi.org/10.1016/j.chemosphere.2014.12.043
- Rawles, M. E. (1960). The uropygial gland. In A. J. Marshall (Ed.), Biology and comparative physiology of birds (pp. 211–212). Academic Press.
- Reynolds, K. D., Skipper, S. L., Cobb, G. P., & McMurry, S. T. (2004). Relationship between DDE concentrations and laying sequence in eggs of two passerine species. Archives of Environmental Contamination and Toxicology, 47(3), 336–340. https://doi.org/10.1007/s00244-004-3157-3
- Sagerup, K., Helgason, L. B., Polder, A., Strøm, H., Josefsen, T. D., Skåre, J. U., & Gabrielsen, G. W. (2009). Persistent organic pollutants and mercury in dead and dying glaucous gulls (Larus hyperboreus) at Bjørnøya (Svalbard). The Science of the Total Environment, 407(23), 6009–6016. https://doi.org/10.1016/j.scitotenv.2009.08.020
- Sandilands, V., Powell, K., Keeling, L., & Savory, C. J. (2004). Preen gland function in layer fowls: Factors affecting preen oil fatty acid composition. British Poultry Science, 45(1), 109–115. https://doi.org/10.1080/00071660410001668932
- Shlosberg, A., Wu, Q., Rumbeiha, W. K., Lehner, A., Cuneah, O., King, R., Hatzofe, O., Kannan, K., & Johnson, M. (2012). Examination of Eurasian griffon vultures (Gyps fulvus fulvus) in Israel for exposure to environmental toxicants using dried blood spots. Archives of Environmental Contamination and Toxicology, 62(3), 502–511. https://doi.org/10.1007/s00244-011-9709-4
- Soini, H. A., Whittaker, D. J., Wiesler, D., Ketterson, E. D., & Novotny, M. V. (2013). Chemosignaling diversity in songbirds: Chromatographic profiling of preen oil volatiles in different species. Journal of Chromatography. A, 1317, 186–192. https://doi.org/10.1016/j.chroma.2013.08.006
- Solheim, S. A., Sagerup, K., Huber, S., Byrkjedal, I., & Gabrielsen, G. W. (2016). The black-legged kittiwake preen gland—An overlooked organ for depuration of fat-soluble contaminants? Polar Research, 35(1), 29651. https://doi.org/10.3402/polar.v35.29651
- Sonne, C., Bustnes, J. O., Herzke, D., Jaspers, V. L. B., Covaci, A., Halley, D. J., Moum, T., Eulaers, I., Eens, M., Ims, R. A., Hanssen, S. A., Erikstad, K. E., Johnsen, T., Schnug, L., Riget, F. F., & Jensen, A. L. (2010). Relationships between organohalogen contaminants and blood plasma clinical-chemical parameters in chicks of three raptor species from Northern Norway. Ecotoxicology and Environmental Safety, 73(1), 7–17. https://doi.org/10.1016/j.ecoenv.2009.08.017
- Sonne, C., Riget, F. F., Leat, E. H. K., Bourgeon, S., Borgå, K., Strøm, H., Hanssen, S. A., Gabrielsen, G. W., Petersen, A., Olafsdottir, K., Magnusdottir, E., Bustnes, J. O., Furness, R. W., & Kjelgaard-Hansen, M. (2013). Organohalogen contaminants and blood plasma clinical-chemical parameters in three colonies of North Atlantic Great skua (Stercorarius skua. )Ecotoxicology and Environmental Safety, 92, 245–251. https://doi.org/10.1016/j.ecoenv.2013.02.012
- Souza, J. S., Pacyna-Kuchta, A. D., Schmauder Teixeira da Cunha, L., Schneider Costa, E., Niedzielski, P., & Machado Torres, J. P. (2021). Interspecific and intraspecific variation in organochlorine pesticides and polychlorinated biphenyls using non-destructive samples from Pygoscelis penguins. Environmental Pollution, 275, 116590. https://doi.org/10.1016/j.envpol.2021.116590
- Su, G., Letcher, R. J., Moore, J. N., Williams, L. L., Martin, P. A., de Solla, S. R., & Bowerman, W. W. (2015). Spatial and temporal comparisons of legacy and emerging flame retardants in herring gull eggs from colonies spanning the Laurentian Great Lakes of Canada and United States. Environmental Research, 142, 720–730. https://doi.org/10.1016/j.envres.2015.08.018
- Sun, J., Bossi, R., Bustnes, J. O., Helander, B., Boertmann, D., Dietz, R., Herzke, D., Jaspers, V. L. B., Labansen, A. L., Lepoint, G., Schulz, R., Sonne, C., Thorup, K., Tøttrup, A. P., Zubrod, J. P., Eens, M., & Eulaers, I. (2019). White-tailed eagle (Haliaeetus albicilla) body feathers document spatiotemporal trends of perfluoroalkyl substances in the northern environment. Environ. Environmental Science & Technology, 53(21), 12744–12753. https://doi.org/10.1021/acs.est.9b03514
- Svendsen, N. B., Herzke, D., Harju, M., Bech, C., Gabrielsen, G. W., & Jaspers, V. L. B. (2018). Persistent organic pollutants and organophosphate esters in feathers and blood plasma of adult kittiwakes (Rissa tridactyla) from Svalbard—Associations with body condition and thyroid hormones. Environmental Research, 164, 158–164. https://doi.org/10.1016/j.envres.2018.02.012
- Tartu, S., Gabrielsen, G. W., Blevin, P., Ellis, H., Bustnes, J. O., Herzke, D., & Chastel, O. (2014). Endocrine and fitness correlates of long-chain perfluorinated carboxylates exposure in arctic breeding black-legged kittiwakes. Environmental Science & Technology, 48(22), 13504–13510. https://doi.org/10.1021/es503297n
- Thorstensen, H., Ruus, A., Helberg, M., Baek, K., Enge, E. K., & Borgå, K. (2021). Common eider and herring gull as contaminant indicators of different ecological niches of an urban fjord system. Integrated Environmental Assessment and Management, 17(2), 402–412. https://doi.org/10.1002/ieam.4340
- Van den Steen, E., Dauwe, T., Covaci, A., Jaspers, V. L. B., Pinxten, R., & Eens, M. (2006). Within-and among-clutch variation of organohalogenated contaminants in eggs of great tits (Parus major. Environmental Pollution, 144(1), 355–359. https://doi.org/10.1016/j.envpol.2005.10.053
- Van den Steen, E., Covaci, A., Jaspers, V. L. B., Dauwe, T., Voorspoels, S., Eens, M., & Pinxten, R. (2007). Experimental evaluation of the usefulness of feathers as a non-destructive biomonitor for polychlorinated biphenyls (PCBs) using silastic implants as a novel method of exposure. Environment International, 33(2), 257–264. https://doi.org/10.1016/j.envint.2006.09.018
- Van den Steen, E., Jaspers, V. L. B., Covaci, A., Neels, H., Eens, M., & Pinxten, R. (2009). Maternal transfer of organochlorines and brominated flame retardants in blue tits (Cyanistes caeruleus). Environment International, 35(1), 69–75. https://doi.org/10.1016/j.envint.2008.08.003
- Verreault, J., Houde, M., Gabrielsen, G. W., Berger, U., Haukås, M., Letcher, R. J., & Muir, D. C. G. (2005). Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctic. Environmental Science & Technology, 39(19), 7439–7445. https://doi.org/10.1021/es051097y
- Verreault, J., Villa, R. A., Gabrielsen, G. W., Skaare, J. U., & Letcher, R. J. (2006). Maternal transfer of organohalogen contaminants and metabolites to eggs of Arctic-breeding glaucous gulls. Environmental Pollution, 144(3), 1053–1060. https://doi.org/10.1016/j.envpol.2005.10.055
- Vicente, J., Sanpera, C., García-Tarrasón, M., Pérez, A., & Lacorte, S. (2015). Perfluoroalkyl and polyfluoroalkyl substances in entire clutches of Audouin’s gulls from the ebro delta. Chemosphere, 119, S62–S68. https://doi.org/10.1016/j.chemosphere.2014.04.041
- Warner, N. A., Sagerup, K., Kristoffersen, S., Herzke, D., Gabrielsen, G. W., & Jenssen, B. M. (2019). Snow buntings (Plectrophenax nivealis) as bio-indicators for exposure differences to legacy and emerging persistent organic pollutants from the Arctic terrestrial environment on Svalbard. The Science of the Total Environment, 667, 638–647. https://doi.org/10.1016/j.scitotenv.2019.02.351
- Wang, J., Caccamise, S. A. L., Woodward, L. A., & Li, Q. X. (2015). Polychlorinated biphenyls in the plasma and preen oil of black-footed albatross (Diomedea nigripes) chicks and adults on Midway Atoll, North Pacific Ocean. PLoS One, 10(4), e0123041. https://doi.org/10.1371/journal.pone.0123041
- Wang, F., Zhao, C., Gao, Y., Fu, J., Gao, K., Lv, K., Wang, K., Yue, H., Lan, X., Liang, Y., Wang, Y., & Jiang, G. (2019). Protein-specific distribution patterns of perfluoroalkyl acids in egg yolk and albumen samples around a fluorochemical facility. The Science of the Total Environment, 650(Pt 2), 2697–2704. https://doi.org/10.1016/j.scitotenv.2018.10.006
- Yamashita, R., Takada, H., Murakami, M., Fukuwaka, M.-A., & Watanuki, Y. (2007). Evaluation of noninvasive approach for monitoring PCB pollution of seabirds using preen gland oil. Environmental Science & Technology, 41(14), 4901–4906. https://doi.org/10.1021/es0701863
- Yamashita, R., Takada, H., Nakazawa, A., Takahashi, A., Ito, M., Yamamoto, T., Watanabe, Y. Y., Kokubun, N., Sato, K., Wanless, S., Daunt, F., Hyrenbach, D., Hester, M., Deguchi, T., Nishizawa, B., Shoji, A., & Watanuki, Y. (2018). Global monitoring of persistent organic pollutants (POPs) using seabird preen gland oil. Archives of Environmental Contamination and Toxicology, 75(4), 545–556. https://doi.org/10.1007/s00244-018-0557-3
- Zhao, Z., Li, Q., Ni, C., & Zhang, L. (2019). Non-destructive bioindicator of little egret (egretta garzetta) to assess the pollution of highly toxic organic pollutants in Poyang Lake wetland. Wetlands, 39(S1), 137–150. https://doi.org/10.1007/s13157-017-0978-1