Abstract
The pollen content of 140 samples collected between 1995 and 2004 was used to characterise the honey and determine the source of nectar that Apis mellifera L. uses in Chubut (Argentinean Patagonia). A diverse spectrum of 139 pollen types from 53 families was identified with the Asteraceae and Fabaceae being most frequent. Forty‐eight per cent of the samples analysed were classified as monofloral, whereas the remaining were multifloral. Predominant pollen types were: Tamarix gallica L. (Tamaricaceae), in twenty‐one samples; Rosaceae and Melilotus spp. (Fabaceae) in nine samples; Trifolium spp. (Fabaceae) in eight; Medicago sativa L. (Fabaceae) in six; Aristotelia chilensis (Molina) Stuntz (Elaeocarpaceae) in four; Colletia/Discaria (Rhamnaceae) in three; Escallonia spp. (Saxifragaceae) in two; Eucalyptus spp. (Myrtaceae), Myrtaceae, Phacelia secunda J. F. Gmel (Hydrophyllaceae), Prosopidastrum globosum (Hook & Arn) Burkart (Fabaceae), and Schinus patagonica (Phil) I. M. Johnst. (Anacardiaceae) in one sample. Thirty per cent of the identified pollen corresponded to native flora. Six native taxa made up 18% of the monofloral honey. They included: Prosopidastrum globosum, Colletia/Discaria‐type, Phacelia secunda, Schinus patagonica, Aristotelia chilensis and Escallonia spp. All of these are new monofloral types in Argentina. Characteristic pollen associations gave a geographical identity to these honeys.
Argentina extends from 22 to 55° S (Figure ), and is one of the main producers and exporters of honey in the world (FAO, 2005). The country proportions, large latitudinal range, and biogeographic complexity can explain why the Argentina's potential for apicultural production has not been fully explored. The most important honey producing areas are located in the centre of the country, mainly in the Pampa region, where agriculture and cattle farming are most intensive. Because of the human impact in this region, the native vegetation has been disturbed, and is poorly represented in the honeys (Tellería, Citation1988, Citation1992, Citation1996a , b; Valle et al., Citation1995; Andrada et al., Citation1998). Recently, the growing demand for contaminant‐free honey products has increased the interest in Argentinean regions with the relatively little human impact. Because of the abundance of native and endemic plant species and a lack of human intervention, areas such as Patagonia offer possibilities for a diverse and contamination‐free apiculture industry.
Patagonia is a macroregion of Argentina that extends south of 39° S latitude, to the tip of Argentina (at 55° S). It includes the mountain range of Andes and the region situated between the Andean foothills and the Atlantic Ocean (León et al., Citation1998; Roig, Citation1998). The characteristic features of Patagonia are aridity, strong winds from the west, and rainfall that is abundant only in a narrow area of the Andean region and decreases sharply towards the east. This rainfall gradient determines a spectrum of physiognomic types of vegetation: forest, grassy steppe, shrubby steppe and semi‐desert (Soriano, Citation1983). The latter being the most extensive type (León et al., Citation1998).
Chubut with an area of 224.686 km2 is the second largest province in Argentinean Patagonia and the third largest administrative province in Argentina. The apiculture industry in Chubut is still in its developmental stage, and it is expanding.
Melissopalynological studies have been already carried out in all melliferous areas of the Chubut province (Forcone & Tellería, Citation1998, Citation2000; Forcone, Citation2003; Forcone et al., Citation2003, 2005).
The aim of this study was to determine the nectariferous plants used by honey bees (Apis mellifera L.), and to characterise the pollen content of honeys from the Chubut province of Patagonia.
Material and methods
Study area
Two physiogeographical regions in the Chubut province are clearly distinguishable: the Andean and the extra‐Andean (Figure ). The Andean region is characterised by its mountainous relief and influenced by glacial activity. The climate varies from template‐cold to cold‐humid, with rains and snowfall during the winter. The average annual temperature does not exceed 13°C, rainfall has a pronounced west‐east gradient, ranging from 2 000 mm near the Chilean frontier to less than 500 mm near the steppe (50 km towards the east). In this narrow area, forests characteristic to the phytogeographical Subantartic Province occur in altitudinal layers (Cabrera, Citation1971). Forests of Nothofagus spp. prevail in the higher parts of the slopes of the mountains, whereas Austrocedrus chilensis (D. Don) Florin & Boutelje, ‘ciprés de la cordillera’, dominates the forest at lower elevations. Xerophytic groves of Lomatia hirsuta (Lam.) Diles., ‘radal’, and Maytenus boaria Molina, ‘maiten’, grow at an even lower elevation.
In the zone between the forest and the steppe there are mosaics of both formations. Crops are mainly exotic forest species (e.g. Pinus ponderosa Lawson & C. Lawson; Pinus radiata D. Don and Pseudotsuga menziesii (Mirbel) Franco; fodder species (Medicago sativa L., Trifolium spp.) and fruit crops (Fragaria spp., Rubus spp., Ribes spp., Prunus spp., and Juglans regia L.).
The extra‐Andean region includes the territory that extends from the Andes mountains in the west to the Atlantic Ocean. This landscape is mainly formed from volcanic and sedimentary rock, which forms a system of cut plateaus descending towards the river valleys. These tabular plateaus are accompanied by low hills, some closed depressions and glacifluvial plains. The climate is arid and ranges from temperate to cold. Annual average temperatures vary between 7.0 and 13.9°C. Precipitation of 200 mm per year is concentrated during the winter months. The wind is strong and constant. Most of the extra‐Andean region is a part of the phytogeographical Patagonian Province (Cabrera, Citation1971; Soriano, Citation1983). This formation presents different physiognomic types, with the semi‐desert (a low shrubby steppe with a high proportion of exposed soil) being the most extensive type. Low, scattered or cushion‐like shrubs and scarce graminoid plants live here. Some of the characteristic species are: Acaena caespitosa Hook. & Arn, Acantholippia seriphioides (A. Gray) Moldenke ‘tomillo’, Chuquiraga avellanedae Lorentz ‘quilimbay’, Mulinum spinosum (Cav.) Pers. ‘neneo’, Nassauvia spp., Stipa spp. and Poa spp. (Soriano, Citation1983; Roig, Citation1998).
Northeast of Chubut, where temperatures are higher and the wind is less intense, pertains to the Monte phytogeographical Province (Figure ). This province is characterised by an open shrubby steppe of variable height and scarce shrubby cover. The predominant species are ‘jarillas’ (Larrea divaricata Cav., Larrea nitida Cav.). Other commonly found shrubs are: Lycium chilense Bertero ‘llaullín’, Chuquiraga erinacea D. Don. ‘chilladora’, Prosopis alpataco Phil. ‘alpataco’, Ephedra ochreata Miers ‘solupe’, Prosopidastrum globosum (Gillies ex Hook. & Arn.) Burkart ‘manca potrillo’ and Bougainvillea spinosa (Cav.) Heimerl ‘mata negra’. In low places with salty soil there are halophytic species, such as Suaeda divaricata Moq. ‘jume’, Atriplex lampa (Moq.) D. Dietr. ‘zampa’, Cyclolepis genistoides D. Don ‘palo azul’ and Lycium ameghinoi Speg. ‘mata laguna’ (Soriano, Citation1983; León et al., Citation1998; Roig, Citation1998).
Due to the extreme aridity of the extra‐Andean region, agriculture is only developed in the irrigated valleys of the Chubut and Senguerr rivers. Among the crops are fodder species (Medicago sativa, Melilotus spp. and Trifolium spp.), followed in order of importance by horticultural and fruit plants (Malus sylvestris Mill., Pyrus communis L., Prunus spp. and Rubus idaeus L.). Abundant vegetation, consisting mainly of weeds, grows next to the water channels. Malvella leprosa (Ortega) Krapov., a native species widely spread in Argentina (Krapovickas, Citation1988), is plentiful in the salty soils of the irrigated area of the lower Chubut River valley.
About 6 500 producing beehives exist in the Chubut province. They are distributed in three melliferous areas: the Andean region, in the northwest of Chubut, the lower Chubut River valley and the plains of the Senguerr River. In the Andean region are situated 3 000 hives. This area is located in the Subantartic phytogeographical Province, includes forest and transitional areas between forest and steppe vegetation. In the lower Chubut River valley, situated in the southern extreme of the Monte, are 3 000 hives, and 500 in the plains of the Senguerr River, an irrigated area of the steppe, located in the phytogeographical Patagonian Province.
Honey analyses
The pollen content of 140 honey samples produced by A. mellifera was studied. The samples, obtained by centrifugation, were provided by beekeepers between 1995 and 2004. Of the total samples, 62 were from the lower Chubut River valley; 58 from the Andean region, and 20 from the plains of the Senguerr River. The amount of each sample depended on the level of apicultural activity.
Qualitative analysis of the samples was carried out following the method described by Louveaux et al. (Citation1978) slightly modified. A sub‐sample of 20 g of honey was dissolved in 100 ml of distilled water, centrifuged for 10 minutes, washed once with distilled water, centrifuged again and acetolysed. Pollen sediment was mounted in glycerine‐gelatine and sealed with paraffin. The samples were centrifuged at 1500 g (3 000 rpm) (Pendleton, Citation2006). Louveaux's method was followed during the whole sampling period to enable the comparison among results. To determine frequency classes (Louveaux et al., Citation1970) 500 pollen grains were counted.
Pollen types were identified by comparing them with a reference collection that was made from the plants in the area surrounding the beehives. Pollen atlases of Heusser (Citation1971) and Markgraf & D'Antoni (Citation1978) were also consulted. The reference collection was donated to the Palynotheque of the Facultad of Ciencias Naturales of the Universidad Nacional de la Patagonia, San Juan Bosco (Sede Trelew). The herbarium specimens are vouchered in the Herbarium of the Museo Botánico Córdoba (CORD) and Herbarium Trelew (HTW).
The pollen types were identified to species whenever possible and otherwise to genus, tribe or family ranks. Three pollen types were assigned to generalised type: Crepis type (Asteraceae) includes Picris spp., Lactuca spp., and Sonchus spp.; Cressa type (Convolvulaceae‐Cuscutaceae) groupes Cressa truxillensis Kunth and Cuscuta indecora Choisy; and Colletia/Discaria‐type (Rhamnaceae) includes Discaria spp. and Colletia hystrix Clos.
Pollen from fruit crops and naturalised species, such as Rosa rubiginosa L. and Rubus ulmifolius Schott were identified to the family rank (Rosaceae). Similarly, in the Andean region, pollen from native taxa, such as Luma apiculata (DC.) Burret ‘arrayán’, Myrceugenia excsucca (DC.) O. Berg ‘pitra’ and some species of Eucalyptus present in isolated plantations were classified as Myrtaceae.
Pollen types were classified into four categories: predominant pollen (⩾45%), secondary pollen (16–45%), important minor pollen (3–15%) and minor pollen (<3%) (Louveaux et al., Citation1970). When one pollen type represented ⩾45% of the total number of pollen grains, the sample was classified as a monofloral honey (Louveaux et al., Citation1978), samples from Eucalyptus spp. and Medicago sativa were considered monofloral when the presence of pollen were in a proportion higher than 70 and 20% respectively (Maurizio & Louveaux, Citation1961; Serra & Cañas, Citation1988). The frequency occurrence of pollen, expressed as a percentage, was calculated by totalling the number of samples in which a taxon occurs and dividing by the total number of samples per melliferous area.
Quantitative analysis followed Moar's methods (Citation1985) by using tablets of Lycopodium clavatum L. spores (Stockmarr, Citation1971). A subsample of 10 g of honey was dissolved in distilled water, and two Lycopodium clavatum tablets (dissolved in 5 ml of 5% hydrochloric acid) were added, each containing 12 000±200 spores. The sediment was concentrated by repeated centrifugation at 1500 g (3 000 rpm) for 10 minutes, mounted in glycerine‐gelatine, and sealed with paraffin. Spores, pollen, and honeydew elements, were counted up to 500 grains of pollen. Honey was classified (Maurizio, Citation1939) per 10 g of honey: Group I (<20 000 grains), Group II (20 000–100 000 grains), Group III (100 000–500 000 grains), Group IV (500 000–1 000 000 grains), Group V (>1 000 000 grains). The honeydew index (HDE/P), ratio of honeydew elements to pollen grains of nectariferous plants, was calculated (Louveaux et al., Citation1978).
Scanning electron micrographs were made of the most frequent native pollen types. Pollen from herbarium samples were placed onto stubs coated with gold palladium and photographed on JEOL‐JSM T‐100 and LEO EVO 40 XVP scanning electron microscopes.
Results
A total of 139 pollen types, belonging to 53 families were identified from the honey samples (Table ). Of this total 58 were identified to species rank, 59 to generic rank, four to tribe and 15 to family. Pollen from the Asteraceae were mainly represented by Baccharis spp. and Grindelia spp.
The families Asteraceae and Fabaceae were the most represented in the pollen of honey, with 26 and 19 types respectively. Out of the total pollen identified, 128 types were from entomophilous taxa, of which 17 were common in all the producing areas. Brassicaceae, Medicago sativa, Melilotus spp. and Trifolium spp. stood out because they had a frequency of occurrence that exceeded 50% (Table ).
Table I. Pollen types identified in 140 honeys analysed and their frequency classes.
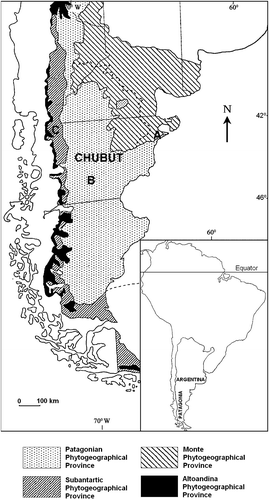
Thirteen types were classified as predominant taxa. They characterised 48% of the studied samples. Twenty‐one (15%) of the honeys were dominated by Tamarix gallica L.; nine (6%) by Rosaceae; eight (6%) by Trifolium spp.; nine (6%) by Melilotus spp.; six (4%) by Medicago sativa; four (3%) by Aristotelia chilensis pollen; three (2%) were dominated by Colletia/Discaria‐type pollen; two (1%) by Escallonia spp.; one (1%) Eucalyptus spp.; one (1%) by Myrtaceae pollen; one (1%) by Phacelia secunda; one (1%) by Prosopidastrum globosum pollen; and one (1 %) by Schinus patagonica (Table , Figure ).
Within each melliferous area the predominant pollen type was very diverse and the Rosaceae was the only common type. In the lower Chubut River valley of the total of 62 samples, 21 (34%) came from Tamarix gallica; six (10%) from Medicago sativa; one (2% each) from Rosaceae, Eucalyptus spp., and Prosopidastrum globosum. In the plains of the Senguerr River, of the 20 samples, nine (45%) were Melilotus spp. and two (10%) Rosaceae. Whereas in the northwest Andean region, eight (16%) out of the 58 samples were Trifolium spp.; six (10%) were Rosaceae; four (7%) Aristotelia chilensis; three (5%) Colletia/Discaria‐type; two (3%) Escallonia spp.; one (2%) ‐ from Myrtaceae, Phacelia secunda and Schinus patagonica each (Figure ).
Among the entomophilous pollen, the native types with an occurrence frequency ⩾50% were: Lycium spp., Larrea spp., Astereae and Malvella leprosa in the lower Chubut River valley; Acaena spp., Astereae and Glycyrrhiza astragalina in the plains of the Senguerr River; and Colletia/Discaria‐type, Aristotelia chilensis, Schinus patagonica, Lomatia hirsuta and Phacelia secunda in the northwest Andean region (Table ).
The most frequent anemophilous type was Plantago spp. This pollen type was followed in order of importance by Chenopodiaceae (in the melliferous areas of the extra‐Andean region) and Nothofagus spp., (in the Andean region) (Table ).
Quantitative analysis of honeys revealed low pollen amounts in honeys from the extra‐Andean region. In the lower Chubut River valley 71% of the samples were classified as Group I (Maurizio's Citation1939 classification), 26% as Group II, and only 3% as Group III. Similarly, in the plains of the Senguerr River 65% of the samples were classified as Group I, 25% as Group II and 10% as Group III. In contrast, honeys from the northwest Andean region were rich in pollen having 56% of the samples classified as Group III, 7% as Group IV, 35% as Group II and 2% as Group I (Figure ).
Figure 2. Honey from Chubut grouped by botanical origin: Multifloral honey – 52%; Monofloral honey – Tamarix gallica (15%), Rosaceae (6%), Trifolium spp. (6%), Melilotus spp. (6%), Medicago sativa (4%), Aristotelia chilensis (3%), Colletia/Discaria (2%), Escallonia spp. (1%), Eucalyptus sp. (1%), Myrtaceae (1%), Phacelia secunda (1%), Prosopidastrum globosum (1%), Schinus patagonica (1%).
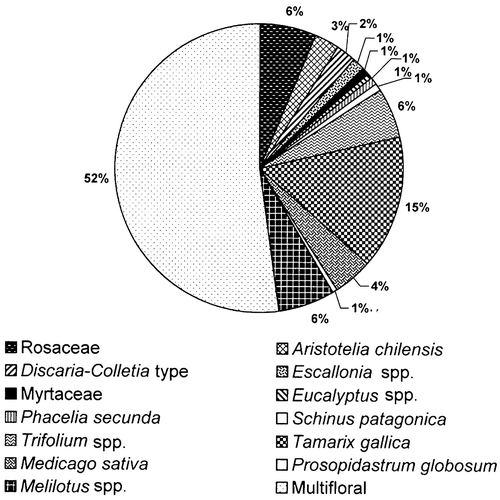
Figure 3. Botanical origin of honeys from Chubut based on geographic regions.Lower Chubut River valley. Multifloral honey – 51.6%; Monofloral honey – Tamarix gallica (34%), Medicago sativa (10%), Rosaceae (2%), Eucalyptus spp., (2%), Prosopidastrum globosum (2%). Plains of the Senguerr River. Multifloral honey – 45%. Monofloral honey – Melilotus spp. (45%), Rosaceae (10%). Northwest Andean region. Multifloral honey – 53%; Monofloral honey – Trifolium spp. (16%). Rosaceae (10%), Aristotelia chilensis (7%), Colletia/Discaria‐type (5%), Escallonia spp. (3%), Phacelia secunda (2%), Myrtaceae (2%), Schinus patagonica (2%).
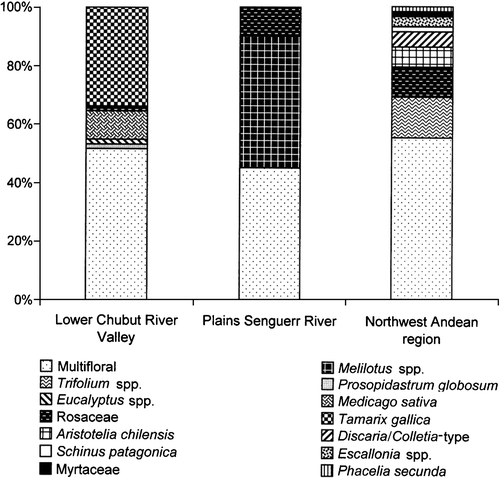
Figure 4. Pollen richness of honey in different melliferous areas of Chubut. Honey grouped by pollen amount in 10 g of sample (Maurizio Citation1939): Group I: <20 000; Group II: 20 000–100 000; Group III: 100 000–500 000; Group IV: 500 000–1 000 000. Lower Chubut River valley: Group I: 71%; Group II: 26%; Group III: 3%. Plains of the Senguerr River: Group I: 65%; Group II: 25%; Group III: 10%. Northwest Andean region: Group I: 2%; Group II: 35%; Group III: 56%; Group IV: 7%.
Honeydew indicators were scarce or absent as the HDE/P ratio was <1 in all the samples. The highest honeydew index value was reached in one sample from the plains of the Senguerr River, where the HDE/P ratio was 0.98.
Discussion
The broad pollen spectrum of honey from Chubut with 139 pollen types shows the large diversity of vegetation in this Argentinean province. Asteraceae and Fabaceae contained here the greatest number of pollen types. The latter family accounts for 35% of monofloral honeys. The melliferous importance of these families has been recognised in Argentina and in other regions of the world (Tellería, Citation1988; Crane, Citation1991; Andrada & Tellería, Citation2002; Fagundez & Caccavari, 2006).
The most important sources of nectar were from cultivated and naturalised plants: Medicago sativa, Melilotus spp., Rosaceae, Tamarix gallica and Trifolium spp. These taxa produce 78% of monofloral honey. However, native plants represented up to 30% of the pollen of the honeys that was analysed. Aristotelia chilensis, Colletia/Discaria‐type, Escallonia spp., Phacelia secunda, Prosopidastrum globosum and Schinus patagonica were found as predominant pollen types.
Honey from the different melliferous areas of Chubut have some characteristics in common. Firstly they share 17 entomophilous pollen types, and secondly they have Rosaceae as the predominant pollen. Finally they have an abundance of pollen from the tribe Trifolieae (Fabaceae). In spite of these similarities, these honeys differ in their composition of predominant pollen and the presence of native communities (Figure ).
Figure 5. Characteristic pollen types of honey from Chubut.A – D. Lower Chubut River valley: (A) Prosopidastrum globosum; (B) Malvella leprosa; (C) Chuquiraga spp.; (D) Lycium chilense. E – G. Plains of the Senguerr River: (E) Acaena sp.; (F) Glycyrrhiza astragalina; (G): Ameghinoa patagonica. H – K. Northwest Andean region: (H) Mutisia spp.; (I, J) Lomatia hirsute; (K) Aristotelia chilensis. Scale bars – 10 µm (A‐I & K); 2 µm (J).
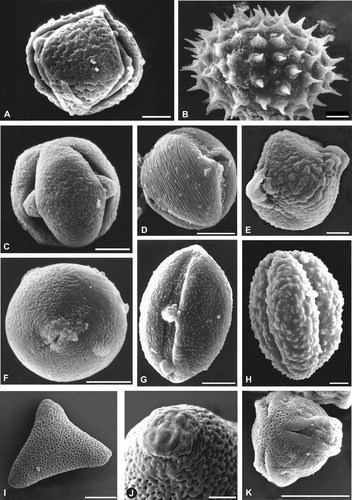
Honey from the lower Chubut River valley are distinguished by the frequent occurrence of pollen types characteristic of the southern Monte and its edaphic communities, such as: Larrea spp., Lycium spp., Chuquiraga spp., Astereae, Prosopidastrum globosum and Prosopis spp. The constant presence of Malvella leprosa is unique to the honey from this area. This halophytic species is widely spread in America (from Mexico to Argentina), but in Chubut it is only found in the eastern part (Krapovickas, Citation1988). Therefore, pollen from Malvella leprosa distinguishes the honey from the lower Chubut River valley. The main nectariferous plants of the lower Chubut River valley are Tamarix gallica and Medicago sativa. Monofloral honey from these two taxa occurred through the whole honey production period (Forcone et al., Citation2003). Other plants that produce monofloral honey are Prosopidastrum globosum and Eucalyptus spp.
The pollen assemblage of the Senguerr River Plains comprised of steppe taxa (Acaena spp., Ameghinoa patagonica, Schinus spp., Senecio spp.) and taxa from the water meadows (Glycyrrhiza astragalina, Cyperaceae). These pollen types together with the abundant pollen from Trifolieae (mainly Melilotus spp.) indicate the origin of these samples. In the Senguerr River Plains Melilotus spp. is the main nectariferous resource, 45% of the honey are monofloral of this genus.
Honeys from the northwest, Andean region of Chubut were represented by taxa from the Subantartic Forest: Nothofagus spp., Lomatia hirsuta, Aristotelia chilensis, Schinus patagonica, Colletia/Discaria‐type, Mutisia spp., and by characteristic plants of the steppe, such as Adesmia spp. and Phacelia secunda. In this region, monofloral honey come from Trifolium spp., Rosaceae, Aristotelia chilensis, Colletia/Discaria‐type, Escallonia spp., Myrtaceae, Phacelia secunda and Schinus patagonica. The major contribution of nectar comes from Trifolium spp. and from Rosaceae.
The pollen richness of the honeys studied varies according to the areas. Low pollen content of honey from the extra‐Andean region may due to contribution of nectar from fodder crops that grow in its irrigated valleys, mainly from Medicago sativa, which is sub‐represented in the pollen content of honey (Louveaux et al., Citation1978). The low honeydew index in all the samples analysed, indicates that nectar from flowers is the main source of honey in Chubut Province (Louveaux et al., Citation1978).
Anemophilous pollen helps to distinguish honey from Chubut. In the melliferous areas of the extra‐Andean region, this type of pollen is abundant; the most frequent types are Plantago spp. and Chenopodiaceae. (Forcone, Citation2003). Whereas in the northwest Andean region, anemophilous pollen is scarce, it is represented mainly by Plantago spp. and Nothofagus spp. (Forcone et al., 2005). The mentioned pollen types have scarce importance in other melliferous regions of Argentina (Tellería, Citation1988; Andrada et al., Citation1998; Andrada & Tellería, Citation2002; Fagúndez & Caccavari, Citation2006).
Seven of the 13 types of monofloral honey that originated in Chubut are new records in Argentina. They include: Aristotelia chilensis; Colletia/Discaria‐type; Escallonia spp.; Phacelia secunda; Prosopidastrum globosum; Rosaceae and Schinus patagonica.
Honey from Tamarix gallica were also frequent in the valley of the Negro River, a melliferous area located in the south of the Monte, 400 km north of Chubut Valley. In contrast, Tamarix gallica honey originating from Chubut did not contain pollen from Capparis atamisquea Kuntze, Cercidium praecox (Ruiz & Pav.) Burkart & Carter, Condalia microphylla Cav., Geoffroea decorticans (Hook. & Arn) Burkart and Monttea aphylla (Miers.) Benth. & Hook. (Tellería & Forcone, Citation2000; Forcone, Citation2003). These results are due to the distribution area of these species, which does not reach the southern limit of the Monte (Roig, Citation1998).
In Argentina, honey from Melilotus spp., Trifolium spp., Medicago sativa, and Eucalyptus spp. are mainly produced in the Pampean phytogeographical Province (Tellería, Citation1988, Citation1992, Citation1996a , b). Honey from Chubut can be differentiated from Pampean honey since they have characteristic pollen associations of the producing areas with lower proportions of Brassicaceae, Helianthus sp. and Lotus sp. All these taxa produce monofloral honey in the Pampean region (Tellería, Citation1988; Citation1996b ; Valle et al., Citation1995). In the honey studied, Brassicaceae has been mainly found as important minor pollen and minor pollen, and it only occurred in four samples as secondary pollen. Whereas Helianthus sp. and Lotus sp. have only been found as minor pollen.
Eucalyptus spp. was registered as predominant pollen in one sample; however, its melliferous importance is secondary compared with the honey from Pampean region, where it frequently occurs as predominant pollen (Telleria 1988, 1992, 1996a; Andrada et al., Citation1998). These results are due to the paucity of Eucalyptus plantations in Argentinean Patagonia.
Honey from the extra‐Andean region of Chubut share some native pollen types with honey from the Espinal phytogeographical Province, such as: Prosopis spp., Schinus spp. and Lycium spp. However, they differ in the lower proportions of Prosopis spp., and in the absence of Condalia microphylla, which are the main sources of nectar in the south of Espinal (Andrada & Tellería, Citation2002).
Conclusions
A diverse spectrum of 139 pollen types from 53 families is present in the honeys from Chubut. The families with a major diversity of morphologic types are Asteraceae and Fabaceae.
Monofloral honey represent 48% of the production, and its main sources are exotic plants: Medicago sativa, Melilotus spp., Rosaceae, Tamarix gallica and Trifolium spp. Native flora occurs as up to 30% in the pollen spectrum of the studied honey, and forms six types of monofloral honey: Prosopidastrum globosum, Colletia/Discaria‐type, Phacelia secunda, Schinus patagonica, Aristotelia chilensis and Escallonia spp. These types were not previously reported in Argentina.
Honey from Chubut can be distinguished from those of other areas of Argentina. Pollen associations from native flora, crops and naturalised plants of the different melliferous areas characterised the honey studied.
Anemophilous pollen contributes to characterise honey from Chubut, in the extra‐Andean region, this type of pollen is abundant, and Plantago spp. and Chenopodiaceae are very frequent types. In contrast, honey from the Andean region has scarse anemophilous pollen; it is represented mainly by Plantago spp. and Nothofagus spp.
The pollen richness of the honey studied varies according to the areas. Honey from the Andean region is remarkably richer in pollen content than those from the extra‐Andean region.
Acknowledgements
I thank Dr María C. Tellería for critically reading the manuscript.
References
- Andrada , A. C. and Tellería , M. C. 2002 . Botanical origin of honey from south of Caldén district (Argentina). . Grana , 41 : 58 – 62 .
- Andrada , A. , Valle , A. , Aramayo , E. and Lamberto , S. 1998 . Espectro polínico de las mieles de la región de Bahía Blanca, Provincia de Buenos Aires, Argentina. . Polen , 9 : 75 – 84 .
- Cabrera , A. L. 1971 . Fitogeografía de la República Argentina. . Bol. Soc. Argent. Bot. , 14 : 1 – 30 .
- Crane , E. 1991 . The plant resources of honeybees. . Apiacta , 26 : 57 – 64 .
- Fagúndez , G. A. and Caccavari , M. A. 2006 . Pollen analysis of honeys from the central zone of the Argentine province of Entre Ríos. . Grana , 45 : 305 – 320 .
- FAO (Food and Agriculture Organization of the United Nations). (2005). www.fao.org .
- Forcone , A. 2003 . Plantas nectaríferas utilizadas por Apis mellifera L. en la Patagonia extra‐andina. . Rev. Mus. Argentino Cien. Nat. , 5 : 363 – 369 .
- Forcone , A. and Tellería , M. C. 1998 . Caracterización palinológica de las mieles del Valle Inferior del Río Chubut (Argentina). . Darwiniana , 36 : 81 – 86 .
- Forcone , A. and Tellería , M. C. 2000 . Caracterización palinológica de las mieles de la llanura del Río Senguerr (Chubut‐Argentina). . Darwiniana , 38 : 267 – 271 .
- Forcone , A. , Bravo , O. and Ayestarán , G. 2003 . Intraannual variations in the pollinic spectrum of honey from the lower valley of the River Chubut (Patagonia, Argentina). . Sp. J. Agricult. Res. , 1 : 29 – 36 .
- Forcone , A. , Ayestarán , G. , Kutschker , A. and García , J. 2005 . Palynological characterization of honeys from the Andean Patagonia (Chubut, Argentina). . Grana , 44 : 202 – 208 .
- Heusser , C. 1971 . Pollen and spores of Chile , Tucson, AZ : Univ. Arizona Press .
- Krapovickas , A. 1988 . “ Malvaceae. ” . In Flora de la Patagonia. P. 5 , Edited by: Correa , M . 126 – 153 . Buenos Aires : INTA . Colecc. Cien. 8
- León , R. , Brand , D. , Collantes , M. , Paruelo , J. M. and Soriano , A. 1998 . Grandes unidades de vegetación de la Patagonia extra andina. . Ecol. Austr. , 8 : 125 – 144 .
- Louveaux , J. , Maurizio , A. and Vorwhol , G. 1970 . Methods of melissopalynology by International Commission for bee Botany or IUBS. . Bee World , 51 : 125 – 138 .
- Louveaux , J. , Maurizio , A. and Vorwhol , G. 1978 . Methods of melissopalynology by International Commission for bee Botany or IUBS. . Bee World , 59 : 139 – 157 .
- Markgraf , V. and D'Antoni , H. 1978 . Pollen flora of Argentina , Tucson, AZ : Univ. Arizona Press .
- Maurizio , A. 1939 . Untersuchungen zur quantitativen Pollenanalyse des Honigs. . Mitt. Geb. Lebensmittelunters. U. Hyg. , 30 : 27 – 69 .
- Maurizio , A. and Louveaux , J. 1961 . Pollens de plantes mellíferes d’Europe. . Pollen Spores , 3 : 219 – 246 .
- Moar , N. T. 1985 . Pollen analysis of New Zealand honey. . N. Zeal. J. Agricult. Res. , 28 : 39 – 70 .
- Pendleton , M. 2006 . Descriptions of melissopalynological methods involving centrifugation should include data for calculating Relative Centrifugal Force (RCF) or should express data in units of RCF or gravities (g). . Grana , 45 : 71 – 72 .
- Roig , F. A. 1998 . “ La vegetación de la Patagonia. ” . In Flora de la Patagonia, P. 1 , Edited by: Correa , M . 48 – 167 . Buenos Aires : INTA . Colecc. Cien. 8
- Serra Bonvehi , J. and Cañas Lloria , S. 1988 . Caratteristiche fisico‐chimiche, composizione e spettro pollinico del miele di Eucalipto (Eucalyptus sp.) prodotto in Spagna. . Apicoltura , 4 : 59 – 81 .
- Soriano , A. 1983 . “ Desert and semidesert of Patagonia. ” . In Temperate deserts and semi‐deserts , Edited by: West , N. E . 423 – 460 . Amsterdam : Elsevier .
- Stockmarr , W. 1971 . Tablets with spores used in absolute pollen analysis. . Pollen Spores , 13 : 615 – 621 .
- Tellería , M. C. 1988 . Analyse pollinique des miels du Nord‐Ouest de la Province de Buenos Aires (République Argentine). . Apidologie , 19 : 275 – 290 .
- Tellería , M. C. 1992 . Caracterización botánica y geográfica de las mieles de la provincia fitogeográfica pampeana (República Argentina) I: Distrito Oriental. . Darwiniana , 31 : 341 – 350 .
- Tellería , M. C. 1996a . Caracterización botánica y geográfica de las mieles de la provincia fitogeográfica pampeana (República Argentina) II Tandilia. . Bol. Soc. Argent. Bot , 32 : 91 – 94 .
- Tellería , M. C. 1996b . Caracterización botánica y geográfica de las mieles de la provincia fitogeográfica pampeana (República Argentina) III: noreste de la Provincia de La Pampa. . Darwiniana , 34 : 245 – 249 .
- Tellería , M. C. and Forcone , A. 2000 . El polen de las mieles del valle de Río Negro, Provincia Fitogeográfica del Monte (Argentina). . Darwiniana , 38 : 273 – 277 .
- Valle , A. F. , Andrada , A. C. , Aramayo , E. M. and Lamberto , S. 1995 . Análisis polínico de las mieles del Sudoeste de la Provincia de Buenos Aires. . Investig. Agrar. Prod. Prot. Veg. , 10 : 375 – 383 .